Infections are the main cause of death after spinal cord injury (SCI). Brommer et al. reveal that an SCI-induced immune deficiency syndrome is responsible for the enhanced infection susceptibility in SCI patients and a mouse model. Lesion level determines the extent of this ‘immune paralysis’.
Keywords: neuroinflammation, myelopathy, spinal cord injury, rehabilitation, mechanisms
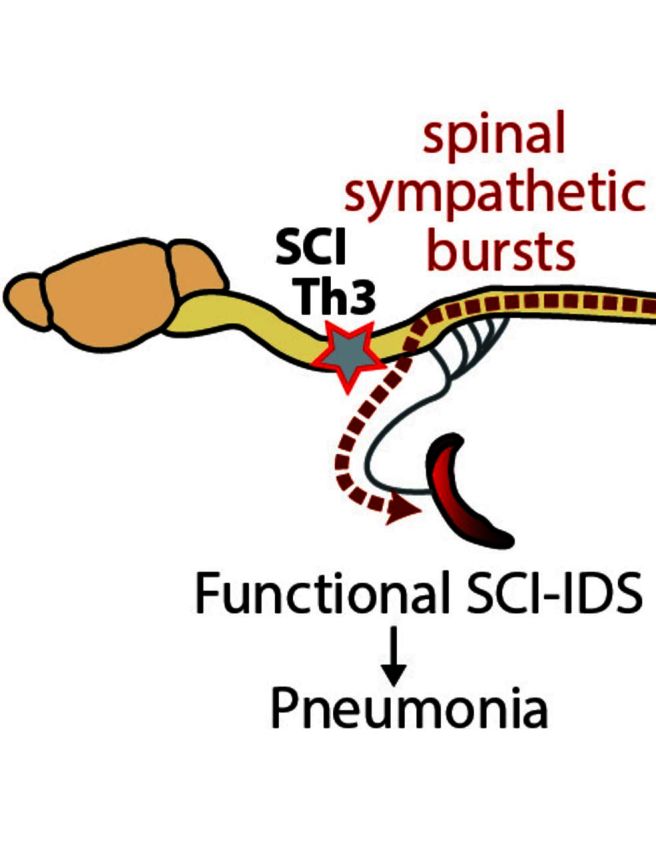
Infections are the main cause of death after spinal cord injury (SCI). Brommer et al. reveal that an SCI-induced immune deficiency syndrome is responsible for the enhanced infection susceptibility in SCI patients and a mouse model. Lesion level determines the extent of this ‘immune paralysis’.
Abstract
Pneumonia is the leading cause of death after acute spinal cord injury and is associated with poor neurological outcome. In contrast to the current understanding, attributing enhanced infection susceptibility solely to the patient’s environment and motor dysfunction, we investigate whether a secondary functional neurogenic immune deficiency (spinal cord injury-induced immune deficiency syndrome, SCI-IDS) may account for the enhanced infection susceptibility. We applied a clinically relevant model of experimental induced pneumonia to investigate whether the systemic SCI-IDS is functional sufficient to cause pneumonia dependent on spinal cord injury lesion level and investigated whether findings are mirrored in a large prospective cohort study after human spinal cord injury. In a mouse model of inducible pneumonia, high thoracic lesions that interrupt sympathetic innervation to major immune organs, but not low thoracic lesions, significantly increased bacterial load in lungs. The ability to clear the bacterial load from the lung remained preserved in sham animals. Propagated immune susceptibility depended on injury of central pre-ganglionic but not peripheral postganglionic sympathetic innervation to the spleen. Thoracic spinal cord injury level was confirmed as an independent increased risk factor of pneumonia in patients after motor complete spinal cord injury (odds ratio = 1.35, P < 0.001) independently from mechanical ventilation and preserved sensory function by multiple regression analysis. We present evidence that spinal cord injury directly causes increased risk for bacterial infection in mice as well as in patients. Besides obvious motor and sensory paralysis, spinal cord injury also induces a functional SCI-IDS (‘immune paralysis’), sufficient to propagate clinically relevant infection in an injury level dependent manner.
Introduction
The CNS controls the immune system through several pathways, among them hardwired fibres of the autonomic nervous system (Steinman, 2004; Meisel et al., 2005; Irwin and Cole, 2011). Sensors within the central and peripheral autonomic nervous systems relay information about the status of the immune system (Bellinger et al., 2008; Elenkov et al., 2000). Disruption of CNS–immune system interaction after injury results in an abrupt and drastic systemic decrease of immune function, termed CNS injury-induced immune deficiency syndrome (CIDS) (Meisel et al., 2005). This is observed after cerebral ischaemia, traumatic brain injury and spinal cord injury (SCI). In SCI patients this is referred to as spinal cord injury-induced immune deficiency syndrome (SCI-IDS) (Riegger et al., 2007, 2009), further aggravating the unspecific stress response after injury referred to as post-aggression syndrome (Desborough, 2000). Different symptoms of SCI-IDS relating to macrophage, natural killer (NK), T and B lymphocyte cell function have been identified (Cruse et al., 1992; Campagnolo et al., 1997; Furlan et al., 2006; Lucin et al., 2007; Held et al., 2010; Riegger et al., 2007, 2009). The exact cause of this syndrome remains unclear but might relate to the attempt of ameliorating the risk of developing overt autoimmunity against CNS antigens (Ankeny et al., 2006; Ibarra et al., 2007; Zajarías-Fainsod et al., 2012) and recent studies indicate possible SCI-specific mechanisms (Ibarra et al., 2007; Lucin et al., 2007). Compared with stroke and traumatic brain injury, SCI offers an advantageous neuroanatomical approach to decipher the underlying neurogenic mechanisms specifically caused by the loss of supraspinal sympathetic innervation of immune relevant organs. It has been demonstrated previously that a high but not a low thoracic SCI causes rapid spleen atrophy, accompanied by an increase in splenic norepinephrine levels (Lucin et al., 2007). Therefore, it seems likely that the deafferentation of efferent sympathetic input to the spleen is involved in propagating a clinically relevant SCI-IDS in terms of enhanced infection susceptibility. One neurogenic sympathetic candidate mechanism by which SCI elicits SCI-IDS is direct deafferentation of supraspinal sympathetic efferents and autonomic afferents (sympathetic decentralization) of lymphatic organs by injury. Another candidate is consecutively enhanced spinally generated sympathetic nerve activity (SNA; Dembowsky et al., 1980; Taylor and Schramm, 1987; Maiorov et al., 1997), which originates distal from the lesion site and becomes disinhibited by the rostral SCI. Episodes of SNA remain present until chronic SCI in humans, including subclinical episodes not meeting the criteria of autonomic dysreflexia, which develops subsequent to acute injury (Wallin and Stjernberg, 1984). As intact immune function is essential to combat infections, a neurogenic, functionally relevant SCI-IDS may facilitate the development of infections. Infections, in particular SCI-associated pneumonia (SCI-AP) (Fig. 1A) are the main cause of death in the acute phase after SCI (DeVivo et al., 1989). Recently, they were also identified as a disease modifying factor imposing an independent risk factor for poor neurological outcome in patients after SCI (Failli et al., 2012). SCI-IDS is aggravated by a variety of non-SCI specific triggers. First, polytrauma and iatrogenic surgical treatment lead to a post-aggression syndrome, followed by a ‘systemic immune response syndrome’ (SIRS) and ‘compensatory anti-inflammatory response syndrome’ (CARS), which are also able to increase incidence of infections even in the complete absence of any CNS injury. Second, motor paralysis is likely to enhance bacterial contamination by propagating aspiration. Third, leucocyte migration might be decreased due to a reduction in blood flow, which is caused by the immobility of bedridden patients after SCI. However, the factors mentioned above cannot sufficiently explain the enhanced susceptibility to infection after SCI as (i) physiological aspiration during sleep does not cause pneumonia; and (ii) patients with isolated vertebrae fracture and/or polytrauma suffer infections less frequently despite sharing the hospital milieu enriched by multi-resistant bacteria. Moreover, SCI patients are on average comparatively young patients with few comorbidities (e.g. diabetes), who would be otherwise expected to mount a robust immune response. Thus, in contrast to the current understanding, attributing enhanced infection susceptibility solely to the patient’s environment and motor dysfunction only, a secondary functional SCI-IDS may account for the enhanced infection susceptibility. This hypothesis is supported by the sequel of the SCI-IDS preceding the onset of infections (Berlly and Shem, 2007; Riegger et al., 2007, 2009).
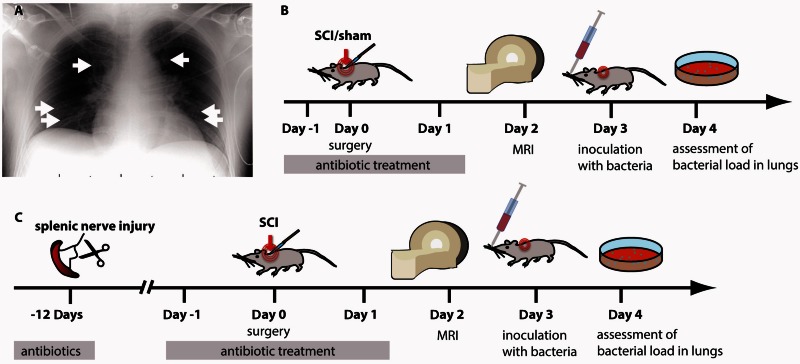
Figure 1.
Human and controlled experimental SCI-associated pneumonia (SCI-AP). (A) Typical SCI-AP occurs with an onset from Day 2–7 (Berlly and Shem, 2007) in humans and is caused by community-acquired pathogens, prevailingly S. pneumonia (white arrows: pneumonic infiltrates). SCI-AP is antedated by onset of SCI-IDS beginning within the first 24 h (Riegger et al., 2007, 2009). (B) Experimental design to investigate whether SCI lesion level enhances the susceptibility to infection after SCI. Mice were treated with antibiotics starting 1 day before surgery until the day before infection to prevent uncontrolled spontaneous infections. Animals received either a laminectomy only (control group) or a transection injury at thoracic level 3 or 9 (Th3, Th9). Animals were inoculated with 500 cfu of S. pneumoniae on Day 3 after surgery by a lung intubation. 24 h after infection, the animals were killed and the organs were harvested and processed. (C) To investigate the origin of the maladaptive signalling after SCI a preceding peripheral sympathetic deafferentation of the spleen (and a laparotomy as appropriate control) occurred 12 days before SCI. Here we investigate whether SCI-IDS is functional relevant and able to propagate the susceptibility for bacterial infections in a lesion dependent manner (neurogenic implication).
To quantify a direct functional neurogenic effect of SCI on infection susceptibility, we applied a controlled experimental model of SCI-AP. We set out to analyse the hypothesis that SCI blunts the ability of the immune system to mount a functional immune response to a clinically relevant infectious challenge and whether this is dependent on the injury level after acute SCI. Specifically, we investigated: (i) whether the lesion-level dependent, neurogenic component of SCI-IDS is sufficient to propagate infections; (ii) whether it is dependent on preganglionic or postganglionic injury; and (iii) how this neurogenic component relates to non-SCI specific factors that likewise contribute to immune suppression, such as post-aggression syndrome. To validate the clinical relevance, we investigated whether the experimental observation of a lesion level dependent, enhanced susceptibility to develop SCI-AP was mirrored after human SCI. In sum, we set out to characterize functional SCI-IDS as a direct consequence of SCI suppressing host defence and its underlying neurogenic mechanism mediated by sympathetic decentralization.
Materials and methods
Study design
In a mouse model of SCI, we investigated the effect of SCI-IDS on susceptibility to infection. Forty-seven mice were randomly assigned to undergo a transection injury of the spinal cord at thoracic 3 (Th3), thoracic 9 (Th9) or received a control surgery without injury of the spinal cord. Two days after injury we assessed the level and severity of the SCI by MRI. Three days after SCI all animals underwent an infection with Streptococcus pneumoniae. The mice were sacrificed 24 h after infection and bacterial load in the lungs was assessed to compare infection susceptibility between different injury groups (Fig. 1B). Twenty-three animals (11 Th3-SCI, seven Th9-SCI, five sham) did not survive the end point of the study or had to be excluded (see below). In the second part of the study, 88 mice were randomly assigned to a deafferentation of the spleen or a control injury, prior to SCI and infection. This was thought to shield the spleen against autonomic dysreflexia in order to investigate the influence of spinally generated nerve activity on spleen size, functional immune suppression and infection susceptibility (Fig 1C). Forty-three mice died before the end point of the study or were sacrificed to prevent inappropriate suffering according to applicable legislation (23 with spleen deafferentation, 20 with laparotomy).
Animals
Male C57Bl6/J mice (Charles River), 8–11 weeks old, weighing between 20 and 25 g, were randomly assigned to different experimental groups, as outlined above. We focused on male mice as male patients have been identified to be at higher risk to develop CNS-injury associated pneumonia (Hoffmann et al., 2012). All mice were housed in groups of five to eight in cages lined with chip bedding and environmental enrichment [mouse tunnel and igloo (Plexx BV), and nesting material) on a 12 h light/dark cycle (change 7 am/pm) and controlled room temperature with ad libitum access to food (standard chow) and water. All mice were cared for in accordance with the published International Health Guidelines under a protocol approved by the Animal Care and Use Committee and the Administration District Official Committee (Landesamt für Gesundheit und Soziales, Berlin, Germany), and performed in accordance with the European directive on the protection of animals used for scientific purposes and the respective German legislation. All mice were weighed daily and euthanized if their weight loss exceeded 20% of the preoperative weight. This excluded seven animals from the first part of the study. In the second part of the study, with two successive surgeries and subsequent infection, several animals had to be sacrificed ahead of study end due to poor health. Also, several animals died shortly after the infection. The combined number of animals that did not survive until the end of the study in the second part was 45 mice. The reporting in this manuscript is in agreement with the ARRIVE (Kilkenny et al., 2010) guidelines.
Experimental model of induced murine pneumonia
To mimic clinically relevant infections we employed a model of experimental pneumonia (SCI associated pneumonia, SCI-AP). The SCI-AEP model introduces a defined number of bacteria directly into the lungs of mice. This, in contrast to nasal application, excludes the influence of differences in aspiration between treatment groups. D39 capsular type 2 S. pneumoniae (Rockefeller University) was grown in C+Y medium (Lacks and Hotchkiss, 1960) to an optical density (OD620) of 0.4 to 0.6, and diluted to the appropriate concentration in sterile phosphate-buffered saline (PBS). The bacteria suspension was plated on blood agar plates to retrospectively confirm quantity and viability of the bacteria. The exact concentration of the bacteria suspension was used to normalize the bacterial loads in the lungs. For infection, mice were anaesthetized using midazolam (5.0 mg/kg; Dormicum®, Roche) and medetomidin (0.5 mg/kg; Domitor®, Orion Pharma Janssen Animal Health) intraperitoneally and then suspended at a 60° angle by the two front upper teeth by a wire attached to a plexiglas support. A 22G peripheral venous catheter (Becton and Dickinson) was used as tubus and a 0.5 mm optical fibre (Communication grade plastic fibre #NT02-542, Edmund Optics) attached to a cold light source was used for illumination (MacDonald et al., 2009). The tongue was pulled out with a small spatula (custom build, Medizinisch-technische Labore Charité) and the mouse was intubated. Thirty to fifty microliters of bacterial suspension, corresponding to 500 colony forming units (cfu) of S. pneumoniae, were administered into the lung. The anaesthesia was antagonized by flumazenil (Flumazenil Inversa, Inversa Arzneimittel GmbH, 0.5 mg/kg) and atipamezole (Antisedan®, Orion Pharma Janssen Animal Health, 2.5 mg/kg). Day 3 after SCI was chosen as an ideal time for infection, because SCI-induced immunodepression is most pronounced from Day 1 to 3 after injury indicated by reduced MHC class II expression pattern (Riegger et al., 2007), and the mortality was demonstrated to increase soon thereafter in another model of CNS injury (Prass et al., 2006). To obtain a homogenous population without concomitant spontaneous infections the animals received antibiotics (enrofloxacin, 10 mg/kg, per os, twice daily) in drinking water starting 1 day before SCI (Fig. 1) until 1 day before experimental infection. To ensure antibiotic uptake even in case of reduced drinking after SCI, antibiotics were given by gavage on the day of SCI and 1 day thereafter. Then antibiotic treatment was terminated on Day 2 after SCI to prevent interference of the antibiotics with the controlled SCI-AP on Day 3 post SCI. After the SCI, 0.2 mg of the antibiotic was additionally given by gavage twice daily to ensure antibiotic uptake in case of reduced drinking. Animals were placed in a heated cage supplemented with moistened oxygen to support breathing after infection to decrease mortality. Infections of all experimental rounds and the sacrificing 24 h thereafter were performed at the same time of the day to account for a potential influence of the circadian rhythm.
Experimental spinal cord injury
The preganglionic sympathetic neurons are localized in the lateral funiculus and in the intermediolateral columns corresponding to the dorsal–mid spinal cord. The four-fifth transection model used in this study therefore assured that the soma of the sympathetic preganglionic neurons are damaged (Fig. 2A). Mice received pain medication 1 h before surgery (buprenorphine, Temgesic®, Schering-Plough, 0.05 mg/kg) and were anaesthetized by isoflurane (1.5–2% in 2:3 O2/N2O, inhaled). To prevent xerophthalmia during anaesthesia, both eyes were covered with retinopalmitate (Retinopalmitol®, Ciba). Mice were placed on a heated pad maintaining body temperature at 36°C and their backs were shaved. The skin overlying the vertebral column was incised, and the muscles were detached from the vertebra. A single-level bilaminectomy (complete removal of the dorsal arch of the vertebrae: processus spinosus and bilateral lamina arcus vertebrae) was then performed to expose the spinal cord at level Th3 or Th9, respectively. After opening the dura mater, the dorsal spinal cord was symmetrically lesioned with fine iridectomy scissors (FST), resulting in a ≥ four-fifth hemisection. This was assured by marks on the microscissors correlating with four-fifth spinal cord incision depth. The wound was rinsed with normal saline and closed in layers. All animals were kept in heated cages at 30°C until they recovered from anaesthesia. Control (sham-injured) mice underwent bilaminectomy without injuring the spinal cord. Postoperative care comprised analgesic treatment (buprenorphine, Temgesic®, Schering-Plough, 0.05 mg/kg), manual bladder compression and bathing daily in hand-warm water to prevent urinary tract infections.
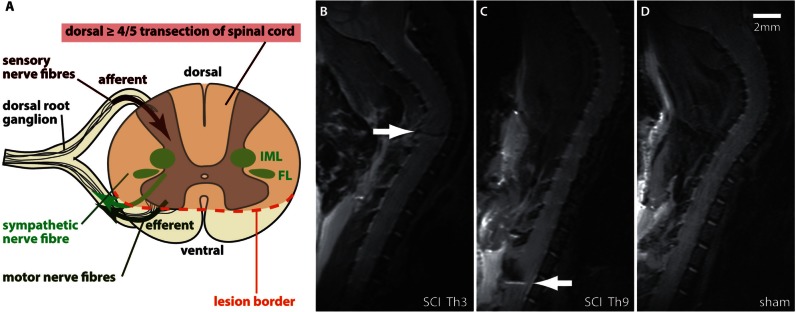
Figure 2.
Experimental SCI with differential lesion levels (Th3 versus Th9) of the sympathetic preganglionic neurons. (A) The CNS controls the immune system through the autonomous nervous system. SCI specifically disconnects sympathetic preganglionic neurons located in the thoracolumbar spinal cord, which control systemic immune function by postganglionic noradrenergic projections to secondary lymphoid tissue including the spleen. Inhibition of spinally generated SNA and reflex excitation through sympathetic preganglionic neurons by baroreceptors and other supraspinal inhibitory systems (bulbo-spinal tracts) is lost after complete SCI above mid-thoracic level (‘isolated sympathetic spinal cord’ by ‘sympathetic decentralization’). Episodes of excessive SNA correlate with injury severity and lesion level. Lesion level dependent injury of sympathetic preganglionic neurons localized to the inter-mediolateral columns (IML) and funciculus lateralis (FL) in the thoracic rodent spinal cord is assured by a dorsal > 4/5 transection SCI. (B–D) Severity, consistency and SCI lesion level were confirmed by MRI 1 day before infection. Animals with spared ventral tissue bridges were excluded. Representative T2 MRI images, illustrating a spinal cord lesion at the respective SCI level Th3 (B) and Th9 (C) (white arrows) compared with the uninjured spinal cord after sham surgery (D).
7 T MRI
MRI was applied for conformation of the lesion level and severity consistency for the criteria of demonstrating a ≥ four-fifth overhemisection 2 days after surgery (Figs. 2B–D). Mice with discrete remaining spared tissue bridges were excluded to assure a complete decentralization of sympathetic efferents. This led to the exclusion of eight animals from the first part of the study. MRI was performed using a 7 T rodent scanner (Pharmascan 70 / 16AS, Bruker BioSpin) with a 16 cm horizontal bore magnet and a 9 cm (inner diameter) shielded gradient with a H-resonance-frequency of 300 MHz and a maximum gradient strength of 300 mT/m. For imaging a 72 mm volume resonator for transmission and a 1H phased array surface coil for mouse heads were used. Data acquisition and image processing were carried out with the Bruker software Paravision 4.0. During the examinations mice were placed on a heated circulating water blanket to ensure constant body temperature. Anaesthesia was induced with 2.5% and maintained with 2.0–1.0% isoflurane (Forene, Abbot) delivered in an O2/N2O mixture (30/70%) via a facemask under constant ventilation monitoring (Small Animal Monitoring & Gating System, SA Instruments). For imaging the mouse spine a T2-weighted 2D turbo spin-echo sequence was used with repetition time / echo time = 3500 / 36 ms, RARE factor 8 and 8 averages. 10 sagittal slices with a slice thickness of 0.5 mm, a field of view of 2.60 cm2 and a matrix of 256 × 256 were positioned over the injury area, reaching from the base of the scull to the 12th thoracic vertebrae.
Organ processing
Twenty-four hours after infection mice were sacrificed and lungs and spleens were removed. The spleen length was measured as the maximum distance between organ margins. Spleen size was normalized according to the weight of a 20 g mouse. The lungs were homogenized in 0.5 ml C+Y media and plated on Columbia blood agar plates in serial dilutions of 1:1, 1:10, 1:100 and 1:1000. The colony forming units were counted after 24 h incubation and the total number of colony forming units per lung was calculated. The assessment of colony forming units in serially diluted samples of homogenized lungs is currently the gold standard in diagnosing experimental pneumonia (Prass et al., 2003). In one round, plates showed massive contamination of other bacterial strains and all eight animals of that round were excluded since the accurate bacterial count was not possible. For histology, lungs were fixated in paraformaldehyde and underwent haematoxylin/eosin staining.
Preventive denervation of the spleen prior to spinal cord injury
In an interventional attempt we intended to investigate the impact of maladaptive sympathetic signaling as a major contributor to SCI-IDS (Zhang et al., 2013). For this purpose we injured splenic nerves, including sympathetic projections (Felten et al., 1987), 12 days before SCI (Fig. 1C). This shielded the spleen from maladaptive, disinhibited, elevated SNA (Maiorov et al., 1997) originating below the lesion site. Mice received pain medication 1 h before surgery (buprenorphine, 0.05 mg/kg), were anaesthetized by isoflurane (induction 1.5–2% in 2:3 O2/N2O, maintenance 1–1.5%) and laid on their back on a heated pad maintaining body temperature at 36°C. To prevent xerophthalmia during anaesthesia, both eyes were covered with retinopalmitate (Retinopalmitol, Ciba). The abdominal cavity was opened with a short cut of skin in the upper left quadrant of the abdomen, ∼5 mm beside the linea alba, followed by muscle layer and peritoneum incision. The wound was opened with a Rigid Rake retractor (blunt prongs) and the spleen was grasped by peritoneum and fat tissue at the hilus. After placing on sterile wet surgical drape the supplying blood vessels (arteria lienalis, vena splenica) were separated carefully from surrounding tissue. By removing all surrounding tissue down to the vessel wall, all nerves that run along the arteries were severed. Completeness of nervous interruption was assured by immunohistochemical analysis of tyrosine hydroxylase staining. The spleen was carefully placed back into the abdominal cavity and the wound was closed in layers with simple interrupted stitches after applying local pain relief (bupivacaine). Control surgeries contained opening of the abdominal cavity and handling of the spleen without injury of the nerves. This control surgery is referred to as laparotomy (lap) hereafter to distinguish from the control surgeries of the SCI model (sham). To prevent mice from harming the wound, they were bandaged with a small dressing and a cohesive bandage (StabiloColor Haft, bart medical) for 24 h. Antibiotic coverage was achieved by marbofloxacin (5 mg/kgintraperitoneally, given daily for 4 days).
Human spinal cord injury: database information and study population
The datasets of patients with SCI were collected in 20 centres contributing to the National Spinal Cord Injury Database (NSCID), Birmingham, Alabama, USA. The measures taken to ensure the quality of data have been previously described in detail (Richards et al., 1995; Stover et al., 1999; DeVivo et al., 2002). The Institutional Review Board of each participating institution approved the database, and enrolment was in accordance with the Declaration of Helsinki. All subjects were informed about the database and its aim and gave their written informed consent. Longitudinal observational data were collected from patients suffering from SCI of acute traumatic aetiology. A total of 10 853 patients SCI admitted within 24 h after SCI and subsequently assigned to longitudinal follow-up had been incorporated in the NSCID at the time of our database query. We selected datasets of patients assessed with the International Standards for Neurological Classification of SCI (ISNCSCI) comprising the American Spinal Injury Association impairment scale (AIS). Thus, comparable neurological assessment was ensured.
Dataset selection was performed in a stepwise manner (Supplementary Fig 1). First, the eligibility criteria for inclusion into the statistical analysis were the patient’s age (16–75 years) and the neurological classification using the AIS. For detection of potential selection bias we compared eligible patients to non-eligible patients in terms of their baseline characteristics. Eligible patients were comparable to non-eligible patients in terms of gender and neurological level. Slight differences were observed in age and race (Supplementary Table 1). Second, to ensure data quality, datasets with missing neurological level for either side of the body and missing AIS at baseline were not included. The AIS was also encoded as ‘missing’ if an associated injury interfered with the correct performance of the neurological examination. Therefore, patients with confounding severe concomitant injuries such as traumatic brain injury were excluded. Furthermore, datasets with missing records regarding pneumonia during acute care and inpatient rehabilitation were excluded. A missing data analysis was performed comparing the sociodemographic baseline characteristics between datasets with and without missing data. Datasets removed because of missing key parameters, were not different from included datasets in terms of sociodemographic baseline characteristics (Supplementary Table 2). Finally, the analysis was restricted to patients with the neurological level Th1–Th12. Thus, cervical SCI patients with potentially severe respiratory malfunction and risk for aspiration were excluded (Supplementary Fig. 1).
Baseline data and patient characteristics were obtained at admission to acute care. The neurological assessments were performed according to the ISNCSCI also referred to as the American Spinal Injury Association (ASIA) classification (Marino et al., 2003). For the study population the occurrence of pneumonia during acute care and inpatient rehabilitation following SCI was assessed. Pneumonia was defined as an inflammation of lung tissue of infectious aetiology with radiographic demonstration of parenchymal disease during acute care and/or inpatient rehabilitation. Furthermore, complications related to the development of pneumonia, such as the dependency from mechanical ventilation and pulmonary embolism occurring until discharge from inpatient rehabilitation, were assessed. The analysis strategy was to consider the role of disturbed innervation of the major splanchnic nerve (Th5–Th9) as connected to alterations of the immune system function. Therefore, we categorized the single neurological level into lesions: (i) Th1–Th4; (ii) Th5–Th8; and (iii) Th9–Th12. Furthermore, to investigate the impact of disrupted motor function as determined by the degree of autonomic dysfunction (Previnaire et al., 2009) we stratified the patients into: (i) motor complete SCI (AIS A and B); and (ii) motor incomplete SCI (AIS C and D). Within the strata we adjusted for the AIS grade as a measure of severity of SCI. The study is reported according to the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) statement (Elm von et al., 2007).
Statistical analyses
The experimental data are shown on a logarithmic scale with lines indicating the median in case of bacterial loads of lungs and as box plots with whiskers reaching from minimum to maximum for spleen sizes. Treatment allocation of mice was performed randomly (prepared lists or schemes) and remained completely blinded for the examiners. A one-way ANOVA analysis with Bonferroni post-test was used to compare spleen lengths between three groups and a t-test for comparisons between two groups. As distributions did not pass the normality test for Gaussian distribution, the Kruskal-Wallis analysis with Dunn’s post-test was used to compare infection rates between three groups and the Mann-Whitney test between two groups. The effect sizes for changes in bacterial load were calculated based on the logarithmized original data, according to Cohen’s d (Sullivan and Feinn, 2012). The statistical analyses were performed with Prism for MacOS version 5.0 (GraphPad Software Inc.). A priori sample size calculation was performed with G*Power 3.0 (Faul et al., 2007) assuming a change in bacterial burden by 100-fold as relevant.
For the patient data, the distribution of continuous variables was described as mean ± standard deviation (SD) or confidence intervals (CI) or median and interquartile range (IQR). The two-tailed student’s t-test was used to compare between the groups. Categorical variables were reported as frequencies, including percentages and 95% confidence interval. Here, the chi-square test was applied for statistical comparison. Logistic regression analysis was performed using the occurrence of pneumonia during acute care and/or inpatient rehabilitation as dependent variable. The analysis was stratified for motor completeness of SCI. Independent variables were age, gender, race, neurological level categories, AIS, penetrating injury, spinal surgery, pulmonary embolism, and mechanical ventilation. Goodness of fit was assessed using the Hosmer-Lemeshow test. All tests were two-sided and the level of significance was 0.05. The statistical analyses of human data were performed with SPSS Statistics for MacOS, version 19.0 (IBM) using a syntax file allowing for the exact reproduction of the analysis based on the dataset.
Results
Induced experimental bacterial pneumonia is a feasible and clinically relevant model to investigate SCI-IDS
We developed and established a controlled model of SCI-associated experimental pneumonia (SCI-AEP) (Figs 1–3). Similar to the clinical scenario, pneumonia is the most prominent infection in mice after CNS injury (Hetze et al., 2013). The main pathogen causing pneumonia in humans early after SCI is S. pneumonia (Berlly and Shem, 2007). Experimental pneumonia by S. pneumonia in rodents matches clinical, histopathological and microbiological findings of lobar pneumonia in humans (Candiani et al., 1997). We inoculated 500 cfu of the coagulase negative S. pneumonia by lung intubation as a defined immune challenge. The dose of 500 cfu was calculated as 2.5-fold of the lowest colony forming unit dosage able to induce pneumonia after CNS injury (Prass et al., 2006) to ensure a clinically relevant and reliable infection rate. Histological examination of the lungs derived from SCI animals demonstrated characteristic signs of bacterial pneumonia such as massive infiltration of granulocytes and monocytes, breakdown of endothelial barriers, and oedema formation (Fig. 3C). The homogenization of lung tissue and subsequent assessment of colony forming units on blood agar plates with serially diluted samples is the gold standard in diagnosing experimental pneumonia (Prass et al., 2003) and enabled us to quantify the amount of bacterial load as a surrogate for infection susceptibility. We therefore propose that this experimental model of controlled pneumonia (SCI-AEP) is a feasible and reliable model to investigate underlying mechanisms of human SCI-associated infections in quantitative terms. To determine the neurogenic effect of segmental sympathetic nervous system ablation on bacterial susceptibility, we exposed SCI animals to this model of experimental pneumonia. SCI resulted in a ≥ four-fifth transection at the respective levels Th3 and Th9 as verified by MRI analysis 2 days after SCI. Animals with inconsistent lesion severity (tissue bridges) and lesion level were removed from the study. This assured a complete interruption of supraspinal efferents to sympathetic preganglionic neurons (intermediolateral columns and funiculus lateralis), thereby disconnecting autonomic afferents (Fig. 2A).
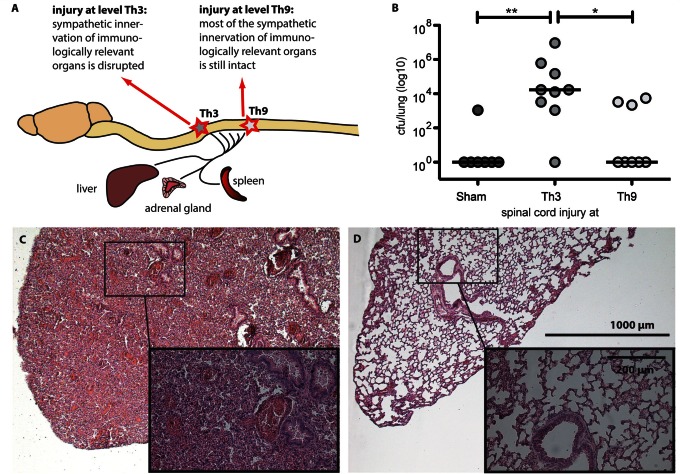
Figure 3.
Differential sympathetic deafferentation level determines infection susceptibility. (A) We assessed the infection susceptibility after Th3-SCI and Th9-SCI compared to sham operated animals differing in terms of sympathetic decentralization. (B) The elevated bacterial load in the lung 24 h after inoculation demonstrated an increased susceptibility for bacterial infection in SCI animals (11 of 17, 65 %) compared to sham animals (1 of 7, 14%). Th3 level SCI (eight of nine, 89%) triggered a significantly elevated susceptibility compared to Th9 level SCI (three of eight, 38%). Besides obvious motor and sensory paralysis SCI induces a functional SCI-IDS (‘immune paralysis’) causally relevant for clinical prevalent infections. Lines indicate median. Kruskal-Wallis analysis with Dunn’s post-test, *P < 0.05, **P < 0.01. (C) Haematoxylin and eosin staining of lung tissue from a Th3-injured mouse within the first 24 h after infection, demonstrating histopathological hallmarks of human pneumonia such as massive infiltration of immune cells and oedema formation. (D) Tissue of uninjured, uninfected mouse for comparison.
Spinal cord injury increases susceptibility to pneumonia in a neurogenic manner, independent of non-specific post-traumatic stress response
One of the aims of this study was to investigate whether SCI-IDS elevates susceptibility to infection more than a non-SCI injury. We investigated whether SCI-IDS translates into an elevated susceptibility to infection in a clinically relevant model. We analysed whether SCI mice were less able to mount a host response to combat a controlled infection compared to sham mice which are altered by a non-neurogenic, post-aggression syndrome only. Based on the low infection load used in our study, the majority (six of seven sham animals, 86%) was able to clear inoculated bacteria completely from their lung within 24 h (Fig. 3B). In contrast, after SCI this ability was reduced to 35% (six of 17) and the majority (65%) of SCI animals of different lesion levels displayed elevated bacterial loads in infected lungs after 24 h and frequently demonstrated characteristic hallmarks of infection such as lethargia or ruffled fur.
Spinal cord injury increases susceptibility to pneumonia in an injury-level dependent manner
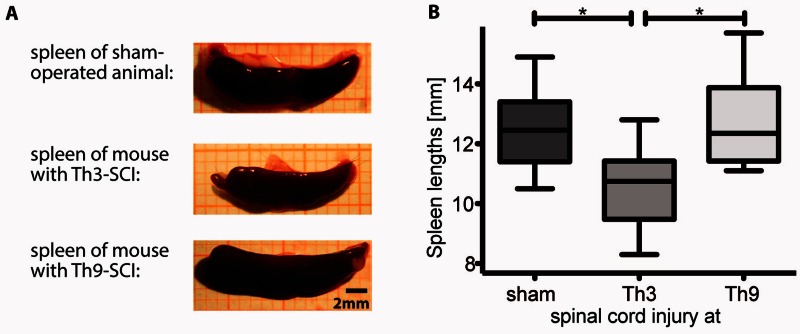
To assess whether the susceptibility to pneumonia is dependent on SCI level we measured the bacterial load in lung homogenates after Th3 compared to Th9 level SCI, following the experimental schedule as depicted in Fig. 3A. We focused on the two lesion levels Th3 and Th9 as they allow us to analyse the impact of sympathetic immune system innervation based on their differential impact on secondary lymphoid organs, including the spleen. Whereas Th3-SCI disrupts the sympathetic innervation to the spleen, Th9-SCI spares the sympathetic innervations almost completely (Fig. 3A). We observed a robust impact of SCI level on susceptibility to infection. Mice with a Th3-SCI (n = 10) accumulated a significantly higher bacterial load 24 h after infection than mice with Th9-SCI (n = 8, P ≤ 0.05, effect size = 1.5) (Fig. 3B). Correspondingly, histological analysis of lung specimens after Th3-SCI and subsequent bacterial inoculation demonstrated typical histological signs of pneumonia including massive leucocyte infiltration and hyper-cellularity (Fig. 3C). The spleens, harvested concomitantly at Day 4 after the experimental injury, demonstrated a reduction in spleen size after Th3-SCI but not after Th9-SCI (Fig. 4). This was in line with the observations of others (Lucin et al., 2007; Zhang et al., 2013) and raised the question whether the increased infection susceptibility is dependent on the injury-induced loss of supraspinal control on sympathetic efferent innervation. Therefore, we proceeded to specifically dissect the role of sympathetic innervation of the spleen. Of note sympathetic fibres of the spleen also incorporate rostral signalling from the vagus nerve, which converges at the level of the ganglion coeliacum, thereby integrating also putative, compensatory afferent or efferent effects of the vagus nerve, which remain fully intact after SCI. The spleen is a site for storage and rapid deployment of monocytes (Swirski et al., 2009) and is a main SCI-IDS effector organ characterized by leucocyte sequestering and apoptosis early after SCI (Lucin et al., 2007, 2009). In particular we aimed to identify the origin of the maladaptive sympathetic activity contributing to impaired host defence.
Figure 4.
Spleen atrophy is only caused after high thoracic but not after low thoracic SCI. To investigate the relevance of efferent sympathetic innervation of secondary lymphoid organs we focused on the spleen as the largest secondary lymphoid organ and investigated spleen atrophy as a macroscopic marker associated with systemic immune dysfunction. Shown are representative images of spleens derived from sham animals compared with mice after Th3 and Th9 SCI. (A) Spleen atrophy is induced dependent on sympathetic decentralization by Th3 SCI but not by Th9 SCI with spared efferent sympathetic innervation. (B) Data are presented as box plots with whiskers showing minimum to maximum. Th3- and Th9-level SCI mice do not differ in terms of vagus nerve innervation, which is derived from supra-lesional brainstem regions and is reported to also exert immune-modulatory effects. One-way ANOVA analysis with Bonferroni post tests, n as in Fig. 3; *P < 0.05, **P < 0.01.
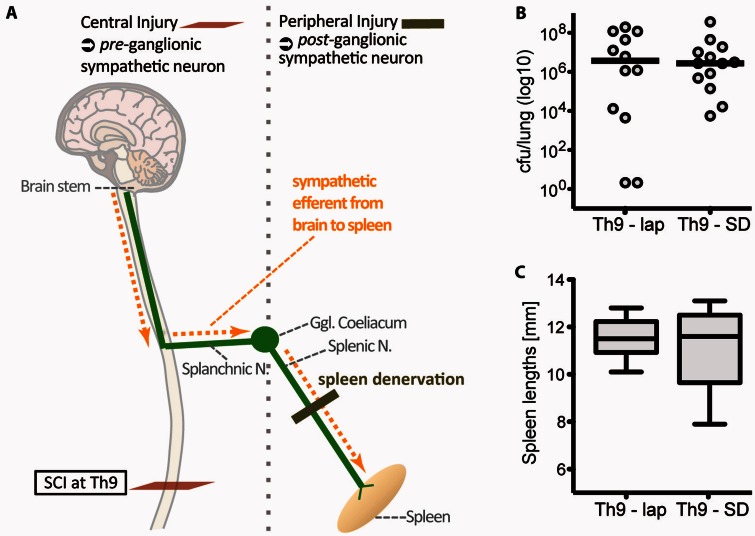
The decentralization of the spleen does not cause functional SCI-IDS
We investigated the hypothesis that immune deficiency is directly mediated by sympathetic decentralization of large secondary lymphatic organs like the spleen. In this case, Th9-SCI in combination with peripheral, postganglionic deafferentation of the spleen would likewise increase infection susceptibility as it results in a loss of sympathetic spleen innervation comparable to a Th3-SCI (Fig. 5A). We observed that infection susceptibility was not increased in Th9-SCI after a decentralization of the splenic nerve (Fig. 5B). Also, the spleen size remained unaltered (Fig. 5C). Thus, increased infection susceptibility is not caused by simply disconnecting sympathetic efferent innervation to the spleen. It seems that peripheral postganglionic sympathetic injury is not able to propagate functional SCI-IDS. Functional SCI-IDS can therefore only be induced by central injury e.g. due to SCI. Our results reject the hypothesis that interrupted sympathetic efferent spleen innervation is a main underlying cause of enhanced infection susceptibility.
Figure 5.
Functional SCI-IDS depends on central (preganglionic) lesion site and is not caused by postganglionic sympathetic deafferentation. (A) We investigated whether deafferentation of sympathetic efferents per se causes enhanced immune susceptibility or whether functional immune suppressive effects depend on its injury site [central (preganglionic) versus peripheral (postganglionic sympathetic injury); vertical grey dotted line demarcates the border between pre- and postganglionic sympathetic innervation, localized at the ganglion coeliacum]. As shown above, a high-thoracic preganglionic injury causes functional SCI-IDS (Fig. 3) and spleen atrophy (Fig. 4). However, postganglionic deafferentation (peripheral injury to the splenic nerve prior to a Th9-SCI, which itself does not cause SCI-IDS; Th9-SD) does not enhance bacterial load comparing with intact peripheral spleen innervation (Th9-lap) (B) and does not affect spleen size (C). Thus, postganglionic sympathetic deafferentation of large lymphoid organs does not propagate infection susceptibility. Bacterial load in lungs are displayed on a logarithmic scale with lines indicating medians. Spleen lengths are displayed as box plots with whiskers reaching from minimum to maximum. SD = spleen deafferentation; Lap = laparotomy (sham surgery).
Disinhibited sympathetic neural bursts of spinal origin from below the injury side cause functional SCI-IDS
We analysed whether enhanced infection susceptibility is mediated by a central mechanism due to pre-ganglionic injury rather than postganglionic injury. As a consequence of loss of supra-spinal control on sympathetic firing, consecutive disinhibition elicits elevated spinally generated SNA originating distal from the lesion site. SNA-mediated systemic effects depend on whether excitatory sympathetic bursts can enter peripheral immunological organs, such as the spleen (Fig. 6).
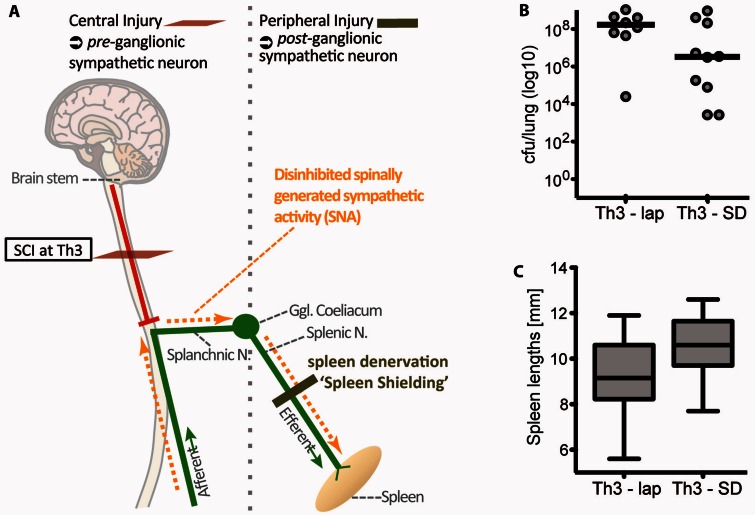
Figure 6.
Maladaptive sympathetic signalling triggering functional SCI-IDS after high thoracic SCI originates from below the lesion site. (A) Given that enhanced infection susceptibility (functional SCI-IDS) is induced by central (preganglionic) and high (Th3-SCI) injury only, we analysed whether the underlying mechanism is dependent on disinhibited spinally generated SNA originating distal to the lesion site with unrestricted access into the spleen. Therefore, we analysed the effect of blocking SNA signalling to the spleen by deafferentation of the splenic nerve. (B) Peripheral, postganglionic sympathetic deafferentation of the spleen prior Th3-SCI (Th3-SD, ‘spleen shielding’ from SNA) decreases the bacterial load in the lung 24 h after infection (improved host defence) compared to sham/laparotomy operated Th3-SCI animals (Th3-lap) (Mann-Whitney test, Th3-SD versus Th3-lap, P = 0.06). (C) Congruently, it attenuates spleen size atrophy after Th3-SCI (t-test, Th3-SD versus Th3-lap P = 0.06). The fact that compensatory signals cannot reach the ganglion coeliacum and the spleen in case of Th3 SCI, supports the hypothesis that the origin of maladaptive sympathetic signalling is below the injury (A). Bacterial loads in lungs are displayed on a logarithmic scale with lines indicating medians. Spleen lengths are displayed as box plots with whiskers reaching from minimum to maximum. SD = spleen deafferentation; Lap = laparotomy (sham surgery). Vertical grey dotted line demarcates the border between pre- and postganglionic sympathetic innervation localized at the ganglion coeliacum.
To unravel the role of preganglionic sympathetic injury on infection susceptibility, the spleen was shielded from post-injury sympathetic signalling (Fig. 6A). Mice received either a peripheral postganglionic sympathetic deafferentation of the spleen by transecting the splenic nerve, (‘spleen shielding’) 12 days before SCI or a laparotomy as control procedure. Of note, the overall bacterial load 24 h after infection was generally elevated in groups with two surgical procedures within 2 weeks compared to groups with only one surgery. This is likely due to increased stress levels. Despite higher background stress levels due to two surgeries (Fig. 1C) Th3-SCI consistently resulted in higher susceptibility to SCI-AEP compared with Th9-SCI (cf Figs 5B and 6B).
However, after acute Th3-SCI, known to propagate sympathetic splenogastric bursts (Taylor and Schramm, 1987), the preventive ‘sympathetic shielding’ of the spleen (n = 10) but not laparotomy (n = 8) ameliorated bacterial load in the lung (effect size = 0.9, P = 0.06, Fig. 6A). ‘Sympathetic shielding’ also corresponded with prevention of spleen size atrophy (effect size = 0.8, P = 0.06, Fig. 6C). The experimental paradigm identifies enhanced infection susceptibility to be caused by preganglionic, central mechanisms, and not by postganglionic peripheral mechanism. Central disinhibited sympathetic signalling appears to be decisive and pathophysiologically relevant, whereas deafferentation of peripheral lymphatic organs is not.
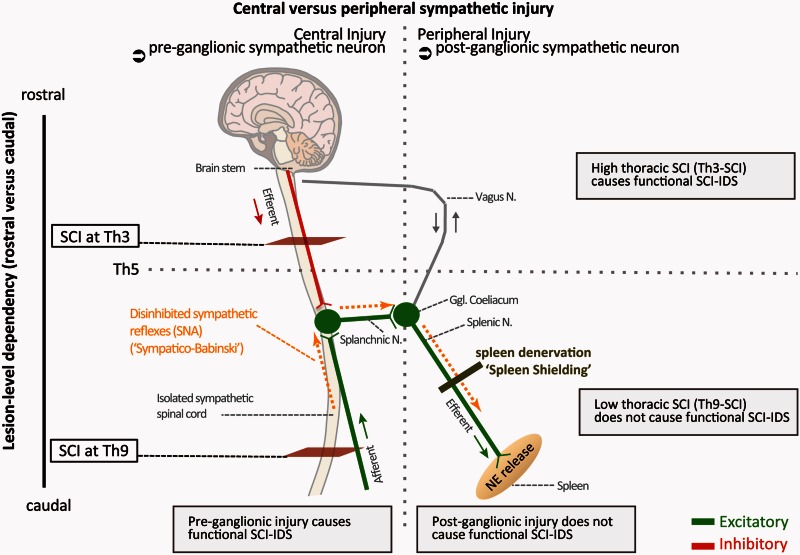
Central disinhibition mediated effects are restricted to SCI lesion levels above Th5, which allow efferent sympathetic bursts to enter the spleen via the splanchnic and splenic nerves. Central disinhibition effects after Th9 SCI are not able to contribute to functional SCI-IDS (Fig. 5) as they cannot signal to the spleen or other secondary lymphoid organs that receive sympathetic innervation by the ganglion coeliacum. Hence, the maladaptive increased spinally generated SNA, known to originate from below the lesion site into the spleen, is a main underlying cause for functional SCI-IDS. We conceptualize that disinhibited SNA contributes to increased splenic norepinephrine levels after Th3-SCI, causing leucocyte apoptosis, spleen atrophy and resulting in increased infection susceptibility (Fig. 8).
Figure 8.
Neurogenic pathophysiology and targets of maladaptive sympathetic signalling perpetuating functional SCI-IDS after SCI. Under physiological conditions excitatory sympathetic signals are controlled by supraspinal inhibition. After SCI, loss of supraspinal control leads to spinally generated sympathetic nerve activity (SNA). SNA originates below SCI injury and leads to maladaptive efferent sympathetic activity signalling to the spleen. It is able to culminate in large excitatory spinal sympathetic reflexes. Spinal sympathetic reflexes occur in analogy to pathological Babinksi motor reflexes, caused by a loss of supraspinal (bulbospinal) tonic inhibition (‘Sympathico-Babinski’). Disinhibited spinally generated sympathetic nerve activity after high thoracic (Th3) SCI leads to ad hoc induction of excitatory SNA burst entering the splenic nerve (Dembowsky et al., 1980; Taylor and Schramm, 1987) associated with increased levels of norepinephrine (NE) in the spleen, which in turn causes apoptosis of immune cells in the spleen (Lucin et al., 2007, 2009). This results in a decrease of spleen size and an elevated susceptibility to infection. Blocking SNA signaling to the spleen (‘shielding’) by preceding peripheral denervation of the splenic nerve ameliorates functional SCI-IDS and bacterial load after Th3-SCI. Moreover, here we identify the peripheral splenic nerve as a target for immunomodulation after SCI in order to restore impaired host defense after SCI (SCI-IDS) by blocking SNA to the spleen. Spleen shielding may be an interventional strategy to ameliorate functional SCI-IDS to prevent infections in patients at risk.
Thoracic lesion-level is an independent risk factor for pneumonia in motor complete patients with spinal cord injury
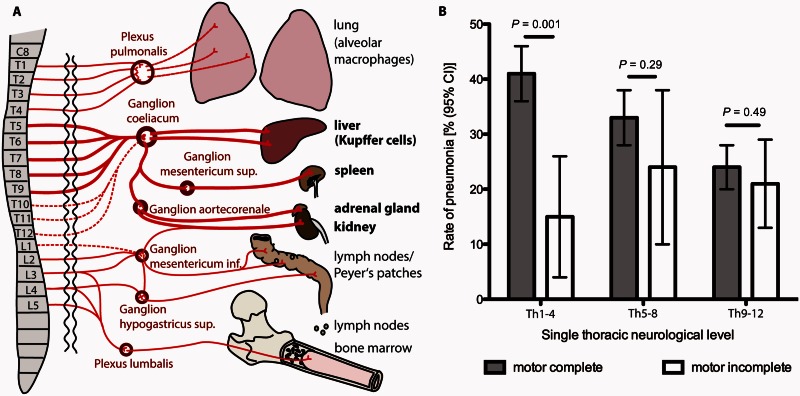
To determine whether the susceptibility to develop pneumonia is lesion level dependent after human SCI, we analysed data from the NSCID (Fig. 7). After application of the eligibility and selection criteria, 1221 datasets from patients AIS A–D, enrolled between September 1993 and September 2006 (Supplementary Fig. 1) were analysed with respect to the occurrence of pneumonia and its dependency on the injury level regarding the thoracic segments (Th1–Th4 versus Th5-–Th8 versus Th9–Th12) (Fig. 7A). The groups with and without pneumonia exhibited similar baseline and clinical characteristics in terms of gender, race, percentage of penetrating injury, spinal surgery and pulmonary embolism. The groups were different in age, and use of mechanical ventilation. Furthermore, they differed in the thoracic neurological level and the motor completeness of the injury as measured with the AIS (Supplementary Table 3). Pneumonia occurring between admission to acute care within 24 h and discharge from inpatient rehabilitation 55 days (39–78) [median (IQR)] post SCI is injury severity dependent and most frequent in patients with AIS A injury (33.0%, 290 of 878). Its frequency decreases with injury severity (AIS B 22.4%, 36 of 161; AIS C 24.6%, 29 of 118; and AIS D 10.9%, 7 of 64 patients).
Figure 7.
Thoracic lesion level and severity after human SCI are independently associated with enhanced ‘neurogenic’ risk of SCI-associated pneumonia (SCI-AP). (A) Sympathetic innervation (preganglionic, postganglionic) of immunological relevant organs in humans. SCI rostral to Th5 (Th1–Th4) will result in preganglionic injury to sympathetic efferents whereas Th5–Th8 SCI spares some and SCI Th9–Th12 most sympathetic efferents to the ganglion coeliacum. (B) We compared motor complete (AIS A and B patients, grey bars, given the excellent correspondence between somatic and complete sympathetic lesions in this population) with motor incomplete patients (AIS C and D, white bars) with enhanced sparing of sympathetic outflow. Relative frequency of pneumonia is shown in motor complete versus motor incomplete SCI patients, categorized as high (Th1–Th4), mid (Th5–Th8) or low (Th9–Th12) thoracic injury level. Within the group of Th1–Th4 patients, pneumonia rates are significantly different between motor complete and incomplete patients, whereas no differences between the groups were detectable in lower thoracic levels. Error bars represent 95% CI. Differences within the neurological level categories were assessed with the chi-square test. Patient numbers: motor complete Th1–Th4, n = 317, Th5–Th8 n = 271, Th9–Th12 n = 451; motor incomplete Th1–Th4, n = 41, Th5–Th8 n = 34, Th9–Th12 n = 107.
In motor complete patients (AIS A and B, interrupted sympathetic efferents) the rate of pneumonia is highest in the neurological level Th1–Th4 group (41.3%, 131 of 317) where it differs significantly from the motor incomplete (AIS C and D, sparing of the sympathetic efferents) stratum (14.6%, 6 of 41, P = 0.001). Within the level categories Th5–Th8 [motor complete 88 of 271 (32.5%) versus motor incomplete 8 of 34, (23.5%) P = 0.29] and Th9–Th12 [motor complete 107 of 451 (23.7%) versus motor incomplete 22 of 107 (20.6%) P = 0.49] no differences were detectable (Fig. 7B).
The results of the multiple logistic regression analysis in motor complete SCI patients (n = 960) verified that high thoracic injury (Th1–Th4) relative to mid (Th4–Th8) and mid relative to low thoracic injury (Th9–Th12) is independently associated with an enhanced pneumonia risk as represented by an odds ratio (OR) of 1.35 [95% confidence interval (CI) 1.14–1.60, P < 0.001, Supplementary Table 4]. The groups with and without pneumonia were different in the rate of mechanical ventilation, as a relevant parameter associated with the development of pneumonia. However, multiple logistic regression analysis confirmed that patients with motor complete SCI of high or mid thoracic neurological level—related to restriction of the outflow to the major splanchnic nerve—are at risk to develop pneumonia independently from mechanical ventilation and pulmonary embolism and other outcome relevant factors (injury/AIS severity). Patients with motor incomplete SCI reveal no association of the lesion level to the development of pneumonia. Here factors such as age, penetrating injury, and—most pronounced—mechanical ventilation were identified as risk factors (Supplementary Table 5). Thoracic lesion level and severity are independently associated with a ‘neurogenic’ mediated risk for pneumonia (OR 1.35).
Discussion
Patients with SCI are 37-times more likely to die of pneumonia than individuals in the general population (DeVivo et al., 1993). The most common pathogen in the acute phase of SCI is S. pneumonia, which is also the major cause for rehospitalization in patients with SCI. A better understanding of the underlying mechanisms for the high prevalence of SCI-AP is required to develop more effective treatment strategies to reduce mortality and to improve neurological outcome. This medical need is emphasized further in light of the recent sobering findings that SCI mortality has increased during the past three decades (Shavelle et al., 2015). Moreover, SCI-AP and wound infections have recently been identified as independent risk factor for poor neurological recovery associated with significantly reduced gains of AISA motor score (up to −50%), impaired gains of neurological segments (−2) and reduced rates of AIS conversions in motor complete (AIS A and B) patients (Failli et al., 2012).
Following up on reports of fluctuations and activation of immune cells contributing to SCI-IDS (Cruse et al., 1992; Campagnolo et al., 1997; Furlan et al., 2006; Lucin et al., 2007, 2009; Riegger et al., 2007, 2009), we assessed whether SCI-IDS is functionally relevant for prevalent clinical infectious disease. The susceptibility for bacterial pneumonia was significantly increased in mice after Th3-SCI compared to Th9-SCI, whereas the post-aggression stress response was not able to propagate pneumonia in our model. These findings were mirrored in motor-complete SCI patients who displayed a significantly elevated susceptibility for pneumonia with higher thoracic SCI-levels. Here, we identify the ‘neurogenic’ SCI-IDS to directly facilitate the development of infections. We specifically focused on thoracic SCI to minimize other lesion level-dependent effects, which may independently enhance the risk of pneumonia not related to sympathetic innervation. First, brain stem-mediated functions leading to swallowing defects and enhanced risk of aspiration can be excluded. Second, intact cervical innervation will allow for ventilation in both groups, as the major respiratory muscles [M. diaphragmaticus (C3–C5)], responsible for up to 80% of breathing, remain unaffected after high or low thoracic SCI. Likewise, this holds true for the accessory respiratory muscles [M. scalenus (C3–C8), M. sterno-cleidomastoideus (C2–C4) and M. trapezius (C1–C4) that pull up the chest opposing the M. diaphragmaticus] and for M. pectoralis major (C5–7), a major cough muscle (Lane, 2011). Third, the degree of immobilization is similar in all groups, as both Th3- and Th9-SCI result in paraparesis with spared forelimb locomotion. Fourth, brain stem derived innervation of the vagus nerve, which also attributes to immune modulatory effects (Tracey, 2009), remains unaffected. Fifth, non-neurogenic triggers causing a post-aggression syndrome due to trauma/surgical intervention are represented in the spine-operated sham group and can therefore be differentiated from neurogenic effects of SCI. Thus, increased infection susceptibility after SCI can be specifically attributed to neurogenic effects mediated by deafferentation of the sympathetic nervous system, resulting in the loss of supraspinal sympathetic control.
Next, we investigated the underlying lesion-level dependent mechanism of functional SCI-IDS. We pursued two main approaches to localize the origin of maladaptive sympathetic signaling as a cause of functional SCI-IDS: first, effects due to direct deprivation of lymphoid organs (spleen) from sympathetic innervation, and second, disinhibited enhanced spinally generated SNA starting early after SCI and originating from below the lesion site getting unrestrained access to the spleen (Maiorov et al., 1997; Dembowsky et al., 1980; Taylor and Schramm, 1987). Based on our findings we conclude that direct sympathetic deprivation of the spleen alone is unable to cause functional SCI-IDS, as post-ganglionic deafferentation of the splenic nerve did not increase bacterial load. Alternatively, a central (preganglionic) sympathetic mechanism may propagate functional SCI-IDS. This pathophysiological concept is founded on prolonged, maladaptive episodes of elevated spinally generated sympathetic hyperactivity occurring early after SCI (Dembowsky et al., 1980; Wallin and Stjernberg, 1984; Taylor and Schramm, 1987). Sympathetic bursts can propagate to the spleen by the efferent splanchnic/splenic nerve. This was shown by elegant electrophysiological experiments, which demonstrated splenogastric nerve activities doubles after acute SCI (Taylor and Schramm, 1987). Splenogastric sympathetic nerve activity serves as a marker for postganglionic sympathetic activity distal to the ganglion coealiacum. Exacerbated or prolonged, maladaptive exposure of the spleen to sympathetic activity induces lymphocyte apoptosis and monocyte deactivation caused by increased epinephrine levels. Moreover, spinally generated SNA has been verified by other groups to culminate in large excitatory spinal sympathetic reflexes due to loss of supraspinal inhibitory control after acute SCI (Maiorov et al., 1997). Consecutively, spontaneous autonomic dysreflexia develops (Maiorov et al., 1997), which has been demonstrated to further decrease leucocyte function and spleen size (Zhang et al., 2013). If a central preganglionic cause is decisive for the development of functional SCI-IDS the blockade of elevated spinally generated sympathetic signalling to the spleen would attenuate functional SCI-IDS.
Shielding the spleen from SNA after high thoracic injury by spleen denervation attenuated functional SCI-IDS and reduced bacterial loads in mice with a large effect size of 0.9. Therefore, it can be considered biologically meaningful, although only a statistically non-significant association could be demonstrated (Fig. 6). Additional verification of this hypothesis is the observed spleen atrophy after Th3-SCI, which is partially prevented when spleens are shielded by postganglionic denervation. Lastly, as independent proof there is no difference in spleen size between Th9-SCI mice with or without spleen denervation as there is no SNA signalling to the spleen in low thoracic SCI.
Thus ‘spleen shielding’ effects are confined to conditions ofloss of tonic supraspinal control and consecutively triggered SNA getting unrestricted access to the splanchnic nerve (Fig. 6). Of note, denervation of the splenic nerve prior to Th3-SCI did not fully protect animals (Fig. 6B), implying additional lesion-level independent mechanisms. Moreover, although we specifically focused on the effects of the sympathetic nervous system in this study, concomitant effects of the hypothalamic-adrenal-pituitary gland (HPA)-axis are likely aggravating SCI-IDS (DeVivo et al., 1989; Lucin et al., 2007). Based on our findings, we conclude that functional SCI-IDS is caused by a central (preganglionic) injury mechanism (sympathetic disinhibition) but not by the decentralization of sympathetic input from the spleen per se.
To improve the translational impact of our findings and validate our results in humans, we extended our investigation to SCI-AP susceptibility in patients with distinct thoracic injury levels. We focused on the risk of SCI-AP in motor complete (somatic) AIS A and B patients given the excellent to good correspondence between somatic motor complete (ASIA A and B) and complete sympathetic lesions in this population (Previnaire et al., 2009). Motor complete patients were compared to a stratum of motor incomplete SCI in which autonomic dysfunction is principally less pronounced (Previnaire et al., 2009). The thoracic lesion level was associated with elevated risk of SCI-AP, independent from other important risk factors such as mechanical ventilation, pulmonary embolism and age as confirmed by multiple logistic regression analysis.
Our clinical observations have the limitation that they are not population based. However, as there is no population based large data registry worldwide, the results are based on the best available data. Also, previously published outcome data from the NSCID are in line with data from other national and international studies (Fawcett et al., 2007). Furthermore, the applied eligibility criteria are unlikely to represent a source of bias in this study, as included patients are largely comparable to excluded patients (Supplementary Table 1). Another limitation of the study is that the lower rate of pneumonia in low thoracic SCI might be not exclusively related to a less pronounced functional SCI-IDS but also caused by better bacterial clearance of the respiratory tracts due to residual intercostal and abdominal motor function.
In summary, acute SCI-IDS (i) impairs host immune defence; (ii) propagates infectious diseases such as pneumonia in a lesion level dependent manner; and (iii) is mediated by a central neurogenic mechanism caused by injury to preganglionic neurons. This pathomechanism occurs already in the acute stage after SCI, even before spontaneous full blown autonomic dysreflexia develops, which has previously been shown to also induce immune dysfunction after chronic SCI (Zhang et al., 2013). Analogous to pathological Babinksi motor reflexes, loss of supraspinal tonic inhibition (sympathetic decerebration) causes spinal sympathetic reflexes as spinally generated SNA originating from below the lesion side (‘Sympatico-Babinski’). We propose SNA to causally affect spleen function when gaining access to the spleen as immunological effector organ, thereby propagating functional SCI-IDS. Thus, besides obvious motor and sensory paralysis, SCI induces a functional SCI-IDS (neurogenic immune paralysis) causally relevant for clinical prevalent infections. Inhibition of excessive spinally generated sympathetic nerve activity originating from below the lesion site (isolated sympathetic spinal cord) is a causal target to treat SCI-IDS to decrease infection and associated mortality after acute SCI.
Supplementary Material
Acknowledgements
We would like to thank Yvonne Amoneit and Heike Lerch for excellent technical support.
Glossary
Abbreviations
- AIS
American Spinal Injury Association impairment scale
- CIDS
CNS injury-induced immune deficiency syndrome
- NSCID
National Spinal Cord Injury Database
- SCI
spinal cord injury
- SCI-AEP
SCI-associated experimental pneumonia
- SCI-AP
SCI-associated pneumonia
- SCI-IDS
spinal cord injury-induced immune deficiency syndrome
- SNA
(spinally generated) sympathetic nerve activity
Funding
The project has received funding from the German Research Council (DFG; Cluster of Excellence NeuroCure, PR 1274/2-1), the German Academic Exchange Service (DAAD, D/10/43923), the Wings for Life Spinal Cord Research Foundation (WfL-DE-006/1) and the W.E Hunt and C.M. Miller Endowment (to J.M.S.). R.W. is an awarded scholar of the ‘Studienstiftung des deutschen Volkes’ (Grant Number 186392). The NSCID is funded by the National Institute on Disability and Rehabilitation Research (NIDRR, Grant number H133A110002), U.S. Department of Education, USA.
Supplementary material
Supplementary material is available at Brain online.
References
- Ankeny DP, Lucin KM, Sanders VM, McGaughy VM, Popovich PG. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J Neurochem 2006; 99: 1073–87. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, et al. Sympathetic modulation of immunity: relevance to disease. Cell Immunol 2008; 252: 27–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med 2007; 30: 309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of Staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil 1997; 76: 276–80. [DOI] [PubMed] [Google Scholar]
- Candiani G, Abbondi M, Borgonovi M, Williams R. Experimental lobar pneumonia due to penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae in immunocompetent and neutropenic rats: efficacy of penicillin and teicoplanin treatment. J Antimicrob Chemother 1997; 39: 199–207. [DOI] [PubMed] [Google Scholar]
- Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res 1992; 11: 104–16. [DOI] [PubMed] [Google Scholar]
- Dembowsky K, Czachurski J, Amendt K, Seller H. Tonic descending inhibition of the spinal somato-sympathetic reflex from the lower brain stem. J Auton Nerv Syst 1980; 2: 157–82. [DOI] [PubMed] [Google Scholar]
- Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000; 85: 109–17. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil 1993; 74: 248–54. [PubMed] [Google Scholar]
- DeVivo MJ, Go BK, Jackson AB. Overview of the national spinal cord injury statistical center database. J Spinal Cord Med 2002; 25: 335–8. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Cause of death for patients with spinal cord injuries. Arch Intern Med 1989; 149: 1761–6. [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 2000; 52: 595–638. [PubMed] [Google Scholar]
- Elm von E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–7. [DOI] [PubMed] [Google Scholar]
- Failli V, Kopp MA, Gericke C, Martus P, Klingbeil S, Brommer B, et al. Functional neurological recovery after spinal cord injury is impaired in patients with infections. Brain 2012; 135: 3238–50. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–91. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007; 45: 190–205. [DOI] [PubMed] [Google Scholar]
- Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res 1987; 18: 28–36. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Krassioukov AV, Fehlings MG. Hematologic abnormalities within the first week after acute isolated traumatic cervical spinal cord injury: a case-control cohort study. Spine 2006; 31: 2674–83. [DOI] [PubMed] [Google Scholar]
- Held KS, Steward O, Blanc C, Lane TE. Impaired immune responses following spinal cord injury lead to reduced ability to control viral infection. Exp Neurol 2010; 226: 242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetze S, Engel O, Römer C, Mueller S, Dirnagl U, Meisel C, et al. Superiority of preventive antibiotic treatment compared with standard treatment of poststroke pneumonia in experimental stroke: a bed to bench approach. J Cereb Blood Flow Metabol 2013; 33: 846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Malzahn U, Harms H, Koennecke H-C, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43: 2617–23. [DOI] [PubMed] [Google Scholar]
- Ibarra A, Jiménez A, Cortes C, Correa D. Influence of the intensity, level and phase of spinal cord injury on the proliferation of T cells and T-cell-dependent antibody reactions in rats. Spinal Cord 2007; 45: 380–6. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 2011; 11: 625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S, Hotchkiss RD. A study of the genetic material determining an enzyme activity in Pneumococcus. Biochim Biophys Acta 1960; 39: 508–18. [DOI] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 2011; 179: 3–13. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol 2007; 207: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Popovich PG. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J Neurochem 2009; 110: 1409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald KD, Chang H-YS, Mitzner W. An improved simple method of mouse lung intubation. J Appl Physiol 2009; 106: 984–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorov DN, Weaver LC, Krassioukov AV. Relationship between sympathetic activity and arterial pressure in conscious spinal rats. Am J Physiol 1997; 272: H625–31. [DOI] [PubMed] [Google Scholar]
- Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26 (Suppl 1): S50–6. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005; 6: 775–86. [DOI] [PubMed] [Google Scholar]
- Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke 2006; 37: 2607–12. [DOI] [PubMed] [Google Scholar]
- Prass K, Meisel C, Hoflich C, Braun JS, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 2003; 198: 725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previnaire JG, Soler JM, Masri El W, Denys P. Assessment of the sympathetic level of lesion in patients with spinal cord injury. Spinal Cord 2009; 47: 122–7. [DOI] [PubMed] [Google Scholar]
- Richards JS, Go BK, Rutt RD, Lazarus PB. Spinal cord injury: clinical outcomes from the model systems. 1995 edn Gaithersburg, MD: Aspen Publishers; 1995. [Google Scholar]
- Riegger T, Conrad S, Liu K, Schluesener HJ, Adibzahdeh M, Schwab JM. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur J Neurosci 2007; 25: 1743–7. [DOI] [PubMed] [Google Scholar]
- Riegger T, Conrad S, Schluesener HJ, Kaps H-P, Badke A, Baron C, et al. Immune depression syndrome following human spinal cord injury (SCI): a pilot study. Neuroscience 2009; 158: 1194–9. [DOI] [PubMed] [Google Scholar]
- Shavelle RM, DeVivo MJ, Brooks JC, Strauss DJ, Paculdo DR. Improvements in long-term survival after spinal cord injury? Arch Phys Med Rehabil 2015; 96: 645–51. [DOI] [PubMed] [Google Scholar]
- Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol 2004; 5: 575–81. [DOI] [PubMed] [Google Scholar]
- Stover SL, DeVivo MJ, Go BK. History, implementation, and current status of the National Spinal Cord Injury Database. Arch Phys Med Rehabil 1999; 80: 1365–71. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ 2012; 4: 279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RF, Schramm LP. Differential effects of spinal transection on sympathetic nerve activities in rats. Am J Physiol 1987; 253: R611–8. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nat Rev Immunol 2009; 9: 418–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Stjernberg L. Sympathetic activity in man after spinal cord injury. Outflow to skin below the lesion. Brain 1984; 107 (Pt 1): 183–98. [DOI] [PubMed] [Google Scholar]
- Zajarías-Fainsod D, Carrillo-Ruiz J, Mestre H, Grijalva I, Madrazo I, Ibarra A. Autoreactivity against myelin basic protein in patients with chronic paraplegia. Eur Spine J 2012; 21: 964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci 2013; 33: 12970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.