Summary
Background
The potassium‐competitive acid blocker vonoprazan (VPZ) has potent acid‐inhibitory effects and may offer clinical advantages over conventional therapy for acid‐related disorders.
Aim
To investigate the efficacy and safety of VPZ in patients with erosive oesophagitis (EO).
Methods
In this multicentre, randomised, double‐blind, parallel‐group, dose‐ranging study, patients ≥20 years with endoscopically confirmed EO [Los Angeles (LA) grades A−D] received VPZ 5, 10, 20 or 40 mg, or lansoprazole (LPZ) 30 mg once daily for 8 weeks. The primary endpoint was the proportion of healed EO subjects as shown by endoscopy at week 4.
Results
A total of 732 subjects received VPZ or LPZ. The proportion of healed EO subjects at week 4 was 92.3%, 92.5%, 94.4%, 97.0% and 93.2%, respectively, with VPZ 5, 10, 20 and 40 mg and LPZ 30 mg. All VPZ doses were non‐inferior to LPZ when adjusted for baseline LA grades A/B and C/D. Among those with LA grades C/D, the proportions of healed EO subjects were 87.3%, 86.4%, 100%, 96.0% and 87.0%, respectively, with VPZ 5, 10, 20 and 40 mg and LPZ 30 mg. The incidence of adverse events was similar across the groups.
Conclusions
Vonoprazan was effective and non‐inferior to LPZ in healing EO. VPZ 20 mg or higher was highly efficacious for severe EO (LA grades C/D). VPZ was associated with no safety concern during this 8‐week study, while there was a dose‐dependent increase in serum gastrin. Once‐daily VPZ 20 mg is the recommended clinical dose for treating EO.
Introduction
Proton pump inhibitors (PPIs) are the cornerstone for the treatment of acid‐related disorders, such as gastro‐oesophageal reflux disease (GERD), providing superior symptom relief of reflux oesophagitis and healing of the disease compared with H2 antagonists (H2RAs).1, 2 Published reports suggest, however, that erosive oesophagitis (EO) may remain unresolved in a subset of patients after standard‐dose/‐duration PPI therapy3, 4 or that it may relapse within 6 months after healing.5 This may be accounted for in part by the inadequate control of night‐time gastric acidity with PPIs,6, 7, 8 and/or the metabolism of PPIs by polymorphic cytochrome CYP2C19, which could result in varying plasma drug concentrations and acid‐inhibitory effects between extensive and poor metabolisers.9, 10 Also, improvements are required in the slow cumulative onset of PPI action at therapeutic doses,11, 12, 13 especially in terms of achieving faster symptom relief for patients with EO.
To provide faster, more potent and sustained gastric acid suppression, thereby delivering more effective symptom relief and EO healing than standard PPIs, alternative compounds with acid‐suppressing properties have been investigated. Of these, potassium‐competitive acid blockers (P‐CABs) have shown potential as an alternative to PPIs.
Vonoprazan (VPZ) is a novel, orally active P‐CAB discovered and synthesised by Takeda Pharmaceutical Company Ltd., Japan. Nonclinical studies14, 15, 16, 17, 18 have shown that this compound exerts more potent, sustained suppression of gastric acid secretion than the PPI lansoprazole (LPZ) or a prototype P‐CAB (SCH28080). The therapeutic potential of VPZ may be derived from its ability to accumulate at high concentrations in the gastric parietal cell canaliculi, become slowly cleared from the gastric glands, and exert its effects acid‐independently,16, 17 thus exerting its potent and sustained action. Furthermore, unlike earlier P‐CABs, VPZ has no imidazopyridine ring, which has been associated with reversible increases in liver transaminases.19 VPZ may thus offer a favourable safety profile with additional clinical advantages over conventional and emerging gastric acid suppressants.
Phase I single ascending‐dose20 and multiple repeat‐dose21 studies in Japan and the UK showed that VPZ was well tolerated at single doses (1–120 mg) as well as at repeat doses (10–40 mg). In addition, very strong, dose‐dependent gastric acid suppression was observed with VPZ at doses between 10 and 40 mg. In the phase I multiple repeat‐dose study in Japan, the percentages of time that the subjects had a gastric acid pH ≥4 were 85.3% and 100.0% on days 1 and 7, respectively, after multiple dosing with VPZ 40 mg. Similarly, the percentages of time that the subjects had a gastric acid pH ≥ 4 during night‐time (21:00–09:00) were 86.5% and 100.0% on days 1 and 7, respectively, after multiple dosing with VPZ 40 mg.21
Based on these results, a phase II multicentre, randomised, double‐blind, parallel‐group, dose‐ranging study was designed and conducted in Japan to evaluate the efficacy and safety of once‐daily VPZ vs. once‐daily LPZ in Japanese patients with endoscopically confirmed EO (LA grades A−D).
Methods
Subjects and study design
This was a multicentre, randomised, double‐blind, parallel‐group, dose‐ranging, 8‐week study (TAK‐438/CCT‐001) comparing the efficacy and safety of daily oral VPZ at 5, 10, 20 and 40 mg vs. daily oral LPZ 30 mg. The study was conducted at 66 sites in Japan in accordance with the Declaration of Helsinki, the International Conference on Harmonisation guideline for Good Clinical Practice, and Japanese regulatory requirements. The study was also approved by the ethics committee of each study site. Written informed consent was obtained from all subjects before the initiation of any study procedure. The study was registered at JapicCTI with the identifier JapicCTI‐090928.
The study consisted of a ‘run‐in’ period, followed by an 8‐week treatment period. Following the ‘run‐in’ period, which was intended to evaluate all patients for study eligibility and varied in length between 3 and 7 days to accommodate varying schedules, eligible subjects with EO were randomised at a ratio of 1:1:1:1:1 according to a computer‐generated randomisation schedule to receive an 8‐week treatment with one of the study drugs. The subjects were stratified by the baseline Los Angeles (LA) grades A/B or C/D, which has been widely used as an endoscopic grading system for oesophagitis,22 and were randomised to one of the study treatments. Five visits to the study site were required by all subjects for assessment: at the start of the ‘run‐in’ phase (visit 1), before initiating study drug administration (week 0; visit 2), at week 2 (visit 3), at week 4 (visit 4) and at week 8 (visit 5).
All subjects were screened for study entry within 4–8 days prior to the start of study drug administration (visit 1). After obtaining informed consent for study participation, demographic data, medical history as well as information on concomitant medications, concurrent medical conditions and pre‐treatment events were collected from all subjects. Examinations performed at visit 1 included a measurement of body weight, height and body mass index as well as an evaluation of physical health and vital signs. In addition, an electrocardiogram (ECG), clinical laboratory tests, a pregnancy test, a Helicobacter pylori immunoglobulin G antibody blood test, serum gastrin and pepsinogen I/II measurements, and endoscopy were performed for all subjects. Eligible subjects returned for final eligibility assessment (visit 2) 1 day prior to the start of the study treatment. Physical health, vital signs, pre‐treatment events and concomitant medications were assessed before being randomised to a study drug.
All subjects returned to the study site at weeks 2, 4 and 8 for physical examinations, vital sign assessments, clinical laboratory tests, blood sampling for pharmacokinetic measurements, serum gastrin and pepsinogen I/II measurements, a pregnancy test, and blood sampling for CYP2C19 genotyping (for consenting subjects at week 2 only) and pharmacogenomic analysis (for consenting subjects at week 4 only).
During these visits, all subjects were questioned about any adverse events experienced, their drug compliance status and concomitant medication use.
Endoscopic assessments were performed at weeks 2 and 4 in all subjects as well as at week 8 in subjects not healed at week 4. All endoscopic photographs were reviewed by the study's Central Adjudication Committee (CAC). The CAC was responsible for standardised reviews of endoscopic EO grading by the investigators.
Erosive oesophagitis‐related symptoms (heartburn, regurgitation, nausea, abdominal bloating, burping/belching, coughing, hoarseness, difficulty falling asleep, number of nocturnal awakenings, and feeling tired upon waking) were assessed for severity score (e.g. for heartburn, 0 – No symptom, 1 – Very mild, 2 – Mild, 3 – Moderate, 4 – Severe, 5 – Very severe) using a patient's diary that each patient filled out twice daily throughout the ‘run‐in’ period and the treatment period. Entries in the patient's diaries were confirmed at weeks 0, 2, 4 and 8.
The subjects were not allowed to concomitantly use any medication or therapy likely to influence the outcome of this clinical trial. These included: agents affecting digestive organs (PPIs, H2 receptor antagonists, muscarinic receptor 3 antagonists, gastrointestinal prokinetic agents, anti‐cholinergic agents, prostaglandins, antacids, anti‐gastrin agents, gastric mucosal protective agents), CYP3A4 inhibitors, CYP2C19 inhibitors, CYP inducers, medications contraindicated for use in combination with LPZ or similar agents (atazanavir), medications with reported drug–drug interactions with LPZ (warfarin and clopidogrel) and H. pylori eradication therapy.
Inclusion and exclusion criteria
Subjects eligible for the study had endoscopically confirmed EO (LA grades A to D) at visit 1, and were aged 20 years or older at the time of providing informed consent.
The main exclusion criteria were factors affecting efficacy and safety evaluation: (i) absence of endoscopically confirmed EO at visit 1; (ii) enrolment in a previous clinical trial using VPZ; (iii) presence of coexisting diseases of the oesophagus (e.g. eosinophilic oesophagitis, oesophageal varices, scleroderma, viral or fungal infection and oesophageal stenosis); (iv) a history of radiation therapy or cryotherapy to the oesophagus; (v) presence of caustic or physiochemical oesophageal trauma; (vi) a history of surgery or any treatment that could induce gastro‐oesophageal reflux or affect the stomach or duodenum (excluding endoscopic removal of benign polyps); (vii) presence of acute upper gastrointestinal haemorrhage or a gastric/duodenal ulcer (active stage A1/A2 or healing stage H2) within 30 days prior to visit 1 (with the exception of gastric or duodenal erosions); (viii) a history of Zollinger–Ellison syndrome or any of its complications or any other gastric acid hypersecretory disorder; (ix) a history of hypersensitivity or allergy to VPZ (or any of its pharmaceutical excipients) or PPIs; (x) presence of complications of any significant neurological, cardiovascular, pulmonary, hepatic, renal, metabolic, gastrointestinal, urological, endocrinological or haematological disorder; (xi) a history of malignancy (except for basal cell carcinoma of the skin) within 3 years prior to visit 1; (xii) treatment with any PPI within 14 days prior to visit 1 or any H2RA within 7 days prior to visit 1; or (xiii) abnormal laboratory test results at visit 1, i.e. (a) serum creatinine level >2 mg/dL, (b) alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level >2 × upper limit of normal (ULN), (c) ALT or AST level >1.5 × ULN, and total bilirubin level >2 mg/dL.
Treatment
The subjects were enrolled by the participating sites and randomised to receive VPZ 5, 10, 20 or 40 mg once daily (hereinafter VPZ5, VPZ10, VPZ20 and VPZ40 respectively) or LPZ 30 mg once daily (hereinafter LPZ30). Drugs were taken after breakfast for 8 weeks. The subjects were randomised 1 day prior to the first administration of the study drug. Study drug administration was initiated on the morning of day 1, and all subjects received two tablets (VPZ 5, 10, 20 mg, or placebo tablet) and one capsule (LPZ 30 mg or placebo capsule) according to the double dummy method.
Efficacy assessments
The primary efficacy endpoint was the proportion of healed EO subjects as shown by endoscopy at week 4. Week 4 was selected as the time point for primary evaluation as the onset of acid‐inhibitory effect of VPZ is faster than that of LPZ based on data from previous studies,20, 21 and was thus expected to achieve EO healing at an earlier time point, compared with the approved duration of LPZ treatment for EO in Japan (8 weeks). Endoscopic healing was defined as absence of endoscopically confirmed mucosal breaks on the basis of outcome reviews by the CAC. The secondary efficacy endpoint was the proportion of healed EO subjects as shown by endoscopy at weeks 2 and 8. Other efficacy endpoints were subjective symptoms associated with EO (e.g. heartburn and regurgitation) which were entered in the patient's diary. The plasma trough concentrations of VPZ and its major metabolites (M‐I, M‐II, M‐III and M‐IV‐Sul) were measured as pharmacokinetic parameters at weeks 2, 4 and 8 before study drug administration on that day.
Safety assessments
Safety and tolerability assessments were based on adverse events recorded at each study visit using standard medical terminology and terms in the Medical Dictionary for Regulatory Activities (MedDRA) Ver. 13.1, clinical laboratory test values, ECG findings and vital signs. Other measurements included serum gastrin and pepsinogen I/II levels.
Sample size and statistical analysis
Determination of sample size
The proportion of healed EO subjects at week 4 was assumed to be 91.5%, 91.5% and 90.5% for subjects receiving VPZ20, VPZ40 and LPZ30 respectively. On this basis, 138 subjects were required for each treatment group to achieve 80% joint power with a 10% non‐inferiority margin to demonstrate that both VPZ40 and VPZ20 were comparable to LPZ30. Therefore, after accounting for a predicted 5% drop‐out rate after randomisation, the sample size required for randomisation was determined to be 145 subjects for each treatment group, with a total of 725 study subjects.
Statistical and analytical plans
The full analysis set (FAS), which was used for the efficacy analysis, was defined as all subjects who were randomised and received at least one dose of the study drug. The safety analysis set (SAS) was defined as all subjects who received at least one dose of the study drug. The following is a summary description of the pre‐planned efficacy and the safety analyses.
The proportion of healed EO subjects at week 4 (primary endpoint) was calculated as the percentage of subjects with healed EO in the FAS in each treatment group. Point estimates and two‐sided 95% confidence intervals were calculated for each treatment group. The proportion of healed EO subjects at week 4 as assessed by CAC was stratified by the baseline LA grade. Each VPZ group was compared with the LPZ30 group by conducting a non‐inferiority Cochran–Mantel–Haenszel test23 adjusted for baseline LA grades A/B and C/D using a 10% non‐inferiority margin for the risk difference. The proportion of healed EO subjects at weeks 2 and 8 (secondary endpoints) were also summarised.
The adverse events reported were coded in accordance with the MedDRA system organ class (SOC) and descriptively summarised. The incidences of individual treatment‐emergent adverse events (TEAEs) were summarised in accordance with SOC and preferred term (PT) by treatment group. TEAEs were also summarised by their severity, time of onset and relationship with the study drug. TEAEs occurring in 2% or more of subjects in any treatment group were summarised by SOC/PT. In addition, TEAEs resulting in study drug discontinuation and serious TEAEs were summarised by treatment group.
Results
Subject disposition
A total of 733 subjects (mean age, 57.4 years; males/females, 551/182) were randomised to treatment, and of the 732 subjects who received treatment (VPZ or LPZ), 703 completed the study (Figure S1). One randomised subject did not receive the study drug because of a major protocol deviation (use of an excluded medication). Twenty‐nine subjects who received treatment prematurely discontinued their participation in the study (3, 5, 9, 7 and 5 subjects in the VPZ5, VPZ10, VPZ20, VPZ40 and LPZ30 groups respectively). The reasons for study drug discontinuation were adverse events (16 subjects), voluntary withdrawal (6 subjects), major protocol deviations (2 subjects), loss to follow‐up (1 subject), lack of efficacy (1 subject), and ‘others’ (3 subjects). The FAS comprised 731 subjects (148, 145, 154, 145 and 139 subjects respectively), and the SAS was consistent with the FAS. One subject who received treatment in the VPZ40 group was excluded from the FAS and the SAS due to a major GCP violation (conduct of an investigational procedure without a clinical contract).
There were no notable differences in baseline demographic characteristics among the treatment groups (Table 1). The distribution of baseline LA grades was similar among the treatment groups (grade A, 20.7–23.0%; B, 36.3–40.7%; C, 26.6–28.8%; and D, 3.4–8.8%).
Table 1.
Demographic and other baseline characteristics (randomised subjects)
| LPZ 30 mg | VPZ 5 mg | VPZ 10 mg | VPZ 20 mg | VPZ 40 mg | |
|---|---|---|---|---|---|
| Number of subjects | 140 | 148 | 145 | 154 | 146 |
| Age (years) | 55.8 ± 13.92 | 57.9 ± 12.96 | 57.3 ± 13.01 | 58.3 ± 13.86 | 57.6 ± 12.83 |
| Gender | |||||
| Male | 99 (70.7) | 110 (74.3) | 113 (77.9) | 115 (74.7) | 114 (78.1) |
| Female | 41 (29.3) | 38 (25.7) | 32 (22.1) | 39 (25.3) | 32 (21.9) |
| Height (cm) | 164.5 ± 9.80 | 163.8 ± 10.37 | 165.0 ± 9.92 | 163.8 ± 8.97 | 165.4 ± 9.32 |
| Weight (kg) | 67.08 ± 12.655 | 67.47 ± 12.751 | 67.62 ± 12.200 | 66.60 ± 13.511 | 67.67 ± 10.795 |
| Baseline LA grade | |||||
| O | 7 (5.0) | 5 (3.4) | 11 (7.6) | 9 (5.8) | 11 (7.5) |
| A | 29 (20.7) | 34 (23.0) | 32 (22.1) | 35 (22.7) | 31 (21.2) |
| B | 57 (40.7) | 54 (36.5) | 57 (39.3) | 59 (38.3) | 53 (36.3) |
| C | 38 (27.1) | 42 (28.4) | 39 (26.9) | 41 (26.6) | 42 (28.8) |
| D | 9 (6.4) | 13 (8.8) | 5 (3.4) | 9 (5.8) | 9 (6.2) |
| A/B | 86 (61.4) | 88 (59.5) | 89 (61.4) | 94 (61.0) | 84 (57.5) |
| C/D | 47 (33.6) | 55 (37.2) | 44 (30.3) | 50 (32.5) | 51 (34.9) |
| X* | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.6) | 0 (0.0) |
| Oesophageal hiatal hernia | |||||
| ≥2 cm | 33 (23.6) | 50 (33.8) | 49 (33.8) | 56 (36.4) | 49 (33.6) |
| <2 cm | 65 (46.4) | 53 (35.8) | 61 (42.1) | 56 (36.4) | 66 (45.2) |
| No | 42 (30.0) | 45 (30.4) | 35 (24.1) | 42 (27.3) | 31 (21.2) |
| Helicobacter pylori status | |||||
| Positive | 16 (11.4) | 25 (16.9) | 18 (12.4) | 23 (14.9) | 18 (12.3) |
| Negative | 124 (88.6) | 123 (83.1) | 127 (87.6) | 131 (85.1) | 128 (87.7) |
| CYP2C19 genotyping | |||||
| EM† | 112 (83.6) | 118 (83.1) | 127 (91.4) | 118 (78.1) | 114 (82.0) |
| PM‡ | 22 (16.4) | 24 (16.9) | 12 (8.6) | 33 (21.9) | 25 (18.0) |
Data are represented as mean ± s.d. or number of subjects with percentages in parentheses.
LPZ, lansoprazole; VPZ, vonoprazan.
*Unable to judge; †extensive metabolisers; ‡poor metabolisers.
Efficacy analysis
The proportion of subjects who reached the primary endpoint, ‘endoscopic EO healing at week 4’, was 92.3%, 92.5%, 94.4%, 97.0% and 93.2% in the VPZ5, VPZ10, VPZ20, VPZ40 and LPZ30 groups respectively (Table 2). Each VPZ dose was shown to be non‐inferior to LPZ30 in all FAS populations adjusted for baseline LA grades A/B and C/D (P = 0.0026, 0.0038, 0.0006 and <0.0001 respectively; Table 3). There was no statistically significant difference between each VPZ group and the LPZ group. Among the subjects with baseline LA grades A/B, the proportion of healed EO subjects at week 4 was 95.5%, 95.5%, 91.5%, 97.6% and 96.5% respectively. Among the more severe C/D subjects, the corresponding proportions were 87.3%, 86.4%, 100%, 96.0% and 87.0% respectively. Among those with baseline LA grades C/D, the proportions were numerically higher in the VPZ20 and VPZ40 groups compared with the LPZ30 group. The point estimates for the differences were 13.0% and 9.0% respectively.
Table 2.
Proportion of healed EO subjects as shown by endoscopy at week 4 as assessed by Central Adjudication Committee (full analysis set)
| LA grade | LPZ 30 mg | VPZ | |||
|---|---|---|---|---|---|
| 5 mg | 10 mg | 20 mg | 40 mg | ||
| Overall | 123/132 (93.2) | 132/143 (92.3) | 123/133 (92.5) | 136/144 (94.4) | 130/134 (97.0) |
| A/B | 83/86 (96.5) | 84/88 (95.5) | 85/89 (95.5) | 86/94 (91.5) | 82/84 (97.6) |
| C/D | 40/46 (87.0) | 48/55 (87.3) | 38/44 (86.4) | 50/50 (100) | 48/50 (96.0) |
Data are represented as number of subjects with percentages in parentheses. Subjects who had no mucosal breaks or who were not evaluable on the basis of reviews by the CAC at baseline were excluded from the analysis.
LPZ, lansoprazole; VPZ, vonoprazan.
Table 3.
Non‐inferiority Cochran–Mantel–Haenszel test for VPZ 5–40 mg to LPZ 30 mg at week 4 after adjustment for baseline LA grades A/B and C/D
| Non‐inferiority Cochran–Mantel–Haenszel test | ||
|---|---|---|
| Z‐value | P‐value | |
| 5 mg VPZ–30 mg LPZ | 2.7919 | 0.0026 |
| 10 mg VPZ–30 mg LPZ | 2.6664 | 0.0038 |
| 20 mg VPZ–30 mg LPZ | 3.2617 | 0.0006 |
| 40 mg VPZ–30 mg LPZ | 4.1122 | <0.0001 |
LPZ, lansoprazole; VPZ, vonoprazan.
At week 2, the proportions of healed EO subjects were numerically higher in the VPZ10, VPZ20 and VPZ40 groups (93.2%, 93.8% and 94.8% respectively) compared with the LPZ30 (88.6%) and VPZ5 (86.0%) groups (Table 4a). This trend was more notable among those with baseline LA grades C/D, with the proportions of healed EO subjects at week 2 tending to be higher in the VPZ10, VPZ20 and VPZ40 groups (88.6%, 96.0% and 96.0% respectively) compared with the LPZ30 (82.6%) and VPZ5 (78.2%) groups.
Table 4.
Proportion of healed EO subjects as shown by endoscopy at week 2 (a) and week 8 (b) as assessed by Central Adjudication Committee (full analysis set)
| LA grade | LPZ 30 mg | VPZ | |||
|---|---|---|---|---|---|
| 5 mg | 10 mg | 20 mg | 40 mg | ||
| (a) | |||||
| Overall | 117/132 (88.6) | 123/143 (86.0) | 124/133 (93.2) | 135/144 (93.8) | 127/134 (94.8) |
| A/B | 79/86 (91.9) | 80/88 (90.9) | 85/89 (95.5) | 87/94 (92.6) | 79/84 (94.0) |
| C/D | 38/46 (82.6) | 43/55 (78.2) | 39/44 (88.6) | 48/50 (96.0) | 48/50 (96.0) |
| (b) | |||||
| Overall | 126/132 (95.5) | 138/143 (96.5) | 127/133 (95.5) | 139/144 (96.5) | 130/134 (97.0) |
| A/B | 83/86 (96.5) | 86/88 (97.7) | 86/89 (96.6) | 89/94 (94.7) | 82/84 (97.6) |
| C/D | 43/46 (93.5) | 52/55 (94.5) | 41/44 (93.2) | 50/50 (100) | 48/50 (96.0) |
Data are represented as number of subjects with percentages in parentheses. Subjects who had no mucosal breaks or who were not evaluable on the basis of reviews by the CAC at baseline were excluded from the analysis. LPZ, lansoprazole; VPZ, vonoprazan.
In contrast, at week 8, the proportions of healed EO subjects were similar between either VPZ group and the LPZ30 group among those with LA grades A/B (94.7–97.7% vs. 96.5%) and among those with LA grades C/D (93.2–100% vs. 93.5%) (Table 4b).
Assessment of the mean severity score of all EO‐related symptoms on the basis of entries in the patient's diaries revealed a decreasing trend in all treatment groups but no significant differences between the groups (Table 5). The proportion of subjects without EO‐related symptoms increased in all treatment groups, with no significant differences observed between the treatment groups.
Table 5.
Mean severity score of heartburn symptoms
| LPZ 30 mg | VPZ 5 mg | VPZ 10 mg | VPZ 20 mg | VPZ 40 mg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | n | Mean (s.d.) | n | Mean (s.d.) | n | Mean (s.d.) | |
| Daytime | ||||||||||
| Baseline | 140 | 1.285 (1.0502) | 148 | 1.345 (0.9908) | 145 | 1.254 (0.9520) | 154 | 1.203 (1.0446) | 146 | 1.295 (0.9868) |
| Days 29–56 | 109 | 0.291 (0.4856) | 130 | 0.286 (0.5334) | 123 | 0.259 (0.4300) | 121 | 0.210 (0.3938) | 118 | 0.213 (0.3939) |
| Night‐time | ||||||||||
| Baseline | 140 | 1.047 (0.9334) | 148 | 1.015 (0.9004) | 145 | 1.030 (0.9200) | 154 | 0.887 (0.8933) | 146 | 1.087 (0.9568) |
| Days 29–56 | 102 | 0.237 (0.4241) | 119 | 0.280 (0.5179) | 107 | 0.256 (0.4252) | 106 | 0.213 (0.5093) | 117 | 0.187 (0.3588) |
LPZ, lansoprazole; VPZ, vonoprazan.
Mean plasma concentrations of VPZ and its major metabolites (M‐I, M‐II, M‐III and M‐IV‐Sul) increased with dose and were almost constant within each dose group throughout the treatment period.
Safety analysis
Of the 731 subjects, 310 (42.4%) experienced TEAEs. Of these, 53 (7.3%) experienced TEAEs that were thought to be related to the study drug. The incidence of TEAEs was similar among the treatment groups (Table 6). Nasopharyngitis was the most commonly reported TEAE in all treatment groups. The incidence of diarrhoea was slightly higher in subjects administered VPZ ≥10 mg; however, most of these cases were assessed and determined by the investigators to be unrelated to the study drug. Serious adverse events were reported in seven subjects, all of which were determined by the investigators to be unrelated to the study drug (Table 7). Serum gastrin levels increased following administration of the study drug in all treatment groups, and were significantly higher in subjects receiving VPZ ≥10 mg than in those receiving LPZ30 at weeks 2, 4 and 8 (Figure 1a). For those receiving VPZ5 and those receiving LPZ30, serum gastrin levels were comparable at all assessed time points. Serum gastrin levels increased most rapidly over the first 2 weeks of the study treatment and more slowly thereafter. Pepsinogen I and II levels also increased in all treatment groups. As with serum gastrin, both pepsinogen I and II levels were significantly higher in subjects receiving VPZ ≥10 mg compared with those receiving LPZ30 at weeks 2, 4 and 8 (Figure 1b,c), while they were comparable between those receiving VPZ5 and those receiving LPZ30 at all assessed time points. No clinically significant changes in the other laboratory test values, including liver function tests, were observed during the study.
Table 6.
Summary of treatment‐emergent adverse events (safety analysis set)
| LPZ 30 mg (n = 139) | VPZ | ||||
|---|---|---|---|---|---|
| 5 mg (n = 148) | 10 mg (n = 145) | 20 mg (n = 154) | 40 mg (n = 145) | ||
| TEAEs | 61 (43.9) | 59 (39.9) | 62 (42.8) | 73 (47.4) | 55 (37.9) |
| Drug‐related TEAEs | 8 (5.8) | 9 (6.1) | 13 (9.0) | 16 (10.4) | 7 (4.8) |
| TEAEs leading to discontinuation | 4 (2.9) | 1 (0.7) | 5 (3.4) | 11 (7.1) | 2 (1.4) |
| Serious AEs | 1 (0.7) | 1 (0.7) | – | 3 (1.9) | 2 (1.4) |
| TEAEs reported by ≥2% of subjects in any treatment group by preferred term | |||||
| Nasopharyngitis | 14 (10.1) | 12 (8.1) | 15 (10.3) | 15 (9.7) | 12 (8.3) |
| Diarrhoea | 1 (0.7) | 2 (1.4) | 4 (2.8) | 4 (2.6) | 5 (3.4) |
| Constipation | 2 (1.4) | – | 2 (1.4) | 5 (3.2) | 1 (0.7) |
| Abdominal pain upper | 3 (2.2) | 2 (1.4) | 2 (1.4) | 1 (0.6) | – |
| Seasonal allergy | 3 (2.2) | 2 (1.4) | – | 2 (1.3) | 2 (1.4) |
| Pharyngitis | 2 (1.4) | 1 (0.7) | 2 (1.4) | 3 (1.9) | 3 (2.1) |
| Gastroenteritis | 4 (2.9) | – | 3 (2.1) | 1 (0.6) | 1 (0.7) |
| Blood triglycerides increased | 3 (2.2) | 4 (2.7) | 3 (2.1) | 4 (2.6) | 3 (2.1) |
| Blood creatine phosphokinase increased | 2 (1.4) | 3 (2.0) | – | 3 (1.9) | 4 (2.8) |
| Eosinophil count increased | 1 (0.7) | 1 (0.7) | 4 (2.8) | 1 (0.6) | 3 (2.1) |
| Blood glucose increased | 4 (2.9) | 1 (0.7) | 2 (1.4) | 1 (0.6) | 1 (0.7) |
| Blood uric acid increased | 3 (2.2) | 1 (0.7) | 3 (2.1) | – | 1 (0.7) |
| Protein urine present | – | – | 3 (2.1) | – | – |
| Upper respiratory tract inflammation | 3 (2.2) | 1 (0.7) | 3 (2.1) | 1 (0.6) | 1 (0.7) |
Data are represented as number of subjects with percentages in parentheses. TEAEs, treatment‐emergent adverse events; LPZ, lansoprazole; VPZ, vonoprazan.
Table 7.
Serious treatment‐emergent adverse events by preferred term (safety analysis set)
| LPZ 30 mg (n = 139) | VPZ | ||||
|---|---|---|---|---|---|
| 5 mg (n = 148) | 10 mg (n = 145) | 20 mg (n = 154) | 40 mg (n = 145) | ||
| Number of subjects | 1 (0.7) | 1 (0.7) | – | 3 (1.9) | 2 (1.4) |
| Number of events | 2 | 1 | – | 3 | 2 |
| Coronary artery stenosis | – | – | – | – | 1 (0.7) |
| Colonic polyp | – | 1 (0.7) | – | – | – |
| Urinary tract infection | – | – | – | 1 (0.6) | – |
| Enterocolitis bacterial | – | – | – | – | 1 (0.7) |
| Subdural haematoma | 1 (0.7) | – | – | – | – |
| Brain contusion | 1 (0.7) | – | – | – | – |
| Putamen haemorrhage | – | – | – | 1 (0.6) | – |
| Arteriosclerosis obliterans | – | – | – | 1 (0.6) | – |
Data are represented as number of subjects with percentages in parentheses. LPZ, lansoprazole; VPZ, vonoprazan.
Figure 1.
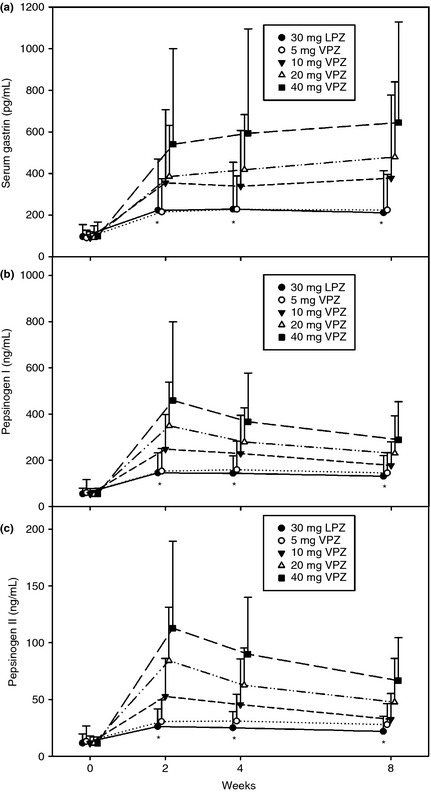
Time course of arithmetic mean serum gastrin concentration (a), and pepsinogen I and II concentrations (b, c) (SAS). LPZ, lansoprazole; VPZ, vonoprazan. *P < 0.0001 for VPZ 10, 20 and 40 mg vs. LPZ 30 mg. Each bar shows the standard deviation.
Discussion
In this double‐blind, parallel‐group, multicentre, dose‐ranging study performed in Japan, the safety profile and dose–response of VPZ were evaluated in patients with EO at doses of 5, 10, 20 and 40 mg once daily for 8 weeks and compared with those receiving LPZ 30 mg once daily for 8 weeks. The proportion of healed EO subjects in each group was evaluated at week 4 (the primary endpoint) as well as at weeks 2 and 8 (secondary endpoints). In addition to these efficacy endpoints, VPZ and LPZ were compared for their effects on EO‐related symptoms on the basis of patient diary entries made twice daily. Given that those with LA grades C/D are assumed to be more refractory to treatment than those with LA grades A/B, it was ensured that the distribution of baseline LA grades was balanced among the treatment groups, so that the differences in distribution would not affect the efficacy evaluations. Indeed, those with baseline LA grades A/B and C/D were almost equally distributed among the treatment groups (A/B, 57.5–61.4%; C/D, 30.3–37.2%).
The PPI, LPZ 30 mg was chosen as the active comparator against VPZ because PPIs remain the standard for EO treatment and the use of placebo was considered clinically inappropriate and ethically unacceptable. In previous phase I studies in healthy volunteers,20, 21 VPZ was shown to suppress gastric acid levels with once‐daily dosing to levels which would likely result in EO healing, while LPZ is approved for clinical use at a dose of 30 mg once daily for the treatment of EO. Therefore, once‐daily VPZ and once‐daily LPZ 30 mg were compared for their efficacy in treating EO.
A 4‐week course of daily VPZ was shown to be non‐inferior at all doses tested to LPZ 30 mg for the treatment of EO on the basis of outcome analyses adjusted for baseline LA grades. Among those with severe grades C/D, the proportion of healed EO subjects was numerically higher after 4‐week treatment with VPZ20 (100%) or VPZ40 (96.0%) than after 4‐week treatment with VPZ5 (87.3%), VPZ10 (86.4%) or LPZ30 (87.0%), although these differences did not reach statistical significance. This suggests that the clinical benefits of VPZ may be greater when administered at higher doses to patients with more severe disease. Furthermore, the proportion of healed EO subjects was numerically higher at week 2 in those administered VPZ ≥10 mg (93.2–94.8%) than in those administered LPZ30 (88.6%), although the differences among the treatment groups did not reach statistical significance. Similarly, the proportion of healed EO subjects was higher at week 2 among those with baseline LA grades C/D administered VPZ20 (96.0%) or VPZ40 (96.0%), compared with those administered LPZ30 (82.6%), although there were no significant differences among the treatment groups.
These data are comparable to those from a systematic review of eight randomised comparative trials of PPIs,24 where the proportion of healed EO patients ranged from 47.5% to 81.7% at week 4 and from 77.5% to 95.5% at week 8. Our efficacy findings are also supported by the results of two earlier phase I studies in healthy volunteers20, 21 that demonstrated the rapid, potent and sustained acid‐inhibitory effect of VPZ as measured by the 24 h percentages of time with intragastric pH > 4 on days 1 and 7 (85.3% and 100.0%) and the percentages of time with pH > 4 at night on days 1 and 7 (86.5% and 100.0%) for the 40 mg daily dose. These data are in agreement with the observation that endoscopic healing of EO is achieved when the 24 h percentage of time with intragastric pH > 4 is sustained over a period of 16 h/day or more (67% of the time).25, 26, 27 Notably, the proportion of subjects healed with VPZ ≥10 mg at week 2 (93.2–94.8%) suggests that the rapid onset of VPZ effects may indeed translate into a shorter healing time in patients with EO, particularly in those with more severe LA grades C/D.
In Japan, the prevalence of EO has increased in the last few decades because of the westernisation of lifestyle, ageing of society and decreasing incidence of H. pylori infection.28 Moreover, the proportion of EO patients healed with PPIs is significantly lower among those with more severe baseline EO grades.29 Against this background, our study findings suggest that VPZ may offer an advantage over PPIs with respect to EO healing, particularly in patients with severe baseline EO (LA grades C/D), while long‐term studies are required to confirm these findings.
Eight‐week treatment with VPZ was safe and well tolerated at all doses studied, and the incidence and nature of the adverse events observed with VPZ were similar to those observed with LPZ30. In addition, no liver function abnormalities were noted with VPZ, unlike an earlier P‐CAB prototype, AZD0865 which contained an imidazopyridine ring structure.19
Serum gastrin levels increased in a dose‐dependent manner for VPZ, and the increase with VPZ5 was similar to that with LPZ30, whereas the gastrin level following VPZ20 treatment exceeded twice that following LPZ30 treatment. These increases may result from a feedback mechanism, whereby gastrin‐producing cells increase gastrin secretion in response to elevated intragastric pH, as predicted from the potent pharmacodynamic effect of VPZ on gastric acid secretion.21 While this may raise concern over the implications of hypergastrinaemia, particularly the potential development of gastric carcinoids, with long‐term use of VPZ (gastric carcinoids are variably reported to occur30 or not to occur31 with the long‐term use of PPIs), it is assumed in the literature that gastric carcinoids are not induced by hypergastrinaemia alone and that some other factors, such as MEN‐1 gene deletions, may be required for their development.32 In addition, pepsinogen I/II levels were increased in a VPZ dose‐dependent manner, with these increases being significantly larger with VPZ10, VPZ20 or VPZ40 compared to LPZ30. However, given that increases in gastrin and pepsinogen I/II levels are also reported with acid suppression with PPIs,33 the clinical implications of these increases with VPZ remain to be explored.
The limitation of this study was, therefore, that the duration of the study (8‐week treatment) was too short to explore the clinical implications of the gastrin and pepsinogen increases with VPZ, and long‐term studies are required to evaluate the effect of elevated gastrin and pepsinogen levels, including their histopathological effects on the gastric mucosa, in patients receiving VPZ.
Conclusions
Once‐daily VPZ was effective at all doses studied and non‐inferior to once‐daily LPZ 30 mg for healing EO. In addition, VPZ 20 mg or higher was efficacious for EO healing at weeks 2 and 4 for subjects with severe EO at baseline (LA grades C/D). There was no safety concern associated with VPZ during this 8‐week study, while there was a dose‐dependent increase in serum gastrin and pepsinogens. VPZ 20 mg once daily was determined to be the recommended clinical dose for the treatment of EO on the basis of efficacy and safety assessments performed at week 4.
Authorship
Guarantor of the article: Kiyoshi Ashida.
Author contributions: Kiyoshi Ashida, Tsutomu Chiba, Yuuichi Sakurai and Akira Nishimura were involved in study conception and design. Tsutomu Chiba served as the coordinating investigator. Naoki Hiramatsu served as Medical Expert. Eiji Umegaki, Katsuhiko Iwakiri and Kiyoshi Ashida served as the Central Adjudication Committee. Kentarou Kudou conducted statistical analysis. All authors were involved in the drafting and critical revision of the manuscript. All authors approved the final version of the manuscript, including the authorship list.
Supporting information
Figure S1. Disposition of subjects. LPZ, lansoprazole; VPZ, vonoprazan.
Acknowledgements
Declaration of personal interests: Kiyoshi Ashida, Naoki Hiramatsu, Eiji Umegaki, Katsuhiko Iwakiri and Tsutomu Chiba are all paid consultants to Takeda Pharmaceutical Company Ltd. Yuichi Sakurai, Akira Nishimura and Kentarou Kudou are employees of Takeda Pharmaceutical Company Ltd.
Declaration of funding interests: This study (TAK‐438/CCT‐001) was funded in full by Takeda Pharmaceutical Company Ltd. Writing support was provided by Hiroaki Itoh of Interface, Kanagawa, Japan, and was funded by Takeda Pharmaceutical Company Ltd, Japan. The authors thank Richard Jenkins, Nigel Brayshaw and Göran Hasselgren from Takeda Development Centre, Europe, and Caterina Hatzifoti on behalf of Takeda Pharmaceuticals International GmbH for reviewing the manuscript.
This article was accepted for publication after full peer‐review.
References
- 1. Chiba N, De Gara CJ, Wilkinson JM, et al Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta‐analysis. Gastroenterology 1997; 112: 1798–810. [DOI] [PubMed] [Google Scholar]
- 2. van Pinxteren B, Numans ME, Lau J, et al Short‐term treatment of gastroesophageal reflux disease. J Gen Intern Med 2003; 18: 755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards SJ, Lind T, Lundell L, et al Systematic review: standard‐ and double‐dose proton pump inhibitors for the healing of severe erosive oesophagitis – a mixed treatment comparison of randomized controlled trials. Aliment Pharmacol Ther 2009; 30: 547–56. [DOI] [PubMed] [Google Scholar]
- 4. Fennerty MB, Johanson JF, Hwang C, et al Efficacy of esomeprazole 40 mg vs. lansoprazole 30 mg for healing moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2005; 21: 455–63. [DOI] [PubMed] [Google Scholar]
- 5. Vakil NB, Shaker R, Johnson DA, et al The new proton pump inhibitor esomeprazole is effective as a maintenance therapy in GERD patients with healed erosive oesophagitis: a 6‐month, randomized, double‐blind, placebo‐controlled study of efficacy and safety. Aliment Pharmacol Ther 2001; 15: 927–35. [DOI] [PubMed] [Google Scholar]
- 6. Sachs G, Shin JM, Hunt R. Novel approaches to inhibition of gastric acid secretion. Curr Gastroenterol Rep 2010; 12: 437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katz PO, Castell DO, Chen Y, et al Intragastric acid suppression and pharmacokinetics of twice‐daily esomeprazole: a randomized, three‐way crossover study. Aliment Pharmacol Ther 2004; 20: 399–406. [DOI] [PubMed] [Google Scholar]
- 8. Johnson DA, Katz PO. Nocturnal gastroesophageal reflux disease: issues, implications, and management strategies. Rev Gastroenterol Disord 2008; 8: 98–108. [PubMed] [Google Scholar]
- 9. Chong E, Ensom MHH. Pharmacogenetics of the proton pump inhibitors: a systematic review. Pharmacotherapy 2003; 23: 460–71. [DOI] [PubMed] [Google Scholar]
- 10. Furuta T, Shirai N, Sugimoto M, et al Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor‐based therapies. Drug Metab Pharmacokinet 2005; 20: 153–67. [DOI] [PubMed] [Google Scholar]
- 11. Andersson K, Carlsson E. Potassium‐competitive acid blockade: a new therapeutic strategy in acid‐related diseases. Pharmacol Ther 2005; 108: 294–307. [DOI] [PubMed] [Google Scholar]
- 12. Cederberg C, Lind T, Röhss K, et al Comparison of once‐daily intravenous and oral omeprazole on pentagastrin‐stimulated acid secretion in duodenal ulcer patients. Digestion 1992; 53: 171–8. [DOI] [PubMed] [Google Scholar]
- 13. Dammann HG, Burkhardt F. Pantoprazole versus omeprazole: influence on meal‐stimulated gastric acid secretion. Eur J Gastroenterol Hepatol 1999; 11: 1277–82. [PubMed] [Google Scholar]
- 14. Arikawa Y, Nishida H, Kurasawa O, et al Discovery of a novel pyrrole derivative 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methymethanamine fumarate (TAK‐438) as a potassium‐competitive acid blocker (P‐CAB). J Med Chem 2012; 55: 4446–56. [DOI] [PubMed] [Google Scholar]
- 15. Shin JM, Inatomi N, Munson K, et al Characterization of a novel potassium‐competitive acid blocker of the gastric H,K‐ATPase, 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol 3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438). J Pharmacol Exp Ther 2011; 339: 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsukawa J, Hori Y, Nishida H, et al A comparative study on the modes of action of TAK‐438, a novel potassium‐competitive acid blocker, and lansoprazole in primary cultured rabbit gastric glands. Biochem Pharmacol 2011; 81: 1145–51. [DOI] [PubMed] [Google Scholar]
- 17. Hori Y, Matsukawa J, Tekeuchi T, et al A study comparing the antisecretory effect of TAK‐438, a novel potassium‐competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther 2011; 337: 797–804. [DOI] [PubMed] [Google Scholar]
- 18. Hori Y, Imanishi A, Matsukawa J, et al 1‐[5‐(2‐fluorophenyl)‐1‐(pyridin‐3‐ylsulfonyl)‐1H‐pyrrol‐3‐yl]‐N‐methylmethanamine monofumarate (TAK‐438), a novel and potent potassium‐competitive acid blocker for the treatment of acid‐related diseases. J Pharmacol Exp Ther 2010; 335: 231–8. [DOI] [PubMed] [Google Scholar]
- 19. Dent J, Kahrilas PJ, Hatlebakk J, et al A randomized, comparative trial of a potassium‐competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol 2008; 103: 20–6. [DOI] [PubMed] [Google Scholar]
- 20. Sakurai Y, Nishimura A, Kennedy G, et al Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK‐438 (Vonoprazan) doses in healthy male Japanese/non‐Japanese subjects. Clin Transl Gastroenterol 2015; 6: e94. doi:10.1038/ctg.2015.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jenkins H, Sakurai Y, Nishimura A, et al Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK‐438 (vonoprazan), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther 2015; 41: 636–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Armstrong D, Bennett JR, Blum AL, et al The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996; 111: 85–92. [DOI] [PubMed] [Google Scholar]
- 23. Yanagawa T, Tango T, Hiejima Y. Mantel‐Haenszel‐type tests for testing equivalence or more than equivalence in comparative clinical trial. Biometrics 1994; 50: 859–64. [PubMed] [Google Scholar]
- 24. Kalaitzakis E, Björnsson E. A review of esomeprazole in the treatment of gastroesophageal reflux disease (GERD). Ther Clin Risk Manag 2007; 3: 653–63. [PMC free article] [PubMed] [Google Scholar]
- 25. Yu KS, Bae KS, Shon JH, et al Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol 2004; 44: 73–82. [DOI] [PubMed] [Google Scholar]
- 26. Kahrilas PJ, Dent J, Luaritsen K, et al A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol 2007; 5: 1385–91. [DOI] [PubMed] [Google Scholar]
- 27. Bell NJV, Burget D, Howden CW, et al Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion 1992; 51(Suppl. 1): 59–67. [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol 2009; 44: 518–34. [DOI] [PubMed] [Google Scholar]
- 29. Higuchi K, Joh T, Nakada K, et al Is proton pump inhibitor therapy for reflux esophagitis sufficient?: a large real‐world survey of Japanese patients. Intern Med 2013; 52: 1447–54. [DOI] [PubMed] [Google Scholar]
- 30. Jianu CS, Fossmark R, Viset T, et al Gastric carcinoids after long‐term use of a proton pump inhibitor. Aliment Pharmacol Ther 2012; 36: 644–9. [DOI] [PubMed] [Google Scholar]
- 31. Thomson AB, Sauve MD, Kassam N, et al Safety of the long‐term use of proton pump inhibitors. World J Gastroenterol 2010; 16: 2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bordi C. Neuroendocrine pathology of the stomach: the Parma contribution. Endocr Pathol 2014; 25: 171–80. [DOI] [PubMed] [Google Scholar]
- 33. Agréus L, Storskrub T, Aro P, et al Clinical use of proton‐pump inhibitors but not H2‐blockers or antacid/alginates raises the serum levels of amidated gastrin‐17, pepsinogen I and pepsinogen II in a random adult population. Scand J Gastroenterol 2009; 44: 564–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Disposition of subjects. LPZ, lansoprazole; VPZ, vonoprazan.


