Abstract
Behavioural phenotypes may provide a means for identifying individuals that disproportionally contribute to disease spread and epizootic outbreaks. For example, bolder phenotypes may experience greater exposure and susceptibility to pathogenic infection because of distinct interactions with conspecifics and their environment. We tested the value of behavioural phenotypes in larval amphibians for predicting ranavirus transmission in experimental trials. We found that behavioural phenotypes characterized by latency-to-food and swimming profiles were predictive of disease susceptibility and infectiousness defined as the capacity of an infected host to transmit an infection by contacts. While viral shedding rates were positively associated with transmission, we also found an inverse relationship between contacts and infections. Together these results suggest intrinsic traits that influence behaviour and the quantity of pathogens shed during conspecific interactions may be an important contributor to ranavirus transmission. These results suggest that behavioural phenotypes provide a means to identify individuals more likely to spread disease and thus give insights into disease outbreaks that threaten wildlife and humans.
Keywords: super-spreader, ranavirus, amphibian, emerging disease, epidemiology
1. Introduction
While individual variation in susceptibility to pathogens is an important driver of disease dynamics, some individuals are much more effective at transmitting infections and thus can disproportionally contribute to the risk of disease outbreaks. A priori identification of such individual hosts is challenging, but essential to understanding and potentially managing disease outbreaks. These hosts are expected to have greater probabilities of (i) infection given pathogen exposure (susceptibility), (ii) transmission of pathogens per contact, (iii) contacts, (iv) harbouring or shedding greater pathogen populations and (v) persistence with infection (tolerance) [1–3]. Because host behaviour and physiology underlie such disease dynamics, functional traits that integrate and represent characteristic phenotypes could be valuable in the a priori identification of ‘super-spreaders’—individuals with a disproportionate potential to spread disease [1,2,4].
Animals often exhibit a spectrum of behavioural phenotypes ranging from bolder-aggressive individuals to passive individuals [5,6]. Relatively bolder individuals often forage more to support faster development and growth rates, compared with passive and slower-growing individuals [7–10]. For bolder animals, these behaviours may induce greater exposure to pathogens, as they exploit more habitat space and food sources [11]. For example, larval amphibians exhibit consistent bold phenotypes associated with high foraging, growth and development rates, whereas passive larvae exhibit solitary swimming behaviours and lower foraging rates [8,9]. In larval amphibians, such behavioural phenotypes are also predictors of behaviours to resist macroparasites [12]. How behavioural phenotypes influence infectious disease transmission, however, is unclear. Behavioural phenotypes could underlie disease transmission, because variation among individuals in activity and conspecific interactions could influence both exposure to pathogens and host infectiousness. Infectiousness is defined as the capacity of an infected host to transmit an infection by contact with a susceptible host [1,2,13]. Here, we measured infectiousness as the proportion of susceptible animals infected after exposure to an infected conspecific. In addition, associated growth and developmental traits could also influence susceptibility and infectiousness of differing host phenotypes if these life-history patterns influence metabolic and immunity trade-offs [11,14–16].
Here, we used a larval amphibian and ranavirus disease system to test the hypothesis that behavioural phenotypes are predictive of both susceptibility to infection and infectiousness. To characterize behavioural phenotypes of individuals, we established replicate groups in which we measured foraging, movement patterns, growth and development in association with latency-to-food, a repeatable behavioural assay of competitiveness [8]. We then exposed these groups to conspecifics experimentally infected with ranavirus, an emerging disease of ectotherms [17]. Through manipulation and quantification of disease dynamics, we show that behavioural phenotypes can be a predictive tool for identifying individuals that disproportionately contribute to disease outbreaks.
2. Material and methods
Larvae from wild collected wood frog (Lithobates sylvaticus) eggs (IDNR permit no. NH12.5566) were sorted into and maintained in 20 replicate groups of eight larvae per tank (SIU IACUC no. 12-011). Individuals (n = 160 total) were tagged with unique subcutaneous elastomer tags (Northwest Marine Technology, Inc.) [18]. We quantified behavioural phenotypes in all animals by measuring time spent foraging, swimming and resting during 3 min observations repeated three times over a week. We also measured latency-to-food (how long all individuals took to find food) after a 24 h food restriction (referred to as ‘latency’ hereafter) (see the supplementary methods). These traits characterize boldness (short latency, low swimming rates) and passive (long latency, high solitary swimming rates) phenotypes in larval wood frogs [8], and were tested as predictors of disease transmission in trials.
(a). Infection and transmission trials
A focal animal from each group (smallest or largest per tub, by mass) was infected by 24 h exposure to a lethal dose (104 pfu ml−1) of FV3-like ranavirus [17], and then reintroduced to their groups for 24 h transmission trials. Circulating water past UV filters inactivated free virions, restricting infections to direct transmission. To test how variance in focal phenotypes influenced infectiousness via direct transmission, focal contacts, along with the time focals spent aggregated within one body length of non-focals, were quantified. Three focals died and their tanks were excluded. Immediately after trials, focal animals were placed in 40 ml of water for 1 h to test if shed virus was associated with infectiousness. Focal animals were euthanized and the livers extracted. Non-focal animals were placed in individual 500 ml containers and monitored for survival until metamorphosis. Infection status was quantified via quantitative polymerase chain reaction (qPCR) of all livers. The infectiousness of the focal animals was measured as the proportion of infected non-focals.
(b). Viral shedding and viral load quantification
Viral DNA was extracted from water samples (following [19]) and livers (Puregene; Life Technologies), and quantified by qPCR using standard primers and probes for the ranavirus major capsid protein in a StepOnePlus™ System (Applied Biosystems) [17].
(c). Statistical analyses
To test if infectiousness was predicted by mean swimming, foraging and latency-to-food behaviours of focals, we used a general liner model (GLM) with Gosner developmental stage as a covariate. Note that focal size class had no association with disease outcomes. For focal behaviours during transmission, a GLM was used to test for associations between focal Gosner stage, viral shedding rates, contacts and time aggregated with non-focals. To assess if susceptibility to infection in non-focals was predicted by their latency-to-food, we used mixed-effects logistic regression with tub as a random factor. Logistic regression was also used to test if developmental rates were associated with the probability of death due to infection, because development is an important factor in ranavirus infection [17]. Cox proportional hazards models estimated the effects of development and growth rates, latency-to-food, and liver viral loads on time to death and metamorphosis. All analyses were conducted in JMP v. 11.0 [20].
3. Results
Focal larvae infectiousness (proportion of non-focals infected) was negatively associated with time spent swimming (figure 1a; F1,12 = 7.8, p = 0.02; n = 17) and latency-to-food (F1,12 = 8.1, p = 0.02) behaviours measured prior to infection; foraging and Gosner developmental stage had no effect. While there was no multicollinearity, these behaviours were correlated (swim × forage: r = −0.38, p < 0.001; latency × forage: r = −0.25, p < 0.01; latency × swim: r = 0.23, p < 0.01). The proportion of non-focals infected per tub tended to be positively associated with focal viral shedding rates (figure 1b; F1,12 = 4.3, p = 0.06) and negatively associated with focal contact rates (figure 1c; F1,12 = 7.4, p = 0.02); but not time aggregated with non-focals (F1,12 = 3.0, p = 0.1); Gosner stage was not associated. Focal contacts were negatively associated with their liver viral loads (slope = −5.34, F1,13 = 4.8, p = 0.05).
Figure 1.
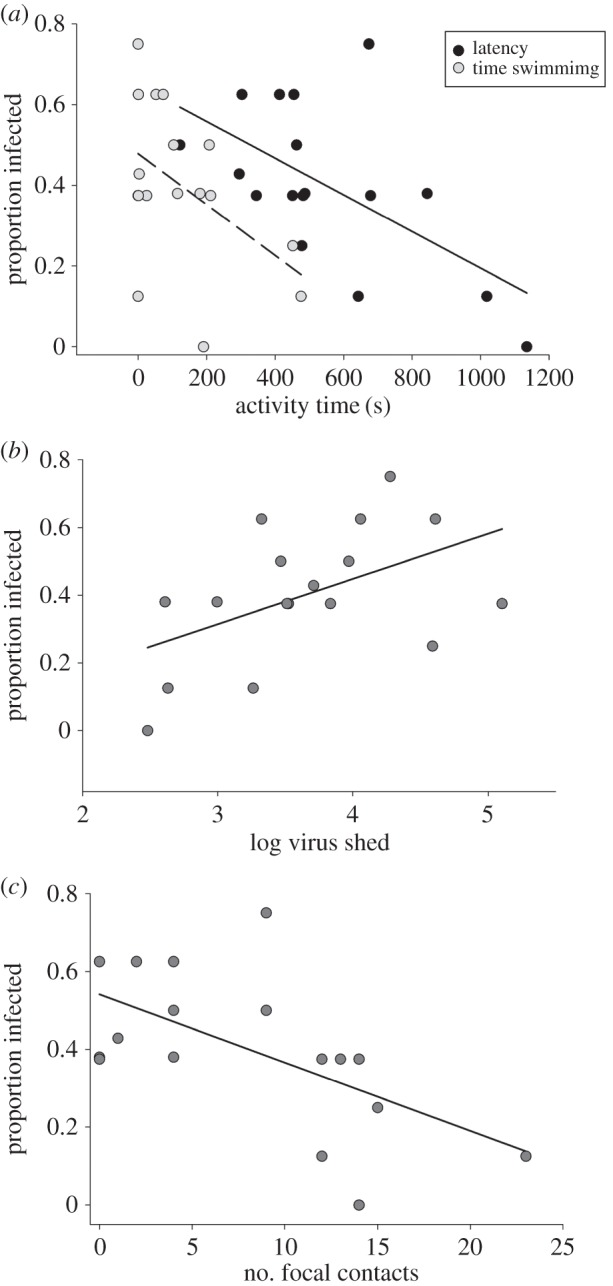
Focal infectiousness (percentage non-focals infected) was predicted by focal latency-to-food and swimming behaviours (measured prior to transmission trials) (a), and was associated with focal viral shedding rates (shed viral DNA imputed for two focals) (b) and contacts (c).
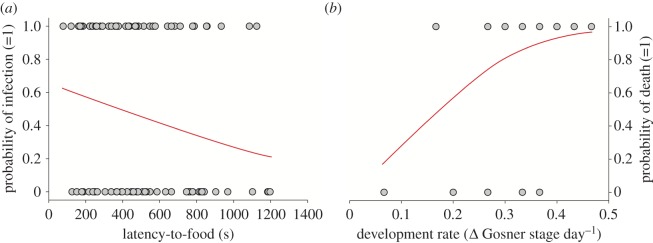
For non-focals, latency but not swimming or foraging predicted their susceptibility to infection (figure 2a; χ2 = 4.2, p = 0.04; n = 119). When infected, the probability of death was associated with development (figure 2b; χ2 = 3.7, p = 0.05), but not growth (χ2 = 0.01, p = 0.92); viral load increased time to death 0.5 times (p < 0.0001). Infection delayed metamorphosis 0.5 times among survivors (χ2 = 6.4, p = 0.01).
Figure 2.
Non-focal susceptibility to infection (1 = infected) was predicted by their latency-to-food (a), and mortality (1 = dead) when ‘infected’ was associated with ‘development rate’ (b). (Online version in colour.)
4. Discussion
Our study demonstrates that behavioural phenotypes can provide a predictive framework to identify infectious and susceptible individuals. Identifying such individuals, and the traits that contribute to their exposure, susceptibility and infectiousness can provide insights into disease spread [1–3,11,21,22]. We found that larval amphibians exhibit behaviours that, when measured prior to pathogen exposure, can predict infectiousness and susceptibility to ranavirus.
Shorter latency-to-food and lower swimming rates predicted greater infectiousness, suggesting bolder larvae that more aggressively accessed food are more likely to contribute to a ranavirus outbreak. Transmission may be via greater interactions with conspecifics near food sources, whereas more passive larvae exhibit more solitary swimming in the water column. However, contrary to our expectations, we found an inverse relationship where more contacts led to fewer infections. This result could be due to sickness behaviours such as lethargy. Focals in this experiment exhibited a negative association between viral loads and contact rates, suggesting greater pathogen loads reduced activity and conspecific interactions. This result may reflect variation in tolerance to infection in these larvae, which if present could play an important role in the capacity for individuals to act as ‘super-spreaders’ [1]. Because viral shedding was also predictive of transmission, these results suggest that if traits such as metabolic rate and immunocompetence influence pathogen shedding rates then associated host physiological phenotypes may also be important contributors to ranavirus transmission.
Our finding that latency phenotypes also predicted infection risk among non-focal animals corroborates behavioural phenotypes as a forecasting tool. Bolder non-focal animals were more likely to become infected than solitary–passive larvae, suggesting higher viral exposure via conspecific interactions, and potentially greater susceptibility. Susceptibility to infection may vary with behavioural phenotypes if, for example, bolder individuals have reduced immunocompetence owing to allocation trade-offs with factors such as rapid growth [11,16]. Animal personality studies often find bolder phenotypes also have relatively higher metabolic, growth and development rates that trade-off with other life-history traits [10,23,24]. Indeed, in a follow-up study, we have found that metabolic rates of larval wood frogs are associated with the behavioural phenotypes described here (unpublished data, 2015). Taken together, these prospects suggest studies that testing for mechanistic links between behavioural phenotypes, resource allocation and physiological function could provide new insights into the factors underlying resistance and tolerance of infection. Given that these aspects of immunity are central to the capacity of ‘super-spreaders' to transmit infections, this approach may provide a means to identify individuals more likely to spread disease and thus give insights into disease outbreaks that threaten wildlife and humans alike.
Supplementary Material
Acknowledgements
Our thanks to laboratory assistants and all reviewers.
Data accessibility
The dataset supporting this article is available via Dryad at http://dx.doi.org/10.5061/dryad.sd6jq [25].
Ethics
Animals were collected under state permit (IDNR no. NH12.5566). All institutional and national guidelines for the care and humane use of laboratory animals were followed and approved by the SIU IACUC (no. 12-011).
Authors' contributions
A.A. and L.K. conducted the experiments. A.A., L.K. and R.W.W. conceived, designed, analysed the data, and equally drafted the manuscript. A.A., L.K. and R.W.W. approve final publication. A.A., L.K. and R.W.W. are accountable for all aspects of the work and ensuring accuracy.
Competing interests
The authors have no competing interests.
Funding
This research was supported by an SIUC start-up grant and a SIUC Seed Grant to R.W.W.
References
- 1.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. 2005. Superspreading and the effect of individual variation on disease emergence. Nature 438, 355 ( 10.1038/nature04153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cronin JP, Rúa MA, Mitchell CE. 2014. Why is living fast dangerous? Disentangling the roles of resistance and tolerance of disease. Am. Nat. 184, 172–187. ( 10.1086/676854) [DOI] [PubMed] [Google Scholar]
- 3.De Leo GA, Dobson AP. 1996. Allometry and simple epidemic models for microparasites. Nature 379, 720–722. ( 10.1038/379720a0) [DOI] [PubMed] [Google Scholar]
- 4.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct. Ecol. 25, 48–60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 5.Dingemanse NJ, Kazem AJ, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 6.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 7.Wilson ADM, Krause J. 2012. Personality and metamorphosis: is behavioral variation consistent across ontogenetic niche shifts? Behav. Ecol. 23, 1316–1323. ( 10.1093/beheco/ars123) [DOI] [Google Scholar]
- 8.Warne RW, Kardon A, Crespi EJ. 2013. Physiological, behavioral and maternal factors that contribute to size variation in larval amphibian populations. PLoS ONE 8, e76364 ( 10.1371/journal.pone.0076364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urszán T, Török J, Hettyey A, Garamszegi L, Herczeg G. 2015. Behavioural consistency and life history of Rana dalmatina tadpoles. Oecologia 178, 129–140. ( 10.1007/s00442-014-3207-0) [DOI] [PubMed] [Google Scholar]
- 10.Le Galliard J-F, Paquet M, Cisel M, Montes-Poloni L. 2013. Personality and the pace-of-life syndrome: variation and selection on exploration, metabolism and locomotor performances. Funct. Ecol. 27, 136–144. ( 10.1111/1365-2435.12017) [DOI] [Google Scholar]
- 11.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koprivnikar J, Gibson CH, Redfern JC. 2012. Infectious personalities: behavioural syndromes and disease risk in larval amphibians. Proc. R. Soc. B 279, 1544–1550. ( 10.1098/rspb.2011.2156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beldomenico P, Begon M. 2010. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 25, 21–27. ( 10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 14.Martin L, Hasselquist D, Wikelski M. 2006. Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575. ( 10.1007/s00442-005-0314-y) [DOI] [PubMed] [Google Scholar]
- 15.Cable J, Enquist B, Moses M. 2007. The allometry of host–pathogen interactions. PLoS ONE 2, e0001130 ( 10.1371/journal.pone.0001130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemelä PT, Vainikka A, Hedrick AV, Kortet R. 2012. Integrating behaviour with life history: boldness of the field cricket, Gryllus integer, during ontogeny. Funct. Ecol. 26, 450–456. ( 10.1111/j.1365-2435.2011.01939.x) [DOI] [Google Scholar]
- 17.Warne RW, Crespi EJ, Brunner JL. 2011. Escape from the pond: stress and developmental responses to ranavirus infection in wood frog tadpoles. Funct. Ecol. 25, 139–146. ( 10.1111/j.1365-2435.2010.01793.x) [DOI] [Google Scholar]
- 18.Warne RW, Crespi EJ. 2015. Larval growth rate and sex determine resource allocation and stress responsiveness across life stages in juvenile frogs. J. Exp. Zool. A 323, 191–201. ( 10.1002/jez.1911) [DOI] [PubMed] [Google Scholar]
- 19.Hall EM, Crespi EJ, Goldberg CS, Brunner JL. 2016. Evaluating environmental DNA-based quantification of ranavirus infection in wood frog populations. Mol. Ecol. Resour. 16, 423–433. ( 10.1111/1755-0998.12461) [DOI] [PubMed] [Google Scholar]
- 20.SAS I. 1999. SAS/STAT user's guide, vers. 8. Cary, NC: SAS Institute Inc. [Google Scholar]
- 21.Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM, Prins HHT. 2013. Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8, e0054341 ( 10.1371/journal.pone.0054341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Civitello DJ, Rohr JR. 2014. Disentangling the effects of exposure and susceptibility on transmission of the zoonotic parasite Schistosoma mansoni. J. Anim. Ecol. 83, 1379–1386. ( 10.1111/1365-2656.12222) [DOI] [PubMed] [Google Scholar]
- 23.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mittelbach GG, Ballew NG, Kjelvik MK. 2014. Fish behavioral types and their ecological consequences. Can. J. Fish. Aquat. Sci. 71, 1–18. ( 10.1139/cjfas-2013-0558) [DOI] [Google Scholar]
- 25.Araujo A, Kirschman L, Warne RW. 2016. Data from: Behavioural phenotypes predict disease susceptibility and infectiousness. Dryad Digital Repository: 10.5061/dryad.sd6jq. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting this article is available via Dryad at http://dx.doi.org/10.5061/dryad.sd6jq [25].