Jacoby et al. [1] recently proposed that the cruise swim speeds (S) of ram-ventilating sharks can be predicted by body mass (M), which they use as ‘a surrogate for metabolic rate’ (R; i.e. oxygen uptake rate). They reasoned that ‘overall scaling of swim speed can be predicted by metabolic need’ based on their ‘expectation that swim speed will increase with increasing body size in order to meet higher whole-body metabolism relative to gill surface area’ (Ag). However, the authors assumed that for ram-ventilating sharks Ag scales with M raised to the power of 0.667. Here I propose that if a more appropriate exponent is used (0.84; electronic supplementary material, table S1), then the authors' theoretical predictions of S are not valid.
The authors presumed that if it is known how both R, and Ag scale with M, as described by the allometric equation Y = aMb, then S can be reasonably predicted by M following from the theoretical relationship they developed in which  . Although the authors justifiably assumed that R scales with M where b = 0.84 (according to [2]), it is unclear why they assumed that Ag scales with M in direct proportion to body surface area (b = 0.667) because this assumption is not substantiated by the available evidence [3], including the study that the authors referenced in support of their assumption [4]. If the scaling exponent for Ag (as a function of M) was less than that for R, then there would be an increasing mismatch between oxygen demand and supply with increasing M, which led the authors to presume that S needs to increase with increasing M to maintain ram ventilation rate (F) to maintain R. However, this does not appear to be the case for the few sharks for which data are available (including data from [4]) where the mean mass scaling exponent for Ag is 0.84 (electronic supplementary material, table S1), identical to the value for R [3]. Thus, the theoretical relationship would be modified to
. Although the authors justifiably assumed that R scales with M where b = 0.84 (according to [2]), it is unclear why they assumed that Ag scales with M in direct proportion to body surface area (b = 0.667) because this assumption is not substantiated by the available evidence [3], including the study that the authors referenced in support of their assumption [4]. If the scaling exponent for Ag (as a function of M) was less than that for R, then there would be an increasing mismatch between oxygen demand and supply with increasing M, which led the authors to presume that S needs to increase with increasing M to maintain ram ventilation rate (F) to maintain R. However, this does not appear to be the case for the few sharks for which data are available (including data from [4]) where the mean mass scaling exponent for Ag is 0.84 (electronic supplementary material, table S1), identical to the value for R [3]. Thus, the theoretical relationship would be modified to 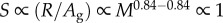 , indicating that S would not need to increase with increasing M to maintain F as proposed by the authors.
, indicating that S would not need to increase with increasing M to maintain F as proposed by the authors.
The swim speed data used by Jacoby et al. [1] and the confidence interval around the slope of their empirical model span a wide range at a given mass. Consequently, it is difficult to justify the predictive insight of their model, and unconvincing to implicate a respiratory dependence on swim speed as a physiological causality between swim speed and mass. Furthermore, the authors did not provide an intercept for their linear model and conceded that ‘further information would be needed to predict the intercept’. However, without this parameter the empirical model cannot be used to estimate swim speeds. This was inadvertently demonstrated by the authors when, ‘as a proof of concept,’ they appear to have incorrectly derived the speed for megalodon by using just the slope from their linear model.
An alternative hypothesis predicts that cruise swimming speeds are optimal where the total cost of transport (i.e. the energy required to move one unit distance) is minimized [5,6]. Within species of ram-ventilating sharks the cost of transport decreases with increasing mass [7]. Thus, at cruising speeds it is less energetically costly for a large shark to swim a given distance compared with a smaller shark of similar body morphology. In support of this, the exponent determined for a recent empirical model [8] that relates shark cruising speed to length (S = aLengthb) is very similar to that of Weihs's [5] theoretical bioenergetics model, in which b = 0.43 (exponent range: 0.35–0.5). Therefore, for ram-ventilating sharks the hypothesis that S is driven by energetics is more physiologically rational than the authors' hypothesis that S is driven by a mismatch between R and Ag with increasing M, which does not appear to exist.
Supplementary Material
Supplementary Material
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rsbl.2016.0502.
Competing interests
I declare I do not have competing interest.
Funding
This study was funded by Natural Sciences and Engineering Research Council of Canada (grant no. 6564).
References
- 1.Jacoby DMP, Siriwat P, Freeman R, Carbone C. 2015. Is the scaling of swim speed in sharks driven by metabolism? Biol. Lett. 11, 20150781 ( 10.1098/rsbl.2015.0781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sims DW. 2000. Can threshold foraging responses of basking sharks be used to estimate their metabolic rate? Mar. Ecol. Prog. Ser. 200, 289–296. ( 10.3354/meps200289) [DOI] [Google Scholar]
- 3.Wegner NC. 2015. Elasmobranch gill structure. In Physiology of elasmobranch fishes. Fish physiology series, vol. 34A (eds Shadwick RE, Farrell AP, Brauner CJ). New York, NY: Academic Press. [Google Scholar]
- 4.Emery SH, Szczepanski A. 1986. Gill dimensions in Pelagic elasmobranch fishes. Biol. Bull. 171, 441–449. ( 10.2307/1541685) [DOI] [Google Scholar]
- 5.Weihs D. 1977. Effects of size on sustained swimming speeds of aquatic organisms. In Scales effects in animal locomotion (ed. Pedley TJ.), pp. 333–338. London, UK: Academic Press. [Google Scholar]
- 6.Videler JJ, Nolet BA. 1990. Costs of swimming measured at optimum speed: scale effects, differences between swimming styles, taxonomic groups and submerged and surface swimming. Comp. Biochem. Physiol. A Physiol. 97, 91–99. ( 10.1016/0300-9629(90)90155-L) [DOI] [PubMed] [Google Scholar]
- 7.Carlson JK, Goldman KJ, Lowe CG. 2004. Metabolism, energetic demand, and endothermy. In Biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR), pp. 203–224. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Ryan LA, Meeuwig JJ, Hemmi JM, Collin SP, Hart NS. 2015. It is not just size that matters: shark cruising speeds are species-specific. Mar. Biol. 162, 1307–1318. ( 10.1007/s00227-015-2670-4) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


