Abstract
Background:
Refractory status epilepticus is often treated with third-line therapy, such as pentobarbital coma. However, its use is limited by side effects. Recognizing and preventing major and minor adverse effects of prolonged pentobarbital coma may increase good outcomes. This study retrospectively reviewed direct and indirect medical and surgical pentobarbital coma.
Methods:
Retrospective chart review of all patients with refractory status epilepticus treated with pentobarbital over a 1 year period at a large tertiary care center. We collected baseline data, EEG data, and complications that were observed.
Results:
Overall, nine patients [median age 46.4 (IQR 21.7, 75.5) years] were induced with pentobarbital coma median 11 (IQR 3, 33) days after seizure onset for a median of 9 (IQR 3.5, 45.4) days. A total of four to eight concurrent antiepileptics were tried prior to the pentobarbital coma. Phenobarbital, due to recurrence of seizures on weaning pentobarbital coma, was required in seven patients. Observed complications included peripheral neuropathy (77.8%), cerebral atrophy (33.3%), volume overload (44.4%), renal/metabolic (77.8%), gastrointestinal (66.6%), endocrine (55.6%), cardiac/hemodynamic/vascular (77.8%), respiratory (100%), and infectious (77.8%). The number of complications trended with duration of induced coma but was nonsignificant. Median ICU length of stay was 40 (IQR 28, 97.5) days. Overall, five patients were able to follow commands after a median 37 (IQR 25.5, 90) days from coma onset. There were eight patients that were discharged from hospital with three remaining in a prolonged unresponsive state. There was one patient that died prior to discharge.
Conclusions:
This study highlights the high morbidity in patients with refractory status epilepticus requiring pentobarbital coma. Anticipating and addressing the indirect and direct complications in prolonged pentobarbital coma may improve survival and functional outcomes in patients with refractory status epilepticus.
Keywords: cerebral atrophy, pentobarbital coma, refractory status epilepticus
Introduction
Status epilepticus is a commonly encountered neurological emergency with significant morbidity and mortality [Lowenstein and Alldredge, 1998; Pugin et al. 2014; Legriel et al. 2010]. It is initially treated with benzodiazepines and one or more antiepileptic drugs. Seizures persisting despite these measures are termed refractory status epilepticus, which occurs in 23–43% of cases [Novy et al. 2010]. Once status epilepticus is refractory, induction with deep sedation with intravenous pentobarbital is acceptable [Pugin et al. 2014]. Unfortunately, high doses of barbiturates have adverse effects that may limit their use, particularly if needed for prolonged period [Schalen et al. 1992a, 1992b; Claassen et al. 2002; Stover and Stocker, 1998; Pugin et al. 2014; Yaffe and Lowenstein, 1993]. Recognizing and addressing direct and indirect adverse effects may improve good outcomes from pentobarbital coma. This retrospective review of consecutive critically ill patients with refractory status epilepticus aims to identify observed direct and indirect medical and surgical complications that occur during pentobarbital-induced coma. This study highlights the ability to use pentobarbital for a prolonged period.
Methods
We retrospectively reviewed the electronic medical records over a 1-year period at a large tertiary referral center for adult cases (i.e. ⩾18 years of age) of refractory status epilepticus treated with pentobarbital coma. The institutional review board approved this study.
Patient management
Continuous electroencephalography (CEEG) was recorded using 21 electrodes placed according to the International 10–20 System by certified EEG technologists and interpreted by board-certified electroencephalographers. CEEG seizures were defined as evolving rhythms in frequency, distribution, or morphology at ⩾2 Hertz (Hz) for ⩾10 seconds duration. Status epilepticus was classified as being convulsive (continued seizure activity for ⩾5 minutes or multiple seizures without return of consciousness) or nonconvulsive (continuous ictal pattern lasting >30 minutes or ictal pattern present in >50% of 1 hour of CEEG). Refractory status epilepticus was defined as continuous seizure activity, clinically or electrographically, despite treatment with a minimum of two antiepileptics. Withdrawal seizures were defined as occurring within 48 hours of weaning pentobarbital coma. Patients were treated for status epilepticus at the discretion of the treating physician following proposed algorithms using a benzodiazepine followed by anticonvulsant(s) (Lowenstein and Alldredge, 1998). Refractory status epilepticus was treated with pentobarbital. Pentobarbital is an anesthetic composed of 50 mg pentobarbital sodium in a propylene glycol vehicle (40%), alcohol (10%) and water to volume with an adjusted pH of 9.5.
Data acquisition
Electronic medical records were reviewed for patient demographic data, past medical history/prior history of epilepsy, status epilepticus etiology, inpatient medications, discharge data, and medical and surgical complications that occurred while in pentobarbital coma. Complications were identified by the following systems: neurological (peripheral neuropathy, cerebral atrophy on serial neuroimaging), respiratory (prolonged ventilation, chest tube, pulmonary embolus, requiring tracheostomy/percutaneous endoscopic gastrostomy), electrolytes (lactic acidosis), head/ears/eyes/nose/throat (HEENT)/skin (anasarca/tongue swelling/penile swelling, exposure keratoconjunctivitis), vascular [deep vein thrombosis (DVT)], cardiac (myocardial dysfunction, hypotension requiring pressor), endocrine (adrenal insufficiency, hypothermia, hypothyroidism), gastrointestinal [ileus, requiring total parenteral nutrition (TPN)], transaminitis [i.e. twice upper limit of normal for aspartate transaminase (AST) and alanine transaminase (ALT)], infectious [yeast in urine/lungs, sepsis, pneumonia, urinary tract infection (UTI)], and renal [acute renal failure, requiring continuous veno–veno hemodialysis (CVVHD)]. This study was approved by the institutional review board.
Data analysis
Data were analyzed with descriptive statistics. Linear regression was used to analyze the complication rate and time in pentobarbital coma. Statistics were performed with GraphPad InStat version 3.00 for Windows 95 (GraphPad Software, San Diego, CA, USA).
Review of literature
A review of the published, English literature was performed through MEDLINE using text words or medical subject headings containing ‘pentobarbital status epilepticus’, ‘pentobarbital complication’, ‘pentobarbital safety’, and ‘refractory status epilepticus’. We excluded manuscripts that were individual case reports, pediatrics, animal studies, review articles, guidelines, and surveys. Extracted data included number of patients, duration of pentobarbital coma, complications (cardiac, respiratory, infectious, and other), and mortality.
Results
Patient characteristics
Overall, nine patients with a median age 46.4 [interquartile range (IQR) 21.7, 75.5] years were identified as having refractory status epilepticus (Table 1). The etiologies for the underlying seizures included remote stroke, viral encephalitis, hypoxic ischemic encephalopathy, liver failure, meningioma, N-methyl-D-aspartate (NMDA) limbic encephalitis, midbrain structural lesion from prior surgery, and medically refractory epilepsy in two (patients: cryptogenic and febrile seizure with hippocampal sclerosis).
Table 1.
Patient characteristics.
| No. | Age (yrs) | Etiology | Duration of seizure prior to coma (days) | Outcome |
|---|---|---|---|---|
| 1 | 80.2 | Remote CVA | 9 | LTAC |
| 2 | 21.0 | Viral Enceph | 2 | LTAC |
| 3 | 55.0 | Liver failure | 45 | Expired |
| 4 | 42.9 | Midbrain lesion | 78 | SNF |
| 5 | 85.7 | HIE | 2 | LTAC |
| 6 | 70.8 | Meningioma | 11 | LTAC |
| 7 | 19.1 | Cryptogenic | 11 | LTAC |
| 8 | 46.4 | Febrile sz/HS | 4 | LTAC |
| 9 | 22.4 | NMDA LE | 21 | LTAC |
| Median (IQR) | 46.4 (21.7, 75.5) | 11 (3, 33) |
CVA, cerebrovascular accident; HIE, hypoxic ischemic encephalopathy; HS, hippocampal sclerosis; IQR, interquartile range; LTAC, long-term acute care; NMDA, ; LE, limbic encephalitis; SNF, skilled nursing facility; sz, seizure.
Anticonvulsant treatment
Overall, 4–8 antiepileptics were tried prior to the start of the pentobarbital coma (Table 2). All patients were induced in a pentobarbital coma [median bolus 10 (IQR 5, 10) mg/kg; median maintenance of 3 (IQR 1.5, 3.5) mg/kg/hr] with an aim to burst suppression at a rate of 1–2 bursts every 10 seconds. Pentobarbital coma was initiated a median of 11 days (IQR 3, 33) after seizure onset. There was one patient that required midazolam infusion after pentobarbital secondary to a nationwide shortage of pentobarbital. Pentobarbital coma was maintained for a median of 9 (IQR 3.5, 45.5) days with a maximum of 105 days. Overall, seven of the nine patients needed institution of phenobarbital due to failure to successfully wean pentobarbital coma (i.e. withdrawal seizures).
Table 2.
Sequential therapies provided for treatment of refractory status epilepticus.
| Patient |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Antiepileptic | 1st | PHT | PHT | PHT | PHT | LEV | PHT | PHT | ZON | PHT |
| 2nd | VPA | LEV | LEV | ZON | Propofol | LTG | VPA | Primidone | Propofol | |
| 3rd | MDZ | VPA | LCM | LCM | LZM | LCM | Propofol | VPA | LCM | |
| 4th | LEV | Propofol | Propofol | LEV | LCM | PGB | LV | LEV | LEV | |
| 5th | LCM | LCM | PB | KLO | PHT | VPA | PB | MDZ | MDZ | |
| 6th | PB | MDZ | PHB | PB | PHT | LCM | PB | PB | ||
| 7th | PHB | PB | PB | LEV | TPM | LCM | PHB | |||
| 8th | PHB | Propofol | Ketogenic diet | PHB | FBM | |||||
| 9th | FBM | PB | PHB | MDZ | ||||||
| 10th | PHB | FBM | ||||||||
FBM, felbamate; KLO, klonopin; LCM, lacosamide; LEV, levetiracetam; LZM, lorazepam injection; MDZ, midazolam infusion; PB, pentobarbital infusion; PGB, pregabalin; PHB, phenobarbital; PHT, phenytoin; Propofol, propofol infusion; VPA, valproic acid; ZON, zonegran.
Safety
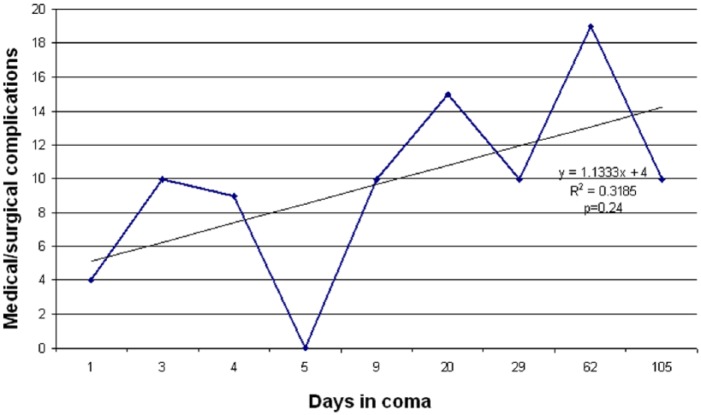
The indirectly or directly observed medical and surgical complications observed in patients in pentobarbital coma included peripheral neuropathy (n = 7), volume overload (pleural effusions requiring chest tubes, n = 2; anasarca, n = 3; and massive tongue swelling, n = 3), renal/metabolic (hemodialysis, n = 4; lactic acidosis, n = 7, with a propylene glycol level of 58mg/dl; none had other significant electrolyte disturbance), gastrointestinal (ileus, n = 5; diverting colostomy for sacral ulcer, n = 1; significant transaminitis, n = 3), endocrine (adrenal insufficiency, n = 2; persistent hypothermia, n = 3; thyroid function abnormalities, n = 1), cardiac/hemodynamic/vascular (hypotension requiring pressors, n = 7; cardiomyopathy with myocardial dysfunction/heart failure defined by ejection fraction (EF) < 40% or prolonged corrected QT cardiac interval (i.e. QTc) > 520 ms, n = 2; DVT, n = 2; pulmonary embolism, n = 1), respiratory (need for prolonged mechanical ventilation, n = 9, of which 6 required ventilation > 30 days; tracheostomy and gastrostomy, n = 7), and infections from various sources (n = 7, with 1 developing septic shock). Median intensive care unit (ICU) length of stay was 40 (IQR 28, 97.5) days. There were three patients that had cerebral atrophy seen on follow-up brain magnetic resonance imaging (MRI). These three patients were placed in a pentobarbital coma median 11 (range 2–21) days after seizure onset and maintained in burst suppression for a median of 62 (range 29–105) days. Initial brain MRI [performed median 12 (range 1–14) days after seizure onset] did not show cerebral atrophy. Subsequent imaging [median 53 (IQR 23, 93) days from seizure onset] showed persistent generalized cerebral atrophy (Figure 1). Table 3 summarizes the medical and surgical complications observed in patients with refractory status epilepticus maintained in a pentobarbital coma. The absolute number of complications positively trended with duration of induced coma, but was nonsignificant (p = 0.24; Figure 2).
Figure 1.
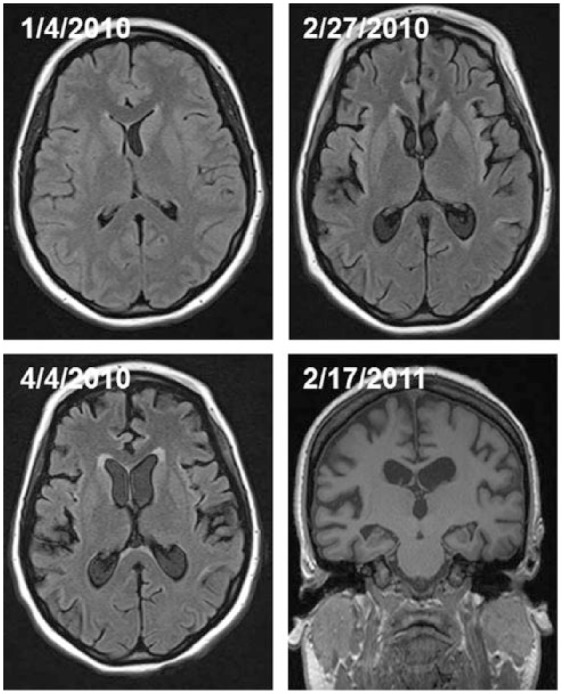
Fluid attenuated inversion recovery sequences (FLAIR) from brain magnetic resonance imaging (MRI) illustrating the persistent cerebral atrophy observed in a patient in a prolonged pentobarbital coma.
Table 3.
Observed direct and indirect medical and surgical complications in patients maintained in a pentobarbital coma for refractory status epilepticus.
| Patient |
Total | % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| ICU length of stay, days | 39 | 143 | 6 | 17 | 40 | 40 | 80 | 61 | 115 | |||
| Length of pentobarb coma, days | 4 | 29 | 1 | 5 | 3 | 9 | 62 | 20 | 105 | |||
| Neurological | ||||||||||||
| Peripheral neuropathy | * | * | * | * | * | * | * | 7 | 77.8 | |||
| Cerebral Atrophy | * | * | * | 3 | 33.3 | |||||||
| Respiratory | ||||||||||||
| Trach/PEG | * | * | * | * | * | * | * | 7 | 77.8 | |||
| Ventilator > 30 days | * | * | * | * | * | * | 6 | 66.7 | ||||
| Chest tube | * | * | 2 | 22.2 | ||||||||
| Pulmonary embolus | * | 1 | 11.1 | |||||||||
| Electrolyte Abnormality | ||||||||||||
| Lactic acidosis | * | * | * | * | * | * | * | 7 | 77.8 | |||
| HEENT/Skin | ||||||||||||
| Anasarca | * | * | * | 3 | 33.3 | |||||||
| Tonge swelling | * | * | * | 3 | 33.3 | |||||||
| Penile edema | * | 1 | 11.1 | |||||||||
| Exposure keratoconjunctivitis | * | * | * | 3 | 33.3 | |||||||
| Vascular | ||||||||||||
| DVT | * | * | 2 | 22.2 | ||||||||
| Cardiology | ||||||||||||
| Myocardial Dysfunction | * | * | 2 | 22.2 | ||||||||
| Hypotension | * | * | * | * | * | * | * | 7 | 77.8 | |||
| Endocrine | ||||||||||||
| Adrenal insufficiency | * | * | 2 | 22.2 | ||||||||
| Hypothermia | * | * | * | 3 | 33.3 | |||||||
| Hypothyroidism | * | 1 | 11.1 | |||||||||
| Gasroenterology | ||||||||||||
| Ileus | * | * | * | * | * | 5 | 55.6 | |||||
| TPN | * | * | 2 | 22.2 | ||||||||
| Transaminitis | * | * | * | 3 | 33.3 | |||||||
| Infectious Disease | ||||||||||||
| Yeast in urine/lungs | * | * | * | * | 4 | 44.4 | ||||||
| Sepsis | * | 1 | 11.1 | |||||||||
| PNA | * | * | * | * | 4 | 44.4 | ||||||
| UTI | * | * | * | * | 4 | 44.4 | ||||||
| Renal | ||||||||||||
| CVVHD | * | * | * | 3 | 33.3 | |||||||
| Acute renal failure | * | 1 | 11.1 | |||||||||
| Sum | 9 | 10 | 4 | 0 | 10 | 10 | 19 | 15 | 10 | |||
CVVHD, continuous veno-veno hemodialysis; DVT, deep venous thrombosis; HEENT, head/ears/eyes/nose/throat; PEG, percutaneous endoscopic gastrostomy; PNA, pneumonia; TPN, total parenteral nutrition; UTI, urinary tract infection.
Figure 2.
Graphical representation illustrating the trend of absolute number of medical and surgical complications with respect to duration of pentobarbital coma.
Outcome
Overall, one (11.1%) patient died within 24 hours of initiating coma due to fulminant liver failure from another etiology. There were three (33.3%) patients that stayed in a persistent state of unresponsiveness after the induced coma (range 136–306 days). Overall, five (55.6%) patients recovered consciousness and regained ability to follow commands after a median of 37 (IQR 25.5, 90) days from coma onset. Of those discharged, six (66.7%) were discharged to long term acute care (LTAC), one (11.1%) to a subacute nursing facility (SNF), and one (11.1%) transferred to another hospital where she died 15 days after discharge (Table 1).
Literature review
A total of 46 articles were identified through database searching. Overall, 41 articles were excluded. The excluded articles were case reports (n = 9), pediatric articles (n = 10), animal articles (n = 3), review articles (n = 16), guidelines (n = 2), and a survey (n = 1). The five included articles have 73 total patients. Mean ± standard deviation duration of anesthesia was 69.2 ± 47.3 hrs. The most commonly observed complication was respiratory failure with need for mechanical ventilation (100%) followed by hypotension/cardiac complications (79.3%). The mortality ranged from 11.8% to 77% (Table 4).
Table 4.
Systematic review of literature of observed complications and outcomes in pentobarbital coma for refractory status epilepticus.
| Author, Year | Design | Total number of patients | Duration of anesthesia (hrs) | Hypotension/ cardiac complications | Mechanical ventilation/ respiratory complications | Infectious complications | Other complications | Mortality |
|---|---|---|---|---|---|---|---|---|
| Rashkin et al. 1987 | Retrospective observation | 9 | 31.6 | 100.0% | NA | NA | NA | 77.0% |
| Lowenstein et al.1987 | Retrospective Observation (n=8); prospective (n=6) | 14 | 62.6 | 64.3% | 100.0% | 7.1% | 87.5% (cognitive); 7.1% (renal); 14.3% (neuropathy) | 42.9% |
| Osorio and Reed, 1989 | Retrospective Observation | 12 | 80.6 | 100.0% | 100% (16.7% unable to wean) | 66.7% | 16.7% (anemia), 100.0% (constellation of weakness, confusion, ataxia, visual disturbance) | 11.8% |
| Van Ness, 1990 | Retrospective observation | 7 | 27.1 | 100.0% | 100.0% | NA | NA | 42.9% |
| Pugin et al. 2014 | Retrospective observation | 31 | 144 | 32.0% | 100.0% | 45.0% | 10.0% (DVT), 10.0% (ileus), 3.2% (peripheral neuropathy) | 42.0% |
DVT, deep vein thrombosis; NA, not applicable.
Discussion
Our study highlights the high morbidity of patients with refractory status epilepticus treated with pentobarbital coma. It also highlights the ability to safely use pentobarbital for extended amounts of time. This study was not intended to infer a direct causality of barbiturate coma to complications. Rather, it highlights direct and indirect complications that were observed in critically ill patients treated with a prolonged duration of pentobarbital coma. Recognizing potential medical and surgical complications in patients with refractory status epilepticus requiring barbiturate coma may lead to improved outcomes [Wittman and Hirsch, 2005; Young et al. 1996].
A total of seven patients had an infection from various sources. Increased propensity to infection may be related to the immunosuppressant effects of pentobarbital coma [Schmutzhard and Pfausler, 2011]. Barbiturates cause immunosuppression by reduction of phagocytic activity of leukocytes, decreasing activation of peripheral lymphocytes, and depression of chemotactic migration of white blood cells [Parviainen et al. 2007; Neuwelt et al. 1982; Kress et al. 1989; Kress and Segmuller, 1987]. Immunosuppression increases the risk of line-associated infections and ventilator-associated pneumonia. Pentobarbital also reduces gastric motility putting a patient at potential risk for transmural translocation of intestinal bacteria and sepsis from intestinal organisms [Schmutzhard and Pfausler, 2011]. Altered gastric motility also makes absorption of orally-available antiepileptics less reliable and compromises nutritional status. We also observed hemodynamic instability and myocardial dysfunctions (i.e. heart failure and prolongation of QTc) resulting in hypotension and volume overload [Schmutzhard and Pfausler, 2011; Ji et al. 2009; Claassen et al. 2001, 2002]. Patients in pentobarbital coma have longer mechanical ventilation times and require aggressive venous thrombosis prophylaxis due to prolong immobility. Indeed, two patients in our study developed DVTs and one with a pulmonary embolus. It has been suggested that since barbiturates redistributes in tissue in a nonlinear fashion after prolonged use, accumulation occurs leading to prolonged recovery [Parviainen et al. 2007]. Pentobarbital coma also is known to either contribute directly to the development of a peripheral polyneuropathy or its development may be a manifestation of prolonged critical illness [Hermans et al. 2008; Stevens et al. 2007]. Overall, seven of our patients had had clinical signs of polyneuropathy and myopathy with one having an electromyography (EMG) / nerve conduction study (NCS) verifying the clinical suspicion.
Lactic acidosis is also encountered in prolonged pentobarbital comas [Ji et al. 2009; Miller et al. 2008]. This may be secondary to the propylene glycol base (40% by volume) used with pentobarbital [Ji et al. 2009]. Propylene glycol is predominantly metabolized in the liver to lactate, acetate, and pyruvate with the remainder excreted unchanged through the kidney [Bledsoe and Kramer, 2008]. A total of seven patients in our series had prolonged lactic acidosis (median 9 days). Overall, one patient had a propylene glycol level of 58 mg/dl with no associated renal failure (Bledsoe and Kramer, 2008). It has been suggested that propylene glycol decreases renal clearance by saturating the proximal tubule [Speth et al. 1987; Zar et al. 2007]. A propylene glycol level was available only in one patient, but four other patients required hemodialysis for acute renal failure with metabolic abnormalities or volume overload.
Generalized cerebral atrophy was seen in 33% of our patients. The progression of cerebral atrophy in patients with refractory status epilepticus is unclear. It may be a reflection of the etiology causing the refractory status epilepticus or the status epilepticus itself with its subsequent treatment and critical illnesses. Cross-sectional studies of this phenomenon are complicated by the possibility of cortical damage from the initial ictal insult, nonlesional MRI, possibility of cerebral atrophy from prolonged critical illness and associated multisystem/metabolic abnormalities, and effects of prolonged high dose multi-antiepileptic therapy [DeGiorgio et al. 1999]. It has been proposed that prolonged sedation with anesthetics disrupts the ascending reticular activating system allowing for decoupling from the posterior parietal cortex, medial temporal lobe, and prefrontal cortex [Gunther et al. 2007]. This prolonged decoupling may lead to excitotoxicity and ultimately apoptosis [Gunther et al. 2007]. This could be one hypothesis to explain the cerebral atrophy. This hypothesis further highlights the urgency of aggressive intervention in patients who present with status epilepticus.
There is considerable amount of controversy on the use of continuous infusion of intravenous antiepileptics for treating refractory status epilepticus. Controversy exists as to the most efficacious agent to use, preference of high monotherapy over combination antiepileptics, therapeutic dose ranges, duration of coma, depth of coma, thresholds for treating abnormal rhythms on EEG, and how continuous should the electroencephalography monitoring be [Corry et al. 2008; Rossetti and Lowenstein, 2011; Jordan and Hirsch, 2006]. A systematic review of the literature by Claasen and colleagues showed that outcomes of patients in status epilepticus are poor [Claassen et al. 2002]. Additionally, there is no difference in outcome on choice of antiepileptic or titration goal [Claassen et al. 2002]. However, they did find that pentobarbital coma is associated with less short-term treatment failure, breakthrough seizures, and the need to switch to another antiepileptic [Claassen et al. 2002]. However, the use of pentobarbital coma has significant morbidity and mortality [Rashkin et al. 1987; Lowenstein et al. 1988; Osorio and Reed, 1989; Van Ness, 1990; Pugin et al. 2014]. We did not analyze comparative efficacy of various antiepileptic therapies for refractory status epilepticus. Overall, four of our nine patients needed further addition of antiepileptics (other than phenobarbital) after instituting the pentobarbital coma. Only one patient required a midazolam infusion.
In conclusion, this study highlights the observed complications of prolonged pentobarbital coma for treatment of refractory status epilepticus in critically ill patients from a single center. It also highlights the ability to safely use prolonged pentobarbital coma in critically ill patients. Anticipating and addressing complications in prolonged pentobarbital coma, whether directly or indirectly related to the coma or critical illness, can improve survival and functional outcomes in patients with medically refractory status epilepticus. A prospective study comparing continuous infusions of anticonvulsants (i.e. pentobarbital, midazolam, and propofol) in comparison to combination multi-antiepileptic agents is necessary to determine what strategies can improve outcomes in the critically ill patients with refractory status epilepticus with minimal systemic complications.
Acknowledgments
Christopher R. Newey, Dolora Wisco, Premkumar Nattanmai, and Aarti Sarwal contributed equally to the writing of the case and formatting the images.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Christopher R. Newey, University of Missouri, Department of Neurology, 5 Hospital Drive, CE 540, Columbia, MO 65211, USA.
Dolora Wisco, Cleveland Clinic, Department of Neurology, Cleveland, OH, USA.
Premkumar Nattanmai, University of Missouri, Department of Neurology, Columbia, MO, USA.
Aarti Sarwal, Wake Forest University School of Medicine, Neurology and Critical Care, Winston Salem, NC, USA.
References
- Bledsoe K., Kramer A. (2008) Propylene glycol toxicity complicating use of barbiturate coma. Neurocrit Care 9: 122–124. [DOI] [PubMed] [Google Scholar]
- Claassen J., Hirsch L., Emerson R., Bates J., Thompson T., Mayer S. (2001) Continuous EEG monitoring and midazolam infusion for refractory nonconvulsive status epilepticus. Neurology 57: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Claassen J., Hirsch L., Emerson R., Mayer S. (2002) Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 43: 146–153. [DOI] [PubMed] [Google Scholar]
- Corry J., Dhar R., Murphy T., Diringer M. (2008) Hypothermia for refractory status epilepticus. Neurocrit Care 9: 189–197. [DOI] [PubMed] [Google Scholar]
- DeGiorgio C., Heck C., Rabinowicz A., Gott P., Smith T., Correale J. (1999) Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology 52: 746–749. [DOI] [PubMed] [Google Scholar]
- Gunther M., Jackson J., Ely E. (2007) Loss of IQ in the ICU brain injury without the insult. Med Hypotheses 69: 1179–1182. [DOI] [PubMed] [Google Scholar]
- Hermans G., De Jonghe B., Bruyninckx F., Van den Berghe G. (2008) Clinical review: critical illness polyneuropathy and myopathy. Crit Care 12: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T., Zubkov A., Wijdicks E., Manno E., Rabinstein A., Kotagal S. (2009) Massive tongue swelling in refractory status epilepticus treated with high-dose pentobarbital. Neurocrit Care 10: 73–75. [DOI] [PubMed] [Google Scholar]
- Jordan K., Hirsch L. (2006) In nonconvulsive status epilepticus (NCSE), treat to burst-suppression: pro and con. Epilepsia 47(Suppl 1): 41–45. [DOI] [PubMed] [Google Scholar]
- Kress H., Eberlein T., Horber B., Weis K. (1989) Suppression of neutrophil migration and chemiluminescence is due to the sulphur atom in the thiobarbiturate molecule. Acta Anaesthesiol Scand 33: 122–128. [DOI] [PubMed] [Google Scholar]
- Kress H., Segmuller R. (1987) Intravenous anesthetics and human neutrophil granulocyte motility in vitro. Anaesthesist 36: 356–361. [PubMed] [Google Scholar]
- Legriel S., Azoulay E., Resche-Rigon M., Lemiale V., Mourvillier B., Kouatchet A., et al. (2010) Functional outcome after convulsive status epilepticus. Crit Care Med 38: 2295–2303. [DOI] [PubMed] [Google Scholar]
- Lowenstein D., Alldredge B. (1998) Status epilepticus. N Engl J Med 338: 970–976. [DOI] [PubMed] [Google Scholar]
- Lowenstein D., Aminoff M., Simon R. (1988) Barbiturate anesthesia in the treatment of status epilepticus: clinical experience with 14 patients. Neurology 38: 395–400. [DOI] [PubMed] [Google Scholar]
- Miller M., Forni A., Yogaratnam D. (2008) Propylene glycol-induced lactic acidosis in a patient receiving continuous infusion pentobarbital. Ann Pharmacother 42: 1502–1506. [DOI] [PubMed] [Google Scholar]
- Neuwelt E., Kikuchi K., Hill S., Lipsky P., Frenkel E. (1982) Barbiturate inhibition of lymphocyte function. Differing effects of various barbiturates used to induce coma. J Neurosurg 56: 254–259. [DOI] [PubMed] [Google Scholar]
- Novy J., Logroscino G., Rossetti A. (2010) Refractory status epilepticus: a prospective observational study. Epilepsia 51: 251–256. [DOI] [PubMed] [Google Scholar]
- Osorio I., Reed R. (1989) Treatment of refractory generalized tonic-clonic status epilepticus with pentobarbital anesthesia after high-dose phenytoin. Epilepsia 30: 464–471. [DOI] [PubMed] [Google Scholar]
- Parviainen I., Kalviainen R., Ruokonen E. (2007) Propofol and barbiturates for the anesthesia of refractory convulsive status epilepticus: pros and cons. Neurol Res 29: 667–671. [DOI] [PubMed] [Google Scholar]
- Pugin D., Foreman B., De Marchis G., Fernandez A., Schmidt J., Czeisler B., et al. (2014) Is pentobarbital safe and efficacious in the treatment of super-refractory status epilepticus: a cohort study. Crit Care 18: R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashkin M., Youngs C., Penovich P. (1987) Pentobarbital treatment of refractory status epilepticus. Neurology 37: 500–503. [DOI] [PubMed] [Google Scholar]
- Rossetti A., Lowenstein D. (2011) Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol 10: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalen W., Messeter K., Nordstrom C. (1992a) Complications and side effects during thiopentone therapy in patients with severe head injuries. Acta Anaesthesiol Scand 36: 369–377. [DOI] [PubMed] [Google Scholar]
- Schalen W., Sonesson B., Messeter K., Nordstrom G., Nordstrom C. (1992b) Clinical outcome and cognitive impairment in patients with severe head injuries treated with barbiturate coma. Acta Neurochir (Wien) 117: 153–159. [DOI] [PubMed] [Google Scholar]
- Schmutzhard E., Pfausler B. (2011) Complications of the management of status epilepticus in the intensive care unit. Epilepsia 52(Suppl 8): 39–41. [DOI] [PubMed] [Google Scholar]
- Speth P., Vree T., Neilen N., de Mulder P., Newell D., Gore M., et al. (1987) Propylene glycol pharmacokinetics and effects after intravenous infusion in humans. Ther Drug Monit 9: 255–258. [DOI] [PubMed] [Google Scholar]
- Stevens R., Dowdy D., Michaels R., Mendez-Tellez P., Pronovost P., Needham D. (2007) Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 33: 1876–1891. [DOI] [PubMed] [Google Scholar]
- Stover J., Stocker R. (1998) Barbiturate coma may promote reversible bone marrow suppression in patients with severe isolated traumatic brain injury. Eur J Clin Pharmacol 54: 529–534. [DOI] [PubMed] [Google Scholar]
- Van Ness P. (1990) Pentobarbital and EEG burst suppression in treatment of status epilepticus refractory to benzodiazepines and phenytoin. Epilepsia 31: 61–67. [DOI] [PubMed] [Google Scholar]
- Wittman J., Hirsch L. (2005) Continuous electroencephalogram monitoring in the critically ill. Neurocrit Care 2: 330–341. [DOI] [PubMed] [Google Scholar]
- Yaffe K., Lowenstein D. (1993) Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology 43: 895–900. [DOI] [PubMed] [Google Scholar]
- Young G., Jordan K., Doig G. (1996) An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology 47: 83–89. [DOI] [PubMed] [Google Scholar]
- Zar T., Yusufzai I., Sullivan A., Graeber C. (2007) Acute kidney injury, hyperosmolality and metabolic acidosis associated with lorazepam. Nat Clin Pract Nephrol 3: 515–520. [DOI] [PubMed] [Google Scholar]