With their large brains and long lives, sheep offer advantages over rodents for studying progressive neurodegenerative disease in timeframes relevant to humans. Perentos et al. demonstrate the validity of an ovine model of Batten disease by revealing in vivo EEG abnormalities that resemble those in affected children.
Keywords: brain atrophy, lysosomal storage disease, neurodegeneration, experimental models, epilepsy, seizures, neuronal ceroid lipofuscinosis, NCL
Abstract
Creating valid mouse models of slowly progressing human neurological diseases is challenging, not least because the short lifespan of rodents confounds realistic modelling of disease time course. With their large brains and long lives, sheep offer significant advantages for translational studies of human disease. Here we used normal and CLN5 Batten disease affected sheep to demonstrate the use of the species for studying neurological function in a model of human disease. We show that electroencephalography can be used in sheep, and that longitudinal recordings spanning many months are possible. This is the first time such an electroencephalography study has been performed in sheep. We characterized sleep in sheep, quantifying characteristic vigilance states and neurophysiological hallmarks such as sleep spindles. Mild sleep abnormalities and abnormal epileptiform waveforms were found in the electroencephalographies of Batten disease affected sheep. These abnormalities resemble the epileptiform activity seen in children with Batten disease and demonstrate the translational relevance of both the technique and the model. Given that both spontaneous and engineered sheep models of human neurodegenerative diseases already exist, sheep constitute a powerful species in which longitudinal in vivo studies can be conducted. This will advance our understanding of normal brain function and improve our capacity for translational research into neurological disorders.
Introduction
Neuroscientists rely on using animals, particularly mice, as models in which to study normal brain function, disease-related neuropathophysiology and drug action on the brain. Modelling of progressive neurological diseases such as Alzheimer’s and Parkinson's disease, however, remains particularly challenging, with mice either not developing the relevant pathology or doing so at such a late stage in their lives that they cannot be used effectively (LaFerla and Green, 2012; Pouladi et al., 2013; Webster et al., 2014). The value of using large-brained animals, particularly for the development of new therapies, is recognized increasingly (Morton and Howland, 2013; Dolezalova et al., 2014). In the case of CLN5 Batten disease, for example, while transgenic mice develop some pathologies relevant to Batten disease (Kopra et al., 2004), the genetically equivalent CLN5−/− affected Batten disease sheep (Jolly et al., 2002; Frugier et al., 2008), and cattle (Harper et al., 1988; Houweling et al., 2006) models show pronounced pathologies that more closely recapitulate the human disease (for review, see Jolly et al., 1992).
Many potential treatments, particularly growth factors and gene therapies, depend on direct delivery to the brain and distribution within the brain by diffusion. Although such therapies may work well in mice where the distance from delivery site to destination is small, many fail to reach their target in the large human brain (Schubert et al., 2008). Therefore, a large-brained animal model is an important stepping-stone for developing therapies that will necessitate direct delivery routes.
Although non-human primates are considered superior to other laboratory animals for translational studies, experimentation with these poses challenges, not only in the ethical domain, but also in practical management (Morton and Howland, 2013). Maintaining the animals safely, high costs and difficulty in managing the large numbers that may be required to generate appropriate statistical power already restrict the use of non-human primates to specialist laboratories, where only small numbers can be used to good effect. Thus, opportunities for preclinical work on large-brained animals are limited, unless we consider using other species.
Sheep have been used as models for deep brain stimulation (Stypulkowski et al., 2011), in basic brain function studies (Kendrick et al., 2001), and as models of brain injury (Grimmelt et al., 2011). A number of neurodegenerative diseases have been found in sheep, including three flocks with different forms of the neuronal ceroid lipofuscinoses (hereafter referred to as Batten disease; Jolly et al., 2002; Frugier et al., 2008; Bond et al., 2013). Other sheep models include experimentally-induced models such as transmissible spongiform encephalopathies (Table 1), and the recently developed transgenic Huntington’s disease model (Jacobsen et al., 2010).
Table 1.
Sheep models of human neurological disorders
| Human disease | Gene | Description |
|---|---|---|
| Neuronal ceroid lipofuscinosis (Variant late-infantile Batten diseases) | CLN5, CLN6 | Naturally-occurring neuronal ceroid lipofuscinoses |
| Neuronal ceroid lipofuscinosis variant (congenital) | CTSD (cathepsin D) | Naturally-occurring neuronal ceroid lipofuscinosis |
| McArdle disease | PYMG | Naturally-occurring metabolic muscle disorder |
| Hereditary lissencephaly and cerebellar hypoplasia | RELN | Naturally occurring lissencephaly |
| Neuroaxonal dystrophy | ? | Naturally-occurring muscular dystrophy |
| Gaucher disease | β-glucocerebrosidase | Naturally-occurring lysosomal storage disease |
| Adult-onset Alexander disease | GFAP | Naturally-occurring hypereosinophilic, intra-astrocytic inclusions (Rosenthal fibres) |
| Tay-Sachs disease | G(M2) activator protein | Naturally-occurring GM2 gangliosidosis |
| Huntington’s disease | HTT | Transgenic sheep model (OVT73) |
| Hereditary cerebellar ataxia (Murrurindi disease) | ? | Naturally-occurring encephalopathy |
| Brachygnathia, cardiomegaly and renal hypoplasia syndrome | ? | Naturally-occurring multisystem disorder |
| Chronic binge alcohol-induced cerebellar injury | - | Induced model of foetal alcohol syndrome |
| Middle cerebral artery occlusion | - | Induced model of stroke |
| Transmissible spongiform encephalopathy | PRNP | Infectious model of bovine spongiform encephalopathy, variant Creutzfeld Jacob disease and other prion diseases |
| Head trauma | - | Induced model of shaken baby syndrome |
| Schizophrenia | - | Infectious model of in utero endotoxaemia |
| Type 2 diabetes | - | Induced model of diabetes |
| Pre-natal global hypoxia | - | Induced model of hypoxic brain damage |
? = gene not known.
Sheep are an attractive species for the study of brain function (Morton and Howland, 2013). Like non-human primates, they have large brains with convoluted neocortices and human-like basal ganglia with anatomically distinct caudate and putamen. They are domesticated, accustomed to human handling and their husbandry is straightforward. Being social animals, they are normally kept in flocks allowing relatively easy ‘scale-up’ of groups to large numbers. Finally, their relatively long life span (>10 years) makes them more appropriate than short-lived rodents for studies of progressive human neurological diseases, such as Alzheimer’s disease and Huntington’s disease.
In this study we investigated the feasibility of using sheep as in vivo models for chronic, untethered monitoring of brain activity, both in normal sheep and sheep with clinically apparent Batten disease. We used clinically affected CLN5 Batten disease sheep because they show abnormal behaviour and neurological features (Jolly et al., 2002; Frugier et al., 2008), that recapitulate those seen in human sufferers (Åberg et al., 2011). We hypothesized that brain activity abnormalities such as sleep deficits and epileptiform activity might also be observed in CLN5−/− affected Batten disease sheep. Techniques were developed for longitudinal in vivo recordings of electroencephalography (EEG) in naturalistic environments in the presence of conspecifics. Twenty-four hour recordings were used to classify vigilance states and characteristic mammalian hallmark sleep signatures were quantified. It is notable that patients with Batten disease suffer from feeding and swallowing abnormalities as well as other motor-related abnormalities (Åberg et al., 2011). Accordingly, we also investigated the potential for using rumination (a physiological process that heavily relies on coordinated regurgitation, mastication and swallowing and is readily observed in sheep) as a read-out for centrally controlled complex motor behaviour.
Materials and methods
Animals
CLN5 affected (CLN5−/−) and heterozygous control (CLN5+/−) Borderdale sheep with a c.571+1G>A splice site mutation (Frugier et al., 2008) were obtained from Lincoln University, New Zealand (NZ), where they had been bred and reared under procedures approved by the Lincoln University Animal Ethics Committee in compliance with the NZ Animal Welfare Act (1999) and in accordance with US National Institutes of Health guidelines. Sheep were shipped by air from NZ to the UK, where experiments were started after a 3-month acclimation period. All procedures were conducted in accordance with the UK Animals Scientific Procedures Act (1986) and the University of Cambridge ethical review board. Because such EEG studies in sheep have never been previously conducted, power analyses could not be performed. We determined the number of animals to use empirically. Five healthy Welsh Mountain ewes were used to establish the techniques (mean age at implantation 10 ± 0.8 months), and five homozygous (CLN5−/−) and two heterozygous (CLN5+/−) Borderdale sheep carrying the CLN5 gene mutation were used to investigate electrophysiological phenotypes associated with the genetic mutation (mean age at implantation 14.3 ± 0.5 months). The two (unaffected) CLN5+/− sheep were age-matched flock mates of the CLN5−/− affected Batten disease sheep. They were included to exclude the possibility that there were major breed or age differences that could account for the differences between the normal Welsh mountain sheep and the CLN5−/− Batten disease affected sheep.
Sheep were housed outdoors in a paddock until implantation, at which point they were transferred to a barn with windows and supplementary artificial light (6 am to 6 pm). All sheep had access to hay feed and water ad libitum and an additional pellet supplement was supplied between the hours of 8–9 am. Sheep behaviour was observed daily, and abnormal behaviours recorded. Sheep were weighed at least once a week.
Surgery
Food was withheld for a period of 12 h before surgery. Anaesthesia was induced intravenously using Alfaxalone (Alfaxan®, Jurox) at 3 mg/kg and maintained using an isoflurane/oxygen/nitrous mixture delivered through a Manley ventilator (stroke volume 300 ml and ventilation rate 15–20/min). Isoflurane was maintained at 2–3%, end-tidal CO2 at 25–30 mmHg and mean arterial blood pressure at 70–90 mmHg. Intravenous fluids were supplied at a rate of 5 ml/kg/h (lactated Ringers, Hartmann’s Solution 11 by Aquapharm). Vital functions were recorded at 5-min intervals, and blood gases sampled every 30 min throughout the procedure.
Under aseptic conditions, a midline incision was made from the midpoint of the interaural line to the occipital end of the skull. Excessive bleeding was stemmed using electrocautery. Using blunt dissection the cranial skin was retracted and the scalp scraped to remove all connective tissue. The skull was cleaned with 3% hydrogen peroxide, and immediately rinsed with sterile saline spray. Craniotomies were drilled 10 mm either side of the midline on both hemispheres, at positions 30, 20 and 10 mm anterior and 10 mm posterior to ‘bregma’ (Fig. 1A). We defined bregma as being the intersection of the midline skull suture separating the frontal bones, and the transverse suture between the frontal and parietal bones i.e. where frontal and posterior cranial bones meet, that would be equivalent to bregma in a primate. Intracortical needle electrodes were then inserted and secured with plastic screws. Two electrodes were placed in the neck muscle for EMG recording and two electrodes at the outer medial canthi of the left eye for electrooculogram (EOG) recording. EOG electrodes were tunnelled subcutaneously and EMG wires were sutured to the neck muscles. All EEG electrodes were secured in place with sterile bone cement containing gentamicin (Depuy CMW2, Johnson & Johnson, UK). Electrodes were bundled together and terminated at an Omnetics Micro circular connector (Omnetics Corporation) that was exteriorized through a purse suture opening at the neck area. The rest of the incision was sutured and the whole incision was sealed with a wound plaster spray (Kruuse Wound Plast). After surgery, the animals were allowed to recover for 7–10 days in their home environment, at which time sutures were removed and recordings commenced.
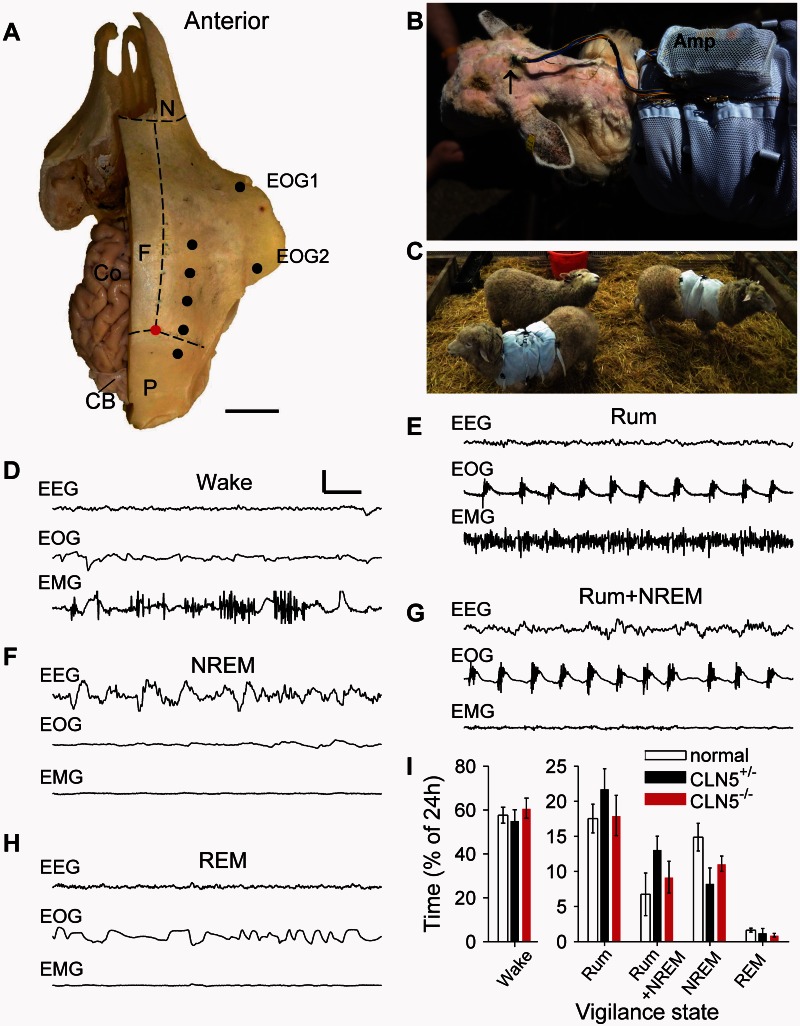
Figure 1.
Implantation technique and example EEG traces from each vigilance state in sheep. (A) Intracortical needles were implanted and secured on the skull on both hemispheres, at the points shown by the black dots on the right side of the skull and equivalent positions on the left hand side. Coordinates were determined with reference to the intersection (red dot) of the transverse suture that separates the frontal (F) and parietal bones (P) and the midline suture that separates the frontal bones. Suture positions are indicated by the dotted lines. Part of the left side of the skull has been removed to show the brain in situ: the dorsal surface of the cortex (Co) and the cerebellum (CB) are partially visible. Bipolar EOGs were obtained from electrodes placed at the inner and outer canthi of the right eye (EOG1 and EOG2), while bipolar EMGs were attached to the dorsal neck musculature (not shown). (B) Electrodes were exteriorized on the back of the neck and terminated in a single connector (black arrow). The amplifier (Amp) was housed within a pouch on the sheep jacket. (C) Recordings were made from animals in their home pens in the presence of conspecifics. Example traces (D–H) obtained from a normal sheep show characteristic EEG (upper trace), EOG (middle trace) and EMG (lower trace) associated with the vigilance states of Wake (D), non-REM (F) and REM sleep (H) that are typically seen in non-human primates. Additional sheep-specific states were identified: rumination (Rum; E) and rumination with concurrent slow-wave EEG activity (Rum+non-REM; G). (I) The amount of time spent in each vigilance state is shown. Data are means ± SEM. Scale bars: A = 3 cm; D = 0.5 s; D–H = 300 μV.
Data acquisition
Sheep were fitted with jackets that carried a paediatric ambulatory EEG amplifier (Siesta 802, Compumedics) (Fig. 1B). Total weight of instrumentation was 1.2 kg (compared to the average body weight of sheep at this age of ∼40 kg). Previous work has established that similar harness equipment carried by sheep did not affect sheep locomotion (Hobbs-Chell et al., 2012). All channels were sampled at 256 Hz and hardware-filtered between 0.15–128 Hz. Twenty-four hour recordings were obtained and stored on board the amplifier. Infrared video recordings were collected through a wall-mounted camera covering most of the accessible environment.
Data analysis
Sleep EEG and rumination analysis
The criteria for vigilance state classification are detailed in Supplementary Table 1. Wake activity was characterized by fast, low amplitude (<80 μV) EEG, and large amplitude variable EMG and eye movements as measured by the EOG. Non-REM sleep was characterized by EEG with large amplitude (>100 μV) slow-wave oscillations in the delta frequency band (0.5–4 Hz), a reduction of EMG tone and a flat EOG. REM sleep was characterized by a further reduction in muscle tone, a wake-like EEG and occasional eye movements. Only epochs that followed the non-REM state were classified as REM. Rumination was characterized by wake-like EEG, EMG free of movement artefact and clear rhythmic chewing in the EOG. Rumination + non-REM was characterized by EEG consistent with non-REM sleep, an artefact-free EMG and an EOG consistent with rumination.
EEG, EOG and EMG channels were used for vigilance state classification in conjunction with behavioural monitoring through video recording. EEG, EMG and EOG channels were bandpass-filtered between 0.5–40 Hz. The 24-h recordings were analysed in 10-s intervals (epochs) and each assigned a vigilance state according to the criteria listed in Supplementary Table 1. Spectral analyses of non-REM epochs were performed using multi-tapered spectral analysis methods as realized in the Chronux Toolbox (http://chronux.org/) with a time-bandwidth product of three and five tapers.
Automated detection of slow-waves, spindle and seizure events
Automated detection routines as described by Phillips et al. (2012) were used. Briefly, spindle-band filtered and rectified waveforms were standardized and an amplitude threshold detection routine, realized in Matlab® (R2010B, Mathworks) was used to detect events exceeding the threshold of 1.5 standard deviations. Additional heuristic criteria were used to select a subpopulation of these events (presence of an autocorrelation peak in the range 0.0625–0.117 s, length smaller than 600 ms and amplitude not exceeding ± 100 μV) and all events were confirmed to be spindles through visual inspection. Slow-waves were detected with a similar detection algorithm (amplitude threshold of two standard deviations) but with EEG waveforms filtered in the 0.5–3 Hz band and an additional rejection criterion (amplitude not exceeding ± 300 μV). Spindle band activity around slow-waves was calculated by forming averages of spindle-band filtered, rectified and smoothed waveforms centred on the peaks of slow-wave events (from −1 to +1 s around corresponding slow-waves). Seizures were automatically detected by the algorithm used for spindle detection but with filtering in the 4–6 Hz frequency band and a threshold of two standard deviations. A subselection of events was chosen using two component Gaussian mixture clustering of features extracted within the threshold-detected events (features: raw signal autocorrelation, root mean square amplitude, ratio of power in 4–6 Hz to the remaining spectrum and absolute power in the 4–6 Hz band). The clustering step facilitated the separation of threshold-detected events into seizures and artefacts.
Verifying concurrent rumination and non-REM sleep using independent component analysis
Because of occasional EEG contamination by rumination-related chewing, we performed independent component analysis on all acquired channels (EEG, EMG and EOG) to verify whether rumination and slow-wave activity were linearly separable. Using the ‘runica’ algorithm of the EEGLAB toolbox (Delorme and Makeig, 2004) independent components associated with rumination were visually identified and then subtracted from all EEG channels (but not from EMG or EOG channels).
Rumination
Epochs marked as rumination were subsequently analysed in terms of numbers of episodes, episode durations, bout length variability and the variability in duration of intervals between consecutive chewing events. Bout lengths were calculated for all sheep. We drew a random sample of 300 bout events from each sheep and calculated standard deviations as a measure of variability. A similar approach was followed for the assessment of inter-chewing intervals but with a sample of 12 000 events from each sheep.
Statistical analysis
T-tests were used where the homogeneity of variance was met (Levene’s test of equality of variance). To assess diurnality in normal and CLN5−/− Batten disease affected sheep, pairwise t-tests were used to compare the amount of time spent in the wake states during the day versus the night. To compare the time spent in each stage for each vigilance state, a two-group (normal versus CLN5−/− Batten disease affected sheep) by five-vigilance states (wake, rumination, rumination + non-REM, non-REM and REM) multivariate analysis of variance (MANOVA) was used. The slow wave characteristics of amplitude and peak frequency, the spindle amplitude and spindle density and slow-wave locked spindle band activity during non-REM sleep were compared using t-tests. Slow wave amplitude data were inversely-transformed to achieve normality. The number of rumination episodes, variability in rumination bout lengths and variability in inter-chewing intervals were assessed using t-tests. Statistical tests were considered significant at P < 0.05.
Results
Sheep are easily managed experimental subjects for chronic brain implants
The large skull and brain size of sheep allow many electrodes to be surgically implanted (Fig. 1A). Sheep recovered well from surgery. Within a few minutes from the end of anaesthesia sheep were eating and within an hour they were standing up. They were then returned to their normal group housing. No overt behavioural changes were observed after recovery from surgery. Animals were instrumented with jackets that held standard ambulatory paediatric EEG recording devices (Fig. 1B), so EEGs could be recorded from sheep in their home pen in the presence of conspecifics (Fig. 1C). The instrumentation was well tolerated and long continuous recordings could be collected. We were able to collect good quality 24-h data for up to 280 days after implantation (Supplementary Fig. 1).
Sheep are diurnal and exhibit five states of vigilance
Vigilance states were visually classified using electrophysiological measures and further corroborated using behavioural criteria obtained through 24-h video monitoring (Supplementary Table 1). All sheep exhibited the characteristic mammalian vigilance states of wake, REM and non-REM sleep (Fig. 1D–H). Sheep are ruminants and so spend a significant amount of time chewing previously ingested and regurgitated food. They ruminate when free from threat and almost exclusively while seated. Thus, we classified rumination as a distinct vigilance state (Fig. 1E). In line with previous observations of ruminant sleep (Bell and Itabisashi, 1973; Ruckebusch, 1975), we identified an additional state of rumination accompanied by EEG slow-wave oscillations consistent with non-REM sleep (rumination + non-REM; Fig. 1G, see also Supplementary Videos 1 and 2 for distinct behaviours during these two states).
Visual inspection of the EEG traces revealed slow wave activity temporally overlapping with chewing-induced artefacts arising from mastication activity during rumination (Supplementary Fig. 2A and B). As it was occasionally difficult to discern whether these two processes were independent of each other, we used independent components analysis to assess whether slow wave oscillations and rumination artefacts were linearly separable. Through independent component analysis decomposition, the identified rumination related independent components were subtracted from the EEG traces leaving an artefact-free EEG. As a result, both wake-like (Supplementary Fig. 2G) and non-REM-like (Supplementary Fig. 2H) EEG activity could be detected. Artefact-free EEG channels remained so after subtraction of rumination components (Supplementary Fig. 2C and D before subtraction; I and J post subtraction). Thus, slow wave-like oscillations during rumination intervals arise from statistically independent processes.
Behavioural and neurological phenotypes of CLN5−/− Batten disease affected sheep
As previously described (Jolly et al., 2002; Frugier et al., 2008), CLN5−/− Batten disease affected sheep show a slowly progressing neurological phenotype that includes deterioration of vision, reduced awareness (manifest by self-segregation from the flock and irruptive bumping into obstacles), tremor (observed in eyelids, ears and hind limbs) and motor deficits (locomotion difficulties and stumbling). Additional behavioural changes that we noted included an unusually low head carriage, stargazing, repetitive behaviours (teeth grinding and compulsive circling), and feeding difficulties (slow, inefficient eating and dribbling). These resembled behavioural signs reported for CLN6 Batten disease affected sheep (Cook et al., 2002). It should be emphasized that the CLN5−/− Batten disease affected sheep used here showed only mild overt signs at the end of the study period. Furthermore, they had not lost significant body weight. Indeed, their body weight profile was similar to their heterozygote counterparts. Linear regression on average weights collected for each genotype group during the study revealed similar positive slopes (0.132 for CLN5−/− and 0.187 for CLN5+ /− ), indicating that both groups of sheep were still growing at similar rates.
CLN5−/− Batten disease affected sheep show reduced sleep and decreased peak EEG delta power
Clinically, sleep phenotypes are characterized in terms of sleep architecture (the relative proportions and timing of different sleep stages) and sleep neurophysiology, both of which are disrupted in a range of brain diseases (Wulff et al., 2010). We therefore translated these measures into the sheep model. Quantification of vigilance states (Fig. 1I) showed that normal and CLN5−/− Batten disease affected sheep exhibited diurnal behaviour consistent with previous reports of sheep housed under similar conditions (Tobler et al., 1991; Piccione et al., 2008) with significantly more wakefulness during day-time (μnorm = 81.4 ± 2.1%; μCLN5−/− = 89.0 ± 2.9%) than during night-time (μnorm = 55.3 ± 7.2%; μCLN5−/− = 67.3 ± 4.6%; normal: t = 4.590, df = 4, P = 0.010; CLN5−/−: t = 8.470, df = 4, P = 0.001).
A two-group (normal versus CLN5−/− Batten disease affected sheep) by five-vigilance states (wake, rumination, rumination + non-REM, non-REM and REM) MANOVA revealed that the time spent in wake and rumination (rumination and rumination + non-REM together) did not differ between groups. However, the time spent in non-REM and REM states was significantly shorter for the CLN5−/− sheep (non-REM: μnorm = 16.2% ± 1.7% and μCLN5−/− = 10.1% ± 1.6%; f = 7.782, df = 1, P = 0.024 and REM: μnorm = 2.2% ± 0.4% and μCLN5−/− = 0.7% ± 0.2%, f = 9.012, df = 1, P = 0.017).
EEG during sleep features ‘hallmark’ neural network oscillations with dissociable origins and frequencies. Quantification of these oscillations provides a direct metric of circuit function and dysfunction during sleep. The most prominent EEG features during non-REM sleep include slow-wave activity (0.1–4 Hz), reflecting synchronized transitions between cortical UP and DOWN states, and thalamocortical spindle oscillations (8–15 Hz) that shape ensemble activity and plasticity (Diekelmann and Born, 2010).
We examined slow-wave activity during non-REM sleep using detection of individual slow-wave events alongside Fourier analyses (Fig. 2). Figure 2A shows typical examples of high amplitude EEG during non-REM sleep from normal, CLN5+/− and CLN5−/− sheep. The latter group clearly displays lower slow-wave amplitude. Accordingly, non-REM peak slow-wave band (0.5–4 Hz) power was significantly lower in CLN5−/−sheep (μnorm = 737.9 ± 164.9 μV2 and μCLN5−/− = 169.3 ± 61.2 μV2; t = 4.378, df = 8, P = 0.002; Fig. 2B and C). Furthermore, the non-REM peak frequency in slow-wave band activity was significantly higher in CLN5−/− sheep than in normal sheep (μnorm = 0.98 ± 0.11 Hz and μCLN5−/− = 1.28 ± 0.08 Hz; t = 2.326, df = 8, P = 0.049). In CLN5+/− sheep, both peak slow-wave band power and frequency, and slow-wave amplitudes were qualitatively more similar to normal sheep than CLN5−/−. Because of the small sample size of CLN5+/− animals, statistical comparisons were not attempted.
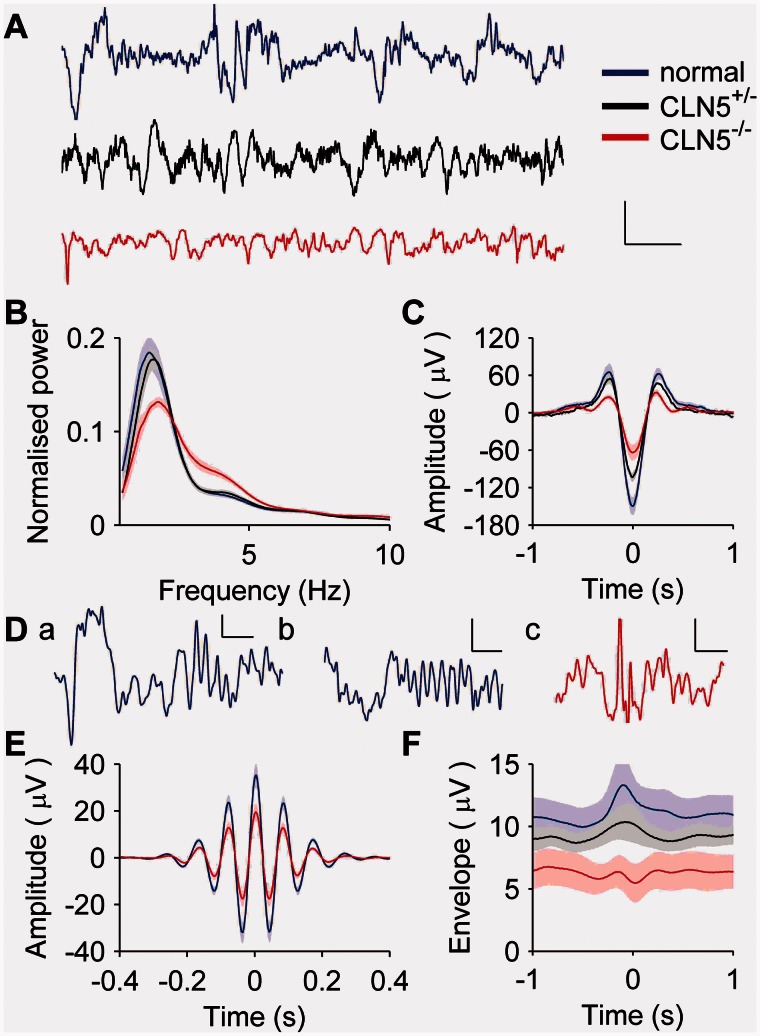
Figure 2.
Power spectra and sleep spindles recorded during non-REM sleep are abnormal in CLN5−/− sheep. (A) Example EEG traces show high-amplitude non-REM sleep recorded from a normal (blue), heterozygous CLN5+/− (black) and homozygous CLN5−/− (red) sheep. Large slow-waves are visible in recordings from normal and CLN5+/− sheep (black trace), but not in those recorded from CLN5−/− sheep (red trace). (B) Power spectra from recordings during non-REM sleep show lower peak delta amplitude and increased activity in the range of 3–5 Hz for CLN5−/− sheep (red trace) compared to both normal (blue trace) or CLN5+/− sheep (black trace). (C) Slow-waves detected automatically had lower amplitudes in CLN5−/− compared to either normal or CLN5+/− sheep. (D) Sleep spindles during non-REM sleep were observed in all sheep. Examples show spindle events from normal (a and b) and CLN5−/− sheep (c), either with (a and b) or without (c) an adjacent slow-wave. (E) Average detected spindles in CLN5−/− sheep (red trace) seemed to have smaller amplitudes than those of normal sheep (blue trace), but the difference did not reach statistical significance. Spindles from CLN5+/− sheep were similar to those from normal sheep (not shown for clarity). (F) Slow wave-triggered and rectified spindle band activity showed an increase in the vicinity of slow waves in normal (blue) and CLN5+/− sheep (black) that was not seen to the same extent in CLN5−/− sheep (red). The scale bar in A applies to all three traces and represents 1 s (x-axis) and 200 μV (y-axis); scales in D are 0.25 s (x-axis) and 50 μV (y-axis).
Sheep sleep is rich in sleep spindles and these remain relatively unaffected in CLN5−/− Batten disease affected sheep
Spindle band activity is a hallmark of light non-REM sleep and, critically, is implicated in the memory-consolidating function of sleep (Eschenko et al., 2006). In humans, changes in sleep spindles have been identified as phenotypic markers in neurological conditions such as Huntington's disease (Wiegand et al., 1991), schizophrenia (Ferrarelli et al., 2007), Alzheimer's disease (Rauchs et al., 2008), and Parkinson's disease (Christensen et al., 2014). Here, we characterized the nature of sleep spindles and their temporal relationship with slow-wave activity in both healthy and diseased sheep.
EEG from non-REM epochs was band-pass filtered at the spindle frequency range (10–16 Hz) and spindle events were detected using a threshold detection algorithm (see ‘Materials and methods’ section). To obtain a representative sample of spindle events (Fig. 2D), 200 spindles were randomly selected from each sheep and the absolute amplitude within the 0.8 s window was integrated (Fig. 2E). There was a trend towards lower spindle amplitude in CLN5−/− Batten disease affected sheep (μnorm = 1636 ± 259 μV and μCLN5−/− = 900 ± 197 μV, t = 2.257, df = 8, P = 0.054). Spindle density and duration did not differ between groups.
We next examined the interrelationships between slow-waves and spindles, which reflect coordinated limbic-cortical activity during non-REM sleep (Hahn et al., 2006; Taxidis et al., 2013). Using similar threshold detection algorithms, slow-wave events (0.5–3 Hz) were detected and the envelope of the spindle band activity around these events was computed (Fig. 2C and E). The absolute slow-wave-associated spindle band activity was significantly lower in CLN5−/− sheep (μnorm = 13.8 ± 2.8 μV and μCLN5−/− = 6.5 ± 0.8 μV, t = 2.452, df = 8, P = 0.040), suggesting disrupted coordination of thalamocortical activity during non-REM sleep. Because of the small sample size (with only two CLN5+/− sheep), we did not compare data statistically. Nevertheless, data from CLN5+/− sheep were qualitatively more similar to normal sheep than they were to CLN5−/− animals. In all, these results are analogous to human neurological findings, suggesting that the study of sleep spindles in sheep may be a useful translational measure in ovine neurodegenerative models.
CLN5−/− Batten disease affected sheep suffer from frequent, short duration, non-manifest seizures
Children affected by CLN5 Batten disease frequently develop epilepsy (Jadav et al., 2014). As well as this, EEG abnormalities during non-REM sleep are seen in patients with Batten disease who do not have convulsive seizures (Lauronen et al., 1999). Overt seizures are not a feature of ovine CLN5 Batten disease, and no manifest seizures have been reported in field observations of >100 CLN5−/− sheep in the founder flock at Lincoln University, a number of which were over 22 months of age. Although none of our sheep showed manifest convulsive seizures, EEG revealed frequent epileptiform activity in all recordings from CLN5−/− Batten disease affected sheep. These events were observed in some sheep as early as 8 months, when recording first started, and were present in all affected sheep. Importantly, none of the recordings from CLN5+/− or normal sheep exhibited these events. Epileptiform events were automatically detected (see ‘Materials and methods’ section for detection routine) in the frequency range of 4–5 Hz. Figure 3A shows examples of epileptiform ictal events recorded from CLN5−/− Batten disease affected sheep. These events were typically non-manifest (i.e. no instances of convulsive seizures were recorded) as revealed by an absence of high amplitude correlated EMG and EOG activity (Fig. 3B). An average polyspike discharge rate of 4.5 Hz was observed (Fig. 3C), with a mean duration of 2.5 s (Fig. 3D). Events were observed in all vigilance states (Fig. 3E). Peak normalized power was similar across vigilance states (Fig. 3F).
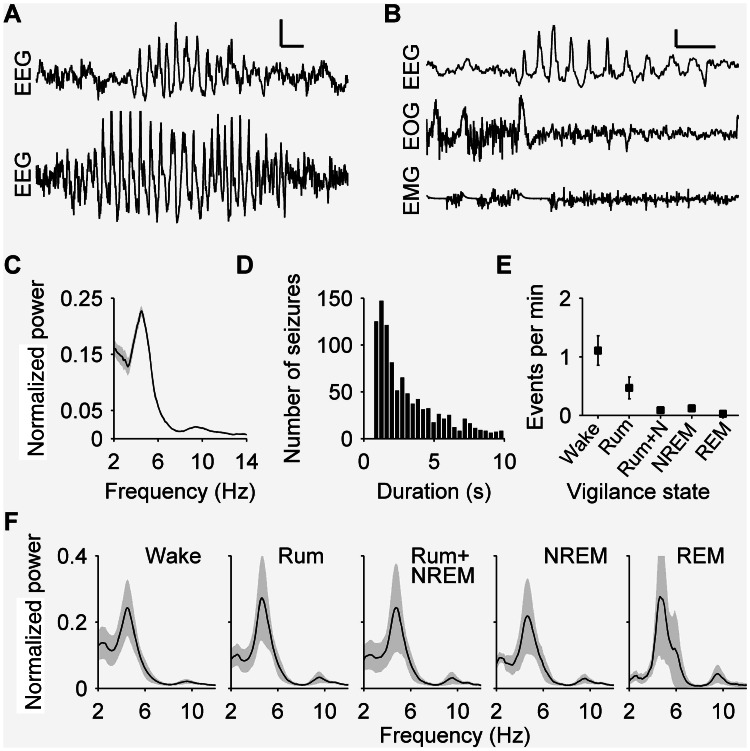
Figure 3.
Epileptiform events were observed exclusively in EEGs from CLN5−/− Batten disease affected sheep. (A) Typical examples of EEG recordings during epileptiform activity. (B) Epileptiform events are seen in the EEG without concomitant EOG or EMG components. (C) Normalized power spectra during epileptiform events show a peak at 4.5 Hz. (D) Epileptiform events were typically short, with a median duration of 2.5 s. (E) Events were seen in all vigilance states at similar rates in each state. (F) Spectral content of these events was similar across vigilance states, with a peak at 4.5 Hz. NREM = non-REM; Rum = rumination.
Abnormalities in rumination may serve as a read-out for motor dysfunction in sheep
Neurodegenerative conditions are often accompanied by symptoms involving motor control manifested as dysphagia or dysarthria (Åberg et al., 2011; Heemskerk and Roos, 2011; Monteiro et al., 2014). Rumination is a complex behaviour requiring both peripheral and central control mechanisms (Baile et al., 1967; Honde and Bueno, 1984): in order to ruminate, sheep must initiate and maintain a complex motor sequence involving regurgitation, mastication and swallowing of food for long periods extending tens of minutes. Given the motor component of rumination, we investigated the use of EMG and EOG activity during rumination as a potential marker of motor dysfunction.
In both normal and CLN5+/− sheep, rumination was found to be highly temporally structured, with consistent bout lengths and chewing rhythmicity (Fig. 4A–C and E). By contrast, rumination in CLN5−/− Batten disease affected sheep was marked by much less consistent bout and chewing intervals (Fig. 4B–D and F). We therefore quantified rumination across three temporal scales (episode, bout and inter-chewing durations). CLN5−/− Batten disease affected sheep had a significantly higher number of rumination episodes (μnorm = 20.2 ± 1.4 and μCLN5−/− = 31.4 ± 3.1, t = 3.317, df = 8, P = 0.011) and these were significantly shorter than those seen in normal sheep (μnorm = 66.8 ± 4.9 min and μCLN5−/− = 38.4 ± 2.9 min, t = 5.02, df = 8, P = 0.001). We calculated the standard deviation of each sheep’s bout length distribution as an index of variability of bout duration. CLN5−/− Batten disease affected sheep had significantly higher bout variability compared to normal sheep (μnorm = 7.3 ± 1.2 and μCLN5−/− = 11.1 ± 2.7, t = 2.86, df = 8, P = 0.021), as illustrated in Fig. 4B, although no difference was detected in the variability of inter-chewing intervals (P = 0.113; Fig. 4C).
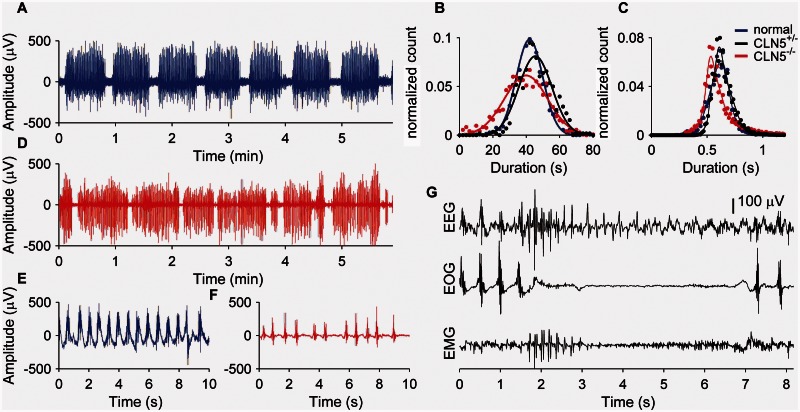
Figure 4.
Rumination is a highly structured temporal process that is abnormal in CLN5−/− affected Batten disease sheep. (A) Typical examples of rumination episodes (EOG) from normal sheep show regular bouts of chewing. The distribution of chewing bout lengths (B) and interchewing intervals (C) in normal (blue) and CLN5+/− sheep (black) are similar. (D) Rumination in CLN5−/− sheep shows increased variability (B) in bout lengths but similar interchewing intervals (C), compared to normal or CLN5+/− sheep. (E) A higher resolution portion of rumination from a normal sheep reveals the rhythmic chewing associated with rumination. (F). Chewing is more variable in the example from a CLN5−/− sheep; overall this was not statistically significant. (G) An example of a myoclonic event with epileptiform spike discharges interrupting rumination.
Together, these results identify rumination as a potential marker for motor dysfunction in sheep. Additionally, the observation that rumination events can be interrupted by epileptiform activity (Fig. 4G) supports the idea that disturbances to an integrated cortico-brainstem network controlling rumination-related motor activity cause the abnormalities.
Discussion
Recording EEGs from a cohort of sheep carrying a natural mutation in CLN5 that causes variant late-infantile Batten disease uncovered neurological abnormalities characteristic of those seen in children with CLN5 or other forms of late infantile Batten disease, including reduced EEG amplitude and frequent epileptiform activity (Harden and Pampiglione, 1982; Veneselli et al., 2000; Jadav et al., 2014). Quantification of the unique feature of rumination showed that CLN5 Batten disease affected sheep have deficits which may arise from motor control abnormalities; therefore this marker may serve as a useful readout of disease progression in sheep with Batten or other neurological diseases.
All of the sheep EEGs were collected via surgically implanted electrodes, because artefacts from the large muscles made it impossible to collect EEG from surface or subcutaneous electrodes (unpublished observations, N.P. and A.J.M.). Nevertheless, for humans EEG is particularly appealing as a measure of human brain activity because of its non-invasive nature and low cost. Our analyses concentrated on EEG features that are readily detectable through scalp EEG recordings in humans, ensuring translational relevance. The method has great potential, since EEG findings in humans can now be studied in a large genetically-relevant sheep model. EEG can be used as a longitudinal biomarker, or combined with experimental interventions to provide important directions for therapeutic development. The technique developed here allows sleep-wake EEG recordings to be conducted on unrestrained sheep under naturalistic housing conditions in which they are behaving normally. Recordings can be conducted longitudinally (see Supplementary Fig. 1 for an example of a series of longitudinal recordings from one animal, with comparable signal amplitudes, extending up to 280 days post-implantation). Thus EEG changes might be useful as a marker of disease progression. Further studies will be necessary to establish this possibility.
Using our EEG methods, sheep are able to express their normal sleep-wake profiles, uncontaminated by stress from restraint or removal from home environments, or by the need to carry cumbersome equipment that may affect behaviour. Our 24-h recordings confirmed that sheep are diurnal, with sleep-wake characterized by recognizable vigilant states. They typically had a total amount of sleep of ∼5 h. The small amount of REM sleep identified was surprising, but consistent with earlier studies where total REM in sheep ranged from 1–2.5% (Ruckebusch, 1972; Ruckebusch and Gaujoux, 1976). A hypothesis explaining the low amount of REM sleep in sheep suggested that the ruminant stomach requires an upright position of the thorax for proper functioning, imposing postural constraints on the animal that limit the opportunities for fully reclined sleep (Balch, 1955). The prey nature of the species and its inability to hide effectively from predators may have also exerted evolutionary pressure on its sleep physiology (Allison and Cicchetti, 1976; Capellini et al., 2008).
Our findings of decreased non-REM EEG amplitudes in Batten disease affected sheep are consistent with those reported from patients with the juvenile form of Batten disease, who display a relative increase in the percentage of sleep spent in stage 1 slow-wave sleep while overall sleep quantity is reduced (Kirveskari et al., 2000). Mean age differences between normal and CLN5 Batten disease affected sheep are unlikely to account for the observation of reduced non-REM EEG amplitudes. The heterozygous unaffected sheep recorded at the same age as the CLN5−/− Batten disease affected sheep presented electrophysiological signatures that were much more similar to normal sheep than to CLN5−/− Batten disease affected sheep. The observation of reduced slow-wave amplitudes during non-REM sleep can be interpreted in several ways. First, reduced non-REM amplitudes could be interpreted as a decrease in homeostatic sleep drive reflecting a reduced need for sleep; this would be consistent with the accompanying reduction in the overall amount of non-REM sleep. The synaptic downscaling hypothesis argues that a prominent function of non-REM sleep is to downscale previously potentiated cortical connections that result from daytime experience and that the non-REM slow-wave amplitude reflects this need (Tononi and Cirelli, 2014). We speculate that in CLN5−/− sheep, because of the learning deficits, dementia and blindness normally associated with Batten disease, there is decreased synaptic upscaling during the day and, accordingly, decreased need for synaptic downscaling during sleep. This would result in depressed slow-wave amplitude during non-REM sleep.
Second, it is also likely that the reduced slow-wave amplitude results from neuronal loss or defective recruitment of neurons into the slow-wave oscillation. This possibility is consistent with observations of atrophied cortices in CLN5 patients with Batten disease (Petersen et al., 1996; Tyynelä et al., 1997; Peña et al., 2001) and CLN5−/− affected Batten disease sheep (Jolly et al., 2002). The shift of slow-wave spectra towards faster frequencies seen in CLN5−/− affected Batten disease sheep may arise from an imbalance between inhibition and excitation in cortico-thalamocortical loops. This would be consistent with a selective loss of GABAergic interneurons in the cortex, the reticular thalamic nucleus and other thalamic nuclei as has been described in CLN5 mice (Kopra et al., 2004) and CLN6 sheep (Oswald et al., 2008).
We have characterized sheep sleep and found a number of features that make sheep attractive models for studying sleep, particularly when compared with rodents. Sheep are diurnal animals, as are primates, whereas rodents are nocturnal. We have observed that sheep sleep is rich in spindles, an EEG feature that is prominent in humans, and has been reported in a number of other mammalian species including rats, mice, cats and monkeys (Andrillon et al., 2011). Sleep spindles reflect coordinated corticothalamic activity and are correlated with overnight memory consolidation (Tononi and Cirelli, 2014). Interestingly, spindles are disrupted in many neurological disorders (Wiegand et al., 1991; Ferrarelli et al., 2007; Rauchs et al., 2008; Christensen et al., 2014) including Batten disease (Veneselli et al., 2000). Here, we quantified sleep spindles and uncovered trend-level reductions in spindle amplitudes, but no density change in the Batten disease affected sheep. It is possible that a change in spindle amplitude is part of the mechanism underlying learning deficits in Batten disease.
Although rumination is not directly translational, the control of chewing is highly relevant, given that dysphagia is a common symptom in many human neurodegenerative diseases, including Batten disease, Huntington’s disease, Alzheimer’s disease and Parkinson’s disease (Åberg et al., 2011; Heemskerk and Roos, 2011; Affoo et al., 2013; Monteiro et al., 2014). The complex and highly temporal structure of rumination along with its strong dependence on coordinated motor control (rhythmic chewing, swallowing reflex and regurgitation) could make it a valuable read out for neurological conditions with dysphagia or dysarthria phenotypes. Accordingly, we have found signatures consistent with motor control impairments in CLN5−/− Batten disease affected sheep. The possibility that the observed differences arose from factors other than motor control, such as reduced muscle tone, cannot be excluded using the available data. Nevertheless, rumination warrants further investigation as a potential marker of disease progression. Further investigations will determine if there is a correlation between decline in rumination efficiency and disease progression.
Our study extends the applicability of sheep for studying both animal models of neurodegeneration and more generally, brain function. Sheep should be considered more frequently as a model to bridge the gap between findings in rodents and humans, particularly in those cases where the use of non-human primates is not feasible either due to ethical, practical or technical reasons. When it comes to assessing therapeutic avenues that rely on brains of comparable size and structure to those of humans, for example delivering gene therapies or large molecules directly to the brain, sheep are an especially attractive species. While our study concentrated on EEG, in vivo electrophysiological studies could easily be extended to address more refined questions through the use of high-density recordings of single neuron activity, targeting specific cortical and subcortical structures.
The docile nature of sheep, the ease of training in behavioural paradigms (Morton and Avanzo, 2011), along with their large brains and thick skulls (providing ample opportunity for fixation of complex implants, e.g. microdrives and high counts of electrodes), and limited ability or inclination to interfere with recording equipment, make them an attractive species to use for in vivo electrophysiological recordings. With the advent of wireless neural recording techniques, the opportunities to use sheep to investigate, for example, spatial navigation in large naturalistic environments, are increasingly realistic. Although sheep have been used previously as models for studying brain function, our study demonstrates how they can be used effectively in conjunction with modern electrophysiological techniques thus paving the way for future experiments that are otherwise not possible in small brained rodents or unfeasible in non-human primates. Further development of sheep as a laboratory model has great potential to facilitate brain research in both health and disease.
Acknowledgements
We thank Prof. Derk-Jan Dijk and Dr Raphaelle Winsky-Sommerer for guidance with sleep-related classification, Polly Taylor and Roger Mason, Beth Alison and Prof. Abigail Fowden for assistance with instrumentation, surgery technique and anaesthesia support.
Glossary
Abbreviation
- EOG
electrooculogram
Funding
This work was funded by CHDI Inc. (AJM). Founding the sheep flock, and costs in NZ relating to the rearing and genotyping of the animals were funded by a series of grants from the Neurological Foundation of NZ and the Batten Disease Support and Research Association (DNP, NLM).
Supplementary material
Supplementary material is available at Brain online.
References
- Åberg L, Autti T, Cooper JD, Elleder M, Haltia M, Jalanko A, et al. CLN5. In: Mole S, Williams R, Goebel H, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Oxford: Oxford University Press; 2011. pp. 140–58. [Google Scholar]
- Affoo RH, Foley N, Rosenbek J, Shoemaker JK, Martin RE. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer’s disease: a scoping review of the evidence. J Am Geriatr Soc. 2013;61:2203–13. doi: 10.1111/jgs.12553. [DOI] [PubMed] [Google Scholar]
- Allison T, Cicchetti DV. Sleep in mammals: ecological and constitutional correlates. Science. 1976;194:732–4. doi: 10.1126/science.982039. [DOI] [PubMed] [Google Scholar]
- Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011;31:17821–34. doi: 10.1523/JNEUROSCI.2604-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile CA, Mahoney AW, Mayer J. Placement of electrodes in the hypothalamus of goats. J Dairy Sci. 1967;50:576–8. doi: 10.3168/jds.S0022-0302(67)87469-1. [DOI] [PubMed] [Google Scholar]
- Balch CC. Sleep in ruminants. Nature. 1955;175:940–1. doi: 10.1038/175940a0. [DOI] [PubMed] [Google Scholar]
- Bell FR, Itabisashi T. The electroencephalogram of sheep and goats with special reference to rumination. Physiol Behav. 1973;11:503–14. doi: 10.1016/0031-9384(73)90037-1. [DOI] [PubMed] [Google Scholar]
- Bond M, Holthaus S-MK, Tammen I, Tear G, Russell C. Use of model organisms for the study of neuronal ceroid lipofuscinosis. Biochim Biophys Acta. 2013;1832:1842–65. doi: 10.1016/j.bbadis.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Capellini I, Barton RA, McNamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–76. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JA, Kempfner J, Zoetmulder M, Leonthin HL, Arvastson L, Christensen SR, et al. Decreased sleep spindle density in patients with idiopathic REM sleep behavior disorder and patients with Parkinson’s disease. Clin Neurophysiol. 2014;125:512–9. doi: 10.1016/j.clinph.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Cook RW, Jolly RD, Palmer DN, Tammen I, Broom MF, McKinnon R. Neuronal ceroid lipofuscinosis in Merino sheep. Aust Vet J. 2002;80:292–7. doi: 10.1111/j.1751-0813.2002.tb10847.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dolezalova D, Hruska-Plochan M, Bjarkam CR, Sørensen JCH, Cunningham M, Weingarten D, et al. Pig models of neurodegenerative disorders: utilization in cell replacement-based preclinical safety and efficacy studies. J Comp Neurol. 2014;522:2784–801. doi: 10.1002/cne.23575. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Mölle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–20. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–92. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- Frugier T, Mitchell NL, Tammen I, Houweling PJ, Arthur DG, Kay GW, et al. A new large animal model of CLN5 neuronal ceroid lipofuscinosis in Borderdale sheep is caused by a nucleotide substitution at a consensus splice site (c.571+1G>A) leading to excision of exon 3. Neurobiol Dis. 2008;29:306–15. doi: 10.1016/j.nbd.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmelt AC, Eitzen S, Balakhadze I, Fischer B, Wölfer J, Schiffbauer H, et al. Closed traumatic brain injury model in sheep mimicking high-velocity, closed head trauma in humans. Cent Eur Neurosurg. 2011;72:120–6. doi: 10.1055/s-0031-1271732. [DOI] [PubMed] [Google Scholar]
- Hahn TTG, Sakmann B, Mehta MR. Phase-locking of hippocampal interneurons’ membrane potential to neocortical up-down states. Nat Neurosci. 2006;9:1359–61. doi: 10.1038/nn1788. [DOI] [PubMed] [Google Scholar]
- Harden A, Pampiglione G. Neurophysiological studies (EEG/ERG/VEP/SEP) in 88 children with so-called ceroid lipofuscinosis. In: Armstrong D, Koppang N, Rider JA, editors. Ceroid lipofuscinoses (Batten’s disease) Amsterdam: Elsevier Biomedical Press; 1982. [Google Scholar]
- Harper PA, Walker KH, Healy PJ, Hartley WJ, Gibson AJ, Smith JS. Neurovisceral ceroid-lipofuscinosis in blind Devon cattle. Acta Neuropathol. 1988;75:62–6. doi: 10.1007/BF00686210. [DOI] [PubMed] [Google Scholar]
- Heemskerk A-W, Roos RAC. Dysphagia in Huntington’s disease: a review. Dysphagia. 2011;26:62–6. doi: 10.1007/s00455-010-9302-4. [DOI] [PubMed] [Google Scholar]
- Hobbs-Chell H, King AJ, Sharratt H, Haddadi H, Rudiger SR, Hailes S, et al. Data-loggers carried on a harness do not adversely affect sheep locomotion. Res Vet Sci. 2012;93:549–52. doi: 10.1016/j.rvsc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Honde C, Bueno L. Evidence for central neuropeptidergic control of rumination in sheep. Peptides. 1984;5:81–3. doi: 10.1016/0196-9781(84)90055-x. [DOI] [PubMed] [Google Scholar]
- Houweling PJ, Cavanagh JAL, Palmer DN, Frugier T, Mitchell NL, Windsor PA, et al. Neuronal ceroid lipofuscinosis in Devon cattle is caused by a single base duplication (c.Gdup662) in the bovine CLN5 gene. Biochim Biophys Acta. 2006;1762:890–97. doi: 10.1016/j.bbadis.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Jacobsen JC, Bawden CS, Rudiger SR, McLaughlan CJ, Reid SJ, Waldvogel HJ, et al. An ovine transgenic Huntington’s disease model. Hum Mol Genet. 2010;19:1873–82. doi: 10.1093/hmg/ddq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadav RH, Sinha S, Yasha TC, Aravinda H, Gayathri N, Rao S, et al. Clinical, electrophysiological, imaging, and ultrastructural description in 68 patients with neuronal ceroid lipofuscinoses and its subtypes. Pediatr Neurol. 2014;50:85–95. doi: 10.1016/j.pediatrneurol.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Jolly RD, Arthur DG, Kay GW, Palmer DN. Neuronal ceroid-lipofuscinosis in Borderdale sheep. N Z Vet J. 2002;50:199–202. doi: 10.1080/00480169.2002.36311. [DOI] [PubMed] [Google Scholar]
- Jolly RD, Martinus RD, Palmer DN. Sheep and other animals with ceroid-lipofuscinoses: their relevance to Batten disease. Am J Med Genet. 1992;42:609–14. doi: 10.1002/ajmg.1320420436. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, da Costa AP, Leigh AE, Hinton MR, Peirce JW. Sheep don’t forget a face. Nature. 2001;414:165–6. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- Kirveskari E, Partinen M, Salmi T, Sainio K, Telakivi T, Hämäläinen M, et al. Sleep alterations in juvenile neuronal ceroid-lipofuscinosis. Pediatr Neurol. 2000;22:347–54. doi: 10.1016/s0887-8994(00)00138-7. [DOI] [PubMed] [Google Scholar]
- Kopra O, Vesa J, von Schantz C, Manninen T, Minye H, Fabritius A-L, et al. A mouse model for Finnish variant late infantile neuronal ceroid lipofuscinosis, CLN5, reveals neuropathology associated with early aging. Hum Mol Genet. 2004;13:2893–906. doi: 10.1093/hmg/ddh312. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN. doi: 10.1101/cshperspect.a006320. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med 2012; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauronen L, Munroe PB, Järvelä I, Autti T, Mitchison HM, O’Rawe AM, et al. Delayed classic and protracted phenotypes of compound heterozygous juvenile neuronal ceroid lipofuscinosis. Neurology. 1999;52:360–5. doi: 10.1212/wnl.52.2.360. [DOI] [PubMed] [Google Scholar]
- Monteiro L, Souza-Machado A, Pinho P, Sampaio M, Nóbrega AC, Melo A. Swallowing impairment and pulmonary dysfunction in Parkinson’s disease: the silent threats. J Neurol Sci. 2014;339:149–52. doi: 10.1016/j.jns.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Avanzo L. Executive decision-making in the domestic sheep. PLoS One. 2011;6:e15752. doi: 10.1371/journal.pone.0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton AJ, Howland DS. Large genetic animal models of Huntington’s disease. J. Huntington’s Dis. 2013;2:3–19. doi: 10.3233/JHD-130050. [DOI] [PubMed] [Google Scholar]
- Oswald MJ, Palmer DN, Kay GW, Barwell KJ, Cooper JD. Location and connectivity determine GABAergic interneuron survival in the brains of South Hampshire sheep with CLN6 neuronal ceroid lipofuscinosis. Neurobiol Dis. 2008;32:50–65. doi: 10.1016/j.nbd.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña JA, Cardozo JJ, Montiel CM, Molina OM, Boustany R-M. Serial MRI findings in the Costa Rican variant of neuronal ceroid-lipofuscinosis. Pediatr Neurol. 2001;25:78–80. doi: 10.1016/s0887-8994(01)00284-3. [DOI] [PubMed] [Google Scholar]
- Petersen B, Handwerker M, Huppertz H-I. Neuroradiological findings in classical late infantile neuronal ceroid-lipofuscinosis. Pediatr Neurol. 1996;15:344–7. doi: 10.1016/s0887-8994(96)00224-x. [DOI] [PubMed] [Google Scholar]
- Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, et al. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012;76:526–33. doi: 10.1016/j.neuron.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccione G, Giannetto C, Casella S, Caola G. Circadian activity rhythm in sheep and goats housed in stable conditions. Folia Biol. 2008;56:133–7. doi: 10.3409/fb.56_3-4.133-137. [DOI] [PubMed] [Google Scholar]
- Pouladi MA, Morton AJ, Hayden MR. Choosing an animal model for the study of Huntington’s disease. Nat Rev Neurosci. 2013;14:708–21. doi: 10.1038/nrn3570. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, et al. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. 2008;19:1159–62. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckebusch Y. The relevance of drowsiness in the circadian cycle of farm animals. Anim Behav. 1972;20:637–43. doi: 10.1016/s0003-3472(72)80136-2. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y. The hypnogram as an index of adaptation of farm animals to changes in their environment. Appl Anim Ethol. 1975;2:3–18. [Google Scholar]
- Ruckebusch Y, Gaujoux M. Sleep-inducing effect of a high-protein diet in sheep. Physiol Behav. 1976;17:9–12. doi: 10.1016/0031-9384(76)90260-2. [DOI] [PubMed] [Google Scholar]
- Schubert M, Breakefield X, Federoff H, Frederickson RM, Lowenstein PR. Gene delivery to the nervous system. Mol Ther. 2008;16:640–6. doi: 10.1038/mt.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stypulkowski PH, Giftakis JE, Billstrom TM. Development of a large animal model for investigation of deep brain stimulation for epilepsy. Stereotact Funct Neurosurg. 2011;89:111–22. doi: 10.1159/000323343. [DOI] [PubMed] [Google Scholar]
- Taxidis J, Mizuseki K, Mason R, Owen MR. Influence of slow oscillation on hippocampal activity and ripples through cortico-hippocampal synaptic interactions, analyzed by a cortical-CA3-CA1 network model. Front Comput Neurosci. 2013;7:3. doi: 10.3389/fncom.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler I, Jaggi K, Arendt J, Ravault JP. Long-term 24-hour rest-activity pattern of sheep in stalls and in the field. Experientia. 1991;47:744–9. doi: 10.1007/BF01958833. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyynelä J, Suopanki J, Santavuori P, Baumann M, Haltia M. Variant late infantile neuronal ceroid-lipofuscinosis: pathology and biochemistry. J Neuropathol Exp Neurol. 1997;56:369–75. doi: 10.1097/00005072-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Veneselli E, Biancheri R, Perrone MV, Buoni S, Fois A. Neuronal ceroid lipofuscinoses: clinical and EEG findings in a large study of Italian cases. Neurol Sci. 2000;21:S75–81. doi: 10.1007/s100720070044. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand M, Moller AA, Lauer CJ, Stolz S, Schreiber W, Dose M, et al. Nocturnal sleep in Huntington’s disease. J Neurol. 1991;238:203–8. doi: 10.1007/BF00314781. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–99. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]