Abstract
Upwelling regions are highly productive habitats targeted by wide-ranging marine predators and industrial fisheries. In this study, we track the migratory movements of eight seabird species from across the Atlantic; quantify overlap with the Canary Current Large Marine Ecosystem (CCLME) and determine the habitat characteristics that drive this association. Our results indicate the CCLME is a biodiversity hotspot for migratory seabirds; all tracked species and more than 70% of individuals used this upwelling region. Relative species richness peaked in areas where sea surface temperature averaged between 15 and 20°C, and correlated positively with chlorophyll a, revealing the optimum conditions driving bottom-up trophic effects for seabirds. Marine vertebrates are not confined by international boundaries, making conservation challenging. However, by linking diversity to ocean productivity, our research reveals the significance of the CCLME for seabird populations from across the Atlantic, making it a priority for conservation action.
Keywords: biologging, human impacts, marine protected areas, migration, upwelling, marine conservation
1. Introduction
Upwelling regions are globally important marine biodiversity hotspots. The mixing of nutrient-rich cool water with warm surface layers fuels primary production, driving bottom-up cascades that also support large communities of upper trophic-level consumers [1]. As a result, they are attractive foraging grounds targeted by a wide-range of marine animals throughout the annual cycle [2]. These characteristics make upwelling regions strong candidates for protection, but this is challenging as they often cross national boundaries, occur in international waters and protection may conflict with fisheries interests [3].
Marine environments are facing unprecedented levels of anthropogenic-driven pressure; including climate change, pollution and offshore development [4–6]. The foremost threat to upwelling regions is biodiversity loss through overfishing; upwellings cover less than 1% of the world's ocean by area but provide approximately 20% of global catch [7]. Commercial capture fisheries deplete stocks, remove top-predators through bycatch, and alter the trophic structure of ecosystems [8,9]. The Canary Current Large Marine Ecosystem (CCLME) now incorporates one of the most intensively fished areas on the Earth [8,10], yet also supports large populations of migratory marine vertebrates from breeding populations across the Atlantic [11–13].
Considering the increasing industrialization of fisheries [10], the pervasive threat from bycatch [14] and a paucity of quantitative information on habitat or space use, understanding marine vertebrate distributions in the CCLME and beyond is a key conservation goal [15]. In this study, we use miniaturized light loggers to reconstruct the non-breeding movements of eight migratory seabird species from disparate regions of the Atlantic that have been previously recorded in the CCLME [12]. Our aims are: (i) to map the distribution of these birds and identify areas of high diversity, (ii) to quantify the extent to which each species uses the CCLME, and (iii) to determine the oceanographic characteristics that drive this association. We use our findings to assess the importance of the CCLME as a biodiversity hotspot and discuss the potential conflict between fisheries and seabirds in this region.
2. Material and methods
We collated data on the non-breeding movements of eight seabird species; Cory's shearwaters (Calonectris borealis); Scopoli's shearwaters (C. diomedea); lesser black-backed gulls (Larus fuscus); northern gannets (Morus bassanus); great skuas (Stercorarius skua); south polar skuas (S. maccormicki); common terns (Sterna hirundo) and Sabine's gulls (Xema sabini). While these species have been recorded previously in the CCLME, the true importance of this region for specific populations is unknown. Between 2000 and 2011, 123 birds were tracked using miniaturized light loggers from 12 breeding colonies from the north (75° N) to the south (62° S) of the Atlantic (see the electronic supplementary material). To quantify the extent to which each species uses the CCLME, we calculated the proportion of time each individual spent in this region [16]. To identify areas of high species richness we constructed spatial density maps by binning location data into 200 km diameter tessellated hexagons spanning the Atlantic. We calculated relative richness by summing the number of species occurring in each hexagon during the non-breeding period.
To characterize the marine environment, we extracted winter seasonal climatology composites (December–March, 2002–2010) of sea surface temperature (SST, °C) and chlorophyll a concentration (CHL, mg m−3) from the MODIS instrument onboard the Aqua (EOS PM) satellite (http://oceancolor.gsfc.nasa.gov/) and calculated mean SST and CHL values for each hexagon. We also included a measure of null usage that incorporated both habitat availability and sampling effort, as this was not uniform across species or colonies [17] (see the electronic supplementary material). These data are available via Dryad [18].
We examined correlations between the observed patterns in relative richness and these covariates using generalized additive models fitted with the packages mgcv [19] and MuMIn [20] in R v. 3.1.0 [21]. We log10 transformed CHL prior to use. We included SST, CHL and null usage as covariates in the global model with thin plate regression splines fitted with a maximum of 10 knots; superfluous knots were penalized during model fitting. Variance inflation factors revealed no multicollinearity between covariates (VIF < 3). We also included the central X and Y coordinates of each hexagon as a spatial smooth term implemented with a soap film boundary [22]. The soap film specifies the extent of the predicted surface, preventing smoothing across boundary features such as the Iberian Peninsula. Variograms of model residuals revealed no spatial autocorrelation in final models. Model selection was based on Akaike's information criterion (AIC), with parameters excluded if their inclusion did not improve the model by more than 2 ΔAIC relative to the lowest AIC.
3. Results
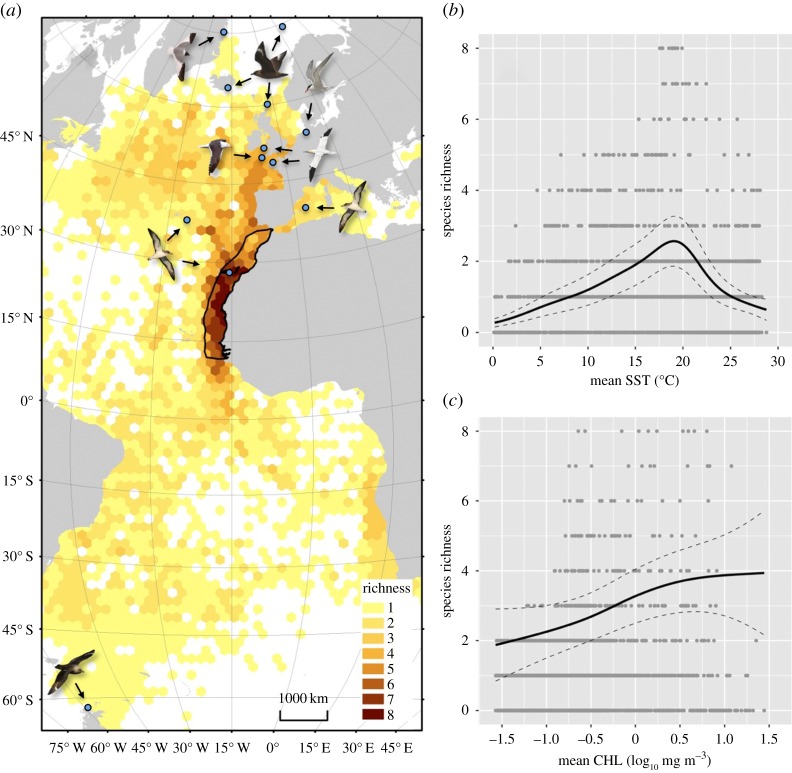
The eight species tracked from 12 colonies over 10 years were widely distributed across the Atlantic during the non-breeding period (figure 1). Highest relative richness was observed in the CCLME, with other hotspots in the Bay of Biscay, Mid-Atlantic Ridge, Brazilian coast and Benguela Current (figure 1). On average, 76.6 ± 28.1% of individuals from each species visited the CCLME, including all Scopoli's shearwaters, Sabine's gulls, south polar skuas and common terns, the majority of lesser black-backed gulls and northern gannets, but only 25% of great skuas (table 1). The proportion of time each species spent in the CCLME was highest for Scopoli's shearwaters (0.35 ± 0.28), northern gannets (0.26 ± 0.29) and common terns (0.24 ± 0.22). There was a high degree of variation both within and among species; individuals may use the CCLME for the entire non-breeding period, only as a staging area, or not at all (table 1).
Figure 1.
Links between (a) relative richness of eight seabird species tracked from pan-Atlantic colonies between 2000 and 2011; and (b) sea surface temperature (SST) and (c) chlorophyll a (CHL). Dark line in (a) represents the boundary of the Canary Current Large Marine Ecosystem; blue dots represent colonies of origin for tracked birds, indicated by arrows. Dark lines in (b) and (c) represent model-estimated response; dashed lines 95% confidence interval; light grey dots indicate the distribution of data. (Online version in colour.)
Table 1.
Summary statistics for tracking by seabird species. (Values represent mean ± s.d. For full methods and description of winter period, see the electronic supplementary material.)
| species | N | % visiting CCLME | winter period (days) | no. locations | locations in CCLME | proportion in CCLME |
|---|---|---|---|---|---|---|
| lesser black-backed gull | 7 | 71.4 | 208.4 ± 27.4 | 151.1 ± 20.8 | 21.0 ± 24.0 | 0.09 ± 0.10 |
| northern gannet | 34 | 58.8 | 93.6 ± 13.4 | 89.6 ± 15.4 | 25.7 ± 29.8 | 0.26 ± 0.29 |
| great skua | 16 | 25 | 92 | 91.9 ± 0.4 | 11.1 ± 21.8 | 0.12 ± 0.24 |
| Cory's shearwater | 19 | 57.9 | 133.9 ± 29.9 | 131.5 ± 27.4 | 13.8 ± 23.2 | 0.10 ± 0.18 |
| Scopoli's shearwater | 9 | 100 | 104.5 ± 40.8 | 102.3 ± 39.6 | 35.4 ± 36.9 | 0.35 ± 0.28 |
| Sabine's gull | 7 | 100 | 287.9 ± 12.7 | 228.6 ± 18.6 | 22.3 ± 3.1 | 0.08 ± 0.01 |
| south polar skua | 19 | 100 | 237.2 ± 35.1 | 176.3 ± 21.6 | 8.7 ± 14.3 | 0.04 ± 0.07 |
| common tern | 12 | 100 | 254.3 ± 67.0 | 181.3 ± 64.0 | 62.8 ± 51.8 | 0.24 ± 0.22 |
Relative richness correlated with SST and CHL; both terms were retained in the top-ranked model along with the soap film smooth term and measure of null usage (table 2). Model-estimates indicated relative richness was highest in areas with SST between 15 and 20°C, and there was a general positive correlation between relative richness and CHL (figure 1).
Table 2.
Model selection testing correlations between relative richness and sea surface temperature (SST) and chlorophyll a (CHL). (The full model included a soap film smooth term (XY) and measure of habitat availability (null). Models shown are those within 6 ΔAIC of the best-supported model. Adj R2 of best-supported model = 0.60.)
| rank | parameters | d.f. | AIC | ΔAIC |
|---|---|---|---|---|
| 1 | SST + CHL + XY + null | 173 | 5573 | 0.00 |
| 2 | SST + XY + null | 175 | 5576 | 2.84 |
| 3 | SST + CHL + XY | 171 | 5578 | 4.47 |
| 4 | SST + XY | 173 | 5579 | 6.28 |
4. Discussion
Here, we demonstrate that the CCLME is an area of high relative species richness for non-breeding seabirds, and detail the environmental conditions that drive this association. More than 70% of individuals from eight species, representing a range of functional groups and originating from breeding colonies across the Atlantic, visited this upwelling region. Relative richness correlated with both SST and CHL. By tracking birds of known origin, our results illustrate the high connectivity between seabird breeding populations across the Atlantic and the CCLME, emphasizing the importance of this upwelling region as a non-breeding destination and migratory stopover site.
This study represents the most comprehensive collation of tracking data for the CCLME to date, but our measure of relative species richness is limited to those populations included in the study. While many other species also visit this region [12], modern developments in biologging are revealing a diversity of migration strategies [23] and highlighting other important areas across the Atlantic. Our measure of relative species richness represents the maximum across the study period and is likely to vary over the annual cycle in response to seasonal differences in environmental conditions. For example, Southern Hemisphere migrants following the austral summer overlap only briefly with Northern Hemisphere migrants in the CCLME (electronic supplementary material, table S1). While our study highlights the CCLME as a hotspot for migratory seabirds, further work is required to understand the significance of other areas across the Atlantic and beyond.
These findings provide evidence of the links between biodiversity and ocean productivity in an eastern boundary upwelling region. Relative richness was highest between 15 and 20°C, and correlated positively with CHL; corroborating previous work on the oceanographic drivers of marine predator diversity in the California Current [2]. This suggests that primary productivity in the CCLME has bottom-up effects that are highly relevant to apex predators. The mechanisms by which animals may target these regions are currently unknown, but frontal density in the CCLME is high and these visible indicators of productivity are known to aggregate marine predators such as seabirds [11,24].
The CCLME attracts some of the highest global fishing effort [8,10], yet there is a paucity of information on the interactions between seabirds and fisheries in this region [15]. Fisheries impact seabirds in three ways; either competing directly for fish, providing food in the form of discarded fish, or posing the threat of bycatch mortality [14,25,26]. More research into fine-scale, species-specific fisheries interactions in the CCLME is required, especially given recent evidence of direct take of seabirds in the region (K. Camphuysen 2013, personal communication); the substantial under-reporting of catch in this area by China's distant-water fleet [27]; and the prevalence of illegal, unreported and unregulated fisheries [28].
Integrating data across multiple species and years highlights the importance of the CCLME as a seabird biodiversity hotspot. Furthermore, environmental conditions such as SST and productivity may offer insights into how distributions could shift in response to global climate change. As marine vertebrates forage across dynamic pelagic systems and are not confined by international boundaries, effective conservation will require multilateral cooperation. Nevertheless, while site fidelity to persistent upwelling regions such as the CCLME could aid conservation, it is unlikely that both a large diversity of marine vertebrates and intense fisheries exploitation can be sustained in this region in the long term.
Supplementary Material
Acknowledgements
We thank all those who supported fieldwork logistics and granted permission for work. Four anonymous reviewers provided comments that greatly improved this manuscript.
Ethics
All animal research was conducted in accordance with relevant in country protocols.
Data accessibility
The species richness and environmental covariate data underlying this study are archived in the Dryad digital repository at http://dx.doi.org/10.5061/dryad.01sd0. Individual species data are archived by BirdLife International (www.seabirdtracking.org).
Authors' contributions
W.J.G. conceived the study, conducted the data analysis and drafted the manuscript; S.C.V. supervised the project; all authors provided data, contributed to article drafts, approved the final version of this manuscript and agree to be held accountable for the content.
Competing interests
The authors declare no competing interests.
Funding
Funding for this work was provided by the Peninsula Research Institute for Marine Renewable Energy, EU INTERREG project CHARM III, NERC (NE/G001014/1), MINECO CGL2013-42585-P, Defra's Darwin, Centre National de la Recherche Scientifique and Ligue Pour la Protection des Oiseaux within the EUINTERREG Project FAME (2009-1/089; 2010-2012).
References
- 1.Mann KH, Lazier JRN. 2005. Dynamics of marine ecosystems, 3rd edn Malden, MA: Blackwell Publishing Ltd; ( 10.1002/9781118687901) [DOI] [Google Scholar]
- 2.Block BA, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. ( 10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 3.Hyrenbach KD, Forney KA, Dayton PK. 2000. Marine protected areas and ocean basin management. Aquat. Conserv. Freshw. Ecosyst. 10, 437–458. () [DOI] [Google Scholar]
- 4.Islam MS, Tanaka M. 2004. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. Pollut. Bull. 48, 624–649. ( 10.1016/j.marpolbul.2003.12.004) [DOI] [PubMed] [Google Scholar]
- 5.Inger R, et al. 2009. Marine renewable energy: potential benefits to biodiversity? An urgent call for research. J. Appl. Ecol. 46, 1145–1153. ( 10.1111/j.1365-2664.2009.01697.x) [DOI] [Google Scholar]
- 6.Sydeman WJ, Poloczanska E, Reed TE, Thompson SA. 2015. Climate change and marine vertebrates. Science 350, 772–777. ( 10.1126/science.aac9874) [DOI] [PubMed] [Google Scholar]
- 7.Pauly D, Christensen V. 1995. Primary production required to sustain global fisheries. Nature 374, 255–257. ( 10.1038/374255a0) [DOI] [Google Scholar]
- 8.Laurans M, Gascuel D, Chassot E, Thiam D. 2004. Changes in the trophic structure of fish demersal communities in West Africa in the three last decades. Aquat. Living Resour. 17, 163–173. ( 10.1051/alr:2004023) [DOI] [Google Scholar]
- 9.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F Jr. 1998. Fishing down marine food webs. Science 279, 860–863. ( 10.1126/science.279.5352.860) [DOI] [PubMed] [Google Scholar]
- 10.Worm B, et al. 2009. Rebuilding global fisheries. Science 325, 578–585. ( 10.1126/science.1173146) [DOI] [PubMed] [Google Scholar]
- 11.Wynn RB, Knefelkamp B. 2004. Seabird distribution and oceanic upwelling off northwest Africa. Br. Birds 97, 323–335. [Google Scholar]
- 12.Camphuysen KCJ, van der Meer J. 2005. Wintering seabirds in West Africa: foraging hotspots off Western Sahara and Mauritania driven by upwelling and fisheries. Afr. J. Mar. Sci. 27, 427–437. ( 10.2989/18142320509504101) [DOI] [Google Scholar]
- 13.Eckert SA. 2006. High-use oceanic areas for Atlantic leatherback sea turtles (Dermochelys coriacea) as identified using satellite telemetered location and dive information. Mar. Biol. 149, 1257–1267. ( 10.1007/s00227-006-0262-z) [DOI] [Google Scholar]
- 14.Lewison RL, Crowder LB, Read AJ, Freeman SA. 2004. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. ( 10.1016/j.tree.2004.09.004) [DOI] [Google Scholar]
- 15.Grémillet D, Peron C, Provost P, Lescroël A. 2015. Adult and juvenile European seabirds at risk from marine plundering off West Africa. Biol. Conserv. 182, 143–147. ( 10.1016/j.biocon.2014.12.001) [DOI] [Google Scholar]
- 16.Claus S, De Hauwere N, Vanhoorne B, Souza Dias F, Hernandez F, Mees J, orpFlanders Marine Institute 2015. http://www.marineregions.org (accessed 23 June 2015).
- 17.Matthiopoulos J. 2003. The use of space by animals as a function of accessibility and preference. Ecol. Modell. 159, 239–268. ( 10.1016/S0304-3800(02)00293-4) [DOI] [Google Scholar]
- 18.Grecian WJ, et al. 2016 Data from: Seabird diversity hotspot linked to ocean productivity in the Canary Current Large Marine Ecosystem. Dryad digital repository: 10.5061/dryad.01sd0. [DOI] [PMC free article] [PubMed]
- 19.Wood SN. 2006. Generalized additive models: an introduction with R. London, UK: Chapman and Hall/CRC. [Google Scholar]
- 20.Bartoń K. 2015. MuMIn: Multi-Model Inference. R package version 1.15.1. See: https://CRAN.R-project.org/package=MuMIn.
- 21.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 22.Wood SN, Bravington MV, Hedley SL. 2008. Soap film smoothing. J. R. Stat. Soc. B 70, 931–955. ( 10.1111/j.1467-9868.2008.00665.x) [DOI] [Google Scholar]
- 23.Costa DP, Breed GA, Robinson PW. 2012. New insights into pelagic migrations: implications for ecology and conservation. Annu. Rev. Ecol. Evol. Syst. 43, 73–96. ( 10.1146/annurev-ecolsys-102710-145045) [DOI] [Google Scholar]
- 24.Scales KL, Miller PI, Hawkes LA, Ingram SN, Sims DW, Votier SC. 2014. On the front line: frontal zones as priority at-sea conservation areas for mobile marine vertebrates. J. Appl. Ecol. 51, 1575–1583. ( 10.1111/1365-2664.12330) [DOI] [Google Scholar]
- 25.Okes NC, Hockey PAR, Pichegru L, Lingen CD, Van Der Crawford RJM, Grémillet D. 2009. Competition for shifting resources in the southern Benguela upwelling: seabirds versus purse-seine fisheries. Biol. Conserv. 142, 2361–2368. ( 10.1016/j.biocon.2009.05.031) [DOI] [Google Scholar]
- 26.Votier SC, et al. 2004. Changes in fisheries discard rates and seabird communities. Nature 427, 727–730. ( 10.1038/nature02315) [DOI] [PubMed] [Google Scholar]
- 27.Pauly D, et al. 2014. China's distant-water fisheries in the 21st century. Fish Fish. 15, 474–488. ( 10.1111/faf.12032) [DOI] [Google Scholar]
- 28.Agnew DJ, Pearce J, Pramod G, Peatman T, Watson R, Beddington JR, Pitcher TJ. 2009. Estimating the worldwide extent of illegal fishing. PLoS ONE 4, e4570 ( 10.1371/journal.pone.0004570) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The species richness and environmental covariate data underlying this study are archived in the Dryad digital repository at http://dx.doi.org/10.5061/dryad.01sd0. Individual species data are archived by BirdLife International (www.seabirdtracking.org).