Baets et al. expand the clinical spectrum of hereditary sensory and autonomic neuropathy type 1E (HSAN1E) by studying nine newly identified kinships, and reveal a potential pathogenic mechanism for causative DNMT1 mutations. Mutant DNMT1 proteins form aggresomes in the cytoplasm, suggesting that aggresome-induced autophagy may contribute to disease pathogenesis.
Keywords: protein aggregation, sensory neuropathy, narcolepsy, REM sleep behaviour disorder, neurodegeneration
Abstract
We report a broader than previously appreciated clinical spectrum for hereditary sensory and autonomic neuropathy type 1E (HSAN1E) and a potential pathogenic mechanism for DNA methyltransferase (DNMT1) mutations. The clinical presentations and genetic characteristics of nine newly identified HSAN1E kinships (45 affected subjects) were investigated. Five novel mutations of DNMT1 were discovered; p.C353F, p.T481P, p.P491L, p.Y524D and p.I531N, all within the target-sequence domain, and two mutations (p.T481P, p.P491L) arising de novo. Recently, HSAN1E has been suggested as an allelic disorder of autosomal dominant cerebellar ataxia, deafness and narcolepsy. Our results indicate that all the mutations causal for HSAN1E are located in the middle part or N-terminus end of the TS domain, whereas all the mutations causal for autosomal dominant cerebellar ataxia, deafness and narcolepsy are located in the C-terminus end of the TS domain. The impact of the seven causal mutations in this cohort was studied by cellular localization experiments. The binding efficiency of the mutant DNMT proteins at the replication foci and heterochromatin were evaluated. Phenotypic characterizations included electromyography, brain magnetic resonance and nuclear imaging, electroencephalography, sural nerve biopsies, sleep evaluation and neuropsychometric testing. The average survival of HSAN1E was 53.6 years. [standard deviation = 7.7, range 43–75 years], and mean onset age was 37.7 years. (standard deviation = 8.6, range 18–51 years). Expanded phenotypes include myoclonic seizures, auditory or visual hallucinations, and renal failure. Hypersomnia, rapid eye movement sleep disorder and/or narcolepsy were identified in 11 subjects. Global brain atrophy was found in 12 of 14 who had brain MRI. EEGs showed low frequency (delta waves) frontal-predominant abnormality in five of six patients. Marked variability in cognitive deficits was observed, but the majority of patients (89%) developed significant cognitive deficit by the age of 45 years. Cognitive function decline often started with personality changes and psychiatric manifestations. A triad of hearing loss, sensory neuropathy and cognitive decline remains as the stereotypic presentation of HSAN1E. Moreover, we show that mutant DNMT1 proteins translocate to the cytoplasm and are prone to form aggresomes while losing their binding ability to heterochromatin during the G2 cell cycle. Our results suggest mutations in DNMT1 result in imbalanced protein homeostasis through aggresome-induced autophagy. This mechanism may explain why mutations in the sole DNA maintenance methyltransferase lead to selective central and peripheral neurodegeneration.
Introduction
The pathogeneses of adult-onset neurodegenerative disorders have been associated with misfolded protein aggregation (Choi et al., 2013; Takalo et al., 2013) and epigenetic dysregulation (Marques et al., 2011). Defects in DNA methylation related genes have been linked with two neurodevelopmental disorders: immunodeficiency centromeric instability-facial anomalies (ICF; DNMT3B) and Rett syndrome (MECP2; Jin and Robertson, 2013; Klein and Benarroch, 2014). The mutations in DNMT3B and MECP2 are either homozygous (ICF) or X-linked (Rett), leading to childhood onset and mortality. In contrast, autosomal dominantly inherited heterozygous mutations of DNMT1 are recently identified as causal for two neurodegenerative diseases with characteristics of adult-onset and age-dependent progression: (i) hereditary sensory autonomic neuropathy with dementia and hearing loss (HSAN1E; OMIM#614116); and (ii) cerebellar ataxia, deafness, and narcolepsy (ADCA-DN; OMIM#604121). A recent study has suggested that narcolepsy may be a common feature of HSAN1E (Moghadam et al., 2014). HSAN1E is a subtype of the HSAN, a group of genetic disorders predominantly affecting the sensory and autonomic neurons of the peripheral nervous system. Genetic causes of HSAN are diverse, with 15 causal genes discovered to date (Rotthier et al., 2012; Rossor et al., 2013). However, each causal gene only accounts for a small percentage of HSAN patients (∼1–12%), and the genetic causes for the majority of patients with HSAN remains to be resolved (Klein et al., 2005; Rotthier et al., 2009; Davidson et al., 2012, Rossor et al., 2013).
DNMT1 is the sole maintenance methyltransferase and an essential component of cellular epigenetic regulation. It is indispensable in embryonic development and performs crucial functions in chromatin structure, neuronal survival and cell cycle regulation. DNMT1 is comprised of a large regulatory N-terminal region and a smaller catalytic C-terminal region. The proper allosteric interaction of N-terminal regulatory region with the catalytic C-terminal region is required for enzymatic function and controls its preference for hemimethylated DNA (Margot et al., 2000). Specifically, the TS domain in the N-terminal regulatory region, where all the causal mutations reside, regulates DNMT1 binding to hemimethylated DNA during S phase and is essential for its persistent association to heterochromatin during G2 phases. DNMT1 has high preference for hemimethylated cytosines on the newly synthesized DNAs, and TS domain serves as a controller to precisely direct DNMT1 to hemimethylated sites (Frauer and Leonhardt, 2011; Song et al., 2011). The TS domain positions itself in the DNMT1 catalytic pocket when the enzyme is inactive, and the activation of DNMT1 is achieved by the intricate interactions of multiple factors that induce the TS domain to exit the enzymatic active pocket and allow hemimethylated CpG sites to enter (Song et al., 2011). Thus, the proper folding of the TS domain is especially crucial for faithful DNA methylation maintenance. The DNMT1 misfolding due to TS domain mutations not only has deleterious impact on its DNA binding and critical interactions with other cellular regulators, but also impairs the faithful maintenance of DNA methylation of the newly synthesized DNA strand, as a significant portion of maintenance methylation occurs during the G2 phase within heterochromatin (Spada et al., 2007).
Herein, we report nine newly recognized kindreds (Fig. 1) with HSAN1E from the USA, Belgium, England, New Zealand and Germany. Five novel mutations in the TS domain are identified and the phenotype–genotype correlations of HSAN1E are expanded. We review their common and unique clinical presentations, and summarize their overlap symptoms with frontotemporal dementia, sleep disorders (including narcolepsy) and myoclonic seizures. Our functional studies indicate that in addition to the aberrant global DNA methylation we previously reported (Sun et al., 2014), DNMT1 mutations also lead to protein homeostasis imbalance due to DNMT1 mutant protein misfolding, implicating aggresome-induced autophagy in the pathogenesis of HSAN1E.
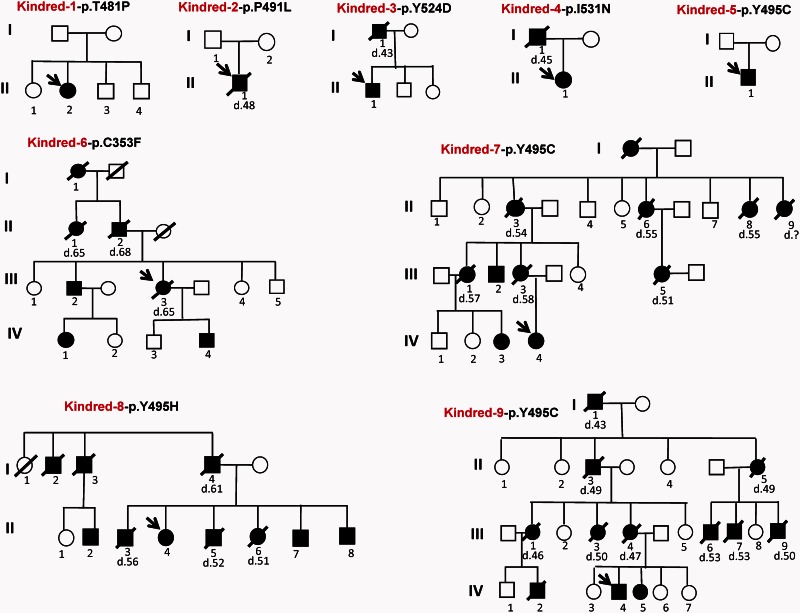
Figure 1.
Pedigrees of nine recently identified HSAN1E kindreds. Kindreds 1–4 and 6 have novel mutations in the TS domain of DNMT1, and four kindreds have mutations at Y495. Black symbols represent affected subjects. The age of death is marked under the deceased patients.
Materials and methods
Kindreds
The Institutional Review Boards of the participating centres approved the study, written informed consent was obtained from all patients and relatives or from their legal representatives prior to enrollment. Nine kindreds from five countries (USA, England, New Zealand, Germany and Belgium) are identified.
Mutation identification
Whole-genome paired-end sequencing was performed in Kindred 6 as described previously (Drmanac et al., 2010; Zimon et al., 2012). Whole-exome sequencing was performed in Kindreds 5 and 7. Library preparation was conducted according to the TruSeq (Illumina) sample-preparation protocol and sequencing (100 bp paired-end reads) was run on HiSeq 2000 (Illumina). The coverage was >90% for at least two reads, encompassing 85% of the targeted regions with coverage >10× sequencing depth. The identified variants in Kindreds 5–7 were validated using Sanger sequencing. The DNMT1 mutations in other kindreds/cases were identified through Sanger sequencing of DNMT1 coding exons. Nucleotide numbering was based on the DNMT1 cDNA sequence (NM_001379).
Expression vectors and mutant constructs
The expression constructs EGFP-human DNMT1 and mRFP-human PCNA were kindly provided by Dr H. Leonhardt (Ludwig Maximilians University Munich, Germany) as previously described (Klein et al., 2011). The pEGFP-hDNMT1 contains a CMV promoter and enhanced green fluorescent protein (GFP) fused into the N-terminal of full length human DNMT1 (NM_001379). Similarly, pmRFP-human PCNA contains a CMV promoter and mRFP cDNA fused into the N-terminal of human PCNA. All mutant constructs were generated using QuikChange0® mutagenesis kit (Stratagene). Primer sequences are available upon request. The full length open reading frames of all DNMT1 constructs were confirmed by Sanger sequencing.
HEK293 transfection and live-cell confocal microscopy
HEK293 cells were seeded on Lab-Tek™ II chambered cover glass 24 h before transfection to reach 50–60% of confluency at the time of transfection. Cells were co-transfected with EGFP-DNMT1 and mRFP-PCNA plasmids using FuGENE® HD (Promega). Thymidine was added to synchronize cells to S phase after 24 h of transfection and released after 12 h. Forty-eight hours after transfection, digital images of live cell microscopy were captured and analysed with Carl Zeiss LSM 780 META laser scanning confocal microscopes. EGFP and mRFP1 were excited sequentially at 488 nm, 547 nm to minimize the crosstalk.
Aggresome fluorescence marker staining
Red fluorescent staining of cellular aggresome was performed using a ProteoStat Aggresome Detection Kit (Enzo Life Science), according to the manufacturer’s protocol. Briefly, HEK293 cells were transfected with wt-EGFP-DNMT1 and various constructs of mutant EGFP-DNMT1. Transfected cells were fixed in 4% formaldehyde and permeabilized by 0.1% Triton™ X-100. Cells were stained with Proteostat Aggresome red-fluorescent dye Detection Reagent (1:4000) and Hoechst 33342 (1:2000), then washed extensively with phosphate-buffered saline. Images of cellular immunofluorescence were acquired using Carl Zeiss LSM 780 META laser scanning confocal microscopes.
GFP-tagged protein quantification assay
The amount of GFP-DNMT1 protein was quantified using GFP Quantitation Kit (BioVision). HEK293 cells were transfected with mutant and wild-type DNMT1 constructs. Forty hours after transfection, cells were treated with cycloheximide to inhibit the protein synthesis and cells were collected and lysed after 8 h of treatment. The quantities of GFP-tagged protein are determined by comparing the fluorescence with that of GFP standards and converted to the percentage of wild-type GFP-DNMT1 after normalizing with total protein concentration. The concentration of total proteins was checked using BCA protein assay (Pierce). The fluorescence value for each well was measured at 510 nm using a Microplate Reader (SpectraMAX Gemini XPS, Molecular Devices). The calculations are in triplicates based on three independent experiments.
Results
Clinical findings and case descriptions
A detailed overview of clinical findings of total 45 patients (21 female, 24 male) from nine Caucasian kindreds is summarized in Table 1, and the pedigrees are shown in Fig. 1. Representative findings on EEG, brain MRI and neuropathology of sural nerve biopsy are shown in Fig. 2.
Table 1.
Clinical features of 45 patients from nine kindreds with DNMT1 mutations
| Kindred, mutation | ID (gender) | Age | Presenting symptom | Hearing loss | Ulcers/amputation | Gait defect | NCS abnormal | Cognitive decline | Brain MRI changes | Sleep disorder | Myoclonus | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kindred-1 p.T481>P | II-2 (F) | 34 | Hearing and behaviour change (19) | Y | Y/N | Y | Y | Y, auditory hallucinations, behaviour change (20s) | Atrophy (G/Ce/F) | Hypersomnia, RBD w/o narcolepsy | Y | Visual/auditory hallucinations diagnosed schizophrenia initially |
| Kindred-2 p.P491>L | II-1 (M) | † (48) | Hearing loss (18) | Y | Y/Y | Y | Y | Y, auditory hallucination, behaviour change (39) | Atrophy (G,Ce) | Hypersomnia, SOREMP | Y | Sural biopsy: axonal neuropathy; EEG: diffuse slowing |
| Kindred-3 p.Y524>D | I-1 (M) | † (43) | - | Y | Y/- | Y | - | - | - | - | - | † Renal failure, amyloid due chronic wound infection |
| II-1 (M) | 46 | Sensory loss (26) | Y | Y/N | Y | Y | N | - | N | - | Sural biopsy: axonal neuropathy | |
| Kindred-4 p.I531>N | I-1 (M) | † (45) | Hearing and sensory loss (25) | Y | - | Y, ataxic | Y | Y | - | OSAS w/o narcolepsy | Y | Spells of postural tone loss + syncope |
| II-1 (F) | 39 | Gait instability (32) | Y | N/N | Y, ataxic | Y | Y | N | OSAS w/o narcolepsy | N | ||
| Kindred-5 p.Y495>C | II-1 (M) | 45 | Sensory loss (30) | Y | Y/N | Y | Y | Y, behaviour change | Atrophy (G) | PLMD, RSWA, MSLTs disturbed | N | Painless fractures |
| Kindred-6 p.C353>F | I-1 (F) | † (75) | - | Y | Y/- | Y | - | - | - | - | - | |
| II-1 (F) | † (65) | - | Y | - | Y | - | - | - | - | - | ||
| II-2 (M) | † (68) | - | Y | Y/Y | Y | - | - | - | - | - | ||
| III-2 (M) | 73 | Hearing loss (42) | Y | Y/Y | Y | Y | Y, hallucination (52) | Atrophy (G) | Hypersomnia | - | Renal failure (dialysis), lymphoedema left leg | |
| III-3 (M) | † (65) | Hearing loss (39) | Y | Y/N | Y | Y | N | - | - | - | Brain haemorrhage (63) | |
| IV-1 (F) | 53 | Gait instability (51) | N | N/N | Y | N | N | - | - | - | ||
| IV-4 (M) | 48 | Hearing loss (48) | Y | N/N | N | N | N | - | - | - | ||
| Kindred-7 p.Y495>C | II-3 (F) | † (54) | Cognitive decline (40) | Y | Y/- | Y | - | Y | - | N | N | FTD |
| II-6 (F) | † (55) | Trophic ulcer (43) | Y | Y/N | Y | Y | Y | - | N | N | Seizures | |
| II-8 (F) | † (55) | Hearing loss (40’s) | Y | N/N | Y | - | Y | - | N | N | ||
| II-9 (F) | † (50s) | Hearing loss (40’s) | Y | N/N | Y | - | Y | - | N | N | ||
| III-1 (F) | † (57) | Hearing loss (40’s) | Y | N/N | Y | Y | Y | Atrophy (G) | N | N | ||
| III-2 (M) | 53 | Impaired balance (36) | Y | Y/N | Y | Y | Y | Atrophy (G),T2 lesions | N | N | EEG: diffuse slowing | |
| III-3 (F) | † (58) | Sensory loss (43) | Y | Y/Y | Y | Y | Y | - | N | N | ||
| III-5 (F) | † (51) | Sensory loss (42) | Y | N/N | Y | Y | Y | Atrophy (G),T2 Lesions | N | N | MS? | |
| IV-3 (F) | 43 | Hearing loss (41) | Y | N/N | Y | Y | N | Atrophy,T2 Lesions | N | Y | Dysarthria | |
| IV-4 (F) | 48 | Foot arthropathy (38) | Y | Y/Y | Y | Y | Y | Atrophy (G) | N | Y | EEG: posterior slowing | |
| Kindred-8 p.Y495>H | I-2 (M) | † (‐) | Neuropathy | Y | - | - | - | - | - | - | - | |
| I-3 (M) | † (‐) | Hearing loss | Y | - | Y | - | - | - | - | - | ||
| I-4 (M) | † (61) | Hearing loss (40s) | Y | Y/N | Y | - | Y | - | Y, (RLS) | - | ||
| II-2 (M) | 50s | Neuropathy (40s) | - | - | Y | Y | - | - | - | - | ||
| II-3 (M) | † (56) | Neuropathy (46) | Y | Y/N | Y | Y | Y, behaviour change | Atrophy (G) | Y, (RLS) | N | Extreme anxiety, FTD, lymphoedema | |
| II-4 (F) | 56 | Neuropathy (45) | Y | N/N | Y, falls | Y | Y, dementia | N | - | N | ||
| II-5 (M) | † (52) | Neuropathy and dementia | Y | Y/Y | Y | Y | Y | - | - | N | FTD | |
| II-6 (F) | † (51) | Neuropathy and infections | Y | Y/Y | Y | Y | Y | - | - | N | Pronounced lymphoedema | |
| II-7 (M) | 52 | Hearing loss (30s) | Y | - | Y | Y | Y | Atrophy (G) | - | N | FTD | |
| II-8 (M) | 50 | Neuropathy | - | - | - | - | - | - | - | - | ||
| Kindred-9 p.Y495>C | I-1 (M) | † (43) | - | Y | Y/Y | Y | Y | Y | - | - | - | |
| II-3 (M) | † (49) | Behaviour changes (30s) | Y | Y/Y | Y | Y | Y | - | Narcolepsy | - | ||
| II-5 (F) | † (48) | Hearing loss (30s) | Y | N/N | Y | Y | Y | - | - | - | ||
| III-1 (F) | † (46) | Hearing loss | Y | Y/- | Y | Y | Y | - | - | - | Normal CT and EEG | |
| III-3 (F) | † (50) | Sensory loss (26) | Y | Y/- | Y | Y | Y | - | - | - | EEG: posterior slowing | |
| III-4 (F) | † (47) | - | Y | N/- | Y | Y | Y | - | Narcolepsy | - | ||
| III-6 (M) | † (53) | - | Y | Y/- | Y | Y | Y | - | - | - | ||
| III-7 (M) | † (53) | - | Y | - | Y | Y | Y | - | - | - | ||
| III-9 (M) | † (50) | - | Y | Y/- | Y | Y | Y | - | - | - | ||
| IV-4 (M) | 47 | Hearing loss (30) | Y | Y/N | Y | Y | Y | Atrophy (G, F) | Narcolepsy | N | ||
| IV-5 (F) | 46 | Hearing loss (30s) | Y | Y/- | Y | Y | Y, hallucination (46) | - | - | - |
ID = individual; NCS = nerve conduction studies; F = female; M = male; Y = yes; N = no; ‘-’ = information not available; R = right; L = left; w/o = without; † = deceased (age of death is indicated between brackets if known); for atrophy on brain MRI: G = global; Ce = cerebellar; F = frontal; RBD = REM-sleep behaviour disorder; SOREMP = sleep-onset REM sleep periods; OSAS = obstructive sleep apnoea syndrome; PLMD = periodic limb movement disorder; RSWA = REM sleep without atonia; MSLT = multiple sleep latency test; RLS = restless legs syndrome; MS = multiple sclerosis; FTD = frontotemporal dementia. For all additional clinical features the age at onset is indicated between brackets if this information was available.
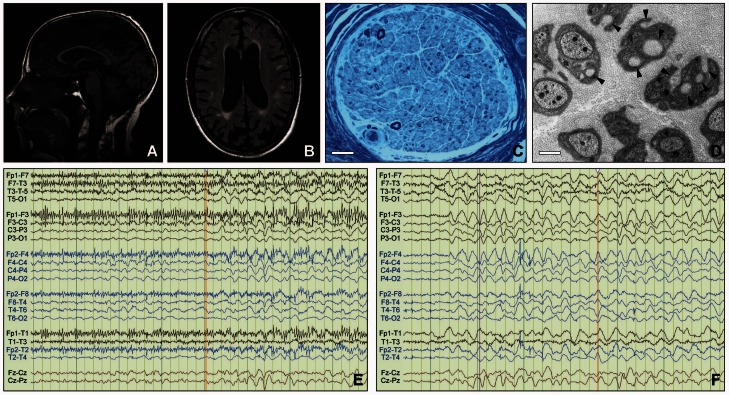
Figure 2.
Imaging results. (A) Brain MRI in Patient III-2 from Kindred 7, a 54-year-old male with significant cognitive impairment and diffuse slowing on EEG: sagittal T1 image showing global supra- and infra-tentorial brain atrophy (Philips, 1.5 T). (B) Transversal FLAIR image showing multiple white matter lesions and global atrophy (Philips, 1.5 T). (C) Morphological analysis of glutaraldehyde-fixed sural nerve biopsy tissue embedded in epoxy resin of Patient II-1 from Kindred 3: showing severely reduced numbers of myelinated nerve fibres. Semi-thin section, toluidine blue. Scale bar = 25 µm. (D) Remak bundles containing several bundles of collagen fibres encircled by Schwann cell processes (collagen pockets, indicated by arrowheads) indicative of unmyelinated axon loss. Electron microscopy of ultrathin section. Scale bar = 1 µm. (E) Twenty-four hours of continuous video-EEG monitoring in Patient II-2 from Kindred 1 showing irregular myoclonic movements associated with runs of higher voltage, irregular theta activity. (F) EEG in the same patient showing abnormal diffuse rhythmic delta frequency (1–4 Hz) background slowing especially in the bifrontal regions, correlated with a mild to moderate diffuse encephalopathy.
Kindred 1 with de novo mutation p.T481P
A 34-year-old female (Patient II-2) had normal development and childhood. In her late teens she started to develop progressive hearing loss and behavioural changes (anger outbursts and social withdrawal). Audiometry revealed moderate to severe bilateral sensorineural hearing loss at age 20. In her early 20s, she developed paraesthesia in the hands and feet with reduced sensation and recurrent foot ulcers. She began hearing negative authoritative voices and schizophrenia was initially considered. At age 31, she was diagnosed with sensory ataxia after being admitted to a hospital with septicaemia from a foot ulcer, and nerve conductions studies confirmed a pure pansensory neuropathy with areflexia.
Her short term memory declined significantly in her 30s and behavioural problems progressed including mood fluctuations and loss of empathy. She also started showing abnormal sleep patterns: sleeping 12–20 h per day and falling asleep while eating. She often acted out her dreams during sleep, with recollection of the dreams on awakening. During wakefulness she has arm and leg myoclonic jerks. On the Epworth Sleepiness Scale she scored 16 points (0 = normal and 24 worst sleepiness) without cataplexy, hypnagogic hallucinations, or sleep paralysis. Electroencephalography (EEG) showed abnormal diffuse delta background with more marked slowing in bifrontal regions, correlating with a mild to moderate diffuse encephalopathy. Twenty-four hour continuous video recordings demonstrated frequent myoclonic extremity movements associated with runs of higher voltage and irregular theta activity potentially epileptic (Fig. 2E and F). No epileptiform activity correlated with dream enactment during recorded REM sleep. She was empirically initiated on lamotrigine and the extremity myoclonic movements abated, supporting myoclonic seizures. Brain MRI showed global atrophy most prominently affecting the frontal lobes, also the cerebellum. Detailed neuropsychiatric testing indicated global difficulties with slowed digit span, processing speed and memory (1 percentile for her age and education). Genetic testing revealed a novel, de novo p.T481P mutation in DNMT1.
Kindred 2 with de novo mutation p.P491L
Patient II-1 presented with progressive perceptive hearing loss since the age of 18 years requiring hearing aids at age 23. Around that same time he developed progressive sensory loss with ulcerations in the feet, later complicated by osteomyelitis requiring amputation of several toes, increasing postural instability caused further gait impairment. Nerve conduction studies showed a severe pure sensory axonal neuropathy, and a sural nerve biopsy revealed loss of myelinated and unmyelinated fibres. He had myoclonic seizures from the age of 24 and mental decline started at the age of 39 with behavioural changes (aggression towards family), disturbances of executive functions and auditory hallucinations. MRI showed global cerebral and cerebellar atrophy and the EEG was diffusely slowed. Late in the disease course, he developed daytime hypersomnolence with abnormal polysomnography identifying multiple sleep onset REM periods. He was transferred to a residential care facility at age 40 and died around the age of 48. A de novo p.P491L mutation in DNMT1 was found using Sanger sequencing. Previously a P491Y mutation affecting the same residues was reported (Klein et al., 2011).
Kindred 3 with mutation p.Y524D
The 46-year-old proband (Patient II-1) developed numbness and pain in his legs with gait unsteadiness at age 26. Due to a pronounced inability to feel pain or temperature, he developed multiple injuries in the feet and hands followed by ulcerations. In addition he developed bilateral hearing loss starting at the age of 33. At the time of last examination (35 years) there was no evidence of cognitive decline. Nerve conduction studies confirmed a sensory neuropathy and a sural nerve biopsy (Fig. 2C and D) was compatible with a chronic axonal neuropathy affecting myelinated and unmyelinated fibres with limited axonal regeneration. The patient’s father also had a history of severe sensory neuropathy with ulcerations and hearing loss. He died at the age of 43 due to chronic renal failure attributed to amyloidosis secondary to chronic wound infection. A novel p.Y524D mutation in DNMT1 was found using Sanger sequencing.
Kindred 4 with mutation p.I531N
The proband (Patient II-1) presented at age 37 with a 5-year history of gait instability that first became apparent with a painless fracture of her left foot with neurogenic arthropathy. Three years earlier, she noted bilateral hearing loss that required hearing aids. Audiometry confirmed sensorineural hearing loss. She had significant and progressive sensory loss resulting in the need to use a walking aid and the loss of the ability to drive. Nerve conduction studies revealed pure sensory axonal neuropathy. She graduated college and worked as an accountant for 10 years. However, at the age of 36 she noted her thinking and communication increasingly became difficult. Clinical examinations revealed normal alertness, comprehension and orientation with moderate impairment in repetition and reduction in fluency. She also exhibited excessive daytime somnolence with sleep attacks. Formal sleep evaluation diagnosed obstructive sleep apnoea with severe dry eyes, but REM onset sleep and narcolepsy were not diagnosed.
Her father passed away at age 45 following 20 years of similar illness that started with distal loss of sensation and hearing loss. He had blacking out spells and episodes of loss of postural tone with generalized atonic seizures. EEG showed frontal slowing but no epileptiform activity. He was diagnosed with chorea and myoclonus and was noted to have gait ataxia. Nerve biopsy revealed a severe sensory axonal neuropathy. A novel DNMT1 mutation p.I531N was identified in the patient, and not found in her mother. The DNA of the deceased father was not available.
Kindred 5 with mutation p.Y495C
The 43-years-old patient (Patient II-1) developed sensory loss and ulcerations in his feet at age 30, followed by progressive hearing loss at 36. Neurological examination showed reduced pinprick and vibration sense and decreased reflexes in the lower limbs. He reported painless left tibia and fibula fractures at the age of 38, also complained of excessive daytime sleepiness. Nerve conduction studies were consistent with a pure sensory axonal neuropathy. Brain MRI showed generalized cerebral and cerebellar atrophy. Detailed neuropsychometric testing showed mild under-functioning in the verbal domain and a severe under-functioning in the performance domain. On focal cognitive tests speed of information processing was extremely low. Polysomnography showed mild periodic limb movement disorder, loss of REM atonia, disturbance of multiple sleep latency tests but not a typical pattern for narcolepsy. He had no family history of neurological diseases, suggesting de novo origin, but the parents’ DNA samples were not available for testing. Whole-exome sequencing identified a previously reported, heterozygous mutation p.Y495C in DNMT1 (Klein et al., 2011).
Kindred 6 with mutation p.C353F
The index patient (Patient III-3) developed progressive perceptive hearing loss at 39 years of age. At age 45 she developed progressive gait difficulties due to balance problems, and poorly healing ulcerations in the feet. She remained cognitively intact until she suffered a haemorrhagic stroke at 63 years. Her elder brother had a similar disease course with onset of hearing loss at 42 years and gait instability 5 years later in combination with foot ulcerations necessitating toe amputations. From the age of 52 onward there was mental decline with disturbance of executive functions in combination with visual and auditory hallucinations. He is currently 73-years-old and undergoes dialysis since renal failure 5 years ago. At that time he was found to have a bilateral renal atrophy on ultrasound, but kidney biopsy was not performed. There was no uncontrolled hypertension or other obvious causes of nephropathy. Lymphoedema affecting the left leg was noted. Nerve conduction studies in both siblings showed a pronounced pure sensory axonal neuropathy. Their father, paternal aunt and paternal grandmother all had a similar disease course. Whole-genome sequencing in Patient III-3 and her elder brother revealed a novel p.C353F mutation. Among the younger generation with the mutation, Patient IV-1 has gait instability at the age of 51. Audiometry showed mild to moderate perceptive hearing loss. Patient IV-4 is currently 48-years-old and has asymmetric perceptive hearing loss. Both showed normal nerve conduction studies. Patients IV-2 and IV-3 do not carry the mutation, and they have no symptoms or signs of the disease and have normal nerve conduction studies and audiometry.
Kindred 7 with mutation p.Y495C
The 48-year-old proband (Patient IV-4) had the first nerve conduction studies at age 33 when she was asymptomatic because her mother had a severe sensory neuropathy, and showed reduced sensory nerve amplitudes. At age 38, she presented with a Charcot neuroarthropathy of the left ankle and subsequently had numerous neuropathic ulcers on both feet. Repeat nerve conduction studies showed absent sensory nerve responses. She required a right below knee amputation for an infected, necrotic foot ulcer at age 42. At age 43, she complained of progressive hearing loss. Audiology revealed moderate to severe sensorineural hearing loss and hearing aids were advised. At age 47, her family raised concerns about her cognitive function and behaviour. She had obvious short-term memory impairment, difficulties caring for her 8-year-old daughter and maintaining the household. On the Addenbrooke’s Cognitive Exam ACE-R, she scored 66/100 (normal > 86/100). She had poor recall, verbal fluency and visuospatial abilities. Her EEG showed paroxysmal predominantly posterior delta wave activity. Her MRI showed severe generalized cerebral and cerebellar atrophy, but no white matter lesions. Many of her relatives also had slowly progressive sensory neuropathy, hearing loss, gait ataxia, and cognitive problems, often with death in their 50s. Her cousin, Patient IV-3 has dysarthria and three subcortical white matter T2 hyperintense lesions in her brain MRI (Fig. 2A and B). Her uncle (Patient III-2) had global atrophy and widespread white matter changes on MRI. Patient III-5 also had an MRI consistent with demyelination. The maternal grandmother was diagnosed with early onset frontal temporal dementia at age 40, along with sensory neuropathy, hearing loss and gait ataxia, and she died at age 54. DNA testing of the index, her affected cousin and uncle revealed the previously reported DNMT1 mutation p.Y495C (Klein et al., 2011).
Kindred 8 with mutation p.Y495H
The index patient (Patient II-4) was first seen at the age of 47 and diagnosed with peripheral sensory neuropathy after experiencing a non-healing cuneiform fracture and loss of sensation in her foot for 2 years, and confirmed by nerve conduction studies. At current age of 56, she has progressed to a severe hearing loss, as documented by audiometry, and showed cognitive decline in addition to her progressive neuropathy. In retrospect, her hearing loss and cognitive decline were likely present at the time of initial evaluation, but not appreciated and reported. Her affected brother Patient II-7 was also evaluated and noted to have severe peripheral neuropathy with foot ulcers, psychiatric complaints, cognitive decline, hearing loss, and lymphoedema. In addition, her other siblings suffered a similar disease course consisting of severe peripheral neuropathy with foot ulcers, psychiatric complaints, cognitive decline, hearing loss, and lymphoedema. The patient’s father, two uncles and three siblings all died in their early 50’s due to various complications of the disease. A previously reported p.Y495H mutation in DNMT1 was found using conventional Sanger sequencing in the index patient and confirmed in one definitely affected sibling (Klein et al., 2013).
Kindred 9 with mutation p.Y495C
The 47-year-old male proband (Patient IV-4) was first noticed with hearing decline around age 30. By 38, he was diagnosed with sensorineural hearing loss and had to wear hearing aids. Around the same time, he was also diagnosed with sleep apnoea and started to use a sleep apnoea machine. Between 39 and 43, his family noticed that he started to change jobs frequently, and his employers mentioned his behaviour became increasingly irrational. His family also noticed that he would spend a lot more time on simple things and became obsessed with small details. His balance declined progressively resulting in a gradual need for walking aids. He had ulcers on his feet and nerve conduction studies confirmed prominent sensory neuropathy. His long-term memory has not been affected, but he had an increasingly difficult time having normal conversations as he cannot stay focused on a topic. FTD was considered as he had predominance of frontal-executive dysfunction and behavioural symptoms (disinhibition, agitation, anger outbursts, hyper-religiosity), but not formally diagnosed. Brain MRI revealed frontally predominant but distributed cerebral cortical and cerebellar atrophy. His sister who started to experience visual hallucinations recently, and many other affected family members, had similar disease course. Death typically occurred in their 40’s. DNA testing found a previously reported DNMT1 mutation p.Y495C in the patient and his affected sister.
Summary
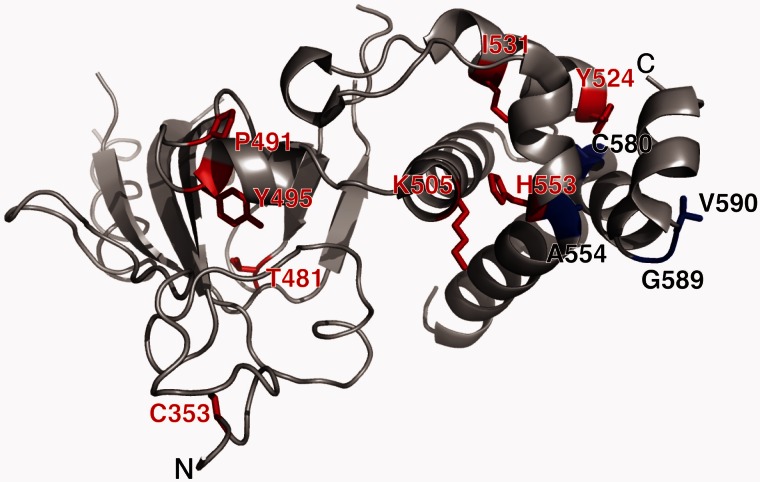
Among these nine kindreds, seven different mutations (C353F, T481P, P491L, Y524D, I531N, Y495C, and Y495H) were found, of which five (C353F, T481P, P491L, Y524D, I531N) are novel. T481P (Kindred 1), and P491L (Kindred 2) arose de novo in the index patients, Y495C in Kindred 5 was also likely de novo as neither parents had any phenotypes. In the other kindreds, the transmitted mutations segregated with the disease. All seven mutations reside in the TS domain of DNMT1, six of them are very close to the previously described hotspot mutant site Y495 (Klein et al., 2013), except C353F which resides at the very edge of the domain (Fig. 3). The other mutations are all positioned at the middle part of TS domain where a highly-conserved region has been identified essential for the binding of heterochromatin (Easwaran et al., 2004). All seven mutations are predicted as ‘deleterious’ by SIFT (score: 0) ‘probably damaging’ by PolyPhen-2 (P = 1), also ‘disease-causing’ by MutationTaster (P = 1).
Figure 3.
Targeting sequence (TS) domain structure with mutations of DNMT1 shown. The TS domain (grey) is the site for all known causal mutations for HSAN1E (red) and ADCA-DN (blue). Images were created using PyMol (www.pymol.org) and crystal structures of the human DNMT1 TS domain.
Using this cohort of 45 affected patients, we estimated the average age of onset is 37.7 years (SD = 8.6, range 18–51), and recognized hearing loss as the most common initial symptom (36%), followed by sensory loss, ulcerations and/or arthropathy (33%), cognitive decline (7%) and gait imbalance (7%). As the disease progresses, hearing loss and ulceromutilating pure sensory neuropathy become obligatory features. Gait difficulties are often present, but are typically attributed to sensory ataxia and/or wound infections rather than muscle weakness. Cerebellar involvement, as demonstrated by cerebellar atrophy, may contribute to the ataxia. The degree of cerebral involvement is also variable; the majority of patients display cognitive decline (89%), ranging from overt (frontal and temporally predominant) dementia to milder cognitive deficit features. In our cohort of 45 patients, only 11% remain free of significant cognitive decline after age 45. Dementia seems to be the main factor driving the reduced life expectancy: the average age of death at 53.6 years (SD = 7.7, range: 43–75). Brain atrophy, was observed in 12 of 14 patients who underwent MRI exam. Most often global atrophy, in some cases selective frontal or cerebellar atrophy, was observed. Additionally, new phenotypic features were identified including myoclonus (in three kinships), seizures (in three kinships), and auditory or visual hallucinations (in four kinships) that lead to consideration of schizophrenia (Table 1).
DNMT1 mutants are mislocalized to the cytosol and form aggregates
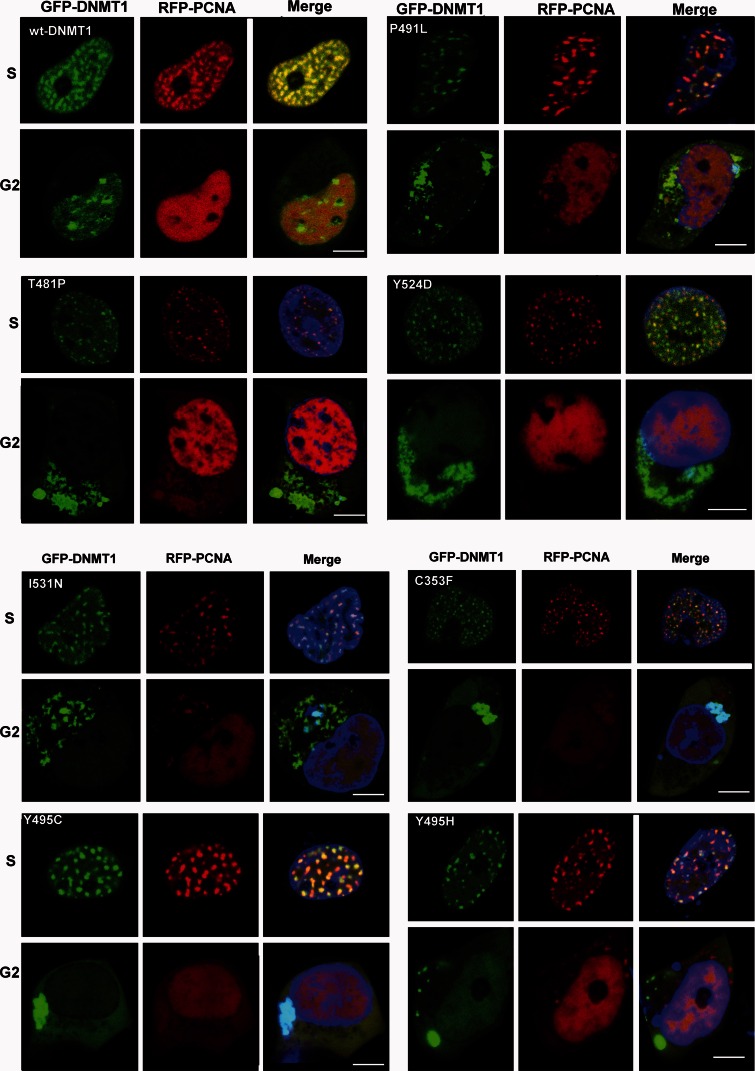
The maintenance of methylation is a continuous process extending to G2 phase, and DNMT1 binding to heterochromatin is essential for the maintenance of DNA methylation (Easwaran et al., 2004; Schermelleh et al., 2007; Spada et al., 2007). During S phase, multiple proteins, such as PCNA and UHRF1, recruit DNMT1 to the replication foci, where DNMT1 functions as an essential component of the DNA replication machinery (Leonhardt et al., 1992). DNMT1 is also continuously loaded onto heterochromatin during G2 phase, and partial deletion of the TS domain abolishes DNMT1 association with heterochromatin (Easwaran et al., 2004). In our previous study using HeLa cells, we demonstrated the heterochromatin binding abilities of Y495C-DNMT1 and P491Y-DNMT1 are abolished during G2 phase, even though these mutants co-localize with PCNA at replication foci during S phase (Klein et al., 2011). To investigate the characteristics of newly identified novel mutants, we used confocal microscopy to analyse HEK293 cells that had been co-transfected to express various mutation constructs of GFP-DNMT1 and S-phase marker RFP-PCNA. Similar to our previous results, we found that C353F, T481P, P491L, Y524D, I531N, Y495C, and Y495H mutants were co-localized with PCNA to replication foci during S phase. After S phase, however, when RFP-PCNA diffuses and is no longer presents in toroid structures within nucleus, these mutants were mislocalized to cytosol, often in aggregates (Fig. 4). We also noticed that RFP-PCNA proteins exist in the aggregates structures, suggesting they are being co-translocated to cytoplasm while still bound to DNMT1.
Figure 4.
Representative images from confocal microscopy in HEK293 cells co-transfected with RFP-PCNA and GFP-wild-type DNMT1, or various mutant GFP-DNMT1. Wild-type and mutant DNMT1 appear in green in the left panels, PCNA appears in red in the middle panels and merged images are shown in the right panels. Scale bars = 5 µm. In the S phase, PCNA locates at the toroidal structures of the replication foci; in the G2 phase, PCNA shows a diffused pattern in the nucleus. Both wild-type DNMT1 and mutant DNMT1 proteins co-localize with PCNA at replication foci during the S phase. However, during G2 phase (when PCNA diffuses) wild-type DNMT1 stays in the nucleus and binds to heterochromatin while mutant DNMT1 proteins mislocated into cytoplasm and formed aggregates.
DNMT1 mutant proteins form aggresomes and are prone to degradation
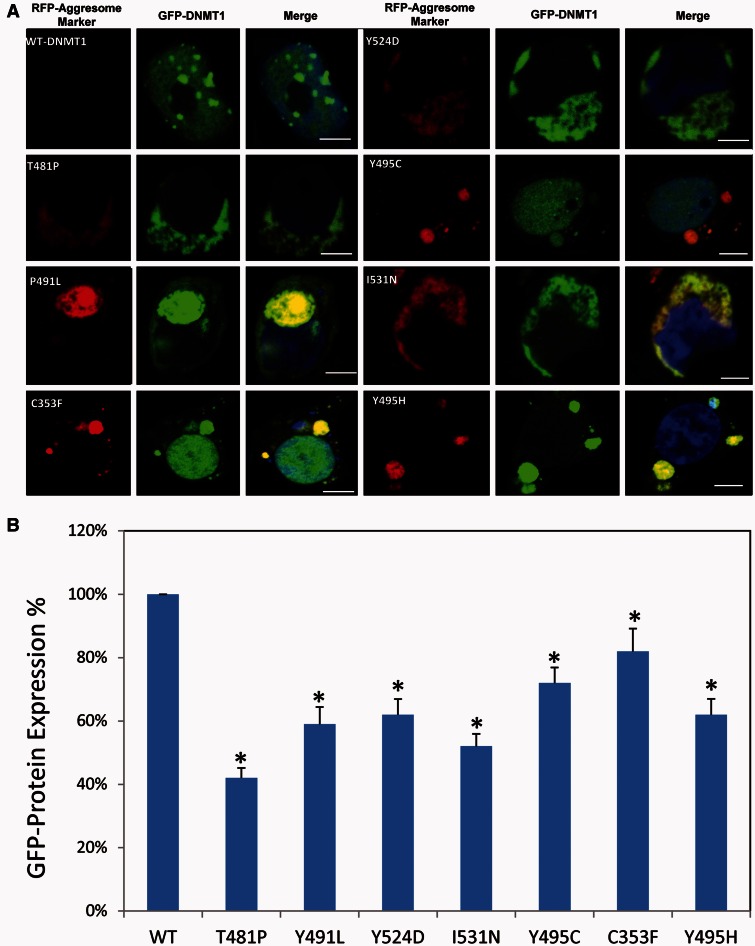
Aggresomes are juxtanuclear inclusion bodies that have been proposed to function as intermediate structures for the disposal of protein aggregates. They are often induced by protein homeostasis imbalance when cells sequester toxic misfolded proteins from the cytoplasm and facilitate their degradation through the autophagy pathway. Aggresomes formed by pathological proteins have been discovered in multiple neurodegenerative diseases, such as synphilin-1 (SNCAIP), HTT and copper-zinc superoxide dismutase (SOD1) in Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis (ALS), respectively (Ross and Poirier, 2004). To determine whether the observed mutant DNMT1 aggregates in cytosol are actual aggresomes, we stained the cells transfected with various constructs of EGFP-DNMT1 with red fluorescent molecular marker (ProteoStat®, Enzo Life Science) that specifically labels aggresome. The results showed that the red-fluorescent aggresomal marker overlaid with the mutant GFP-DNMT1 protein aggregates in the cytoplasm, whereas wild-type DNMT1 stayed within the nucleus. These results demonstrate the DNMT1 mutants form aggresomes (Fig. 5A), suggesting that lysosome-autophagy pathway is likely involved in removing misfolded mutant DNMT1. It has been suggested that the misfolded nucleus proteins are translocated and sequestered in the cytoplasm for degradation to reduce its toxicity (Yamamoto and Simonsen, 2011). DNMT1 mutation-induced translocation of DNMT1 proteins from the nucleus to the cytosol could impose extracellular toxic stress. Previously we reported that Y495C-DNMT1 and P491Y-DNMT1 mutant proteins are prone to early degradation (Klein et al., 2011). To investigate whether the newly identified DNMT1 mutant proteins are also subject to early degradation in cytosol, we treated HEK293 cells with cycloheximide and quantified the DNMT1-GFP fusion protein with a GFP protein quantification assay. The amount of GFP tagged DNMT1 mutants was significantly reduced compared to wild-type DNMT1 (Fig. 5B), ranging from 40–80% compared to wild-type.
Figure 5.
DNMT1 mutants. (A) Representative images from fluorescence aggresomes marker staining of HEK293 cells transfected with either enhanced GFP-wild type DNMT1 or various mutant enhanced GFP-DNMT1. Wild-type DNMT1 stays in the nucleus and binds to heterochromatin whereas mutant DNMT1 aggregates in cytosol overlaid with red fluorescence aggresome markers. Scale bars = 5 µm. (B) Quantification of GFP-DNMT1 proteins in HEK293 cells transfected with eGFP-wild type DNMT1, or various mutant enhanced GFP-DNMT1. All mutant DNMT1 proteins showed degradation comparing to wild-type DNMT protein. Data are derived from three independent experiments, asterisk represents there is statistically significant change between wild-type and mutant proteins.
Discussion
The current study expands the clinical phenotypes of HSAN1E, the number of causal mutations, and provides evidence of a higher than previously expected HSAN1E occurrence in clinical practice. We also identified an additional potential pathogenic mechanism of mutant DNMT1 beyond its impact on genome methylation. The occurrence of HSAN1E was initially thought to be quite low as only four kindreds were found during the initial genetic discovery (Klein et al., 2011). However, after reporting the causal mutations in DNMT1, we quickly identified two more kindreds with HSAN1E, both caused by a mutation at position Y495, suggesting Y495 as a hotspot for mutations (Klein et al., 2013). Subsequently, additional DNMT1 mutations within the TS domain were reported in patients with narcolepsy, expanding DNMT1 mutation-associated phenotypes (Winkelmann et al., 2012). Herein, we report nine newly identified HSAN1E kindreds, totalling 45 patients, all with pathogenic mutations within the TS domain. In addition, five novel mutations in DNMT1 are discovered, further expanding the phenotype–genotype correlation of DNMT1 to HSAN1E.
Kindreds 2, 3, 6 and 8 are part of a previously reported cohort of 100 unrelated HSAN index patients who were systematically screened for the known HSAN genes at that time (Rotthier et al., 2009). This clinically heterogeneous cohort also included patients with early disease onset and recessive inheritance. When only taking those patients with an adult disease onset into account, either in isolated cases or in dominantly transmitting pedigrees, these four kindreds represent 5.9% of the overall cohort. Among all HSAN causal genes with autosomal dominant inheritance, only RAB7A and SPTLC1 were reported with higher frequencies (7% and 12%, respectively), although both are in part overestimated due to founder effects in the studied population. Among the recessively inherited HSAN genes, NTRK1 accounts for a specific recognizable recessive syndrome and is the only one with frequency above 5% in the screened cohort (Rotthier et al., 2009; Davidson et al., 2012). Thus, among all 15 genes causal for HSAN, DNMT1 seems to have a higher occurrence rate.
The marked morbidity and reduced life expectancy underscore the importance of diagnosing HSAN1E, and the unique combination of clinical features should help to make the diagnosis. Previous reports described that patients with HSAN1E have pan-sensory peripheral neuropathy (myelinated and unmyelinated axons), hearing loss and dementia as stereotypic symptoms, and present with different initial symptoms at varied onset ages. In this largest HSAN1E cohort study to date, we recognize that the most frequent presenting symptom is hearing loss, followed by sensory loss and its complications (ulcerations and arthropathy). A minority of patients (7%) presented with changes in mental status, but upon disease progression nearly all patients developed clear cognitive decline that often started with personality change. This study further expanded the clinical spectrum of HSAN1E including auditory and/or visual hallucinations, myoclonic seizures, renal failure and frontotemporal dementia. In addition, diverse parasomnias such as REM sleep disorder were appreciated. Because of the variability and severity of presenting symptoms, HSAN1E patients were first seen in different subspecialty clinics, where the focus was frequently given to the most severe symptom, and the initial diagnosis emphasized the most severe symptoms only. As hearing loss is the common presenting symptom, and the course of sensory neuropathy and cognitive decline is slowly progressive, many patients did not visit a neurologist until they progressed to difficulties in walking, severe ulcerations, or noticeable memory decline, or evident personality change. Subtle changes in behaviour and personality in combination with disturbances of short-term memory usually did not prompt a specific diagnosis in the early stages of the disease. For some patients there were gaps of several years between the appearances of each triad disease element. The diverse clinical presentations are not surprising because methylation maintenance is a fundamental aspect of DNA replication and cell survival, although the predominance of neuronal dysfunction is not explained.
Mutations in the TS domain of DNMT1 have also been linked to ADCA-DN. A recent study reported the occurrence of narcolepsy in two HSAN1E families (Moghadam et al., 2014) suggesting that narcolepsy is a common phenotype for both ADCA-DN and HSAN1E. In our cohort, 11 subjects displayed sleep disorders, most commonly hypersomnolence. The subsequent extensive clinical testing of these 11 patients showed that only three had the clinical feature sufficient to diagnose narcolepsy, and all three had dementia. Establishing a diagnosis of narcolepsy and a progressive encephalopathy, however, is not straightforward. Detailed questioning of patients and their families often did not reveal symptoms of sleep disorders. In addition, excessive daytime sleepiness might be part of the global CNS phenotype, given the high rate of EEG delta slowing in our cohort. In patients with ADCA-DN, a primary diagnosis of narcolepsy might have been easier to establish as many patients were not reported to have significant cognitive decline. It is possible, however, that a more generalized encephalopathy was overlooked, as some patients with ADCA-DN displayed marked atrophy affecting multiple regions of the brain on MRI studies and abnormalities on SPECT imaging. The results of EEGs have not been reported for ADCA-DN patients (Moghadam et al., 2014).
Except for the prominence of the narcolepsy, HSAN1E and ADCA-DN patients have many similar phenotypes. All patients with ADCA-DN had bilateral sensorineural hearing loss that presented around the same time of the narcolepsy diagnosis, and mild to severe neuropathy typically developed later. Both have similar disease onset age and life-expectancy. Cognitive decline is seen in majority of both HSAN1E and ADCA-DN patients by their late 40’s (Table 2). Although severe cognitive decline seems to be the main cause of early death among patients with HSAN1E, the causes for premature death in ADCA-DN patients were not reported. Taken together, these findings suggest that the HSAN1E and ADCA-DN share much more overlapping clinical features than their acronyms suggest, and we propose that a unified disease terminology such as ‘DNMT1-complex disorder’ to better encompass both diseases.
Table 2.
Comparison of the clinical features of all reported families/patients with DNMT1 mutations
| HSAN1E Reports |
ADCA-DN Reports |
||||||
|---|---|---|---|---|---|---|---|
| Baets et al.. (current report) | Klein et al., 2011 | Klein et al., 2013 | Yuan et al., 2013 | Moghadam et al., 2014 | Winkelmann et al., 2012 | Pedroso et al., 2013 | |
| Focus of the study | Large HSAN1E cohort study with in-depth clinical description of nine new HSAN1E families and protein functional assays in cell culture. | Initial genetic discovery of HSAN1E, limited description of clinical features of four HSAN1E families. | Genetic and clinical studies of two new HSAN1E families | Single new HSAN1E case | In-depth clinical description of two ADCA-DN families (also in Winkelmann et al. report) and two new HSAN1E families | Initial genetic discovery for ADCA-DN. Limited clinical description of four ADCA-DN families. | Single new ADCA-DN case |
| Number of kindreds (patients) investigated | 9 (45) | 4 (21) | 2 (6) | 1 (1) | 4 (5) | 4 (9) | 1 (1) |
| Number of mutations (novel) | 7 (5) | 2 (2) | 2 (1) | 1 (1) | 4 (1) | 3 (3) | 1(1) |
| Average age of onset | Late 30’s | Early 40’s | Early 40’s | Late teen | Early 30’s | Mid 30’s to early 40’s | 21 |
| Common presenting symptom | Hearing loss | Hearing loss | Hearing loss | Sensory neuropathy |
|
Narcolepsy | Narcolepsy |
| Hearing loss | Yes, all | Yes, all | Yes, all | Yes | Yes, all | Yes, all | Yes |
| Sensory neuropathy | Yes, all | Yes, all | Yes, all | Yes, | Yes, all | Yes, all | No (age 32) |
| Narcolepsy/cataplexy | 3 diagnosed and 11 reported sleep disorders. | Symptoms not reported by patients, but were not the focus of investigation. | No | Not reported | Yes, all | Yes, all | Yes |
| Cognitive decline or dementia | Yes, 32/45 four have FTD | Yes, 13/21 | Yes, all, one has FTD | Mild mental retardation | Yes, 1/3 | Yes, 4/9 | no |
| Ataxia | Yes, 5/45 | Yes, 4/21 | No | No | Yes, 4/5 | Yes, all | Yes |
| Brain Atrophy by MRI | Yes, 12/14 performed | Yes, 5/5 performed | Yes, 4/4 performed | Yes | Yes, all | Yes, reported in two families | Yes |
| Optic atrophy | Not checked | Not checked | Not checked | Not reported | Yes, all | Yes, 7/9 | no |
| Average age of death | Mid 50’s | Early 60’s | Early 60’s | Alive at 42 | Mid 50’s | Mid 50’s | Alive at 32 |
| EEG/low frequency | Yes, 5 of 6 performed | Not checked | Yes, 1/1 performed | Not reported | Not reported | Not reported | Not reported |
| Hypocretin 1 | Not checked | Not checked | Not checked | Not checked |
|
low | Slightly low |
| Lymphoedema | Yes, 3/45 | No | No | Not reported | No | Yes, 2/9 | Not reported |
Our functional study in cultured cells demonstrates that the mutations in TS domain affect the localization of DNMT1 protein. These DNMT1 mutants translocate out of the nucleus into the cytoplasm during G2 phase, and form aggresomes in the cytoplasm. It has been suggested that binding to replication machinery and hemimethylated DNA require conformation change of the TS domain (Frauer and Leonhardt, 2011). We speculate that DNMT1 binding to replication foci during S phase may have exacerbated the misfolding of mutant DNMT1, causing mutant proteins being recognized by the chaperone factors that are involved in transporting misfolded protein out of nucleus. These findings suggest that mutants have dominant effects by failing to interact with DNA in neurons and by forming cytosolic aggregates. Of interest, although DNMT1 mutations have been shown to cause global DNA hypomethylation and site-specific hypermethylation (Klein et al., 2011; Sun et al., 2014), a phenomenon often seen in cancer, cancer has never been reported in any patients with HSAN1E.
The misfolding and aggregation of DNMT1 mutant proteins could contribute to the pathogenesis of HSAN1E through a toxic gain-of-function, as has been shown for other neurological diseases (Knaevelsrud and Simonsen, 2010). Although the issue of aggresome formation is still under debate pertaining to whether protein misfolding is the mechanism of toxic gain-of-function, and whether aggresomes are part of compensatory cellular process. The nuclear-to-cytoplasm transport of other nuclear proteins, including HTT, ATXN1 and TARDBP mutants, has been proposed as a pathogenic mechanism in their associated neurodegenerative disorders (Levine and Kroemer, 2008). Cytoplasmic accumulated aggresomes often impose toxic cellular stress (Saifi et al., 2003). The post-mitotic neurons and the long length of peripheral nerves could be particularly vulnerable to mutated protein misfolding and the cellular stress arisen from it, and worsening with age (Klein et al., 2013). Our study indicates that the mutations of DNMT1 have deleterious impact on the central and peripheral nervous system, emphasizing the importance of the TS domain for maintaining proper DNMT1 function and its associate epigenetic regulatory mechanism.
All mutations reported to date that cause either HSAN1E or ADCA-DN are missense mutations in the TS domain. Our results better differentiated the location distinction between mutations causal for ADCA-DN (located in the C-terminus end of the TS domain) and for HSAN1E (located in the middle part or N-terminus of TS domain). This large cohort study suggests that the location and nature of the mutations may influence the disease presentations and severity. Two HSAN1E mutations, p.T481P (Kindred 1) and p.P491L (Kindred 2), both from patients with de novo mutations, had young onset age in their late teens, and the disease course progressed rather quickly. Whereas one HSAN1E mutation, p.C353F mutation (Kindred 6), may be associated with a relatively slow disease progression rate: the proband had no apparent cognitive problem until she suffered a stroke at age 63, and the younger generation had later onset and milder symptoms. However, the link between the location of mutation and phenotype diversity is likely complex. The proband’s brother in Kindred 6 had cognitive decline starting at age 52 along with earlier presented hearing loss and sensory neuropathy, illustrating that the phenotypic expressions vary considerably even between siblings.
Our study recognized several new clinical features associated with HSAN1E, including myoclonic seizures, and auditory or visual hallucinations. In addition, two patients had end-stage renal failure. Patient III-2 of Kindred 6 (C353F) is undergoing hemodialysis; the renal failure in Patient I-1 of Kindred 3 (Y524D) was attributed to amyloidosis due to chronic osteomyelitis, but this was not documented. A patient from the previously reported kindred (Klein et al., 2011) with Y495C also died of renal failure after chronic pyelonephritis; the kidneys showed an atrophied cortex and large numbers of nodules. At this point it remains unclear if the renal involvement is an accidental feature or in fact causally linked with DNMT1 mutations. Moreover, lymphoedema was noted in several individuals from Kindred 8 and one individual from Kindred 6. Of interest, several individuals with ADCA-DN were previously reported to have lymphoedema (Winkelmann et al., 2012; Moghadam et al., 2014). The direct causal link of lymphoedema with the DNMT1 mutation remains to be discovered. The diversity of the DNMT1-associated phenotypes certainly suggests the identification of additional cases and warrants further mechanistic investigations. In summary, our study suggests that DNMT1-related disorders appeared to be more common than previously estimated, and the extent of the phenotypic spectrum much broader than currently appreciated, contributing to delayed diagnosis. We hope that this report will lead to more cases of DNMT1-related disorders being recognized and diagnosed by candidate gene testing rather than relatively expensive whole-exome sequencing. At the same time, it will be interesting to see if a greater range of neurodegenerative disorders may also be linked with DNMT1 function as whole-exome sequencing is increasingly being used for genetic screening in clinical setting. Given the complexity of DNMT1 function in mammalian cells, a substantial amount of research is required to unravel its pathogenic mechanisms. DNMT1 mutants seem to exert their damaging impact by two complex pathways. First, epigenetic pathways are impaired, leading to abnormal global methylation in HSAN1E patients. Indeed, the disrupted binding of heterochromatin and the early degradation of mutant Y495C DNMT1 have been shown impairing global genome methylation maintenance (Klein et al., 2011; Sun et al., 2014). Second, misfolded mutant DNMT1 imposes cellular stress by putting protein homeostasis network out of balance, which may eventually lead to cell death. The fact that DNMT1 mutations underlie such a unique yet variable clinical spectrum underscores the super-stringent requirement for the highly conserved DNMT1 protein. This is not entirely surprising as DNMT1 is the sole essential enzyme that keeps the fidelity of methylation inheritance starting from embryogenesis to the very end of life.
Acknowledgements
We thank all patients and their relatives for their willingness to participate in this study.
Glossary
Abbreviations
- ADCA-DN
dominant negative cerebellar ataxia, deafness, and narcolepsy
- HSAN1E
hereditary sensory autonomic neuropathy with dementia and hearing loss
Funding
This study was supported by the National Institute of Health (NIH) (NS065007, DP3DK104394, R01DK064814, U10NS077305), and American Diabetes Association (ADA) 7-11-AEC-23 of USA, the University of Antwerp (UA), the Association Belge contre les Maladies Neuromusculaires (ABMM), the Medical Foundation Queen Elisabeth (GSKE), the agency for Innovation by Science and Technology (IWT) and the EU FP7/2007‐2013 under grant agreement number 2012‐305121 (NEUROMICS) and Judy Seltzer Levenson Memorial Fund for CMT Research. MMR and ML are grateful to the Medical Research Council (MRC), MRC Centre grant (G0601943), and the National Institutes of Neurological Diseases and Stroke and office of Rare Diseases (U54NS065712) for their support. Part of this work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme. J.W. is supported by the German Research Council (DFG WE1406/13‐1). Dr Baets receives research grant from Association Belge contre les Maladies Neuro-Musculaires, Medical Foundation Queen Elisabeth and EU 7th Framework Programme (FP7, ‘NEUROMICS’); Dr Grossman sits on the board of International FTD Society; Dr Dyck receives an honorarium for serving as an Associate Editor of Diabetes, and receives support for teaching of neuropathy examinations from Alnylam and ISIS Pharmaceutical companies; Dr Houlden receives research grant from MRC UK, The Wellcome Trust and NIHR UCL/UCLH BRC; Dr Seeley receives research grant from National Institute of Aging, John D. French Alzheimer's Disease Foundation, Consortium for Frontotemporal Dementia Research, James S. McDonnell Foundation, Alzheimer's Drug Discovery Foundation and serves as a consultant for Bristol-Myers Squibb. Dr Klein, Dr Smith and Dr Scherer receive research grants from National Institute of Neurological Disorders and Stroke. Other co-authors have nothing to disclose.
References
- Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–6. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- Davidson G, Murphy S, Polke J, Laura M, Salih M, Muntoni F, et al. Frequency of mutations in the genes associated with hereditary sensory and autonomic neuropathy in a UK cohort. J Neurol. 2012;259:1673–85. doi: 10.1007/s00415-011-6397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drmanac R, Sparks AB, Callow MJ, Halpern AL, Burns NL, Kermani BG, et al. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2010;327:78–81. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- Easwaran HP, Schermelleh L, Leonhardt H, Cardoso MC. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004;5:1181–6. doi: 10.1038/sj.embor.7400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauer C, Leonhardt H. Twists and turns of DNA methylation. Proc Natl Acad Sci USA. 2011;108:8919–20. doi: 10.1073/pnas.1105804108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin B, Robertson KD. DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol. 2013;754:3–29. doi: 10.1007/978-1-4419-9967-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Benarroch EE. Epigenetic regulation: basic concepts and relevance to neurologic disease. Neurology. 2014;82:1833–40. doi: 10.1212/WNL.0000000000000440. [DOI] [PubMed] [Google Scholar]
- Klein CJ, Bird T, Ertekin-Taner N, Lincoln S, Hjorth R, Wu Y, et al. DNMT1 mutation hot spot causes varied phenotypes of HSAN1 with dementia and hearing loss. Neurology. 2013;80:824–8. doi: 10.1212/WNL.0b013e318284076d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Botuyan MV, Wu Y, Ward CJ, Nicholson GA, Hammans S, et al. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat Genet. 2011;43:595–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Duan X, Shy ME. Inherited neuropathies: clinical overview and update. Muscle Nerve. 2013;48:604–22. doi: 10.1002/mus.23775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Wu Y, Kruckeberg KE, Hebbring SJ, Anderson SA, Cunningham JM, et al. SPTLC1 and RAB7 mutation analysis in dominantly inherited and idiopathic sensory neuropathies. J Neurol Neurosurg Psychiatry. 2005;76:1022–4. doi: 10.1136/jnnp.2004.050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaevelsrud H, Simonsen A. Fighting disease by selective autophagy of aggregate-prone proteins. FEBS Lett. 2010;584:2635–45. doi: 10.1016/j.febslet.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Leonhardt H, Page AW, Weier HU, Bestor TH. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell. 1992;71:865–73. doi: 10.1016/0092-8674(92)90561-p. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margot JB, Aguirre-Arteta AM, Di Giacco BV, Pradhan S, Roberts RJ, Cardoso MC, et al. Structure and function of the mouse DNA methyltransferase gene: Dnmt1 shows a tripartite structure. J Mol Biol. 2000;297:293–300. doi: 10.1006/jmbi.2000.3588. [DOI] [PubMed] [Google Scholar]
- Marques SC, Oliveira CR, Pereira CM, Outeiro TF. Epigenetics in neurodegeneration: a new layer of complexity. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:348–55. doi: 10.1016/j.pnpbp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Moghadam KK, Pizza F, La Morgia C, Franceschini C, Tonon C, Lodi R, et al. Narcolepsy is a common phenotype in HSAN IE and ADCA-DN. Brain. 2014;137(Pt 6):1643–55. doi: 10.1093/brain/awu069. [DOI] [PubMed] [Google Scholar]
- Pedroso JL, Povoas Barsottini OG, Lin L, Melberg A, Oliveira AS, Mignot E. A novel de novo exon 21 DNMT1 mutation causes cerebellar ataxia, deafness, and narcolepsy in a Brazilian patient. Sleep. 2013;36:1257–9. doi: 10.5665/sleep.2898. , 9A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Rossor AM, Polke JM, Houlden H, Reilly MM. Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol. 2013;9:562–71. doi: 10.1038/nrneurol.2013.179. [DOI] [PubMed] [Google Scholar]
- Rotthier A, Baets J, De Vriendt E, Jacobs A, Auer-Grumbach M, Levy N, et al. Genes for hereditary sensory and autonomic neuropathies: a genotype-phenotype correlation. Brain. 2009;132(Pt 10):2699–711. doi: 10.1093/brain/awp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotthier A, Baets J, Timmerman V, Janssens K. Mechanisms of disease in hereditary sensory and autonomic neuropathies. Nat Rev Neurol. 2012;8:73–85. doi: 10.1038/nrneurol.2011.227. [DOI] [PubMed] [Google Scholar]
- Saifi GM, Szigeti K, Snipes GJ, Garcia CA, Lupski JR. Molecular mechanisms, diagnosis, and rational approaches to management of and therapy for Charcot-Marie-Tooth disease and related peripheral neuropathies. J Investig Med. 2003;51:261–83. doi: 10.1136/jim-51-05-14. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Haemmer A, Spada F, Rosing N, Meilinger D, Rothbauer U, et al. Dynamics of Dnmt1 interaction with the replication machinery and its role in postreplicative maintenance of DNA methylation. Nucleic Acids Res. 2007;35:4301–12. doi: 10.1093/nar/gkm432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Rechkoblit O, Bestor TH, Patel DJ. Structure of DNMT1-DNA complex reveals a role for autoinhibition in maintenance DNA methylation. Science. 2011;331:1036–40. doi: 10.1126/science.1195380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada F, Haemmer A, Kuch D, Rothbauer U, Schermelleh L, Kremmer E, et al. DNMT1 but not its interaction with the replication machinery is required for maintenance of DNA methylation in human cells. J Cell Biol. 2007;176:565–71. doi: 10.1083/jcb.200610062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Wu Y, Ordog T, Baheti S, Nie J, Duan X, et al. Aberrant Signature Methylome by DNMT1 hot spot mutation in hereditary sensory and autonomic neuropathy 1E. Epigenetics. 2014;9:1184–93. doi: 10.4161/epi.29676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takalo M, Salminen A, Soininen H, Hiltunen M, Haapasalo A. Protein aggregation and degradation mechanisms in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J, Lin L, Schormair B, Kornum BR, Faraco J, Plazzi G, et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum Mol Genet. 2012;21:2205–10. doi: 10.1093/hmg/dds035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Simonsen A. The elimination of accumulated and aggregated proteins: a role for aggrephagy in neurodegeneration. Neurobiol Dis. 2011;43:17–28. doi: 10.1016/j.nbd.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Higuchi Y, Nagado T, Nozuma S, Nakamura T, Matsuura E, et al. Novel mutation in the replication focus targeting sequence domain of DNMT1 causes hereditary sensory and autonomic neuropathy IE. J Peripher Nerv Syst. 2013;18:89–93. doi: 10.1111/jns5.12012. [DOI] [PubMed] [Google Scholar]
- Zimon M, Baets J, Almeida-Souza L, De Vriendt E, Nikodinovic J, Parman Y, et al. Loss-of-function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nat Genet. 2012;44:1080–3. doi: 10.1038/ng.2406. [DOI] [PubMed] [Google Scholar]