Abstract
Studying the association between fitness and cognition in free-living animals is a fundamental step in the elucidation of the evolution of cognition. We assessed whether survival until the onset of the breeding season was related to reaction time or spatial memory in the African striped mouse Rhabdomys pumilio, a rodent that has to survive summer drought before breeding. We tested a total of 90 individuals at the beginning of summer. Female survival was related to a faster response to predation stimuli. Male survival increased with greater spatial memory, possibly because it is important for males to remember the configuration of the environment during dispersal. This study revealed that individual variation in reaction time and spatial memory can be related to survival probability, which is important for understanding the selection pressures acting on basic cognitive traits.
Keywords: fitness, cognition, drought
1. Introduction
Cognition, defined as neural information processing from the perception of stimuli to the retention and use of information from the environment [1], is involved in guiding a wide range of behaviours. Understanding why animals differ in their cognitive abilities is a crucial challenge in evolutionary biology [2]. To understand the adaptive value of cognitive abilities, we must quantify how and to what extent cognitive variation within species is related to fitness. It has been claimed that basic cognitive traits may prove more useful to test general principles of cognition than specialized and sophisticated cognitive traits [3]. Further, research should focus on cognitive traits that underlie a fitness-determining activity, such as fighting, mating, foraging and predator avoidance [4].
Several studies found evidence that cognition has a positive influence on fitness proxies, such as growth rate in grasshoppers [5], stress resilience in honeybees [6] or number of offspring in male gourami fishes [7]. By contrast, while great tits that were better able to solve a foraging problem laid more eggs, they were at the same time more likely to desert their nest and showed lower competitive ability [8].
We assessed the association between cognitive performance and survival to drought in free-living African striped mice (Rhabdomys pumilio). Neuron numbers can change during harsh seasons [9], which are thus an appropriate time to study cognitive functions. Survival is an excellent proxy of fitness in this species, because between 35% and 86% (61.1 ± 16.8%, n = 8 years) of striped mice disappear during their first dry season and never reproduce [10]. When striped mice detect a predator while foraging in an open field, they search for the safest shelter, which might not be the closest cover. We therefore focused on reaction time and spatial memory as these might be involved in predator detection, foraging and escaping strategies in striped mice.
2. Material and methods
The study was conducted in Goegap Nature Reserve, South Africa. Striped mice were trapped at their nest for three consecutive days and then observed at sunrise and sunset for another three consecutive days, twice a month as part of an ongoing long-term study. Mice were marked using numbered metal ear tags (National Band and Taf Co., Newport, KY) and commercial hair dye (Rapido, Pinetown, South Africa). Striped mice start long-distance, male-biased dispersal (outside of our study area) at the onset of the breeding season in mid-July [11].
Tests were performed during austral summer, from 17 January to 8 April 2014. All individuals tested were juveniles (body mass less than 20 g) at time of first capture during the preceding spring. We estimated the number of days that each tested mouse survived from 1 January (beginning of dry season) to 15 July (onset of the breeding season and before long-distance dispersal). A mouse was considered to have died when it had not been trapped or observed for at least two consecutive months.
We measured reaction time to a moving shadow in 20 males and 30 females from 10 different groups. Striped mice bask as groups in the early morning and late afternoon at a close distance to their nest, which exposes them to predation. Mice were habituated to humans and could be observed within 5 m. The experimenter outstretched one arm in a circular and quick motion, so that the shadow of her arm passed over the mice (details in the electronic supplementary material, S1 and S2). We recorded the reaction time to the moving shadow, using a frame-by-frame video analysis.
We performed two laboratory tests in 30 males and 29 females from nine different groups after trapping them at their nest; 19 mice were also tested for the moving shadow test. Mice were tested for the orientation response test within 1 h, caged individually in a quiet room for 1–2 h, tested for the Barnes maze test and then released at their nest (details in [12]). The orientation response test uses the natural propensity of rodents to orient their head towards a salient stimulus, which is regarded as a behavioural manifestation of attention [13]. We recorded whether and how fast each individual mouse (placed in a transparent box) oriented its head to a raptor-stimulus appearing on a screen, using a frame-by-frame analysis (details in the electronic supplementary material, S1 and S3). Fifteen mice were excluded from the analyses because they showed no orientation response.
The Barnes maze test measures spatial memory by assessing the ability of rodents to relocate a shelter [14]. In brief, mice had six trials to learn the hole giving access to the shelter, followed by an experimental trial in which a bat-like toy was used to mimic a flying predator (details in the electronic supplementary material, S1 and S4). All the mice except five were trapped and retested 8 ± 3 days later in the Barnes maze. Thirteen mice were excluded from the analyses because they never entered the shelter. We used the number of errors made: (i) in the experimental trial of the first session to estimate short-term spatial memory, and (ii) in the first trial of the second session to estimate long-term spatial memory.
All statistics were performed with R v. 3.0.2. We used logistic regression analyses to assess the relationship between survival and performance by building a generalized linear-mixed model (GLMM) for binomial data (glmer function in lme4 package) with survival (survivor or non-survivor) specified as the dependent variable and the performance and sex considered as explanatory variables (table 1). Group identification was entered as a random factor, because family structure and local ecology might be confounding. As male and female striped mice differ in their dispersal strategy, we tested the strength and direction of the correlation between survival (number of days that mice survived) and cognitive performance for each sex separately (Spearman correlation test; table 2).
Table 1.
Results of the logistic regression analyses assessing relationships between survival and performance. (Italicized values: p < 0.05.)
| cognitive trait | cognitive test | fixed effects | sample size (n) | z-value | test statistic (χ2) | p-value |
|---|---|---|---|---|---|---|
| attention | moving shadow | reaction time | n = 50 | −2.36 | 0.56 | 0.453 |
| reaction time × sex | nmales = 20, nfemales = 30 | 2.27 | 5.22 | 0.022 | ||
| orientation response | orientation time | n = 44 | 1.49 | 0.21 | 0.651 | |
| orientation time × sex | nmales = 23, nfemales = 21 | −1.50 | 2.24 | 0.134 | ||
| number of orientations | n = 44 | −1.35 | 1.74 | 0.187 | ||
| number of orientations × sex | nmales = 23, nfemales = 21 | 0.68 | 0.46 | 0.496 | ||
| spatial memory | Barnes maze | number of errors (short-term) | n = 46 | 1.46 | 0.37 | 0.545 |
| number of errors (short-term) × sex | nmales = 24, nfemales = 22 | −1.62 | 2.62 | 0.106 | ||
| number of errors (long-term) | n = 40 | 0.76 | 0.01 | 0.940 | ||
| number of errors (long-term) × sex | nmales = 20, nfemales = 20 | −1.12 | 1.23 | 0.262 |
Table 2.
Spearman correlations between number of days mice survived from 1 January to 15 July and performance in each test for females and males. (Italicized values: p ≤ 0.05.)
| cognitive trait | cognitive test | behavioural measurement | females | males |
|---|---|---|---|---|
| attention | moving shadow (field) | reaction time | −0.35 (n = 30) | 0.23 (n = 20) |
| orientation response | orientation time | 0.24 (n = 21) | −0.19 (n = 23) | |
| number of orientation responses | −0.52 (n = 21) | −0.14 (n = 23) | ||
| spatial memory | Barnes maze | number of errors (short-term) | 0.49 (n = 22) | −0.10 (n = 24) |
| number of errors (long-term) | 0.36 (n = 20) | −0.44 (n = 20) |
3. Results
In the population studied, 149 out of 255 mice (59%) disappeared between January and July 2014. Of the 90 mice tested, 41 were trapped up to 15 July (16 out of 39 males, 25 out of 51 females); they were considered to have survived for 195 days.
We found a significant interaction between sex and reaction time to a moving shadow (GLMM: nmales = 20, nfemales = 30, z = 2.27, χ2 = 5.22, p = 0.022 for first reaction): survival until the onset of the breeding season was significantly related to reaction time in females (GLMM: n = 30, z = −2.16, χ2 = 4.94, p = 0.026), but not in males (GLMM: n = 20, z = 0.36, χ2 = 0.13, p = 0.717). Females that survived reacted significantly faster than females that disappeared (t-test: nsurvived = 12, ndisappeared = 18, t = 3.82, p < 0.001; figure 1), and females that reacted faster tended to survive longer (n = 30, rs = −0.35, p = 0.057).
Figure 1.
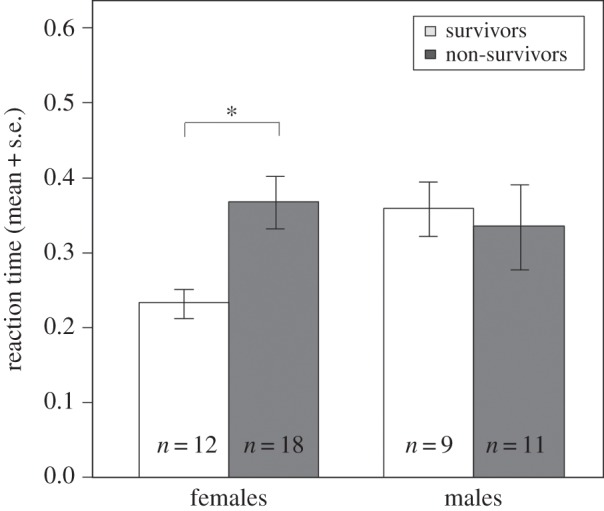
Reaction time (in seconds) measured in the moving shadow test in striped mice that did (white bars) or did not (grey bars) survive until the onset of the breeding season. *p < 0.001.
Survival was not significantly related to the orientation time (GLMM: n = 44, z = 1.49, χ2 = 0.21, p = 0.651 for first response) or the number of orientations to the moving raptor picture (GLMM: n = 44, z = −1.35, χ2 = 1.74, p = 0.187). In females but not males, the number of orientations was negatively correlated with the number of days that mice survived (n = 21, rs = −0.52, p = 0.016).
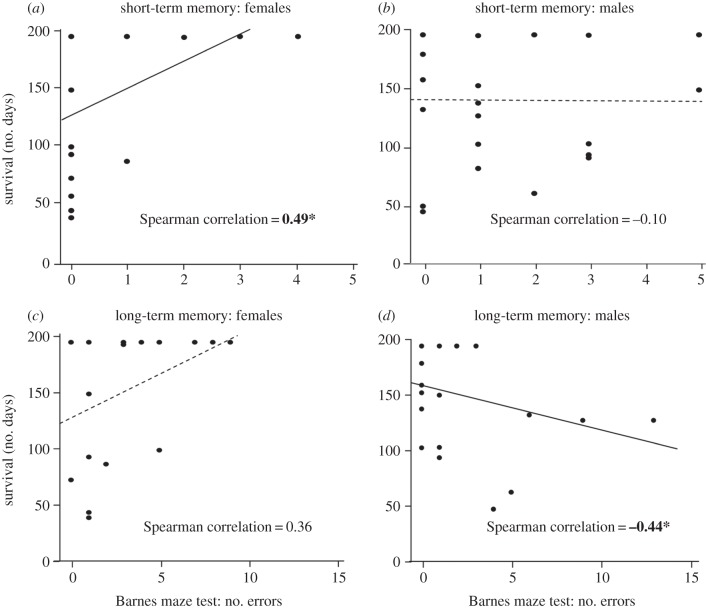
Survival was not significantly related to short-term (GLMM: n = 46, z = 1.46, χ2 = 0.37, p = 0.545) or long-term spatial memory (GLMM: n = 40, z = 0.76, χ2 = 0.01, p = 0.940). The number of errors in the short-term spatial memory trial was negatively correlated with the number of days that males survived (n = 20, rs = −0.44, p = 0.050; figure 2d) but positively correlated with the number of days that females survived (n = 22, rs = 0.49, p = 0.020; figure 2d).
Figure 2.
Correlations between number of days mice survived from 1 January to 15 July and number of errors prior to locating the correct hole in the short-term spatial memory trial for (a) females and (b) males, and in the long-term spatial memory trial for (c) females and (d) males. Lines represent the linear regressions; solid line: p < 0.05, dashed line: p > 0.05. Bold values with asterisks denote Spearman correlations: p < 0.05.
4. Discussion
To the best of our knowledge, this is the first study relating survival to individual variation in cognition in a free-living mammal. The relationship between survival until the onset of the breeding season and reaction time to a moving shadow in the field differed between female and male striped mice. Female survivors reacted faster than those that disappeared, possibly because greater attention to their environment increased the probability of early predator detection. Male survival was not related to reaction time to the same predation stimulus. Females that survived longer reacted less often over 10 consecutive times (but not slower) to a predator-stimulus presented under laboratory conditions, indicating that they habituated and thus reduced vigilance towards a harmless stimulus.
We found evidence that females which survived longer showed poorer short-term spatial memory. We propose that females may improve survival to drought by enhancing attention processes to avoid predation at the cost of decreased spatial learning processes. Trade-offs have been revealed between decision speed and accuracy in many ecologically relevant tasks and species, possibly resulting from developmental constraints on brain growth and functioning [15].
Male survival until the onset of the breeding season was positively related to long-term spatial memory. Male African striped mice need spatial memory to explore surrounding territories. They immediately immigrate if a breeding position becomes vacant, and thus have to learn the spatial structure of their new territory. Otherwise, they disperse at the onset of the breeding season [10], when their spatial performance increases [12]. Male survival thus appears to increase with greater spatial memory, maybe because they have to remember the configuration of their environment during exploration and dispersal, like polygynous voles [16].
5. Conclusion
We found a sex-specific relationship between survival until the onset of the breeding season and reaction time to predation stimuli in striped mice. This study revealed that individual variation in reaction time and spatial memory can be related to survival probability, which is important for understanding the selection pressures on cognition.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Neville Pillay who polished the English and two anonymous referees who helped to clarify the manuscript.
Ethics
Animal ethical clearance was provided by the University of the Witwatersrand, Johannesburg, South Africa (no. 2013/50/2A).
Data accessibility
The data are available as electronic supplementary material.
Authors' contributions
A.M. collected the data and did the statistical analyses. A.M. and C.S. designed the study and drafted the article. Both authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
Funding was provided by the University of Strasbourg Institute for Advanced Study.
References
- 1.Shettleworth SJ. 2001. Animal cognition and animal behaviour. Anim. Behav. 61, 277–286. ( 10.1006/anbe.2000.1606) [DOI] [Google Scholar]
- 2.Thornton A, Lukas D. 2012. Individual variation in cognitive performance: developmental and evolutionary perspectives. Phil. Trans. R. Soc. B 367, 2773–2783. ( 10.1098/rstb.2012.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Waal FBM, Ferrari PF. 2010. Towards a bottom-up perspective on animal and human cognition. Trends Cogn. Sci. 14, 201–207. ( 10.1016/j.tics.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 4.Morand-Ferron J, Cole EF, Quinn JL. 2016. Studying the evolutionary ecology of cognition in the wild: a review of practical and conceptual challenges. Biol. Rev. 91, 367–389. ( 10.1111/brv.12174) [DOI] [PubMed] [Google Scholar]
- 5.Dukas R, Bernays EA. 2000. Learning improves growth rate in grasshoppers. Proc. Natl Acad. Sci. USA 97, 2637–2640. ( 10.1073/pnas.050461497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amdam GV, Fennern E, Baker N, Rascón B. 2010. Honeybee associative learning performance and metabolic stress resilience are positively associated. PLoS ONE 5, e9740 ( 10.1371/journal.pone.0009740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis KL, Pharr VL, Dumas MJ, Britton GB, Field J. 1997. Classical conditioning provides paternity advantage for territorial male blue gouramis (Trichogaster trichopterus). J. Comp. Psychol. 111, 219–225. ( 10.1037/0735-7036.111.3.219) [DOI] [Google Scholar]
- 8.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. 2012. Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808–1812. ( 10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 9.Chancellor LV, Roth TC, LaDage LD, Pravosudov VV. 2011. The effect of environmental harshness on neurogenesis: a large-scale comparison. Dev. Neurobiol. 71, 246–252. ( 10.1002/dneu.20847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schradin C, Lindholm AK. 2011. Relative fitness of alternative male reproductive tactics in a mammal varies between years. J. Anim. Ecol. 80, 908–917. (doi:0.1111/j.1365-2656.2011.01831.x) [DOI] [PubMed] [Google Scholar]
- 11.Solmsen N, Johannesen J, Schradin C. 2011. Highly asymmetric fine-scale genetic structure between sexes of African striped mice and indication for condition dependent alternative male dispersal tactics. Mol. Ecol. 20, 1624–1634. ( 10.1111/j.1365-294X.2011.05042.x) [DOI] [PubMed] [Google Scholar]
- 12.Maille A, Pillay N, Schradin C. 2015. Seasonal variation in attention and spatial performance in a wild population of the African striped mouse (Rhabdomys pumilio). Anim. Cogn. 18, 1231–1242. ( 10.1007/s10071-015-0892-y) [DOI] [PubMed] [Google Scholar]
- 13.Rodriguiz RM, Wetsel WC. 2006. Assessments of cognitive deficits in mutant mice. In Animal models of cognitive impairment (eds Levin ED, Buccafusco JJ). Boca Raton, FL: CRC Press. [PubMed] [Google Scholar]
- 14.Barnes CA. 1979. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104. ( 10.1037/h0077579) [DOI] [PubMed] [Google Scholar]
- 15.Chittka L, Skorupski P, Raine N. 2009. Speed-accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. ( 10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 16.Gaulin SJ, FitzGerald RW, Wartell MS. 1990. Sex differences in spatial ability and activity in two vole species (Microtus ochrogaster and M. pennsylvanicus). J. Comp. Psychol. 104, 88–93. ( 10.1037/0735-7036.104.1.88) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available as electronic supplementary material.