Abstract
In many cooperatively breeding societies, subordinate individuals delay dispersal and independent breeding. The length of time that subordinates delay dispersal (subordinate tenure) is likely to have important implications for both subordinate and dominant fitness. However, quantitative comparisons of the subordinate tenure of males and females are rare, especially with respect to the presence of same- versus opposite-sex close kin. Here, we investigate subordinate tenure and how it is affected by the genetic relationship between subordinates and dominants in the cooperatively breeding southern pied babbler (Turdoides bicolor). We find that for males, longer subordinate tenures result in increased likelihood of attaining dominance. In the presence of an unrelated dominant male, tenure of subordinate males is significantly shorter, indicating nepotism among males. Female tenures are unaffected by the genetic relationship to either the dominant male or female. These results are some of the first to demonstrate that the sex of both the dominants and subordinates, and the genetic relationship between them, can have significant impacts on subordinate tenure and dispersal delays. Nepotism has likely played a vital role in the evolution of cooperative breeding in this species.
Keywords: subordinate tenure, nepotism, delayed dispersal, cooperative breeding
1. Introduction
Many cooperatively breeding societies are characterized by socially subordinate individuals that delay both dispersal and breeding, and then help raise the offspring of breeding individuals [1]. Subordinates may delay dispersal owing to ecological constraints on independent breeding and/or because delaying independent breeding until older is itself adaptive (reviewed by Covas & Griesser [2]). The presence of closely related dominants has been found to affect the likelihood of delayed dispersal of subordinates via changes in nepotistic tolerance or sexual competition [3–6]. For example, in the presence of unrelated dominant males (e.g. Siberian jays, Perisoreus infaustus), females (e.g. western bluebirds, Sialia mexicana) or both (e.g. Florida scrub jays, Aphelocoma coerulescens), subordinates lose nepotistic tolerance and are more likely to disperse [4,6,7]. On the other hand, when unrelated dominants provide adult subordinates with within-group breeding opportunities, sexual competition may result [1,8,9], increasing the likelihood of delayed dispersal (e.g. Seychelles warbler (Acrocephalus sechellensis) subordinate females [10]). However, a quantitative comparison of the length of subordinate tenure of males and females, especially with respect to the presence of same- versus opposite-sex close kin, has not been conducted. Direct comparison of males and females is important because, in most species, sexes vary in dispersal distance, timing or tactics, and the strength of nepotism or the cost of sexual competition may vary with subordinate sex or that of the unrelated dominant [5,6,9,11–13].
The southern pied babbler (Turdoides bicolor) is a cooperatively breeding passerine in which the dominant pair monopolize more than 95% of reproduction and though adult subordinates commonly compete to breed with unrelated dominants, they are rarely successful [14]. When subordinates attempt to breed, intrasexual competition with dominant individuals results; such competition is costly to the reproductive success of dominant females (competitive subordinate females destroy eggs), though not to dominant males [9,13]. Subordinates benefit from delaying dispersal: floaters fare badly compared with when they reside in groups [15], and older subordinates are more likely to attain dominance [12]. Males and females gain dominance at equal rates by filling natal and non-natal breeding vacancies or founding groups; additionally, females sometimes aggressively overthrow other dominant females [12]. Dominants can evict subordinates, but cannot prevent subordinate dispersal (A.R.R. 2016, unpublished data). Dispersal distance is often very short, with subordinates moving to territories that adjoin their natal group [8]. There is no sex-bias in inheritance of dominance or in the age or distance of natal dispersal, but dominant males are more sedentary than dominant females, probably due to the occurrence of aggressive overthrow among females [8,12]. Neither male nor female floaters breed with group members [14].
Here we first ask whether longer subordinate tenures affect the likelihood of attaining dominance. We then ask whether subordinate tenure is affected by group size, subordinate residence on its natal territory, or the subordinate's sex, age or relatedness to dominant individuals. We predict that subordinate sex and genetic relationship to the dominant pair are important in determining tenure, and consider two non-mutually exclusive hypotheses. First, nepotism should result in longer subordinate tenures in the presence of related dominant individuals. Nepotism by dominant males may be more likely because these more sedentary dominant males may benefit more from establishment of kin neighbourhoods. Second, sexual competition should result in shorter subordinate female tenures in the presence of unrelated dominant males, because reproductive activity by subordinate females is costly to dominant females. Sexual competition is not expected to affect male tenures because such competition is not costly to dominant males.
2. Material and methods
Data were collected from 2003 to 2014 from a habituated, colour-ringed population of southern pied babblers at the Kuruman River Reserve (26°58′ S; 21°49′ E) in the Kalahari Desert, South Africa. Babbler groups typically comprised an unrelated dominant pair with grown subordinate offspring of both sexes (mean group size (±s.e.) was 4.4 ± 0.1 adults; range: 2–12), though immigration of an unrelated dominant male or female occurred regularly [14]. Subordinate tenure could end through dispersal (leaving the group, including to become a floater or a subordinate in another group), death or inheritance of dominance. In the following analyses, we excluded birds that may have died (see the electronic supplementary material for details). First, we used a generalized linear mixed model (GLMM) with a binomial error structure to examine whether the length of subordinate tenure affected the likelihood of gaining dominance (1 = dominant, 0 = subordinate) at the end of tenure. Explanatory variables were subordinate sex, tenure (days) and their interaction. Group and subordinate identities were included as random terms, and the dataset included 103 tenures of 90 subordinates at 31 groups. We next investigated subordinate tenure only for subordinates living with one unrelated dominant, thus holding steady any indirect benefits (all subordinates helped). We used a GLMM with a negative binomial error structure. Tenure (the response variable) was measured as the number of days from when the subordinate began living with the unrelated dominant until the subordinate left the group or inherited dominance there (81 subordinate tenures at 27 groups). Explanatory variables included: (i) the age of the subordinate (days) at the beginning of tenure, (ii) natal territory (yes/no), (iii) group size (adults, not including focal subordinate: small = 2 or 3, medium = 4 or 5, large = 6+), (iv) whether the unrelated dominant was the same or the opposite sex, and the interactions of these with (v) subordinate sex. Group identity was included as a random term. Finally, we investigated length of subordinate tenure (days) of all subordinates (n = 134), with respect to their genetic relationship with group dominants (both related, unrelated to opposite-sex, unrelated to same-sex or unrelated to both) using Wilcoxon rank sum tests with Bonferroni's correction for multiple comparisons. In groups where subordinates spent their entire tenure with both parents, we adjusted tenures (subtracted 444 days: mean subordinate age ± s.e.m. at start of tenure with unrelated dominant was 443.6 ± 25.2 days) because tenures of these subordinates began at hatching. We examined males and females separately. See the electronic supplementary material for further details of the study system and statistical methods.
3. Results
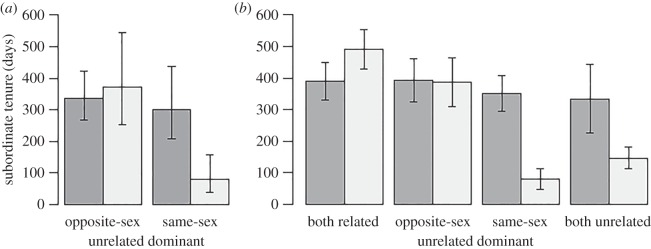
Males were significantly more likely to gain dominance as subordinate tenure became longer, while females were unaffected (electronic supplementary material, table S1; figure 1). When living with an unrelated dominant, there were significant sex differences in subordinate tenure according to the sex of the unrelated dominant. Specifically, males left their groups sooner when an unrelated dominant was the same sex (male), while there was no such effect for subordinate females (figure 2a; electronic supplementary material, table S2). Males living with unrelated dominant males stayed significantly shorter periods of time than did males living with related dominant males; availability of the dominant female as a potential breeding partner had no effect (table 1 and figure 2b). Females displayed no differences in subordinate tenure length with respect to relatedness to either member of the dominant pair (table 1 and figure 2b).
Figure 1.
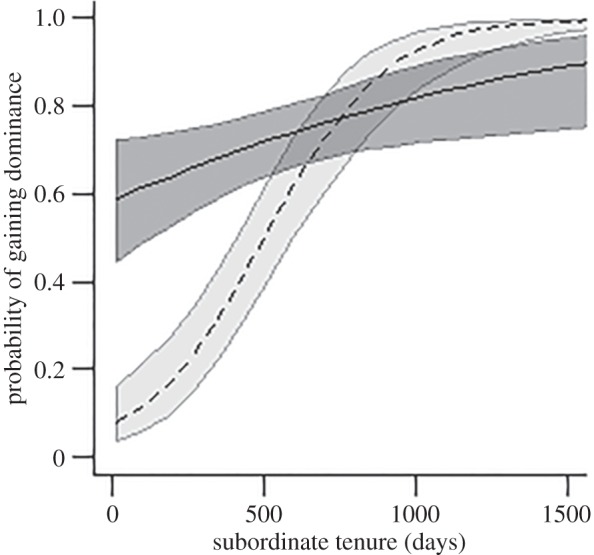
Likelihood of acquisition of dominance by females (dark grey, solid line) and males (light grey, dashed line) at the end of subordinate tenure. Means and standard errors generated from minimal model (electronic supplementary material, table S1).
Figure 2.
Subordinate tenure of females (dark grey) and males (light grey) in days in the presence of related or unrelated dominants. (a) GLMM output: means and standard errors generated from minimal model identified through model averaging (electronic supplementary material, table S2). (b) Raw data (mean ± s.e.m.): subordinate tenures when related to both dominants, unrelated to the opposite-sex dominant, unrelated to the same-sex dominant or unrelated to both dominants.
Table 1.
Intrasexual comparisons of subordinate tenure length with respect to genetic relatedness to the dominant pair, using Wilcoxon rank sum tests with Bonferroni's correction for multiple comparisons.
| female subordinates | unrelated to dominant male | unrelated to dominant female | unrelated to dominants |
|---|---|---|---|
| related to both dominants | 1.000 | 1.000 | 1.000 |
| unrelated to dominant male | 1.000 | 1.000 | |
| unrelated to dominant female | 1.000 | ||
| male subordinates | unrelated to dominant female | unrelated to dominant male | unrelated to dominants |
| related to both dominants | 0.219 | 0.008 | 0.005 |
| unrelated to dominant female | 0.02 | 0.123 | |
| unrelated to dominant male | 0.834 |
4. Discussion
Here, we show that longer subordinate tenure increases the likelihood of gaining dominance for males, but that subordinate tenure length does not affect female acquisition of dominance. We also show that male subordinate tenure is reduced in the presence of unrelated dominant males, but the presence of an unrelated dominant female (potential breeding partner) has no effect. We conclude that nepotism is important in determining tenure length in subordinate males, but that sexual competition is not, probably because such competition poses no cost to dominant male reproduction [13]. Conversely, and contrary to predictions, no clear pattern of nepotism or sexual competition affects subordinate female tenure.
Because dominants monopolize breeding, dominant positions represent very substantial fitness gains [14], and we found that subordinate males are more likely to acquire dominant positions after longer subordinate tenures. This is probably because males gain dominance only through filling a breeding vacancy, founding a new group or inheriting dominance, and must wait for optimal opportunities [12]. Females acquiring dominance through these routes must also wait, but in addition can aggressively oust established dominant females from their positions at any time [12]. Dominance queues, indicated by the presence of an older or same-age, same-sex subordinate in the group, make early dispersal by both male and female subordinates more likely; after dispersal in these circumstances, males are significantly less likely than females to acquire dominance (M.J.N.-F. 2016, unpublished data).
Dominant males gain both indirect and direct benefits from tolerance of their related male subordinates. We suggest that dominant males gain indirect fitness benefits when their own relatives (sons and/or brothers) are nepotistically prioritized in the queue of males waiting in the ‘safe haven’ of the group for local dominance opportunities [16]. Nepotistic tolerance also allows related subordinate males to inherit the dominant position in the event that the dominant male dies, provided the dominant female is unrelated and the subordinate male is the oldest such male [8]. Tolerant dominant males may also improve their direct fitness because there is significantly greater territory overlap when neighbouring dominants are close kin [17]. Because dominant males are more sedentary than dominant females, dominant males may derive greater direct benefits from close kin neighbours.
We expected an effect of decreased nepotistic tolerance on both sexes of subordinate, similar to other nepotistic species [4,6,7]. However, subordinate female tenures are unresponsive to changes in relatedness to the dominant pair (figure 2b and table 1). This is particularly surprising given that these females can impose costs on dominant females through sexual competition [9]. For unrelated dominant males, opportunities for direct fitness may outweigh the benefits of nepotism. Alternatively, male and/or female dominants may have less control over subordinate female dispersal than over subordinate males. Subordinate females may respond aggressively to decreases in nepotistic tolerance (higher aggression levels are found in females [18]), resulting potentially in more aggressive conflict but not decreased tenures. Indeed, increased aggression was observed in groups where subordinate females were unrelated to dominant males [9].
Nepotistic tolerance plays a vital role in shaping group-living societies, and in particular cooperatively breeding societies, when it results in tolerance of related individuals that impose costs through resource or reproductive competition [19]. In theoretical models, nepotism has been proposed to influence the evolution of delayed dispersal and cooperative breeding [16]. Empirically, however, the consequences of nepotism for dispersal are not widely understood, and often not framed as such (e.g. higher rates of eviction for more distant female kin in meerkats, Suricata suricatta [20]). We encourage future research investigating individual variation in subordinate tenure, with specific attention to variation in nepotistic tolerance.
Supplementary Material
Supplementary Material
Acknowledgements
Grateful thanks to Tim Clutton-Brock, Marta Manser, staff at KRR and all colleagues, students and assistants on the babbler project. Thanks also to the Kotzes and the de Bruins, who allowed land access. T. P. Flower and S. A. Kingma as well as two anonymous reviewers provided valuable comments.
Ethics
Research was approved by the Northern Cape Conservation Authority and by the Ethics Committee, UCT (AEC no. 2006/V15/AR).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
M.J.N.-F. helped collect the field data, designed and performed the statistical analyses and wrote the manuscript. A.R.R. habituated the study population, coordinated the field data collection, and helped to write the manuscript. M.J.N.-F. and A.R.R. approved the final version of the manuscript and are accountable for all aspects of the work, and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
We declare we have no competing interests.
Funding
M.J.N.-F.: DST/NRF Centre of Excellence at the PFIAO, University of Cape Town, and the National Science and Engineering Research Council, Canada (PDF 454522-2014). A.R.R.: DST/NRF Centre of Excellence at the PFIAO, University of Cape Town.
References
- 1.Cockburn A. 1998. Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177. ( 10.1146/annurev.ecolsys.29.1.141) [DOI] [Google Scholar]
- 2.Covas R, Griesser M. 2007. Life history and the evolution of family living in birds. Proc. R. Soc. B 274, 1349–1357. ( 10.1098/rspb.2007.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eikenaar C, Richardson DS, Brouwer L, Komdeur J. 2007. Parent presence, delayed dispersal, and territory acquisition in the Seychelles warbler. Behav. Ecol. 18, 874–879. ( 10.1093/beheco/arm047) [DOI] [Google Scholar]
- 4.Ekman J, Griesser M. 2002. Why offspring delay dispersal: experimental evidence for a role of parental tolerance. Proc. R. Soc. B 269, 1709–1713. ( 10.1098/rspb.2002.2082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson JL, Ferree ED, Stern CA, Swift R, Zuckerberg B. 2014. Delayed dispersal in western bluebirds: teasing apart the importance of resources and parents. Behav. Ecol. 25, 843–851. ( 10.1093/beheco/aru042) [DOI] [Google Scholar]
- 6.Goldstein JM, Woolfenden GE, Hailman JP. 1998. A same-sex stepparent shortens a prebreeder's duration on the natal territory: tests of two hypotheses in Florida scrub-jays. Behav. Ecol. Sociobiol. 44, 15–22. ( 10.1007/s002650050510) [DOI] [Google Scholar]
- 7.Dickinson JL, Euaparadorn M, Greenwald K, Mitra C, Shizuka D. 2009. Cooperation and competition: nepotistic tolerance and intrasexual aggression in western bluebird winter groups. Anim. Behav. 77, 867–872. ( 10.1016/j.anbehav.2008.11.026) [DOI] [Google Scholar]
- 8.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Ridley AR. 2012. Inbreeding avoidance mechanisms: dispersal dynamics in cooperatively breeding southern pied babblers. J. Anim. Ecol. 81, 876–883. ( 10.1111/j.1365-2656.2012.01983.x) [DOI] [PubMed] [Google Scholar]
- 9.Nelson-Flower MJ, Hockey PAR, O'Ryan C, English S, Thompson AM, Bradley K, Rose R, Ridley AR. 2013. Costly reproductive competition between females in a monogamous cooperatively breeding bird. Proc. R. Soc. B 280, 20130728 ( 10.1098/rspb.2013.0728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson DS, Burke T, Komdeur J. 2002. Direct benefits and the evolution of female-biased cooperative breeding in Seychelles warblers. Evolution 56, 2313–2321. ( 10.1111/j.0014-3820.2002.tb00154.x) [DOI] [PubMed] [Google Scholar]
- 11.Ekman J. 2002. Fighting to stay: the role of sibling rivalry for delayed dispersal. Anim. Behav. 64, 453–459. ( 10.1006/anbe.2002.3075) [DOI] [Google Scholar]
- 12.Raihani NJ, Nelson-Flower MJ, Golabek KA, Ridley AR. 2010. Routes to breeding in cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol. 41, 681–686. ( 10.1111/j.1600-048X.2010.05211.x) [DOI] [Google Scholar]
- 13.Nelson-Flower MJ, Ridley AR. 2015. Male-male competition is not costly to dominant males in a cooperatively breeding bird. Behav. Ecol. Sociobiol. 69, 1997–2004. ( 10.1007/s00265-015-2011-0) [DOI] [Google Scholar]
- 14.Nelson-Flower MJ, Hockey PAR, O'Ryan C, Raihani NJ, du Plessis MA, Ridley AR. 2011. Monogamous dominant pairs monopolize reproduction in the cooperatively breeding pied babbler. Behav. Ecol. 22, 559–565. ( 10.1093/beheco/arr018) [DOI] [Google Scholar]
- 15.Ridley AR, Raihani NJ, Nelson-Flower MJ. 2008. The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers Turdoides bicolor. J. Avian Biol. 39, 389–392. ( 10.1111/j.2008.0908-8857.04479.x) [DOI] [Google Scholar]
- 16.Kokko H, Ekman J. 2002. Delayed dispersal as a route to breeding: territorial inheritance, safe havens, and ecological constraints. Am. Nat. 160, 468–484. ( 10.1086/342074) [DOI] [PubMed] [Google Scholar]
- 17.Humphries DJ. 2013. The mechanisms and function of social recognition in the cooperatively breeding Southern pied babbler, Turdoides bicolor. PhD thesis, Macquarie University, Sydney, Australia.
- 18.Raihani NJ, Ridley AR, Browning LE, Nelson-Flower MJ, Knowles S. 2008. Juvenile female aggression in cooperatively breeding pied babblers: causes and contexts. Ethology 114, 452–458. ( 10.1111/j.1439-0310.2008.01482.x) [DOI] [Google Scholar]
- 19.Perrin N, Lehmann L. 2001. Is sociality driven by the costs of dispersal or the benefits of philopatry? A role for kin-discrimination mechanisms. Am. Nat. 158, 471–483. ( 10.1086/323114) [DOI] [PubMed] [Google Scholar]
- 20.Clutton-Brock TH, Hodge SJ, Flower TP, Spong GF, Young AJ. 2010. Adaptive suppression of subordinate reproduction in cooperative mammals. Am. Nat. 176, 664–673. ( 10.1086/656492) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.