Abstract
Antimicrobial resistance (AMR) has been detected in the microbiota of many wildlife species, including long-distance migrants. Inadequately treated wastes from humans and livestock dosed with antimicrobial drugs are often assumed to be the main sources of AMR to wildlife. While wildlife populations closely associated with human populations are more likely to harbour clinically important AMR related to that found in local humans and livestock, AMR is still common in remote wildlife populations with little direct human influence. Most reports of AMR in wildlife are survey based and/or small scale, so researchers can only speculate on possible sources and sinks of AMR or the impact of wildlife AMR on clinical resistance. This lack of quantitative data on the flow of AMR genes and AMR bacteria across the natural environment could reflect the numerous AMR sources and amplifiers in the populated world. Ecosystems with relatively simple and well-characterized potential inputs of AMR can provide tractable, but realistic, systems for studying AMR in the natural environment. New tools, such as animal tracking technologies and high-throughput sequencing of resistance genes and mobilomes, should be integrated with existing methodologies to understand how wildlife maintains and disperses AMR.
Keywords: antibiotic resistance, migration, disease transmission, animal dispersal, resistome, sewage treatment
1. Introduction
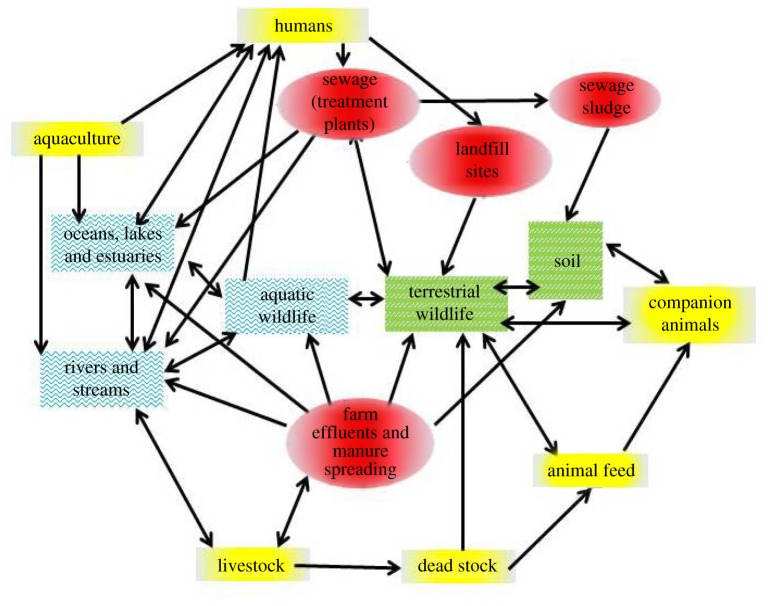
A growing human population and increasing fragmentation of natural habitats inevitably forces wildlife into greater contact, both direct and indirect, with humans and their livestock, thereby increasing the opportunities for transmission of infection between and within populations [1]. While some progress has been made in understanding the epidemiology of multi-host infections involving wildlife [2], less attention has been paid to the role of wild animals in the ecology and evolution of antimicrobial resistance (AMR) [3,4]. Although AMR is considered one of the greatest challenges to global health security [5], to date, most AMR research has been based in clinical settings [6]. Relatively little is known about the flow and fate of AMR in the natural environment [7], particularly in highly mobile species that could act as efficient AMR dispersers [3,4] (figure 1). In this review, we discuss the possible role of wildlife in the dissemination of AMR, specifically how wildlife might acquire and transport AMR and the potential for them to transmit AMR to humans and livestock.
Figure 1.
Dispersal of AMR across the landscape: between human communities, hospitals, sewage treatment plants, farms and the wider environment including via wildlife (adapted from [6]). (Online version in colour.)
2. Antimicrobial resistance
Antimicrobial drugs have saved millions of human lives and improved animal health and welfare globally [6]. Consequently, the evolution and dispersal of AMR is considered to be a major problem facing medical science and food security [5]. AMR is an ancient phenomenon, having evolved in dynamic microbial communities within which antimicrobials are produced by environmental bacteria and fungi naturally living in soil, water, etc. [6]. Such AMR, plus AMR as a side effect of selection of other properties, including efflux pumps for removing environmental stressors such as heavy metals, is often referred to as ‘intrinsic’ AMR. By contrast, ‘acquired’ AMR is the result of exposure to antimicrobial drugs which promotes resistance by selecting bacteria within a population with genetic traits conferring resistance. Thus, the selection of AMR in both pathogens and the normal gut microbiota of livestock and humans is believed to be largely a consequence of increased selective pressure provided by clinical antimicrobial use: recent hospitalization, for example, is a risk factor for shedding antibiotic-resistant Escherichia coli in both horses [8] and humans [9]. In many parts of the world, antimicrobials are still used, not just in clinical settings, but as ‘growth promoters’ in food-producing animals, an activity banned in the European Union owing to concerns about the selection of AMR [10]. So, while wildlife could provide a reservoir of intrinsic genetic determinants for resistance, it has usually been assumed that AMR detected in wildlife samples is acquired AMR resulting directly or indirectly from antibiotic-treated humans or livestock [11].
The ecology of AMR is complicated by the horizontal spread of the genes encoding AMR through communities of different species and even genera of bacteria, via mobile genetic elements such as plasmids (extra-chromosomal DNA molecules). These mobile genetic elements often encode multiple genes, providing resistance to antimicrobials and, indeed, other environmental chemical stressors including metals and disinfectants. Consequently, exposure to one antimicrobial (or other stressor) can select for all co-encoded genes and thus the rapid emergence of multi-drug resistance [6]. Thus, wildlife and other environmental bacteria that have never been found to infect humans can, through horizontal gene transfer, exchange resistance mechanisms with human pathogens [11,12] (but see [13]).
3. Potential sources of antimicrobial resistance in the environment to wildlife
Following selection of resistance within individuals (human or domesticated animals) treated with antimicrobials [10], both resistant bacteria and antimicrobials are subsequently excreted by the patient (figure 1). These can be dispersed in the environment, for example in sewage effluent pumped into rivers [14] and spreading sewage sludge as a fertilizer, or in the faeces of treated livestock and pets [15,16] (figure 2). Effluent and run-off from fields will often end up flowing into the sea, resulting in estuaries, coastal waters and beaches polluted by faecal matter [14] (figure 1). This could be a critical point of contact where humans and marine animals, as well as waders and seabirds, are exposed to AMR [19]. The rapidly expanding aquaculture industry is another source of AMR and antimicrobials to the environment: fish and seafood farmed in some countries where antimicrobial usage is high and poorly regulated are particularly likely to carry medically significant resistant pathogens [4,20].
Figure 2.
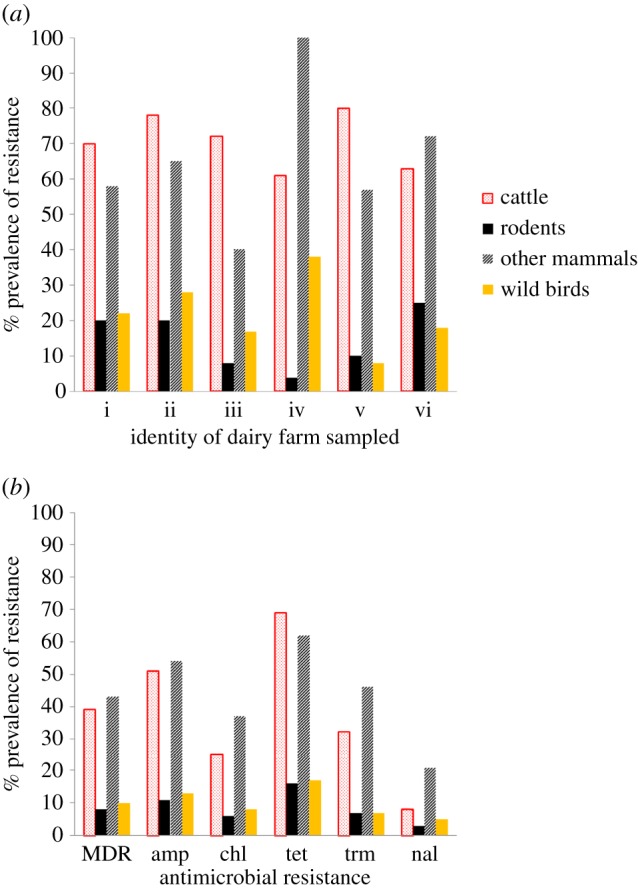
Antimicrobial resistance in wildlife on dairy farms in Cheshire, UK. The resistance patterns of Escherichia coli from the faeces of cattle, rodents (mainly Myodes glaroelus and Apodemus sylvaticus), wild birds (mainly passerines) and other wild mammals (mainly badgers and foxes) were compared. (a) Percentage of faecal samples containing E. coli resistant to at least one antibiotic on six different farms (i–vi). (b) Percentage of E. coli isolated from each group of animals resistant to various antibiotics or multi-drug resistant. Resistance to the following antibiotics was tested: ampicillin (amp), chloramphenicol (chl), tetracycline (tet), trimethoprim (trm) and nalidixic acid (nal) and also MDR (multi-drug resistance defined as resistance to three or more of the antibiotics tested). All susceptibility testing was performed according to the British Society of Antimicrobial Chemotherapy guidelines [17]. See the electronic supplementary material for details of methods. Figure adapted from [18]. (Online version in colour.)
Evolution of AMR does not necessarily stop in the gastrointestinal tract of animals (including humans) undergoing treatment; many antimicrobials can be excreted in an active form and persist in the environment [21]. Thus, ongoing exposure to antimicrobial drugs, for example in sewage, might maintain the selective advantage of AMR and promote the proliferation of resistance determinants and resistant bacteria in the environment. There is an added risk from sites highly contaminated with excreta, such as intensive farms and sewage treatment plants. Places with a high abundance and diversity of bacteria provide a high density of bacterial hosts and excellent conditions for the horizontal transmission of AMR genes from commensal or environmental to pathogenic bacteria [22]. It is clear that, particularly in areas with dense human or livestock populations, there is a myriad of AMR sources and amplifiers. If AMR genes and bacteria are carried in the gut of wildlife, then coupled with inadequate waste management and long-range animal movements, there is potential for wildlife to transport new and emerging AMR genes around the world [14] (figure 1).
4. Patterns of antimicrobial resistance infection in wildlife
With increasing pressure from expanding human populations, wild animals are increasingly forced to forage on resources contaminated by human ‘pathogen pollution’ [2,14]. So it is not surprising that AMR has often been described in peri-domestic wildlife [11]. AMR has been detected, particularly among commensal gut bacteria, in wild mammals, birds, reptiles and fish, with the prevalence and resistance patterns varying across species, locations and possibly time (e.g. [3,20,23–27]). Current data on AMR in wildlife largely consist of series of ‘snap shots’ proving the presence of resistomes (all of the antibiotic resistance genes found in microbes [13]) in those animals, but little else. However, the few studies that identify potential sources of AMR and can make comparisons across sites differing in contamination provide insights into the potential for wildlife to disseminate clinically relevant AMR.
Studies in South America and Africa found AMR to be more common in gut bacteria from non-human primates living close to humans than in those from more isolated populations [28,29]. Ugandan gorilla populations, for example, with home ranges that overlapped human settlements harboured resistant bacteria that were genetically similar to E. coli from those people and livestock, compared with apes more remotely located [28]. In northern elephant seals, Mirounga angustirostris, the probability of shedding antimicrobial-resistant E. coli was found to be directly correlated with the size of local human populations [30]. Similarly, in the Galapagos, molecular markers of AMR were more common in both seawater samples and marine iguanas close to tourist sites compared with those from more pristine conservation areas [25]. There are, however, exceptions to the generally positive relationship between spatial distance to anthropogenic wastes and the detection of clinically important resistance genes. For example, resistance to ciprofloxacin, a relatively recently developed and completely synthetic antimicrobial, was detected even in the most remote groups of monkeys in Mexico [29]. This is suggestive of de novo evolution of resistance, horizontal gene transfer from environmental microbes and/or greater contact with humans than previously thought. Further molecular and ecological investigations are clearly required. In general, however, study sites with relatively low or well-defined AMR inputs enable us to quantify spatial patterns, pathways and processes that drive AMR dissemination at different scales.
In heavily populated areas, high background AMR levels often cloud observations. In the UK, for example, we and others have found that AMR is frequently found in both wild mammals and birds, although the sources and drivers of AMR are often unclear ([18,24,31,32]; figure 2 and table 1). We found that the patterns of AMR in E. coli from the livestock and rodents resident on intensive livestock farms (table 1), and the genes encoding that resistance, were often similar. The E. coli only rarely identified shared genotypes, however, suggesting an important role for the mobilome (all mobile genetic elements in a genome, e.g. plasmids) and horizontal transmission of AMR rather than simple cross-species transmission of resistant bacteria. By contrast, at less intensively farmed sites, such as dairy farms with cattle kept outside, no clear relationships between either patterns or the genetics of AMR were found in livestock and wildlife [18]. As in the African and South American studies, we also found AMR in wildlife in relatively remote and uninhabited (by humans) areas (table 1). Furthermore, sympatric populations of wild mammals, including different species of rodents sharing the same woodland habitat, had different patterns of AMR and/or different temporal dynamics [31]. This strongly suggests that AMR in the bacterial microbiota of wildlife is not simply a matter of recent anthropogenic contamination or selection.
Table 1.
Antimicrobial-resistant Escherichia coli in the faeces of wild rodents collected at sites in the UK varying in predicted exposure to livestock treated with antimicrobial drugs. Resistance to six antibiotics (ampicillin (amp), apramycin (apr), chloramphenicol (chl), tetracycline (tet), trimethoprim (trm) and nalidixic acid (nal)) was investigated [17]. (N.J.W. & M.B. 2011, unpublished data; [32]). See the electronic supplementary material for further details.
| site type | predicted exposure to antimicrobial-treated livestock | rodent species sampleda | prevalence (% samples containing resistant E. coli) of antibiotic resistance in faecal samples of different rodent and livestock populations |
|||||
|---|---|---|---|---|---|---|---|---|
| amp | apr | chl | tet | trm | nal | |||
| uninhabited island | none | water voleb | 10–85 | 0 | 2–65 | 5–75 | 5–85 | 0–35 |
| upland forest | upland sheep | field vole, bank vole and wood mouse | 0–5 | 0 | 0–5 | 0–5 | 0–5 | 0 |
| lowland woodland | reared gamebirds and cattle on adjacent dairy farms | bank vole and wood mouse | 12–22 | 0–1 | 4–6 | 12–18 | 11–18 | 0 |
| fields on dairy farms | cattle on fields | wood mouse | 0–27 | 0 | 0–27 | 0–30 | 0–42 | 0 |
| cattle | 5–75 | 0 | 0 | 8–92 | 5–92 | 0 | ||
| intensive poultry farms | poultry in buildings | house mousec | 47–55 | 0 | 21–25 | 57–75 | 57–68 | 0–10 |
| bank vole and wood mouse | 0–5 | 0 | 0 | 0–9 | 0–12 | 0 | ||
| poultry | 5–8 | 0 | 0 | 15–45 | 3–6 | 0–18 | ||
aRodent species: water vole, Arvicola amphibious; field vole, Microtus agrestis; bank vole, Myodes glareolus; wood mouse, Apodemus sylvaticus; house mouse, Mus musculus.
bWater voles on these islands are fossorial rather than riparian as on the mainland.
cCaptured in and around the buildings housing poultry.
So while most studies in wildlife have assumed that AMR in wildlife is the consequence of spillover of resistant bacteria from domestic animals or people [33,34], there are several non-exclusive alternative hypotheses that challenge this notion of recent transmission. For example, following exposure to wastes containing pharmaceuticals, enteric bacteria present in wildlife evolve resistance through selection of pre-existing environmental AMR genes. These might become ‘naturalized’ in the gut microbiota, but also AMR genes (which have been found in ancient environmental samples [13]) are, and have always been, a normal finding in commensal gut microbiota. Moreover, distinguishing between AMR recently acquired from anthropogenic sources, such as a farm or sewage treatment plants, and ‘intrinsic’ (or at least ‘naturalized’) background AMR will be challenging. Comparing the similarity of sequences of resistance genes collected from sites differing in their connectedness with sources of acquired AMR (e.g. using sequence similarity network approaches [12]) could provide the evidence required.
A particular concern about AMR dispersal is wildlife species that have the capacity for long-range movements. Migratory birds arriving from beyond national boundaries could transfer new or emerging patterns of AMR, but even resident species have the potential to move AMR from hotspots to vulnerable populations. The potential of wild animals to disseminate AMR depends on their AMR ‘infection’ status, their direct and indirect contact with other populations and their movements within the landscape. In communal corvid roosts in Europe and the USA, 2.5–6.0% of faecal samples contained resistance genes for vancomycin, an antimicrobial ‘of last resort’ in human medicine [23,35]. Gulls carrying medically significant AMR are capable of long-range movements and are increasingly found feeding on anthropogenic waste and nesting in urban areas [24,27,36]. Similarly, in aquatic ecosystems, uneaten food and faeces from human sewage, agriculture and aquaculture containing antimicrobials and AMR bacteria can be ingested by wild fish and other organisms, which can travel enormous distances and in some cases enter the human food chain [20]. However, most of these studies on globally moving species are one-off surveys of AMR prevalence with no attempt to identify infection sources (or sinks) [3], which limits our ability to estimate the risk posed by migratory species in disseminating AMR. Finally, it remains unknown whether AMR can be or, more importantly, is transmitted from wildlife to humans or domestic animals, which is the main concern of clinicians and policymakers.
5. Studying antimicrobial resistance dispersal by wildlife
Given the many knowledge gaps, a range of tools and approaches will be needed to identify and characterize transmission routes of AMR in wildlife. At a broad scale, identifying traits that predispose wildlife species or functional groups to transmit AMR could be determined by integrating ecological, biological and life-history datasets for vertebrate hosts with metagenome sequences embedding resistance determinants [12]. While this is an efficient and informative approach, one caveat is that by mining such data, we can only find known resistance determinants. Some evidence from wildlife studies shows that the genes responsible for phenotypic resistance are often not detectable using PCRs (polymerase chain reactions) targeted at common clinical AMR genes. This suggests a greater diversity of resistance genes (many of which will already have been associated with other, non-AMR, functions) in the environment than found in clinical isolates (K.E.A., N.J.W. and M.B. 2011, unpublished data).
At a finer scale, study systems are needed in which clear and measurable transmission routes for AMR exist and the movement of wildlife can be tracked. The discovery of multi-drug resistance in species of high conservation value on oceanic islands [25] and in samples from isolated, relatively untouched points on continents [27] provide ‘natural experiments’ that are ideal for studying patterns and processes in the ecology and evolution of AMR. Monitoring AMR genes within such pristine ecosystems (e.g. Arctic or nature reserves with tight biosecurity) or at their interface with human-influenced areas enables us to estimate the frequency with which genes encoding resistance are exchanged in microbial communities. Such microbial communities can exist within human, domestic animal and wildlife populations, as well as the wider environment [27,37].
When working in the more contaminated ‘natural’ environments common to densely populated areas, distinguishing between AMR acquired from anthropogenic sources, such as a farm or sewage treatment plant, versus naturally occurring or naturalized ‘background’ AMR will be more challenging. One approach is to study the dispersal of relatively rare AMR determinants, currently associated only with human (or particular livestock) populations, through food chains. For example, fluoroquinolone resistance and extended-spectrum β-lactamases (ESBL) (conferring resistance to newer antibiotics used in human medicine) are relatively unusual in livestock and, in our experience, incredibly rare in wildlife. Such resistance might be tracked through high-risk ecosystems, for example from sewage treatment plants or livestock slurry pits into the surrounding environment, at multiple levels: phenotypic resistance, bacterial genotype, mobile elements and individual resistance genes. High-throughput, next-generation sequencing can rapidly provide such detailed forensic trails [13]. Although targeted at a limited range of AMR, this approach would provide a good understanding of the ecology of AMR genes and their ‘resistome’ context. Deeper, metagenomic sequencing studies through these and/or less high-risk ecosystems will be needed to place such targeted AMR studies in a broader perspective, through examining a range of AMR genes across taxa of host bacteria within the same samples. However, metagenomic studies have their own challenges, not least the volume and complexity of bioinformatic data analysis and the cost, which currently limit sample number and interpretation.
Ecological models of AMR transmission involving wildlife need to incorporate indirect rather than just direct host-to-host transmission. Although AMR can be transmitted directly between hosts, for example through predation (food-borne in a clinical context) or grooming and faeco-oral transmission, there is a huge overlap between the microbiota of the normal gut and that of the external environment (e.g. in soil and water) with horizontal transmission of AMR possible in both. Such models could be based on spatial movements in relation to a common environmental source of AMR contamination such as a refuse dump [28]. Sewage treatment plants, for example, are hotspots of AMR, which can provide valuable pockets of semi-natural habitat for birds and bats, attracted by the invertebrates that themselves feed in the sewage [38]. In fragmented landscapes, birds and bats often then move between isolated discrete patches of suitable habitat or food sources [39], such as gardens and farms, enabling the further dispersal of AMR. Ever more powerful and accurate electronic tracking devices and spatial modelling approaches provide the potential to map the movements of animals in both space and time relative to potential sources of AMR pollution and points of contact with humans and livestock [40]. By combining a range of tools including mark–recapture methods, epidemiological modelling, molecular sequencing, behavioural observations and high-tech devices such as GPS trackers, we can start to test empirically hypotheses concerning the dissemination of AMR by wildlife.
6. Consequences of antimicrobial resistance for wildlife
The consequences for wildlife of the evolution of AMR in commensal, or even pathogenic, bacteria are untested [37], but probably small. Unlike avian influenza [41], for example, AMR is not a disease and does not appear to reduce the survival or dispersal capacity of ‘infected’ animals, although this has not been explicitly tested. The clinical issue with AMR in both human and livestock populations is not that it causes disease, but that it threatens the ability to treat infections with antimicrobials, a practice rare in wild-living populations. AMR could compromise the treatment of individual wild animals in captivity, e.g. in wildlife hospitals, or of highly managed populations, especially those immunocompromised due to low genetic diversity (e.g. [42]). This might be exacerbated by conservation management measures such as translocation of rare species that could expedite the spread of novel microbes or antimicrobial genes between isolated populations [43].
The biggest issue for wildlife populations is the management response should they be thought to be significant sources of AMR for humans or livestock (see also table 2). The control of wildlife infections transmissible to humans and livestock relies on three main approaches—separation of, or at least reducing contact with, the wildlife source, vaccination and wildlife population control, often by culling. Vaccination is not possible for AMR control, and the physical separation of wildlife from livestock is difficult, expensive and, except very locally (e.g. keeping rodents or birds out of feed stores), impracticable. Protecting the human food chain from AMR is important but challenging given that wild game, seafood and bushmeat are important both nutritionally and culturally in many human societies [4]. Furthermore, control and mitigation measures such as improved hygiene and restriction on movements cannot be easily implemented, if at all, for free-living animals. For logistical, economic, historical and cultural reasons, culling is often the approach taken: however, the efficacy and efficiency of culling wildlife in controlling disease are at best controversial.
Table 2.
Summary of some of the key outstanding questions, mitigation measures and research approaches regarding the role of wildlife in the transmission of AMR based on the literature reviewed. Suggested research approaches draw upon diverse disciplines including ecology, veterinary science and the social sciences.
| ecology of the host | biology of the host | risks to humans and livestock | mitigation measures | research approaches |
|---|---|---|---|---|
| How do species and climate-driven differences in seasonal population dynamics affect AMR carriage? | How long and far are resistance genes carried and shed by wildlife (particularly migratory) species? | How can we prove the direction of AMR transmission from humans or livestock to/from wildlife? | How can anthropogenic wastes be managed to prevent transmission of AMR to wildlife? | spatially explicit field studies and network modelling to identify key transmission locations, species and individuals |
| Are carnivores and scavengers more likely to harbour AMR than omnivores or herbivores? | Do gut bacteria endemic to wildlife species differ in their propensity to share resistance genes via horizontal transfer? | Are wild animals a direct (bushmeat) or indirect (contaminating livestock food) route by which AMR can enter the human food chain? | Can existing surveillance and monitoring schemes (e.g. WILDCOMS) be used for AMR screening of wildlife? | mine AMR metagenome sequences in public databases to test hypotheses regarding ecology and evolution of AMR transmission |
| Are group-living species or individuals more likely to carry AMR than solitary ones? | Are species with an aquatic life stage or aquatic diet most at risk of acquiring or transmitting AMR? | What is the relative contribution of aquaculture to AMR evolution and transmission in aquatic and marine ecosystems? | Do unmetabolized antibiotics from wastes select for the evolution or maintenance of AMR in the environment? | sensor technology deployed systematically to detect AMR and/or antibiotics in high-risk ecosystems, exposure pathways or species |
| Are urban adaptors or exploiters more likely to disperse AMR than urban avoider species? | Does an individual's immune function affect its propensity to be infected by AMR microbes? | Which agricultural, religious or cultural practices globally expose humans to wildlife disseminated AMR? | What are the alternatives to culling AMR-infected wildlife that pose a risk to humans? | deliberative, stakeholder-driven approaches to developing societal solutions to AMR transmission in the environment |
7. Research and policy priorities
Wildlife clearly is shedding and therefore able to disseminate AMR [2,4]. However, few studies have identified the likely selection factors (including, but not necessarily limited to, sources of antimicrobial exposure), origins of the resistance genes or, importantly, the direction of transmission. Studying infection transmission in wildlife poses a number of challenges, particularly for a complex issue such as AMR that is present in, and can move between, multiple bacterial taxa in multiple hosts and the environment. Approaches used to study and control AMR transmission in the clinical setting are challenging to apply to wildlife systems. Contact between wildlife and sources of AMR and/or antimicrobials often cannot be measured directly but needs to be inferred, for example from molecular ‘fingerprints’ of specific contamination. This can be supplemented with behavioural observations and electronic tracking devices fitted to wild animals.
Interventions that minimize and mitigate the transmission of AMR from livestock or human populations to wildlife need researching alongside investigation of the risk itself, to develop both evidence-based and proportionate protocols and policies (table 2). Pollution control and sewage treatment are likely priority areas for such research, particularly in countries with few controls on either antibiotic usage or release of untreated wastes (which includes both developed and developing countries). Meanwhile ecologists studying wild populations, along with wildlife hospitals and existing programmes designed to monitor pollution, poisoning and diseases in wildlife (e.g. WILDCOMS [44]), might be recruited to collect samples for surveillance. This last approach might be particularly useful in identifying species, key individuals within populations or spatial locations that are ‘super spreaders’ of AMR transmission and could be targeted for focused surveillance, control or mitigation measures [40].
It is important to study AMR in wildlife as a potential hazard to human health and food security, especially given that about 40% of emerging human diseases are thought to have originated in wildlife [1]. Tropical ecosystems and areas in which humans live close to both livestock and wildlife are likely to present heightened, but to date poorly studied, risks for the evolution and transmission of AMR by wildlife (table 2). Furthermore, studies of AMR in wildlife can have wider impact than simply public health risk. First, by stepping outside of the ‘blame game’ of livestock, veterinary and medical systems, they can elucidate fundamental issues in the evolution and transmission ecology of antimicrobial-resistant bacteria and resistance determinants that can be applied back into more clinical settings. Second, a better understanding of the role of wildlife in AMR dissemination should help us decide whether control and mitigation strategies are required and where best to apply them. Finally, while wildlife might be long-distance dispersers of AMR, they can also be sentinels for the abundance and distribution of pathogen pollution in our environment.
Supplementary Material
Acknowledgements
Thank you to D. Graham for discussions about AMR and to L. Al Meslati for permission to reproduce a figure and part of a table from his PhD thesis. Some of the data shown in figure 2 and table 1 were collected through Defra-funded research projects.
Ethics
All works carried out conformed to the legal requirements of the country in which the work was carried out and to all institutional guidelines.
Authors' contributions
All authors contributed to the writing of this review and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
No specific funding was provided for the preparation of this paper.
References
- 1.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. 2015. Global trends in infectious diseases at the wildlife–livestock interface. Proc. Natl Acad. Sci. USA 112, 9662–9667. ( 10.1073/pnas.1422741112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huijbers PMC, Blaak H, de Jong MCM, Graat EAM, Vandenbroucke-Grauls CMJE, Husman AMDR. 2015. Role of the environment in the transmission of antimicrobial resistance to humans: a review. Environ. Sci. Technol. 49, 11 993–12 004. ( 10.1021/acs.est.5b02566) [DOI] [PubMed] [Google Scholar]
- 4.Greig J, Rajic A, Young I, Mascarenhas M, Waddell L, LeJeune J. 2015. A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food chain. Zoonoses Public Health 62, 269–284. ( 10.1111/zph.12147) [DOI] [PubMed] [Google Scholar]
- 5.WHO. 2014. Antimicrobial resistance: global report on surveillance 2014. Geneva, Switzerland: World Health Organisation. [Google Scholar]
- 6.Davies J, Davies D. 2010. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. ( 10.1128/mmbr.00016-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellington EMH, et al. 2013. The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. Lancet Infect. Dis. 13, 155–165. ( 10.1016/S1473-3099(12)70317-1) [DOI] [PubMed] [Google Scholar]
- 8.Maddox TW, Pinchbeck GL, Clegg PD, Wedley AL, Dawson S, Williams NJ. 2012. Cross-sectional study of antimicrobial-resistant bacteria in horses. Part 2: risk factors for faecal carriage of antimicrobial-resistant Escherichia coli in horses. Equine Vet. J. 44, 297–303. ( 10.1111/j.2042-3306.2011.00440.x) [DOI] [PubMed] [Google Scholar]
- 9.Cardoso T, Ribeiro O, Aragao IC, Costa-Pereira A, Sarmento AE. 2012. Additional risk factors for infection by multidrug-resistant pathogens in healthcare-associated infection: a large cohort study. BMC Infect. Dis. 12, 128 ( 10.1186/1471-2334-12-375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 24, 718–733. ( 10.1128/cmr.00002-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vittecoq M, et al. 2016. Antimicrobial resistance in wildlife. J. Appl. Ecol. 53, 519–529. ( 10.1111/1365-2664.12596) [DOI] [Google Scholar]
- 12.Fondi M, Karkman A, Tamminen M, Bosi E, Virta M, Fani R, Alm E, McInerney J. 2016. Every gene is everywhere but the environment selects: global geo-localization of gene sharing in environmental samples through network analysis. Genome Biol. Evol. 8, 1388–1400. ( 10.1093/gbe/evw077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsberg KJ, Patel S, Gibson MK, Lauber CL, Knight R, Fierer N, Dantas G.. 2014. Bacterial phylogeny structures soil resistomes across habitats. Nature 509, 612–616. ( 10.1038/nature13377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DW, Collignon P, Davies J, Larsson DGJ, Snape J.. 2014. Underappreciated role of regionally poor water quality on globally increasing antibiotic resistance. Environ. Sci. Technol. 48, 11 746–11 747. ( 10.1021/es504206x) [DOI] [PubMed] [Google Scholar]
- 15.Leatherbarrow AJH, et al. 2007. Campylobacter lari: genotype and antibiotic resistance of isolates from cattle, wildlife and water in an area of mixed dairy farmland in the United Kingdom. Environ. Microbiol. 9, 1772–1779. ( 10.1111/j.1462-2920.2007.01295.x) [DOI] [PubMed] [Google Scholar]
- 16.Maddox TW, Williams NJ, Clegg PD, O'Donnell AJ, Dawson S, Pinchbeck GL. 2011. Longitudinal study of antimicrobial-resistant commensal Escherichia coli in the faeces of horses in an equine hospital. Prev. Vet. Med. 100, 134–145. ( 10.1016/j.prevetmed.2011.02.006) [DOI] [PubMed] [Google Scholar]
- 17.Anon. 2007. BSAC methods for antimicrobial susceptibility testing (version 6.1). Birmingham, UK: British Society of Antimicrobial Chemotherapy.
- 18.Al-Tunesi LA. 2009. Prevalence of antibiotic-resistant Escherichia coli in faecal samples from domestic animals and wildlife: a cross-sectional study PhD thesis, University of Liverpool. [Google Scholar]
- 19.Leonard AFC, Zhang L, Balfour AJ, Garside R, Gaze WH. 2015. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 82, 92–100. ( 10.1016/j.envint.2015.02.013) [DOI] [PubMed] [Google Scholar]
- 20.Cabello FC, Godfrey HP, Tomova A, Ivanova L, Dölz H, Millanao A, Buschmann AH. 2013. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 15, 1917–1942. ( 10.1111/1462-2920.12134) [DOI] [PubMed] [Google Scholar]
- 21.Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. 2009. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 43, 363–380. ( 10.1016/j.watres.2008.10.047) [DOI] [PubMed] [Google Scholar]
- 22.Heuer H, Schmitt H, Smalla K.. 2011. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 14, 236–243. ( 10.1016/j.mib.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 23.Oravcova V, Ghosh A, Zurek L, Bardon J, Guenther S, Cizek A, Literak I.. 2013. Vancomycin-resistant enterococci in rooks (Corvus frugilegus) wintering throughout Europe. Environ. Microbiol. 15, 548–556. ( 10.1111/1462-2920.12002) [DOI] [PubMed] [Google Scholar]
- 24.Bonnedahl J, Järhult JD. 2014. Antibiotic resistance in wild birds. Ups. J. Med. Sci. 119, 113–116. ( 10.3109/03009734.2014.905663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler E, Hong P-Y, Bedon LC, Mackie RI. 2012. Carriage of antibiotic-resistant enteric bacteria varies among sites in Galapagos reptiles. J. Wildl. Dis. 48, 56–67. ( 10.7589/0090-3558-48.1.56) [DOI] [PubMed] [Google Scholar]
- 26.Gilliver MA, Bennett M, Begon M, Hazel SM, Hart CA. 1999. Enterobacteria: antibiotic resistance found in wild rodents. Nature 401, 233–234. ( 10.1038/45724) [DOI] [PubMed] [Google Scholar]
- 27.Sjölund M, Bonnedahl J, Hernandez J, Bengtsson S, Cederbrant G, Pinhassi J, Kahlmeter G, Olsen B.. 2008. Dissemination of multidrug-resistant bacteria into the Arctic. Emerg. Infect. Dis. 14, 70–72. ( 10.3201/eid1401.070704) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rwego IB, Isabirye-Basuta G, Gillespie TR, Goldberg TL. 2008. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in Bwindi Impenetrable National Park, Uganda. Conserv. Biol. 22, 1600–1607. ( 10.1111/j.1523-1739.2008.01018.x) [DOI] [PubMed] [Google Scholar]
- 29.Cristóbal-Azkarate J, Dunn JC, Day JMW, Amábile-Cuevas CF. 2014. Resistance to antibiotics of clinical relevance in the fecal microbiota of Mexican wildlife. PLoS ONE 9, e107719 ( 10.1371/journal.pone.0107719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoddard RA, et al. 2008. Risk factors for infection with pathogenic and antimicrobial-resistant fecal bacteria in northern elephant seals in California. Public Health Rep. 123, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams NJ, Sherlock C, Jones TR, Clough HE, Telfer SE, Begon M, French N, Hart CA, Bennett M. 2011. The prevalence of antimicrobial-resistant Escherichia coli in sympatric wild rodents varies by season and host. J. Appl. Microbiol. 110, 962–970. ( 10.1111/j.1365-2672.2011.04952.x) [DOI] [PubMed] [Google Scholar]
- 32.Karesh WB, Dobson A, Lloyd-Smith JO, Lubroth J, Dixon MA, Bennett M, Aldrich S, Harrington T, Formenty P, Loh EH, Machalaba CC, Thomas MJ, Heymann DL. 2012. Ecology of zoonoses: natural and unnatural histories. The Lancet 380, 1936–1945. ( 10.1016/S0140-6736(12)61678-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardyn SE, Kauffman LK, Smith TC. 2012. Methicillin-resistant Staphylococcus aureus in Central Iowa wildlife. J. Wildl. Dis. 48, 1069–1073. ( 10.7589/2011-10-295) [DOI] [PubMed] [Google Scholar]
- 34.Porrero MC, et al. 2013. Methicillin resistant Staphylococcus aureus (MRSA) carriage in different free-living wild animal species in Spain. Vet. J. 198, 127–130. ( 10.1016/j.tvjl.2013.06.004) [DOI] [PubMed] [Google Scholar]
- 35.Oravcova V, Zurek L, Townsend A, Clark AB, Ellis JC, Cizek A, Literak I. 2013. American crows as carriers of vancomycin-resistant enterococci with vanA gene. Environ. Microbiol. 16, 939–949. ( 10.1111/1462-2920.12213) [DOI] [PubMed] [Google Scholar]
- 36.Stedt J, Bonnedahl J, Hernandez J, McMahon BJ, Hasan B, Olsen B, Drobni M, Waldenström J. 2014. Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect. Ecol. Epidemiol. 4, 1 ( 10.3402/iee.v4.21565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albrechtova K, et al. 2014. Low rates of antimicrobial-resistant Enterobacteriaceae in wildlife in Tai National Park, Cote d'Ivoire, surrounded by villages with high prevalence of multiresistant ESBL-producing Escherichia coli in people and domestic animals. PLoS ONE 9, e113548 ( 10.1371/journal.pone.0113548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park KJ, Cristinacce A. 2006. Use of sewage treatment works as foraging sites by insectivorous bats. Anim. Conserv. 9, 259–268. ( 10.1111/j.1469-1795.2006.00031.x) [DOI] [Google Scholar]
- 39.Lawton JH, et al. 2010. Making space for nature: a review of England's wildlife sites and ecological network. London, UK: Defra. [Google Scholar]
- 40.Craft ME, Caillaud D. 2011. Network models: an underutilized tool in wildlife epidemiology? Interdiscip. Perspect. Infect. Dis. 2011, 1–12. ( 10.1155/2011/676949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feare CJ. 2010. Role of wild birds in the spread of highly pathogenic avian influenza virus H5N1 and implications for global surveillance. Avian Dis. 54, 201–212. ( 10.1637/8766-033109-ResNote.1) [DOI] [PubMed] [Google Scholar]
- 42.Morris K, Austin JJ, Belov K. 2013. Low major histocompatibility complex diversity in the Tasmanian devil predates European settlement and may explain susceptibility to disease epidemics. Biol. Lett. 9, 20120900 ( 10.1098/rsbl.2012.0900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grange ZL, Gartrell BD, Biggs PJ, Nelson NJ, Marshall JC, Howe L, Balm MGM, French NP. 2015. Using a common commensal bacterium in endangered takahe as a model to explore pathogen dynamics in isolated wildlife populations. Conserv. Biol. 29, 1327–1336. ( 10.1111/cobi.12521) [DOI] [PubMed] [Google Scholar]
- 44.Pereira MG, Chaplow JS, Shore RF. 2015. WILDCOMS (Wildlife Disease & Contaminant Monitoring and Surveillance Network) annual report 2013–2014, p. 19. Lancaster, UK: NERC/Centre for Ecology & Hydrology.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.