Abstract
Noise from shipping activity in North Atlantic coastal waters has been steadily increasing and is an area of growing conservation concern, as it has the potential to disrupt the behaviour of marine organisms. This study examines the impacts of ship noise on bottom foraging humpback whales (Megaptera novaeangliae) in the western North Atlantic. Data were collected from 10 foraging whales using non-invasive archival tags that simultaneously recorded underwater movements and the acoustic environment at the whale. Using mixed models, we assess the effects of ship noise on seven parameters of their feeding behaviours. Independent variables included the presence or absence of ship noise and the received level of ship noise at the whale. We found significant effects on foraging, including slower descent rates and fewer side-roll feeding events per dive with increasing ship noise. During 5 of 18 ship passages, dives without side-rolls were observed. These findings indicate that humpback whales on Stellwagen Bank, an area with chronically elevated levels of shipping traffic, significantly change foraging activity when exposed to high levels of ship noise. This measureable reduction in within-dive foraging effort of individual whales could potentially lead to population-level impacts of shipping noise on baleen whale foraging success.
Keywords: anthropogenic noise, humpback whale, foraging
1. Introduction
Increased levels of anthropogenic noise have become a chronic condition in both terrestrial and marine environments [1,2]. Noise pollution has been shown to alter acoustic communication [3], distribution patterns [4] and stress responses [5,6] in a wide range of taxonomic groups. Noise has also been shown to impact foraging behaviours by masking sound produced by prey movement [7], by eliciting an avoidance response or a cessation of foraging [8], or by altering prey behaviour [9]. These wide ranging effects are raising concerns about the impacts of anthropogenic noise on species survival [1,2].
The impact of noise has been a major focus of cetacean research over the past two decades, as whales and dolphins are highly dependent on sound for critical life functions including communication and foraging [10]. Cetaceans are exposed to a variety of anthropogenic noise sources [10] and have been shown to respond in several ways, including physiological and context-dependent changes in behaviour [5,8,10]. Some evidence suggests that odontocetes (toothed whales and dolphins) may alter foraging behaviour in response to noise exposure [11,12]. However, relatively few studies have investigated the effects of ship noise on foraging behaviour in mysticetes (baleen whales). Many mysticetes are found in coastal areas with high levels of ship traffic, resulting in frequent mortalities from collisions [13]. Investigations of foraging blue (Balaenoptera musculus), finback (Balaenoptera physalus) and humpback whales using surface behavioural observations have found no detectable responses to loud low-frequency noise [14,15]. The advent of multi-sensor tags has allowed for exploration of subsurface behaviours of baleen whales [16]. In response to mid-frequency sonar playbacks, blue whales show termination of feeding events at depth while humpbacks demonstrate avoidance behaviours [17,18]. Preliminary evidence suggests that close ship passage might result in decreased foraging time in blue whales [19].
Humpback whales are generalist predators with diverse diets and foraging behaviours intended to aggregate and engulf small numerous prey [20]. One well-described foraging method used by humpbacks in the Gulf of Maine is bottom side-roll feeding on sand lance (Ammodytes spp.) near the sea floor, particularly during night-time hours [21]. Humpbacks have demonstrated flexible acoustic behaviour in the presence of noise [3,22,23], but few studies have investigated noise effects on their subsurface foraging behaviours. Here, we use subsurface tag data to assess the impacts of shipping noise on this foraging behaviour.
2. Methods
(a). Data collection and analysis
Field data were collected in the southern Gulf of Maine on and around the Stellwagen Bank National Marine Sanctuary (Massachusetts, USA) in June–July from 2006 to 2009 and April 2009. Data were collected using archival digital acoustic recording tags, Dtags [16], to simultaneously record the whale's three-dimensional behaviours and the acoustic environment. Details on field data collection methods are documented in Friedlaender et al. [24].
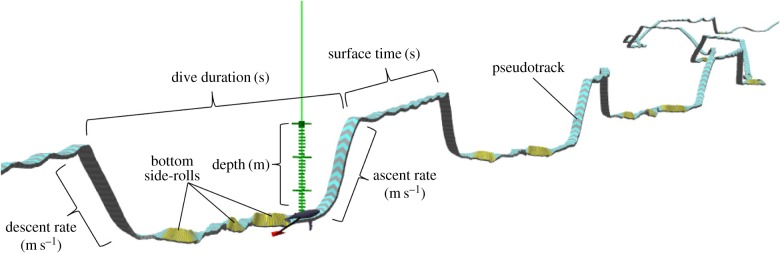
Ten tag deployments were included in the analyses (electronic supplementary material, table S1). These tag records contained data between 1 h after sunset to 1 h before sunrise and included the passage of at least one large ship on the acoustic record. Two of the deployments came from the same individual in different years. A total of 218 dives were analysed: 83 occurred in ship noise exposure periods while 135 occurred with no ship noise. Subsurface behaviours were quantified using the software application TrackPlot (figure 1) [25]. Seven behavioural measurements were extracted from each dive for use as dependent variables in the models: duration, rate of descent and ascent, maximum depth, number of bottom side-roll feeding events, time between dives and surface time immediately following each dive (figure 1).
Figure 1.
TrackPlot still image demonstrating ribbon track and dive measurements for one bottom-feeding dive.
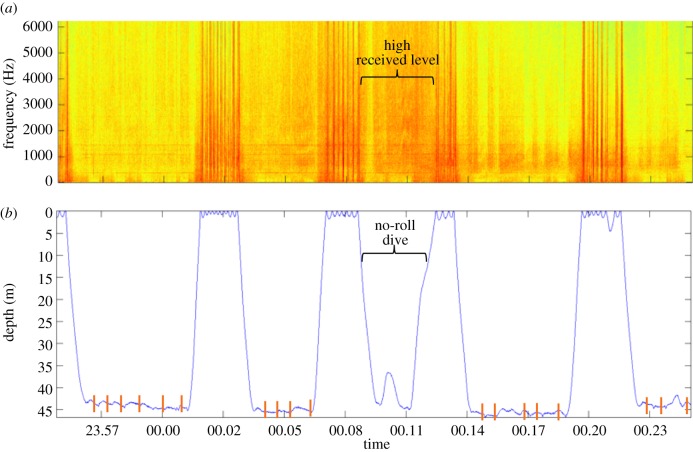
The ship presence was determined through visual and aural detection of ship noise in Dtag audio recordings (figure 2a). To minimize effects of flow noise, we measured the received level (RL) of ship noise within the 2–3 kHz frequency band for a 1 min period during the bottom time of each dive (RavenPro v. 1.5) (electronic supplementary material).
Figure 2.
(a) Spectrogram and (b) depth profile of whale mn08_182a demonstrating atypical dive in the presence of ship noise. Orange tick marks indicate bottom side-rolls.
(b). Statistics
To test whether the ship noise altered bottom foraging behaviours, we performed linear mixed-effects models using the seven dive metrics as dependent variables. Dependent variable data were square root transformed to approximate normality. The two fixed effects included the RL and the presence/absence of ship noise in each dive (SN), with tag deployment as a random effect. Best model fit was evaluated using Akaike's Information Criterion corrected for small sample sizes (AICc) [26]. Variable importance values were calculated by summing Akaike weights (wi) of all models including a particular variable (electronic supplementary material) [26]. All statistical analyses were performed in R (v. 2.15.3).
3. Results
The presence of ship noise significantly affected three of the seven dependent variable metrics tested (table 1; electronic supplementary material, table S2). In the best-fit models, as RL increased, the number of rolls decreased by 29% (t = −2.12, d.f. = 207, p-value = 0.04) (electronic supplementary material, figure S1a), and the descent rate decreased by 14.5% (t = −4.17, d.f. = 207, p-value < 0.01) (electronic supplementary material, figure S1b). Ascent rate also decreased by 12.8% as RL increased (t = −2.40, d.f. = 206, p-value = 0.02); however, ascent rate was faster by 0.002 m s−1 during SN exposure periods than in periods of no ship noise (t = 2.07, d.f. = 206, p-value = 0.04). The interaction of RL and SN was positive but non-significant (t = 0.28, d.f. = 205, p-value = 0.78). RL was the most important variable influencing all three response variables (electronic supplementary material, table S3).
Table 1.
Model coefficients, standard errors, t-values and p-values for best-fit models for descent rate, ascent rate and number of rolls.
| response | model | variables | estimate | s.e. | t-value | p-value |
|---|---|---|---|---|---|---|
| descent rate | RL | intercept | 1.558 | 0.127 | 12.271 | <0.01 |
| RL | −0.006 | 0.001 | −4.171 | <0.01 | ||
| RL, SN | intercept | 1.624 | 0.148 | 10.968 | <0.01 | |
| RL | −0.007 | 0.002 | −3.395 | <0.01 | ||
| SN | 0.013 | 0.019 | 0.858 | 0.392 | ||
| ascent rate | RL, SN | intercept | 1.474 | 0.186 | 7.910 | <0.01 |
| RL | −0.005 | 0.002 | −2.400 | 0.017 | ||
| SN | 0.040 | 0.019 | 2.069 | 0.040 | ||
| RL×SN | intercept | 1.528 | 0.267 | 5.723 | <0.01 | |
| RL | −0.006 | 0.003 | −1.847 | 0.066 | ||
| SN | −0.053 | 0.328 | −0.162 | 0.872 | ||
| RL×SN | 0.001 | 0.004 | 0.283 | 0.778 | ||
| number of rolls | RL | intercept | 3.413 | 0.752 | 4.541 | <0.01 |
| RL | −0.018 | 0.009 | −2.119 | 0.035 | ||
| RL, SN | intercept | 3.984 | 0.879 | 4.531 | <0.01 | |
| RL | 0.025 | 0.010 | −2.457 | 0.015 | ||
| SN | 0.114 | 0.094 | 1.208 | 0.228 |
In five out of nine individuals, one or more dives without bottom side-rolls occurred in the presence of ship noise. All of these responding individuals were adult females: two with their dependent calf, one pregnant and two who were neither pregnant nor lactating. The individual with two deployments showed this response in 2009, but not in 2006. These dives lacked bottom-feeding side-rolls despite a maximum depth near that of usual feeding dives immediately before and after, though some no-roll dives were shallower than the surrounding dives (figure 2). Whales did not demonstrate bottom side-rolls in 11 out of 218 dives. Of the 11 no-roll dives, seven occurred in ship noise exposure periods (7/83) and only four without ship noise (4/135). A McNemar's test with continuity correction indicates that the percentage of dives with no rolls significantly differed in periods of ship noise exposure versus periods of no ship noise (McNemar's χ2 (1, N = 218) = 109.63, p-value < 0.01). Five of 18 ship passages resulted in a no-roll dive. Whales did not compensate by increasing side-rolls following ship passage (Wilcoxon test, p-value > 0.05).
4. Discussion
While numerous studies have demonstrated modifications of acoustic communication in cetaceans exposed to noise, few have assessed changes in baleen whale foraging behaviour. We show that humpback whales decrease the number of bottom-feeding events per dive and reduce feeding dive descent rate as the intensity of ship noise increases, indicating that ship noise can impact foraging rates and efficiency. Ship passages were also correlated with dives without bottom side-rolls, which implies either a cessation of feeding or a switch from bottom side-roll feeding to another method. Our results provide some of the first evidence to show statistically significant alterations in baleen whale foraging behaviour from ship noise exposure.
There are several potential explanations for the observed results. Whales may modify their diving behaviour in response to a perceived threat from ship noise, given that they require surface access to breathe. Ship noise could also affect prey behaviour; in the Gulf of Maine, sand lance seek refuge into sandy seafloor substrate in response to disturbance [24]. If sand lance retreat into substrate in increased ship noise, this could affect the prey availability for foraging whales. Further, if whales coordinate bottom feeding using paired burst vocalizations, which are within the same low-frequency band as ship noise [27], ship noise could cause masking that further reduces foraging efficiency.
Given the adaptability of humpback whales [23,24], we expect the Gulf of Maine population to potentially show habituation to human disturbance from ship noise, as they have been regularly exposed to commercial and whale watching vessels for decades [28]. Therefore, it is especially interesting that alterations to foraging behaviours were detectable in this study, as it suggests that humpbacks are unable to completely adjust to this disturbance. Short and potentially chronic cessations of feeding can result in biologically relevant decreases in balaenopterid foraging efficiency [17], which could manifest to decrease fitness. These behavioural changes were also observed at night when there are fewer ship interactions compared with the daytime hours based on acoustic records; therefore, our results likely reflect the lower limit of disturbance of ship noise on foraging behaviour over the course of 24 h. Humpback whales forage during both day and night, albeit with different strategies dependent on prey behaviour, and each time period is likely critical to help satisfy their large energetic demands [17,24]. Further research on the impacts of noise on daytime foraging activities and variation in the sensitivity to different age and sex classes is needed, as mother–calf pairs are often more sensitive to disturbance [3,10]. Yet, these results are among the first support that ship noise can impact humpback whales’ foraging, making this source of disturbance a management concern. Chronic impacts of even small reductions in foraging efficiency could affect individual fitness and translate to population-level effects on humpback whales exposed to ship noise in critical foraging areas.
Supplementary Material
Acknowledgements
We thank the crew of the research vessels Nancy Foster and Auk for field support, and Jason Fridley and Sam Denes for analysis assistance.
Ethics
All animal use was conducted in accordance with institutional guidelines under IACUC approval at Duke University, the Pennsylvania State University and Syracuse University. Permission was obtained through federal research no. 775-185 (Northeast Fisheries Science Center) and 605-1904 (Whale Center of New England) issued by the United States National Marine Fisheries Service.
Data accessibility
We have deposited the data analysed in this study with the external repository Dryad: http://dx.doi.org/10.5061/dryad.18637 [29].
Authors' contributions
H.B.B. and S.E.P. designed the study; A.S.F., D.N.W. and S.E.P. collected the data; and all authors participated in the data analysis and manuscript preparation. All authors approved the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This research was supported by funding from the Office of Naval Research and the Stellwagen Bank National Marine Sanctuary.
References
- 1.Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. 2010. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 25, 419–427. ( 10.1016/j.tree.2010.04.005) [DOI] [PubMed] [Google Scholar]
- 2.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313. ( 10.1890/120183) [DOI] [Google Scholar]
- 3.Dunlop RA, Cato DH, Noad MJ. 2010. Your attention please: increasing ambient noise levels elicits a change in communication behaviour in humpback whales (Megaptera novaeangliae). Proc. R. Soc. B 277, 2521–2529. ( 10.1098/rspb.2009.2319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mcclure CJW, Ware HE, Carlisle J, Kaltenecker G, Barber JR. 2013. An experimental investigation into the effects of traffic noise on distributions of birds: avoiding the phantom road. Proc. R. Soc. B 280, 20132290 ( 10.1098/rspb.2013.2290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolland RM, Parks SE, Hunt KE, Castellote M, Corkeron PJ, Nowacek DP, Wasser SK, Kraus SD. 2012. Evidence that ship noise increases stress in right whales. Proc. R. Soc. B 279, 2363–2368. ( 10.1098/rspb.2011.2429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tennessen JB, Parks SE, Langkilde T. 2014. Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs. Conserv. Physiol. 2, 1–8. ( 10.1093/conphys/cou032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siemers BM, Schaub A. 2011. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B 278, 1646–1652. ( 10.1098/rspb.2010.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isojunno S, Curé C, Kvadsheim PH, Lam FPA, Tyack PL, Wensveen PJ, Miller PJOM. 2016. Sperm whales reduce foraging effort during exposure to 1–2 kHz sonar and killer whale sounds. Ecol. Appl. 26, 77–93. ( 10.1890/15-0040) [DOI] [PubMed] [Google Scholar]
- 9.Chan AAY-H, Giraldo-Perez P, Smith S, Blumstein DT. 2010. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461. ( 10.1098/rsbl.2009.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowacek DP, Thorne LH, Johnston DW, Tyack PL. 2007. Responses of cetaceans to anthropogenic noise. Mamm. Rev. 37, 81–115. ( 10.1111/j.1365-2907.2007.00104.x) [DOI] [Google Scholar]
- 11.Miller PJO, Johnson MP, Madsen PT, Biassoni N, Quero M, Tyack PL. 2009. Using at-sea experiments to study the effects of airguns on the foraging behavior of sperm whales in the Gulf of Mexico. Deep. Res. Part I Oceanogr. Res. Pap. 56, 1168–1181. ( 10.1016/j.dsr.2009.02.008) [DOI] [Google Scholar]
- 12.Williams R, Lusseau D, Hammond PS. 2006. Estimating relative energetic costs of human disturbance to killer whales (Orcinus orca). Biol. Conserv. 133, 301–311. ( 10.1016/j.biocon.2006.06.010) [DOI] [Google Scholar]
- 13.Laist DW, Knowlton AR, Mead JG, Collet AS, Podestà M. 2001. Collisions between ships and whales. Mar. Mammal Sci. 17, 35–75. ( 10.1111/j.1748-7692.2001.tb00980.x) [DOI] [Google Scholar]
- 14.Croll DA, Clark CW, Calambokidis J, Ellison WT, Tershy BR. 2001. Effect of anthropogenic low-frequency noise on the foraging ecology of Balaenoptera whales. Anim. Conserv. 4, 13–27. ( 10.1017/S1367943001001020) [DOI] [Google Scholar]
- 15.Todd S, Stevick P, Lien J, Marques F, Ketten D. 1996. Behavioural effects of exposure to underwater explosions in humpback whales (Megaptera novaeangliae). Can. J. Zool. 74, 1661–1672. [Google Scholar]
- 16.Johnson MP, Tyack PL. 2003. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean. Eng. 28, 3–12. ( 10.1109/JOE.2002.808212) [DOI] [Google Scholar]
- 17.Goldbogen JA, et al. 2013. Blue whales respond to simulated mid-frequency military sonar. Proc. R. Soc. B 280, 20130657 ( 10.1098/rspb.2013.0657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sivle LD, et al. 2015. Severity of expert-identified behavioural responses of humpback whale, minke whale, and northern bottlenose whale to naval sonar. Aquat. Mamm. 41, 469–502. ( 10.1578/AM.41.4.2015.469) [DOI] [Google Scholar]
- 19.McKenna MF. 2011. Blue whale response to underwater noise from commercial ships. UC San Diego: Oceanography, b7068914. See https://escholarship.org/uc/item/4rv0q1mv.
- 20.Jurasz CM, Jurasz VP. 1979. Feeding modes of the humpback whale, Megaptera novaeangliae, in southeast Alaska. Sci. Rep. Whales Res. Inst. 31, 69–83. [Google Scholar]
- 21.Ware C, et al. 2014. Bottom side-roll feeding by humpback whales (Megaptera novaeangliae) in the southern Gulf of Maine, U.S.A. Mar. Mammal Sci. 30, 494–511. ( 10.1111/mms.12053) [DOI] [Google Scholar]
- 22.Miller PJ, Biassoni N, Samuels A, Tyack PL. 2000. Whale songs lengthen in response to sonar. Nature 405, 903 ( 10.1038/35016148) [DOI] [PubMed] [Google Scholar]
- 23.Dunlop RA. 2016. The effect of vessel noise on humpback whale, Megaptera novaeangliae, communication behaviour. Anim. Behav. 111, 13–21. ( 10.1016/j.anbehav.2015.10.002) [DOI] [Google Scholar]
- 24.Friedlaender AS, Hazen EL, Nowacek DP, Halpin PN, Ware C, Weinrich MT, Hurst T, Wiley D. 2009. Diel changes in humpback whale Megaptera novaeangliae feeding behavior in response to sand lance Ammodytes spp. behavior and distribution. Mar. Ecol. Prog. Ser. 395, 91–100. ( 10.3354/meps08003) [DOI] [Google Scholar]
- 25.Ware C, Arsenault R, Plumlee M, Wiley D. 2006. Visualizing the underwater behavior of humpback whales. IEEE Comput. Graph. Appl. 26, 14–18. ( 10.1109/MCG.2006.93) [DOI] [PubMed] [Google Scholar]
- 26.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer-Verlag. [Google Scholar]
- 27.Parks SE, Cusano DA, Stimpert AK, Weinrich MT, Friedlaender AS, Wiley DN. 2014. Evidence for acoustic communication among bottom foraging humpback whales. Sci. Rep. 4, 7508 ( 10.1038/srep07508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatch L, Clark C, Merrick R, Van Parijs S, Ponirakis D, Schwehr K, Thompson M, Wiley D. 2008. Characterizing the relative contributions of large vessels to total ocean noise fields: a case study using the Gerry E. Studds Stellwagen Bank National Marine Sanctuary. Environ. Manage. 42, 735–752. ( 10.1007/s00267-008-9169-4) [DOI] [PubMed] [Google Scholar]
- 29.Blair H, Merchant N, Friedlaender A, Wiley DN, Parks SE. 2016. Data from: Evidence for ship noise impacts on humpback whale foraging behaviour. Dryad Digital Repository: 10.5061/dryad.18637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We have deposited the data analysed in this study with the external repository Dryad: http://dx.doi.org/10.5061/dryad.18637 [29].