Abstract
Individual recognition is considered to have been fundamental in the evolution of complex social systems and is thought to be a widespread ability throughout the animal kingdom. Although robust evidence for individual recognition remains limited, recent experimental paradigms that examine cross-modal processing have demonstrated individual recognition in a range of captive non-human animals. It is now highly relevant to test whether cross-modal individual recognition exists within wild populations and thus examine how it is employed during natural social interactions. We address this question by testing audio–visual cross-modal individual recognition in wild African lions (Panthera leo) using an expectancy-violation paradigm. When presented with a scenario where the playback of a loud-call (roaring) broadcast from behind a visual block is incongruent with the conspecific previously seen there, subjects responded more strongly than during the congruent scenario where the call and individual matched. These findings suggest that lions are capable of audio–visual cross-modal individual recognition and provide a useful method for studying this ability in wild populations.
Keywords: individual recognition, vocal communication, mammals, playback experiment, cognitive abilities, expectancy-violation paradigm
1. Background
The ability to identify and discriminate between organisms according to their individually distinctive characteristics is known as individual recognition [1] and is an attribute proposed to have been fundamental in driving the evolution of complex social systems [2]. However, while individual recognition is thought to be a widespread ability [3], providing robust scientific support for recognition at the level of the individual has proved difficult [4,5]. ‘True’ individual recognition strictly constitutes the identification of a specific individual, according to individually distinct cues, and the placement of that individual within a society of many others [6]. In empirical terms, it is necessary to demonstrate (i) that recognition occurs at the level of the individual (rather than at a broader level) and (ii) that there is matching of current sensory cues to identity with information stored in memory about that specific individual.
Recent advances in the cognitive sciences have resulted in growing evidence for individual recognition in non-human animals by virtue of demonstrating cross-modal processing of information on identity [7,8]. Cross-modal sensory perception is the ability to integrate information from multiple senses—in the case of individual recognition, this often involves matching vocal and visual cues, which may be demonstrated through experiments in which subjects detect a mismatch when the cues do not correspond [5,7]. While there is now direct evidence for cross-modal recognition in a range of species tested in captive or domestic settings [5,7–9], this ability has not been directly shown in the wild during natural social communication among conspecifics [6]. Such investigations are facilitated by a study species where repeated social interactions lead to important long-term social relationships, in which communication involves multiple sensory modalities, and where communication signals are known to provide familiarity cues as well as potential cues to identity.
Wild African lions (Panthera leo) meet these criteria, as they live within a fluid ‘fission–fusion’ society in which individuals often associate with small sub-groups rather than the entire pride, and use their long-distance calls (termed roars) to communicate with distant group-mates ([10]; see the electronic supplementary material). We investigated individual recognition in lions, using an ‘expectancy violation’ paradigm. A vehicle was used to create a visual block between an individual and the test subject, before roars were played from behind the visual block that either matched this individual (congruent trials) or corresponded to an absent group-mate (incongruent trials). We hypothesized that ‘incongruent trials’ should be followed by increased ‘searching’ behaviour (increased time looking towards call direction, and increased time moving) by the test subject, indicating an attempt to locate the absent group-mate. We also predicted an increased presence of tension-induced ‘displacement’ activities, as these are thought to alleviate stress in socially uncertain situations ([11]; see the electronic supplementary material).
2. Material and methods
Between May 2014 and December 2015, we performed 39 experiments on four male lions and 16 female lions from three prides in the Okavango Delta, Botswana (see the electronic supplementary material). To avoid sexually motivated responses, subjects were selected from a unisex group resting approximately 30 m apart, but still in visual contact. A vehicle was then positioned to create a visual block between two of the adult lions (figure 1). After a short period (30 s to 1 min) designed to ensure that some form of stored information had to be accessed [7], a Tannoy® CPA 12 studio monitor loudspeaker positioned within the vehicle was used to play the roars (standardized to 116 dB at 1 m from the source) either of the appropriate visually blocked individual (congruent trial), or of a lion of the same social group who was currently absent (incongruent trial). The vehicle remained stationary and the test subject was free to approach conspecifics or search for the simulated caller. High-quality recordings of 12 lions roaring alone were used to create the playback stimuli for both treatments, where each recording was standardized to 43 s in length (see the electronic supplementary material). Fourteen (73.7%) subjects were played the same recording in both trials (controlled for as a random effect within the statistical models), which acted as the congruent stimulus in one treatment and incongruent in the other. Only the response of the test subject was video-recorded (using a Bell & Howell® DNV16HDZ video recorder) for analysis.
Figure 1.
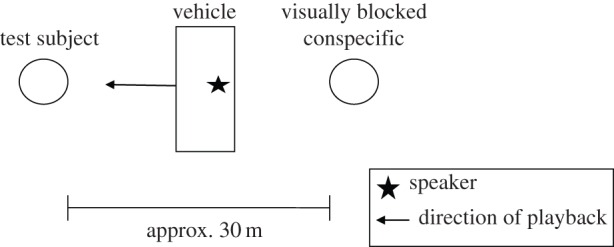
Experimental design: the vehicle (with speaker) was positioned between lions resting approximately 30 m apart. Only two adult lions were present in 79.5% of trials.
To prevent habituation, subjects were tested in both conditions in a random order (50% of subjects were tested with the congruent trial first), at least 9 days after the test subject was last involved in a playback (mean 95.2 days, s.d. ± 86.9). Trials began within 90 min before sunset (average 63.0 min, s.d. ± 21.0), which is a natural time for lions to begin roaring [10], and no experiments took place if the observer had heard roaring in the vicinity of the experiment location during the day. As trials took place in a natural setting, trial length could not be standardized, and key behavioural measures were analysed as proportions or rates (mean trial length 12.45 min, s.d. ± 7.59; see the electronic supplementary material). Trials ended when the test subject rested for at least 1 min after the playback, or began following the movement of a conspecific, whereby the test subject was judged to have ceased responding to the playback. Behavioural responses were analysed frame-by-frame (frame = 0.033/0.034 s) using Avidemux® 2.6.9 video analysis software.
Key responses monitored were latency to respond, time looking towards the call direction, time moving and a range of displacement behaviours as defined in the electronic supplementary material (table SI). Potential displacement behaviours were selected following observations of lions in socially stressful situations. For example, allo-rubbing (head-rubbing) is thought to reduce aggression between felids [12] and could be a key displacement behaviour for lions when stressed. To test inter-observer reliability, a random subset of the videos was double-coded blind in a random order (see the electronic supplementary material). All statistical analyses were conducted using a binomial generalized linear mixed-effects model in R (v. 3.2.5, see the electronic supplementary material), except where the non-parametric Wilcoxon signed-rank test was used owing to violations of the parametric assumptions.
3. Results
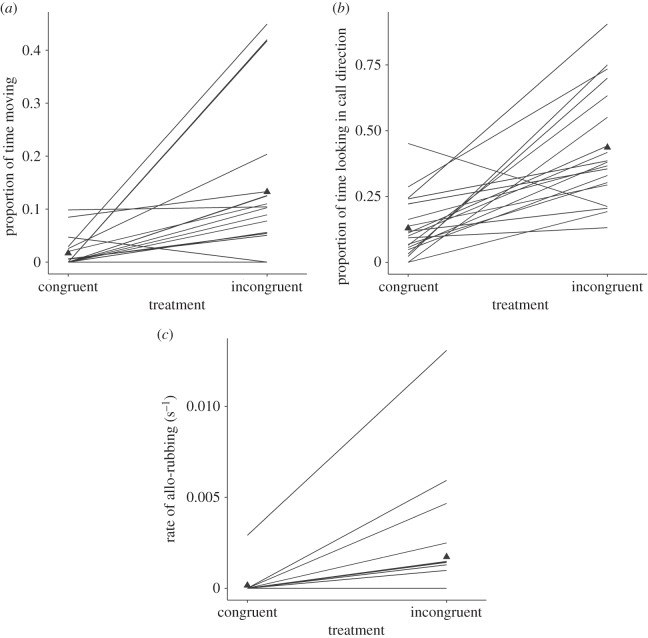
As predicted, lions of both sexes responded to incongruent playbacks by spending a greater proportion of time moving, and also a greater proportion of time looking towards the call direction, before resting again (table 1). In addition, lions initiated more allo-rubbing bouts with conspecifics (per second) following the incongruent playbacks (two-tailed Wilcoxon signed-rank test: Z = −2.96, p = 0.004, r = 0.68). However, there was no difference in any other measurements of potential displacement behaviours, or in the latency to respond (p > 0.0056; refer to the electronic supplementary material, table SIII). Significant behavioural responses to the playbacks are shown in figure 2.
Table 1.
Factors influencing: (1) the proportion of time subject lions spent looking in the call direction and (2) the proportion of time subject lions spent moving, following the playback of an incongruent, rather than congruent, call. Model parameters were generated using model averaging on the optimal GLMMs selected using corrected Akaike information criterion (models 1 : 3; electronic supplementary material, table SII).
| dependent variable | parameter | estimate | s.e | Wald confidence interval: 2.5–97.5% | relative importance | |
|---|---|---|---|---|---|---|
| proportion of time looking in call direction | intercept | −2.105 | 0.368 | 0.06–0.26a | ||
| treatment | congruent | — | — | — | 1.00 | |
| incongruent | 1.905 | 0.040 | 6.19–7.29a | |||
| sex | female | — | — | — | 0.26 | |
| male | −0.263 | 0.793 | 0.15–3.85 | |||
| treatment * sex | female * congruent | — | — | — | 0.06 | |
| male * incongruent | 0.044 | 0.107 | 0.84–1.30 |
| dependent variable | parameter | estimate | s.e | Wald confidence interval: 2.5–97.5% | relative importance | |
|---|---|---|---|---|---|---|
| proportion of time moving | intercept | −4.868 | 0.838 | 0.001–0.04a | ||
| treatment | congruent | — | — | — | 1.00 | |
| incongruent | 2.094 | 0.102 | 6.60–9.99a | |||
| sex | female | — | — | — | 0.27 | |
| male | −0.690 | 1.811 | 0.01–19.89 | |||
| treatment * sex | female x congruent | — | — | — | 0.06 | |
| male x incongruent | −0.141 | 0.207 | 0.57–1.32 |
aSignificant terms.
Figure 2.
Significant behavioural responses of lions to playbacks of congruent and incongruent calls included the proportion of time spent moving (a), proportion of time spent looking at call direction (b), and rate of allo-rubbing initiated by the focal animal after playback (c). Figure shows the response of individual lions (and the overall mean: triangles) to both playback treatments, where responses are represented as proportions (a,b), or rates (c) calculated per second from the raw data.
4. Discussion
Our results suggest that lions have the ability to individually recognize their group-mates, linking unique auditory and visual (and possibly olfactory) cues to identity. On our measures of searching behaviour, lions clearly responded to trials in which the familiar call did not match the familiar lion previously seen by spending more time moving and looking in the direction of the call before resting again. In addition, lions engaged in increased allo-rubbing with conspecifics following the incongruent trial, which may function as a stress-alleviating ‘displacement’ behaviour [13,14]. While other potential displacement measures did not differ between the treatments, it is likely that different species have different displacement signatures, and a wider investigation of stress-related behaviours in carnivores would be an interesting topic for future research.
A previous study on wild meerkats set out to test for individual vocal recognition using an expectancy-violation paradigm based around a physically impossible situation—simulating the presence of the same meerkat in two different places [15]. As the authors acknowledge, the experiment did not investigate whether meerkats were capable of integrating identity cues from multiple modalities, and thus did not test cross-modal individual recognition. We suggest that the experimental paradigm used here, which is based on simulating a natural social context for lions, might provide a useful design for tapping into such abilities in other species in the wild.
A potential alternative explanation for our results is that listeners may have heard the congruent lion roaring more recently than the incongruent lion, and responded more strongly due to the greater novelty of the latter's roars rather than that pride member being recognized across different sensory modalities. We have no way of knowing if the pride had roared the previous night, but we were able to monitor whether roaring occurred on the day of the experiment and no playback took place if this was the case. Furthermore, any roaring that occurred previously may have been joined even by an absent group-mate, as lion roars carry for several kilometres.
In conclusion, we used an ‘expectancy violation’ paradigm, where lions were presented with roars that were either congruent or incongruent with a visually blocked group-mate, to test for cross-modal individual recognition in a wild animal. After hearing an incongruent call that did not match the previously seen conspecific, lions responded by moving and also looking in the direction of the simulated call for a longer proportion of time before resting again, while also initiating a higher rate of allo-rubbing (a potential displacement behaviour thought to alleviate stress)—results that are consistent with the subjects recognizing the auditory–visual mismatch and being capable of cross-modal individual recognition.
Supplementary Material
Acknowledgements
We are grateful to the Sankuyo community and the Botswana Ministry of Environment, Wildlife and Tourism for permission to conduct the research. The fieldwork greatly benefitted through significant support from the Botswana Predator Conservation Trust (BPBT) and the numerous private donors who support the BPCT. Neil Jordan provided valuable comments.
Ethics
Experiments were performed under permits granted from the Botswana Ministry of Environment, Wildlife and Tourism (8/36/4 XXV (8)), and the University of Sussex (Non-ASPA 4-November 2013).
Data accessibility
Raw data have been deposited in Dryad: http://dx.doi.org/10.5061/dryad.6jd59 [16].
Authors' contributions
G.G. and K.M. designed the study. G.G. performed the study. G.G. and K.M. wrote the manuscript, which was corrected by all authors. G.G. and J.V. analysed data. J.W.M. provided significant support for the field research. All authors agree to be held accountable for the content and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This study was funded by the Leverhulme Trust (SAS-2013-044). Additional funding was provided by IDEA WILD and the Chicago Board of Trade Endangered Species Fund.
References
- 1.Dale J, Lank DB, Reeve HK. 2001. Signalling individual identity versus quality: a model and case studies with ruffs, queleas, and house finches. Am. Nat. 158, 75–86. ( 10.1086/320861) [DOI] [PubMed] [Google Scholar]
- 2.Krebs JR, Davies NB. 1978. Behavioural ecology: an evolutionary approach. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- 3.Tibbetts EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 4.Johnston RE, Peng A. 2008. Memory for individuals: hamsters (Mesocricetus auratus) require contact to develop multicomponent representations (concepts) of others. J. Comp. Psychol. 122, 121 ( 10.1037/0735-7036.122.2.121) [DOI] [PubMed] [Google Scholar]
- 5.Kondo N, Izawa EI, Watanabe S. 2012. Crows cross-modally recognize group members but not non-group members. Proc. R. Soc. B 279, 1937–1942. ( 10.1098/rspb.2011.2419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyfarth RM, Cheney DL. 2009. Seeing who we hear and hearing who we see. Proc. Natl Acad. Sci. USA 106, 669–670. ( 10.1073/pnas.0811894106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proops L, McComb K, Reby D. 2009. Cross-modal individual recognition in domestic horses (Equus caballus). Proc. Natl Acad. Sci. USA 106, 947–951. ( 10.1073/pnas.0809127105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sliwa J, Duhamel J-R, Pascalis O, Wirth S. 2011. Spontaneous voice–face identity matching by rhesus monkeys for familiar conspecifics and humans. Proc. Natl Acad. Sci. USA 108, 1735–1740. ( 10.1073/pnas.1008169108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi I, Kuwahata H, Fujita K. 2007. Dogs recall their owner's face upon hearing the owner's voice. Anim. Cogn. 10, 17–21. ( 10.1007/s10071-006-0025-8) [DOI] [PubMed] [Google Scholar]
- 10.Ramsauer S. 2005. Acoustic communication in lions and its use in territoriality. Cogn. Brain. Behav. 9, 539–550. [Google Scholar]
- 11.Maestripieri D, Schino G, Aureli F, Troisi A. 1992. A modest proposal: displacement activities as an indicator of emotions in primates. Anim. Behav. 44, 967–979. ( 10.1016/S0003-3472(05)80592-5) [DOI] [Google Scholar]
- 12.Turner DC, Bateson PPG. 2000. The domestic cat: the biology of its behaviour. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 13.Schulkin J. 2011. Adaptation and well-being: social allostasis. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Gourkow N, Hamon SC, Phillips CJ. 2014. Effect of gentle stroking and vocalization on behaviour, mucosal immunity and upper respiratory disease in anxious shelter cats. Prev. Vet. Med. 117, 266–275. ( 10.1016/j.prevetmed.2014.06.005) [DOI] [PubMed] [Google Scholar]
- 15.Townsend SW, Allen C, Manser MB. 2011. A simple test of vocal individual recognition in wild meerkats. Biol. Lett. 8, 179–182. ( 10.1098/rsbl.2011.0844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilfillan G, Vitale J, McNutt JW, McComb K. 2016. Data from: Cross-modal individual recognition in wild African lions. Dryad Digital Repository: 10.5061/dryad.6jd59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data have been deposited in Dryad: http://dx.doi.org/10.5061/dryad.6jd59 [16].