Abstract
A hallmark of mammalian evolution is a progressive complexity in postcanine tooth morphology. However, the driving force for this complexity remains unclear: whether to expand the versatility in diet source, or to bolster tooth structural integrity. In this study, we take a quantitative approach to this question by examining the roles of number, position and height of multiple cusps in determining sustainable bite forces. Our approach is to use an extended finite-element methodology with due provision for step-by-step growth of an embedded crack to determine how fracture progresses with increasing occlusal load. We argue that multi-cusp postcanine teeth are well configured to withstand high bite forces provided that multiple cusps are contacted simultaneously to share the load. However, contact on a single near-wall cusp diminishes the strength. Location of the load points and cusp height, rather than cusp number or radius, are principal governing factors. Given these findings, we conclude that while complex tooth structures can enhance durability, increases in cusp number are more likely to be driven by the demands of food manipulation. Structural integrity of complex teeth is maintained when individual cusps remain sufficiently distant from the side walls and do not become excessively tall relative to tooth width.
Keywords: contact mechanics, fracture, dental morphology, dental evolution, mammalian origins
1. Introduction
Once mammals came onto the scene in the Late Triassic approximately 200 Ma, diversification of tooth morphology proceeded rapidly [1,2]. One of the hallmark innovations of this diversification was an increasing complexity in the postcanine teeth, most notably in the number and height of individual cusps. This evolving complexity seemingly allowed mammals to invade a broader range of dietary niches so as to increase their intake of nutrients, thereby satisfying a relatively demanding mammalian metabolism. The rate and breadth of diversification increased even further with the adaptive radiation of mammals following the Cretaceous–Paleogene extinction event that caused the loss of non-avian dinosaurs some 65 Ma. However, the underlying driving force behind the enhancement of cuspal complexity remains a topic of debate. Did postcanine teeth evolve to improve the efficiency of food processing by enhancing the ability to manipulate challenging foods in the mouth [3,4], or to increase tooth structural integrity in response to the pressures of high bite forces and prolonged chewing cycles when encountering tougher and harder food items [5]?
Tooth structural integrity can be compromised through both wear and fracture. Tooth wear has been the subject of extensive studies over decades [6–20]. Tooth fracture, on the other hand, has only recently gained significant interest [21–39]. In many omnivorous mammals, it is fracture that most often challenges tooth integrity, especially in the postcanine teeth where the brunt of the bite force is sustained during mastication. The basic form of fracture is a longitudinal crack that propagates stably with steadily increasing or repeating load from the occlusal contact to the gum line (radial crack), or vice versa (margin crack) [28,36]. Longitudinal cracks proliferate in the enamel over time, and can be used as a diagnostic to evaluate bite force and infer the nature of the food source (hard or soft) in mammals with low-crowned (bunodont) postcanines [31]. They are not always readily visible, because they close during unloading and fill up with organic matter from the aqueous oral environment [29,30]. While they may appear to be benign, longitudinal cracks serve as precursors to whole tooth splitting at bite overloads from wedge loading between cusps [40]. Secondary edge chipping can also occur within the enamel [32], especially at cusps loaded too close to a side wall.
To date, the bulk of analytical modelling of tooth fracture has focused on the loading of an idealized unicuspid tooth consisting of a dome shell on a cylindrical base. That modelling, validated by laboratory fracture tests on extracted human molars, has enabled the derivation of analytical relations for predicting the critical loads governing longitudinal fracture in terms of characteristic tooth dimensions [36]. Generally, these critical loads increase with tooth base radius and height and enamel thickness [36,37]. More recent expansion of modelling to molar teeth with a symmetrical four-cusp structure [35,39], although preliminary in its findings, paints the way to a more comprehensive understanding of cuspal complexity. In this study, we address how systematic addition of cusps to an occlusal tooth surface and variation in the height of any individual cusp may influence the structural integrity of postcanine teeth. We hypothesize that the evolution of multiple cusps and subsequent cusp repositioning reflect an essential compromise between the need for the structure to broaden and optimize food processing while maintaining tooth integrity.
2. Biomechanics of tooth fracture
2.1. Cuspal configurations in mammalian teeth
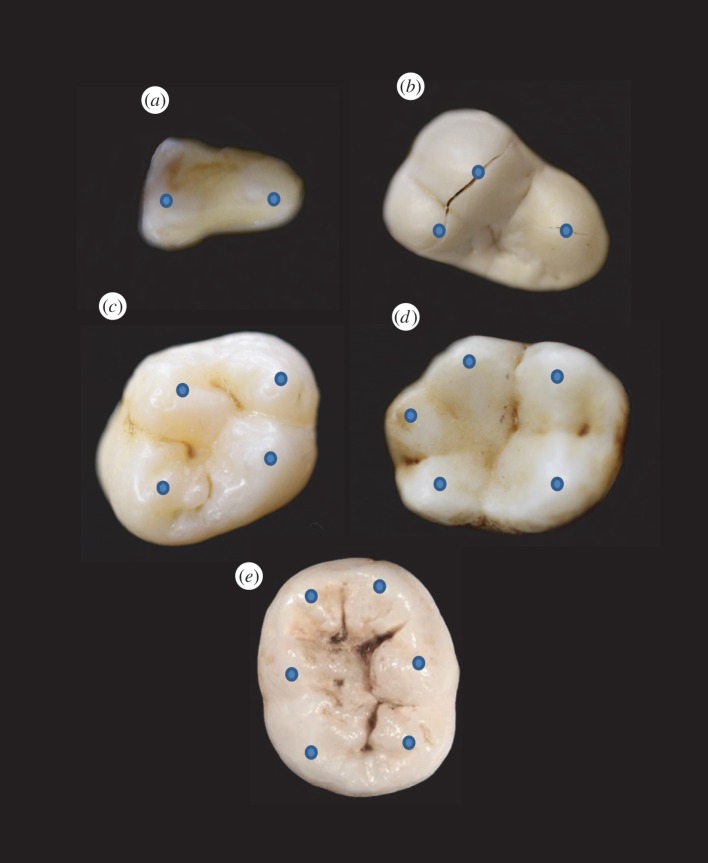
Many mammals feature postcanine teeth with enamel-covered cusps. Such cusps are useful in breaking down food while also shielding the soft dentine–pulp interior from incursive fracture and cumulative wear [5]. One of the earliest changes in mammalian postcanines included transformation from a tricuspid linear (triconodont) to a triangular (tribosphenic) cusp configuration, illustrated by the examples in figure 1. This innovation enabled increased functionality from simple grasping and shearing to crushing and grinding [42]. The transformation was so successful that all living marsupial and placental mammals are believed to have descended from early tribosphenic mammals [43]. Some mammal groups subsequently added a fourth cusp to the upper molars. This configuration can be found in a number of living species including apes, monkeys, raccoons and hedgehogs [44,45]. Over the past 200 Myr, the addition of even more cusps has led to increased morphological diversity as dietary pressures intensified. Examples of various multi-cusp configurations found among living mammals are shown in figure 2. Variations in cuspal morphologies are many, marked among other things by departures from axial symmetry and uniformity in height.
Figure 1.
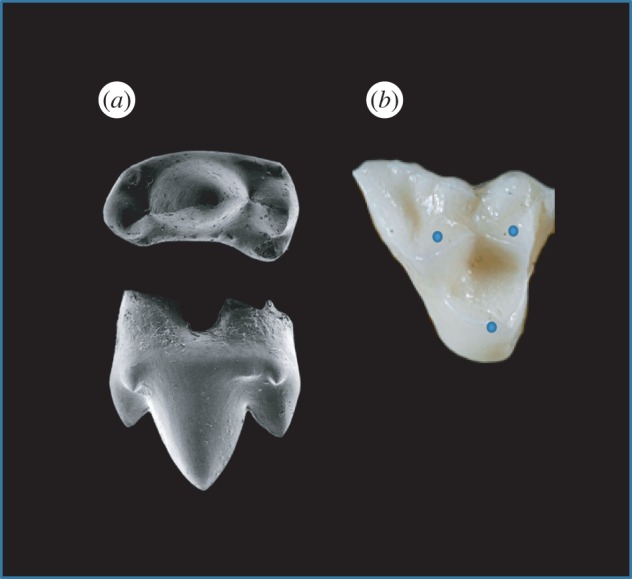
Tooth types of two early mammals. (a) Triconodont tooth from an early mammal from India (Paikasigudodon yadagirii), shown in occlusal (top) and lingual (side) view (adapted from Prasad & Manhas [41]). Note the higher central cusp (protocone). (b) Tribosphenic tooth from a modern opossum (genus Didelphis), shown in occlusal view (adapted from an image taken from Dr Udo Savalli, Arizona State University). Blue dots highlight location of cusp apices. Both teeth are left maxillary molars, anterior to the right. (Online version in colour.)
Figure 2.
Variation in cusp number among mammalian bunodont teeth: (a) 2-cusped slow loris (Nycticebus coucang) premolar, (b) 3-cusped sea otter (Enhydra lutris) molar, (c) 4-cusped human (Homo sapiens) molar, (d) 5-cusped gibbon (Hylobates lar) molar, (e) 6-cusped human molar. Blue dots highlight location of cusp apices. (Online version in colour.)
2.2. Setting up the cusp model
The basis of the tooth modelling has been outlined in earlier articles [35–37,39], and only essential elements are reproduced here. The tooth is considered to consist of an enamel shell with nominal uniform thickness d on a cylindrical base with net height H and base radius R bonded to a dentine interior, depicted in the insets of figure 3. The enamel and dentine are assigned mechanical properties (Young's modulus, Poisson's ratio, fracture toughness) in accordance with previous determination [36]. A vertical load P (bite force) is applied by a hard indenter to the apices of one or more cusp in fixed–displacement mode, meaning that the contact between indenter and specimen at any stage in the loading is held fixed while fracture occurs. The figure illustrates propagating longitudinal cracks (L) in the enamel shell (shaded). The configurations shown in figure 3 are: (a) a single cusp loaded with a point contact either axially (A) or off-axis (O); (b) a quadrate structure loaded on either a single cusp of radius r = R/2 (L1), a pair of opposing cusps (L13), or all 4 cusps (L1234); (c) same quadrate structure as (b) but loaded centrally with a sphere to wedge open a splitting crack (S).
Figure 3.
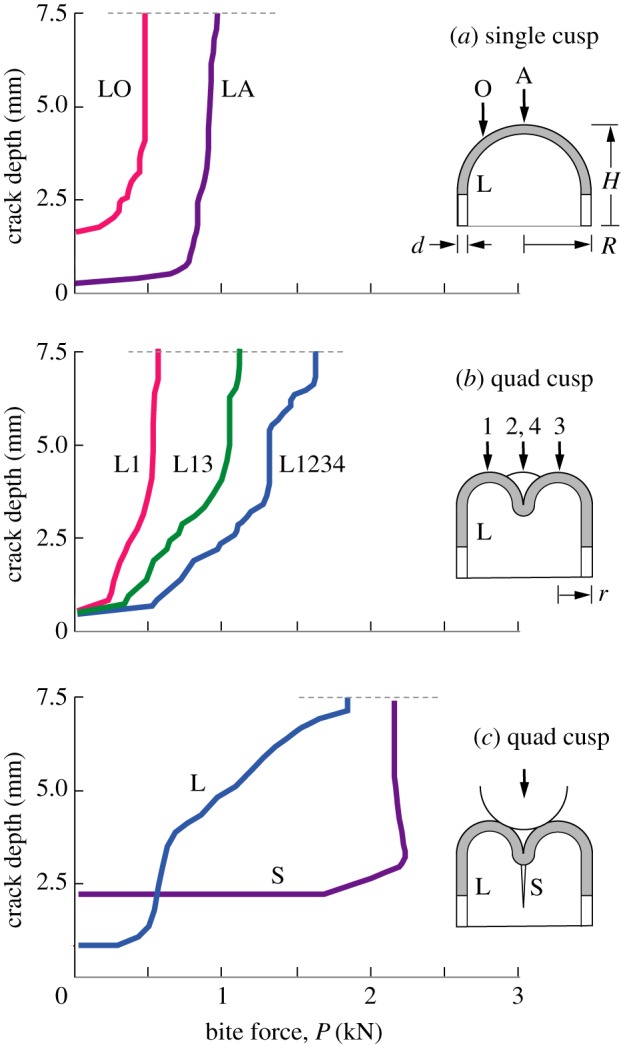
Crack depth measured from top of tooth to maximum penetration, for nominal tooth of base radius R = 5 mm, height H = 7.5 mm and enamel thickness d = 1.5 mm. L denotes longitudinal crack in enamel, S denotes splitting crack. (a) Single cusp dome, loaded axially (A) and off-axis (O). (b) 4-cusp molar loaded on 1, 2 and 4 cusps. (c) Same 4-cusp molar loaded centrally along tooth axis with sphere of radius r = 1.5 mm, contacts at inner cusp surfaces. Longitudinal crack depth measured along enamel side wall, splitting crack on fissure symmetry plane. (Online version in colour.)
Included in figure 3 is the crack propagation history of each loading configuration determined by a full three-dimensional extended finite-element (XFEM) algorithm, for nominal tooth dimensions d = 1.5 mm, H = 7.5 mm and R = 5 mm, representative of human molars. The XFEM algorithm enables one to map out the ensuing step-by-step progress of a small embedded longitudinal starter crack as load P is increased, without the need for repeated re-meshing. The starter crack is representative of pre-existing defects in enamel, such as hypocalcified ‘tufts’—organically filled closed cracks—originating at the enamel/dentine junction, thereby circumventing the need to impose a condition for initiation [29,30]. The criterion for ensuing propagation at increasing load is that the energy release rate for incremental growth should exceed the intrinsic resistance of enamel to fracture, i.e. the ‘toughness’ [36]. The mesh size is refined until the calculations converge [35–37,39]. Crack depth is measured vertically from the tallest cusp apex. Kinks in the crack extension curves in figure 3 reflect the discrete nature of the XFEM mesh. In earlier studies, direct monitoring of crack propagation in vertically loaded extracted human molars has afforded experimental validation of the XFEM predictions [36]. The main features of the crack depth versus applied load histories are as follows:
(a) Single cusp dome model, top-surface loading. Longitudinal cracks grow stably with load until they reach the tooth margin at depth 7.5 mm, which defines the ‘critical load’. For concentrated loading along the axis (curve LA), the critical load P is approximately 1 kN, which is at the high end of bite force in human mastication [31]. But for off-axis contact at a distance R/2 from the axis (LO curve) the critical load is diminished to about one half. Note that the curves become steeper as force increases—the LO curve in particular becomes near-vertical at a critical load, indicating a final unstable propagation to failure. The structure becomes more vulnerable as the load is moved closer to the side wall. Decreasing tooth dimensions d, H and R all exacerbate this vulnerability [36,37], but load offset remains a dominant factor.
(b) Quad-cusp molar model, apical loading. The presence of cusps does not change the nature of longitudinal crack stability, but the response is now dependent on which cusps bear the load [39]. For loading on a single near-cusp apex (curve L1), the critical load is comparable to the off-axis LO configuration in (a), suggesting that it is the distance of the load point from the tooth axis rather than cusp radius that is the dominant factor in the ultimate failure condition. At first sight, the development of cusps might be construed as diminishing load-bearing capacity. On the other hand, loading on two opposite cusps (L13), or better still on all four cusps (L1234), substantially increases the critical load to well above common bite force levels. So again, the implication is that the location of the load points is a dominant factor in tooth vulnerability.
(c) Splitting fracture, axial loading with a spherical contact. The presence of multiple cusps enhances the prospect of tooth splitting. For loading between cusps with a hard round object, a longitudinal crack can be wedged open and penetrate into the dentine sublayer to cause a split [35]. Such a fracture is clearly catastrophic, but the loads to propagate either the precursor longitudinal crack (L) or subsequent splitting crack (S) are typically well beyond normal bite forces, even for smaller indenting objects where wedging forces are greater. On the other hand, the presence of defects or disease in the tooth could cause premature full failures in deleterious instances [40].
2.3. Multiple cusps of uniform height
The data in figure 3 show how the fracture mechanics changes in going from a single to a quadrate cusp configuration. Location of the load points is a critical factor in determining structural integrity. But the question of the role of number of cusps n remains unresolved. To address this question, we consider the mechanics of crack propagation for n = 1, 2, 3, 4, 5 in the model of figure 4, for fixed tooth height H and base radius R and cusp radius r = R/2. In these configurations, the overlap between neighbouring cusps increases as more cusps are ‘squeezed’ onto the tooth upper surface. However, while the distance between cusp apices diminishes at higher n, the distance from the side walls remains constant. Limiting the analysis to five cusp configurations in figure 3 is sufficient to illustrate data trends without undue computation. Inclusion of the simplistic dome configuration at n = 1 from previous studies [36,37] provides a useful comparative base.
Figure 4.
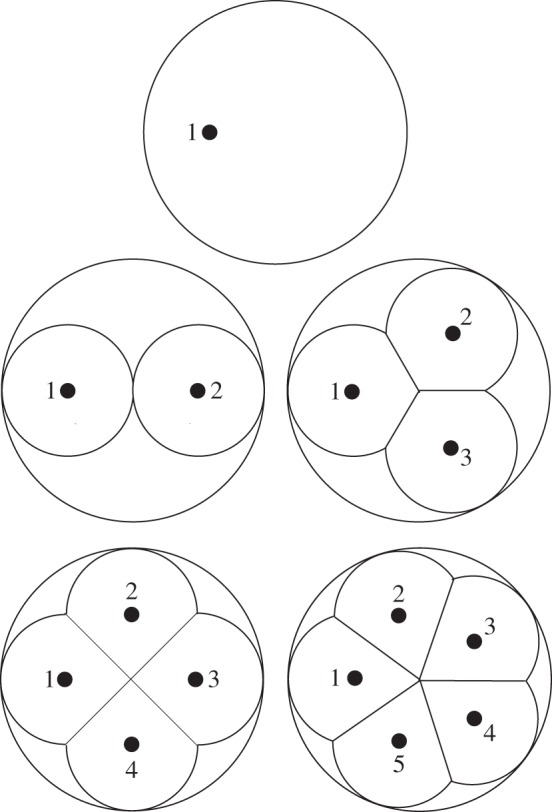
Schematic of tooth of base radius R and height H (not shown in this top view) with 1, 2, 3, 4 and 5 cusps. Individual cusps are modelled as truncated hemispheres of radius R/2, with overlapping multi-cusp neighbours sharing fissure planes (fossae). Black dots indicate apical contact loading points.
The propagation history for each loading configuration in the multi-cusp model is plotted in figure 5, for loading on a single cusp (figure 5a) and simultaneously on all cusps (figure 5b). In this plot, the bite force P is the total force delivered to the tooth, so that in figure 5b the load is distributed, i.e. P/n on individual cusps. The distance of apical contact points from the tooth axis is maintained constant at R/2 for all n, with the curves for n = 1 reproduced from the LO data in figure 3a and for n = 4 from the L1 and L1234 data in figure 3b. Clearly, the effect of increasing n beyond 2 makes little difference to the critical load P approximately 1 kN to drive longitudinal cracks to completion when the load is applied to an individual cusp (or off-axis point at n = 1). Small variations are apparent as a result of changes to geometrical detail as the cusp number is increased, but again it is location of the load point that is determinant. However, if the load is distributed evenly over all cusps, the curves shift perceptibly and systematically with increasing n, suggesting that multi-cusp teeth may be less vulnerable to fracture, provided good occlusion is maintained during mastication.
Figure 5.
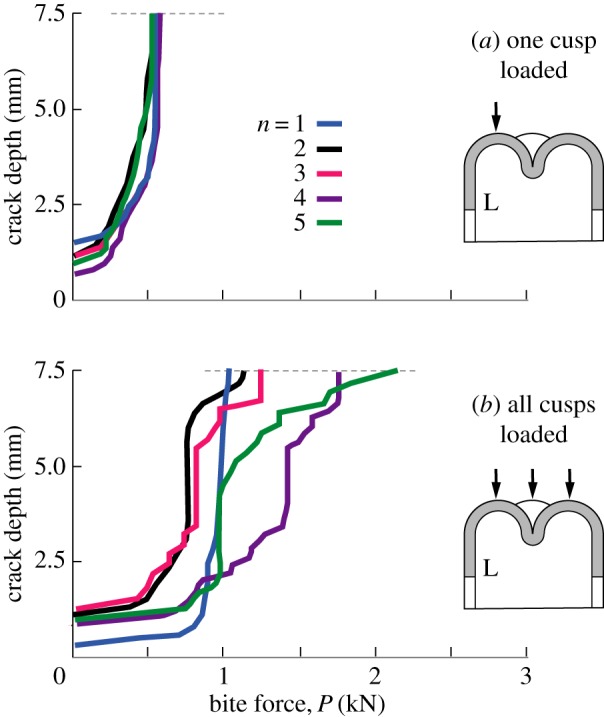
Crack depth measured from top of tooth to maximum penetration, for nominal tooth of base radius R = 5 mm, height H = 7.5 mm and enamel thickness d = 1.5 mm, for number of cusps n = 1, 2, 3, 4 and 5. L denotes longitudinal crack in enamel. Vertical apex loading on (a) one cusp, (b) all cusps. Note insensitivity of crack size to n in (a), but not in (b).
2.4. Multiple cusps of non-uniform height
To investigate the potential effect of different cusp height, consider a quadrate molar as in figure 3 but now with one cusp higher than the other three. Figure 6 plots longitudinal crack extension data for apical loading on cusp 1 of height H = 8.4 mm (L1), relative to loading on a lower cusp 3 of height H = 7.5 mm (L3). In both cases, the crack depth is measured from the crest of the tallest cusp.
Figure 6.
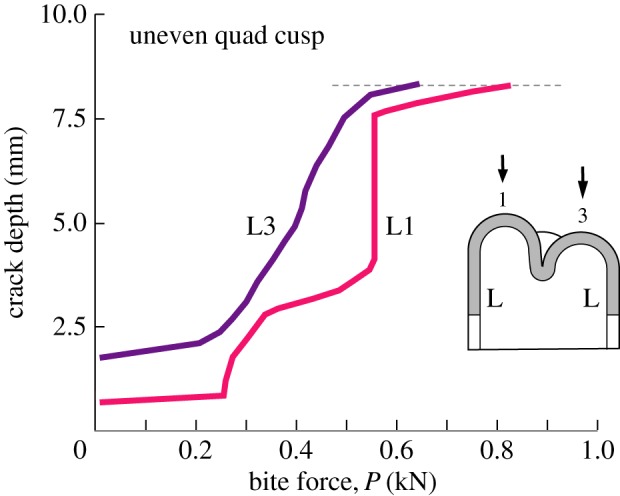
Crack depth measured from top of tallest cusp to maximum penetration, for nominal tooth of base radius R = 5 mm and enamel thickness d = 1.5 mm. Data for tooth with symmetrical quadrate cusps, three at height H = 7.5 mm (as in figure 3), but one higher cusp (cusp 1) at H = 8.5 mm. L denotes longitudinal crack in enamel side wall. Higher load is required to drive crack in taller cusp. (Online version in colour.)
It is apparent that a higher critical load needs to be applied to the tallest cusp in order to complete fracture around a side wall. This result is consistent with an earlier finding for axial loading on single cusp teeth, namely that the critical load scales approximately with tooth elevation, reflecting the fact that longitudinal cracks have to be driven over a greater distance [37]. Thus cusp height, as well as load location, plays a role in determining critical conditions for fracture. The conclusion is that when a given cusp is loaded individually, longitudinal crack propagation is insensitive to the presence of other cusps on the adjacent tooth surface.
3. Dietary pressures in mammalian teeth
3.1. Multi-cusp complexity allows for enhanced food manipulation
From the dental biomechanics considerations in the previous section, it can be concluded that increased cusp complexity did not evolve solely to enhance tooth integrity. It is true that simultaneous contacts on several cusps spread the load, but contact on any one cusp in isolation provides much lower protection from longitudinal cracking. For this reason, the preservation of proper occlusal contact between opposing teeth in multi-cusp teeth is important in the mastication process, so that the bite force may be spread over the tooth crown surface. Small hard foods have localized contact points on teeth, so their consumption may leave the teeth especially vulnerable. However, provided longitudinal cracks remain fully contained within the enamel coat, structural integrity may not be seriously impaired. After all, typical postcanine teeth in mammals contain a myriad of such cracks, and cracked teeth can last a lifetime [31]. Teeth are ‘built to last’ [11]. Nevertheless, such cracks remain precursors to enamel spallation [24] and wholesale splitting [35] in high force mastication, so it is desirable that their incidence be kept to a minimum. Again, proper occlusal function is vital to avoidance of premature fractures.
So why did mammals need more complex teeth in the first place? How was the selection pressure so strong that it caused such an amazing diversity in tooth morphology? A key feature of mammals is their endothermy, i.e. they regulate their body temperature by generating heat from within [46,47]. A major cost of endothermy is the energy required for heat production and dissipation, meaning that mammals have a higher basal metabolic rate than ectothermic (cold-blooded) animals such as fish, amphibians and most reptiles. A multi-cusp dentition for more efficient breakdown of a broad range of foods is just one of the features that mammals have evolved in order to acquire extra energy; enhanced particle size reduction by the teeth can improve digestion by making more surface area available for contact with digestive enzymes [5]. In some mammals, one cusp may become dominant (e.g. protocone, figure 1a), in some instances articulating with the basin of the opposing tooth to create a mortar-and-pestle configuration for enhanced crushing of select food items [48]. As we have seen in §2.4, a taller cusp actually has higher resistance to development of longitudinal cracks, so such a geometrical feature poses little threat to structural integrity, provided the ratio of height to base radius remains sufficiently low as to minimize vulnerability to snap-off transverse fractures [38,49].
It is thereby arguable that the driving force behind cuspal complexity was an improvement in the capacity to manipulate and process a wider range of food items [3,4]. Multiple cusps of variable height confer benefit by allowing for more flexibility in food handling. But the cusps need to be located sufficiently far away from tooth side walls to obviate the risk of fracture and chipping, and not become too tall that any single cusp runs the risk of transverse fracture.
3.2. Do teeth of hard object feeders evolve fewer cusps?
Some mammals consume hard foods as part of their normal diet, a practice known as durophagy [50,51]. The inflexible nature of such objects means that contacts between such objects and teeth will tend to be localized. On a multicusped tooth, contact can be with just a single cusp. From the standpoint of tooth integrity, one might therefore anticipate that hard-food eaters would feature a reduction in cusp number. However, this is not widely observed in mammals, possibly due to developmental constraints [52,53]. Instead, the teeth of durophagous mammals adapt to their increased mechanical loads by becoming larger and more bunodont, and often with thicker enamel [25,33,54–59].
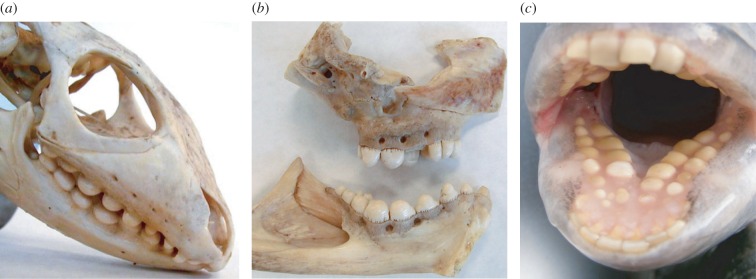
An analogous pattern holds for durophagous non-mammals. While many feature a single bunodont cusp, enhancing fracture resistance by ensuring food-on-tooth contacts closer to the tooth axis (figure 3a), these animals all likely evolved from unicuspid ancestors and so do not furnish evidence for cusp reduction. Nevertheless, it is interesting to note that distantly related species such as the caiman lizard, wolf eel and sheepshead fish have all converged on a similar blunt tooth morphology due to their diets of hard-shelled invertebrate prey including snails, crabs, urchins and bivalves (figure 7) [60–65]. Therefore, both mammals and non-mammals feature similar patterns of dental evolution in response to demanding diets, even though the evolutionary paths differ.
Figure 7.
Unicuspid teeth of durophagous non-mammals. (a) Caiman lizard (genus Dracaena), (b) wolf eel (Anarrhichthys ocellatus) and (c) sheepshead fish (Archosargus probatocephalus). Note dome-like (bunodont) structure of the cheek teeth, suited to a hard-food diet. Images in (a,b) courtesy of Dr Karen Petersen, University of Washington. (Online version in colour.)
4. Conclusion
(i) The mechanics of postcanine tooth fracture can be determined by extended finite-element modelling of a shell-like structure with multiple cusps.
(ii) The defining mode of fracture in bunodont teeth is longitudinal cracking within the enamel shell. This crack mode serves as a precursor to other kinds of fracture, namely splitting and chipping.
(iii) Critical loads to fracture depend on location rather than number of cusps. Cusps closer to the side wall are more vulnerable.
(iv) Simultaneous apical contacts on multiple cusps spread the occlusal load, but contact on a single cusp concentrates it and diminishes strength.
(v) Taller cusps are more resistant to longitudinal fracture.
(vi) A multi-cusp configuration presumably allows for enhanced food manipulation, provided good occlusion is maintained during mastication.
(vii) Evolution of tooth complexity represents a balance between structural integrity and food manipulation.
Acknowledgements
Thanks to Lars Werdelin for earlier discussions on cuspal complexity.
Authors' contributions
All authors designed the study, analysed the data and contributed to writing the paper. A.B. prepared the finite-element models and ran the tooth failure simulations.
Competing interests
There are no conflicts of interest.
Funding
This study was supported by the Australian Research Council (DP130101472).
References
- 1.Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483, 457–460. ( 10.1038/nature10880) [DOI] [PubMed] [Google Scholar]
- 2.Ungar PS. 2010. Mammal teeth: origin, evolution, and diversity. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 3.Berthaume M, Grosse IR, Patel ND, Strait DS, Wood S, Richmond BG. 2010. The effect of early hominin occlusal morphology on the fracturing of hard food items. Anat. Rec. 293, 594–606. ( 10.1002/ar.21130) [DOI] [PubMed] [Google Scholar]
- 4.Berthaume MA, Dumont ER, Godfrey LR, Grosse IR. 2013. How does tooth cusp radius of curvature affect brittle food item processing? J. R. Soc. Interface 10, 20130240 ( 10.1098/rsif.2013.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas PW. 2004. Dental functional morphology: how teeth work. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Molnar S. 1972. Tooth wear and culture: a survey of tooth functions among some prehistoric populations. Curr. Anthropol. 13, 511–526. ( 10.1086/201284) [DOI] [Google Scholar]
- 7.Grine FE. 1981. Trophic differences between ‘gracile’ and ‘robust’ australopithecines: a scanning electron microscope analysis of occlusal events. S. Afr. J. Sci. 77, 203–230. [Google Scholar]
- 8.Janis CM, Fortelius M. 1988. On the means whereby mammals achieve increased functional durability of their dentitions, with special reference to limiting factors. Biol. Rev. 63, 197–230. ( 10.1111/j.1469-185X.1988.tb00630.x) [DOI] [PubMed] [Google Scholar]
- 9.Teaford MF. 1988. A review of dental microwear and diet in modern mammals. Scanning Microsc. 2, 1149–1166. [PubMed] [Google Scholar]
- 10.Rose JC, Ungar PS. 1998. Gross dental wear and dental microwear in historical perspective. In Dental anthropology (eds Alt KW, Rosing FW, Teschler-Nicola M), pp. 349–386. Vienna, Austria: Springer. [Google Scholar]
- 11.Maas MC, Dumont ER. 1999. Built to last: the structure, function, and evolution of primate dental enamel. Evol. Anthropol. 8, 133–152. ( 10.1002/(SICI)1520-6505(1999)8:4%3C133::AID-EVAN4%3E3.3.CO;2-6) [DOI] [Google Scholar]
- 12.Sanson G. 2006. The biomechanics of browsing and grazing. Am. J. Bot. 93, 1531–1545. ( 10.3732/ajb.93.10.1531) [DOI] [PubMed] [Google Scholar]
- 13.Sanson GD, Kerr SA, Gross KA. 2007. Do silica phytoliths really wear mammalian teeth? J. Arch. Sci. 34, 526–531. ( 10.1016/j.jas.2006.06.009) [DOI] [Google Scholar]
- 14.Merceron G, Escarguel G, Angibault J-M, Verheyden-Tixier H. 2010. Can dental microwear textures record inter-individual dietary variations? PLoS ONE 5, e9542 ( 10.1371/journal.pone.0009542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damuth J, Janis CM. 2011. On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733–758. ( 10.1111/j.1469-185X.2011.00176.x) [DOI] [PubMed] [Google Scholar]
- 16.Ungar PS, Sponheimer M. 2011. The diets of early hominins. Science 334, 190–193. ( 10.1126/science.1207701) [DOI] [PubMed] [Google Scholar]
- 17.Kaiser TM, Müller DW, Fortelius M, Schulz E, Codron D, Clauss M. 2013. Hypsodonty and tooth facet development in relation to diet and habitat in herbivorous ungulates: implications for understanding tooth wear. Mammal Rev. 43, 34–46. ( 10.1111/j.1365-2907.2011.00203.x) [DOI] [Google Scholar]
- 18.Lucas PW, et al. 2013. Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J. R. Soc. Interface 10, 20120923 ( 10.1098/rsif.2012.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia J, Zheng J, Huang D, Tian ZR, Chen L, Zhou Z, Ungar PS, Qian L. 2015. New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proc. Natl Acad. Sci. USA 112, 10 669–10 672. ( 10.1073/pnas.1509491112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantino PJ, Borrero-Lopez O, Pajares A, Lawn BR. 2016. Simulation of enamel wear for reconstruction of diet and feeding behavior in fossil animals: a micromechanics approach. BioEssays 38, 89–99. ( 10.1002/bies.201500094) [DOI] [PubMed] [Google Scholar]
- 21.Van Valkenburgh B. 1988. Incidence of tooth breakage among large, predatory mammals. Am. Nat. 131, 291–302. ( 10.1086/284790) [DOI] [Google Scholar]
- 22.Van Valkenburgh B, Hertel F. 1993. Tough times at La Brea: tooth breakage in large carnivores of the late Pleistocene. Science 261, 456–459. ( 10.1126/science.261.5120.456) [DOI] [PubMed] [Google Scholar]
- 23.Rensberger JM. 2000. Pathways to functional differentiation in mammalian enamel. In Development, function and evolution of teeth (eds Teaford MF, Smith MM, Ferguson MWJ), pp. 252–268. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Popowics TE, Rensberger JM, Herring SW. 2001. The fracture behavior of human and pig molar cusps. Arch. Oral Biol. 46, 1–12. ( 10.1016/S0003-9969(00)00102-3) [DOI] [PubMed] [Google Scholar]
- 25.Lucas P, Constantino P, Wood B, Lawn B. 2008. Dental enamel as a dietary indicator in mammals. BioEssays 30, 374–385. ( 10.1002/bies.20729) [DOI] [PubMed] [Google Scholar]
- 26.Bajaj D, Arola DD. 2009. On the R-curve behavior of human tooth enamel. Biomaterials 30, 4037–4046. ( 10.1016/j.biomaterials.2009.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chai H, Lee JJ-W, Constantino PJ, Lucas PW, Lawn BR. 2009. Remarkable resilience of teeth. Proc. Natl Acad. Sci. USA 106, 7289–7293. ( 10.1073/pnas.0902466106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn BR, Lee JJ-W, Constantino PJ, Lucas PW. 2009. Predicting failure in mammalian enamel. J. Mech. Behav. Biomed. Mater. 2, 33–42. ( 10.1016/j.jmbbm.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 29.Myoung S, Lee J, Constantino P, Lucas P, Chai H, Lawn B. 2009. Morphology and fracture of enamel. J. Biomech. 42, 1947–1951. ( 10.1016/j.jbiomech.2009.05.013) [DOI] [PubMed] [Google Scholar]
- 30.Chai H, Lee JJW, Lawn BR. 2010. Fracture of tooth enamel from incipient microstructural defects. J. Mech. Behav. Biomed. Mater. 3, 116–120. ( 10.1016/j.jmbbm.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 31.Lee J-W, Constantino P, Lucas P, Lawn B. 2011. Fracture in teeth—a diagnostic for inferring tooth function and diet. Biol. Rev. 86, 959–974. ( 10.1111/j.1469-185X.2011.00181.x) [DOI] [PubMed] [Google Scholar]
- 32.Constantino PJ, Lee JJ-W, Chai H, Zipfel B, Ziscovici C, Lawn BR, Lucas PW. 2010. Tooth chipping can reveal the diet and bite forces of fossil hominins. Biol. Lett. 6, 826–829. ( 10.1098/rsbl.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constantino P, et al. 2011. Adaptation to hard-object feeding in sea otters and hominins. J. Hum. Evol. 61, 89–96. ( 10.1016/j.jhevol.2011.02.009) [DOI] [PubMed] [Google Scholar]
- 34.Barani A, Bush MB, Lawn BR. 2012. Effect of property gradients on enamel fracture in human molar teeth. J. Mech. Behav. Biomed. Mater. 15, 121–130. ( 10.1016/j.jmbbm.2012.06.014) [DOI] [PubMed] [Google Scholar]
- 35.Barani A, Chai H, Lawn BR, Bush MB. 2015. Mechanics analysis of molar tooth splitting. Acta Biomater. 15, 237–243. ( 10.1016/j.actbio.2015.01.004) [DOI] [PubMed] [Google Scholar]
- 36.Barani A, Keown A, Bush M, Lee JJ-W, Chai H, Lawn BR. 2011. Mechanics of longitudinal cracks in tooth enamel. Acta Biomater. 7, 2285–2292. ( 10.1016/j.actbio.2011.01.038) [DOI] [PubMed] [Google Scholar]
- 37.Barani A, Keown AJ, Bush MB, Lee JJW, Lawn BR. 2012. Role of tooth elongation in promoting fracture resistance. J. Mech. Behav. Biomed. Mater. 8, 37–46. ( 10.1016/j.jmbbm.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 38.Lawn BR, Chai H, Barani A, Bush MB. 2013. Transverse fracture of canine teeth. J. Biomech. 46, 1561–1567. ( 10.1016/j.jbiomech.2013.03.018) [DOI] [PubMed] [Google Scholar]
- 39.Barani A, Bush MB, Lawn BR. 2014. Role of multiple cusps in tooth fracture. J. Mech. Behav. Biomed. Mater. 35, 85–92. ( 10.1016/j.jmbbm.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 40.Chai H, Lee JJ-W, Lawn BR. 2011. On the chipping and splitting of teeth. J. Mech. Behav. Biomed. Mater. 4, 315–321. ( 10.1016/j.jmbbm.2010.10.011) [DOI] [PubMed] [Google Scholar]
- 41.Prasad GVR, Manhas BK. 2002. Triconodont mammals from the Jurassic Kota Formation of India. Geodiversitas 24, 445–464. [Google Scholar]
- 42.Schultz JA, Martin T. 2014. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften 101, 771–781. ( 10.1007/s00114-014-1214-y) [DOI] [PubMed] [Google Scholar]
- 43.Luo Z-X. 2007. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019. ( 10.1038/nature06277) [DOI] [PubMed] [Google Scholar]
- 44.Cope ED. 1886. On lemurine reversion in human dentition. Am. Nat. 20, 941–947. ( 10.1086/274364) [DOI] [Google Scholar]
- 45.Cope ED. 1883. On the trituberculate type of molar tooth in the Mammalia. Proc. Am. Phil. Soc. 21, 324–326. [Google Scholar]
- 46.Rosas A. 1995. Seventeen new mandibular specimens from the Atapuerca/Ibeas Middle Pleistocene hominid sample (1985–1992). J. Hum. Evol. 28, 533–559. ( 10.1006/jhev.1995.1041) [DOI] [Google Scholar]
- 47.Hillenius WJ. 1994. Turbinates in Therapsids: evidence for Late Permian origins of mammalian endothermy. Evolution 48, 207–229. ( 10.2307/2410089) [DOI] [PubMed] [Google Scholar]
- 48.Kay RF, Hiiemae KM. 1974. Jaw movement and tooth use in recent and fossil primates. Am. J. Phys. Anthropol. 40, 227–256. ( 10.1002/ajpa.1330400210) [DOI] [PubMed] [Google Scholar]
- 49.Lawn BR, Bush MB, Barani A, Constantino PJ, Wroe S. 2013. Inferring biological evolution from fracture patterns in teeth. J. Theor. Biol. 338, 59–65. ( 10.1016/j.jtbi.2013.08.029) [DOI] [PubMed] [Google Scholar]
- 50.Constantino PJ. 2007. Primate masticatory adaptations to fracture-resistant foods. PhD thesis, George Washington University, Washington, DC, USA.
- 51.Pregill G. 1984. Durophagous feeding adaptations in an amphisbaenid. J. Herpetol. 18, 186–191. ( 10.2307/1563747) [DOI] [Google Scholar]
- 52.Jernvall J. 2000. Linking development with generation of novelty in mammalian teeth. Proc. Natl Acad. Sci. USA 97, 2641–2645. ( 10.1073/pnas.050586297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jernvall J, Jung H-S. 2000. Genotype, phenotype, and developmental biology of molar tooth characters. Yearb. Phys. Anthropol. 113, 171–190. ( 10.1002/1096-8644(2000)43:31+%3C171::AID-AJPA6%3E3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 54.Kay RF. 1981. The nut-crackers—a new theory of the adaptations of the Ramapithecinae. Am. J. Phys. Anthropol. 55, 141–151. ( 10.1002/ajpa.1330550202) [DOI] [Google Scholar]
- 55.Dumont ER. 1995. Enamel thickness and dietary adaptation among extant primates and chiropterans. J. Mammal. 76, 1127–1136. ( 10.2307/1382604) [DOI] [Google Scholar]
- 56.Lambert JE, Chapman CA, Wrangham RW, Conklin-Brittain N-L. 2004. Hardness of cercopithecine foods: implications for the critical function of enamel thickness in exploiting fallback foods. Am. J. Phys. Anthropol. 125, 363–368. ( 10.1002/ajpa.10403) [DOI] [PubMed] [Google Scholar]
- 57.Tseng ZJ, Wang X. 2010. Cranial functional morphology of fossil dogs and adaptation for durophagy in Borophagus and Epicyon (Carnivora, Mammalia). J. Morphol. 271, 1386–1398. ( 10.1002/jmor.10881) [DOI] [PubMed] [Google Scholar]
- 58.McGraw WS, Pampush JD, Daegling DJ. 2012. Brief communication: enamel thickness and durophagy in mangabeys revisited. Am. J. Phys. Anthropol. 147, 326–333. ( 10.1002/ajpa.21634) [DOI] [PubMed] [Google Scholar]
- 59.Hartstone-Rose A, Stynder DD. 2013. Hypercarnivory, durophagy or generalised carnivory in the Mio-Pliocene hyaenids of South Africa? S. Afr. J. Sci. 109, 0040 ( 10.1590/sajs.2013/20120040) [DOI] [Google Scholar]
- 60.Wilga CD, Motta PJ. 2000. Durophagy in sharks: feeding mechanics of the hammerhead Sphyrna tiburo. J. Exp. Biol. 203, 2781–2796. [DOI] [PubMed] [Google Scholar]
- 61.Tesch FW, White RJ. 2008. The eel. New York, NY: John Wiley & Sons. [Google Scholar]
- 62.Crofts SB, Summers AP. 2014. How to best smash a snail: the effect of tooth shape on crushing load. J. R. Soc. Interface 11, 20131053 ( 10.1098/rsif.2013.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernandez L, Motta P. 1997. Trophic consequences of differential performance: ontogeny of oral jaw-crushing performance in the sheepshead, Archosargus probatocephalus (Teleostei, Sparidae). J. Zool. 243, 737–756. ( 10.1111/j.1469-7998.1997.tb01973.x) [DOI] [Google Scholar]
- 64.Schaerlaeken V, Holanova V, Boistel R, Aerts P, Velensky P, Rehak I, Andrade DV, Herrel A. 2012. Built to bite: feeding kinematics, bite forces, and head shape of a specialized durophagous lizard, Dracaena guianensis (Teiidae). J. Exp. Zool. A 317, 371–381. ( 10.1002/jez.1730) [DOI] [PubMed] [Google Scholar]
- 65.De Massary J, Hoogmoed M, Blanc M. 2000. Comments on the type specimen of Dracaena guianensis Daudin, 1801 (Reptilia: Sauria: Teiidae), and rediscovery of the species in French Guiana. Zool. Meded. 74, 167–180. [Google Scholar]