Highlight
We identified different expression levels of FT5a, an ortholog of FLOWERING LOCUS T, as the molecular basis of a quantitative trait locus that promotes flowering under long-day conditions in soybean.
Key words: FLOWERING LOCUS T, flowering time, near-isogenic line, photoperiod sensitivity, quantitative trait locus, SNP calling, soybean.
Abstract
FLOWERING LOCUS T (FT) is an important floral integrator whose functions are conserved across plant species. In soybean, two orthologs, FT2a and FT5a, play a major role in initiating flowering. Their expression in response to different photoperiods is controlled by allelic combinations at the maturity loci E1 to E4, generating variation in flowering time among cultivars. We determined the molecular basis of a quantitative trait locus (QTL) for flowering time in linkage group J (Chromosome 16). Fine-mapping delimited the QTL to a genomic region of 107kb that harbors FT5a. We detected 15 DNA polymorphisms between parents with the early-flowering (ef) and late-flowering (lf) alleles in the promoter region, an intron, and the 3′ untranslated region of FT5a, although the FT5a coding regions were identical. Transcript abundance of FT5a was higher in near-isogenic lines for ef than in those for lf, suggesting that different transcriptional activities or mRNA stability caused the flowering time difference. Single-nucleotide polymorphism (SNP) calling from re-sequencing data for 439 cultivated and wild soybean accessions indicated that ef is a rare haplotype that is distinct from common haplotypes including lf. The ef allele at FT5a may play an adaptive role at latitudes where early flowering is desirable.
Introduction
Time to flowering and maturation influences the productivity, adaptability, and quality of seed crops. Flowering time is determined by the integration of signals from external stimuli (such as photoperiod and temperature) and internal conditions (such as plant age and the amount of gibberellic acid), which converge on the regulation of FLOWERING LOCUS T (FT), a long-sought systemic floral inducer (Song et al., 2013). Responses of flowering to photoperiod may be one of the major determinants of adaptation to different daylengths, in particular for plants in temperate zones.
Soybean [Glycine max (L.) Merr.], a facultative short-day (SD) plant, is cultivated in a broad range of latitudes, although each cultivar is grown in a very narrow latitude range (Watanabe et al., 2012). This wide adaptability to growing seasons and regions is generated by genetic diversity in flowering responses to various external and internal signals. Ten major genes, E1 to E9 and J, and a number of quantitative trait loci (QTLs) have been reported to be involved in the control of flowering in soybean. The molecular bases of E1–E4 and E9 are known, and their functions in the photoperiod responses of flowering have been characterized (Liu et al., 2008; Watanabe et al., 2009, 2011; Xia et al., 2012; Xu et al., 2015; Zhai et al., 2015; Zhao et al., 2016). E1 encodes a putative transcription factor with a bipartite nuclear localization signal and a region distantly related to the B3 DNA-binding domain; E1 suppresses the expression of the soybean FT orthologs FT2a and FT5a (Xia et al., 2012). Night-break (NB) experiments and experiments with transitions between light and dark phases have revealed that the induction of E1 expression requires light given at the right time in the circadian rhythm (Xu et al., 2015). This light-regulated E1 expression is mediated by two phytochrome A (PHYA) proteins, E3 (GmPHYA3) and E4 (GmPHYA2) (Liu et al., 2008; Watanabe et al., 2009; Xia et al., 2012). In plants with the double-recessive e3/e4 genotype, E1 expression is not induced even under long-day (LD) conditions, resulting in the up-regulation of FT2a and FT5a expression, which induces flowering (Xia et al., 2012). The E2 gene is an ortholog of Arabidopsis GIGANTEA (GI) (Watanabe et al., 2011). GI is a nuclear-localized membrane protein, which interacts with FLAVIN-BINDING, KELCH-REPEAT, F-BOX 1 (FKF1) to up-regulate the expression of CONSTANS (CO) through degradation of CYCLING DOF FACTOR (CDF) (Huq et al., 2000), and which also activates FT expression by directly binding to a cis-element (Sawa et al., 2007). Unlike E1, E2 is not involved in NB responses of soybean (Xu et al., 2015), and therefore E1 and E2 appear to control flowering time via different pathways. Recently, the maturity gene E9 was identified as FT2a; its late-flowering e9 allele has a Ty1/copia-like retrotransposon inserted in the first intron, which attenuates transcript abundance (Zhao et al., 2016).
Different allelic combinations at the above five loci (E1–E4 and E9) produce diverse flowering habits in soybean cultivars (Xu et al., 2013; Kong et al., 2014; Tsubokura et al., 2014; Zhao et al., 2016). Using regression analyses, Tsubokura et al. (2014) found that multi-locus genotypes at E1 to E4 account for 62–66% of natural variation in flowering time among (mainly Japanese) soybean cultivars. Various allelic combinations at the E1, E3, and E4 loci control the absence of or reduced photoperiod sensitivity, which is essential for adaptation to high latitudes, although this trait is also affected by an unknown gene(s) (Xu et al., 2013). Genotyping with functional DNA markers for identified maturity loci has improved our understanding of the relationship between maturity genotypes and flowering habits in various regions at different latitudes and has also uncovered novel genetic variations that affect flowering (Xu et al., 2013; Kong et al., 2014; Tsubokura et al., 2014; Lu et al., 2016).
Many QTLs controlling time to flowering have been reported in soybean (Keim et al., 1990; Mansur et al., 1993; Lee et al., 1996; Orf et al., 1999; Tasma et al., 2001; Yamanaka et al., 2001; Chapman et al., 2003; Wang et al., 2004; Watanabe et al., 2004; Zhang et al., 2004; Funatsuki et al., 2005; Pooprompan et al., 2006; Githiri et al., 2007; Komatsu et al., 2007; Liu et al., 2007; Khan et al., 2008; Liu and Abe, 2010; Cheng et al., 2011; Liu et al., 2011; Yamaguchi et al., 2014; Lu et al., 2016). The molecular dissection of QTLs whose functions remain undetermined is important for better understanding of the molecular mechanisms underlying natural variations of flowering time in soybean, and also for marker-assisted breeding for flowering time.
Here we describe the molecular dissection of a QTL for flowering time detected in two independent crosses between early-maturing soybean cultivars. Fine-mapping and subsequent sequencing and expression analyses have identified FT5a as a gene responsible for this QTL.
Materials and methods
Plant material
Segregating populations of two soybean crosses, Toyoharuka (TH) × 1532-1 (cross A), and a near-isogenic line (NIL) of Harosoy for e3 (H-e3, PI547716) × Jiagedaqi-02 (J02) (cross B), were used in this study. TH and 1532-1 have the same maturity genotype at the E2, E3, and E9 loci (e2/E3/e9), but differ at the E1 and E4 loci: TH has the e1-nl allele, which lacks the entire E1 genomic region, and the e4 allele, whereas 1532-1 has functional E1 and E4 alleles (Yamaguchi et al., 2014). J02 is a breeding line developed at the Agricultural Experimental Station at Jiagedaqi, Heilongjiang Province, China. It has the same maturity genotype as H-e3 at all of the five maturity loci (e1-as/e2/e3/E4/E9), but unlike H-e3 it exhibits a reduced photoperiod sensitivity of flowering under incandescent LD conditions (ILD; Saindon et al., 1989), where natural daylength is extended to 20h by using incandescent lamps with low R:FR ratios (Xu et al., 2013). Recombinant inbred lines (RILs) for each of the two crosses were developed with the single seed descent method. The RIL population consisted of 99 lines homozygous for e1-nl and 62 lines homozygous for E1 in cross A and 79 lines in cross B. NILs for the early-flowering (ef) and late-flowering (lf) alleles at a QTL were developed from the progenies of F6 or F7 plants used for fine-mapping in cross A (NILs #46 and #64) and from the progeny of an F6 plant in cross B (NIL #8).
Field experiment
Flowering time in the RIL population of cross A was evaluated at the Tokachi Agricultural Experiment Station (42°91′N, 143°05′E) in 2010 (for F5) and 2011 (for F5:6) (Yamaguchi et al., 2014). The progeny test was carried out in an experimental field at Hokkaido University, Sapporo (43°07′N, 141°35′E) in 2013 and 2014. Segregation in cross B was examined in an ILD field at Hokkaido University where natural daylength was extended to 20h by using incandescent lamps set at 2 m height for F2 in 2012 and for the RIL population (F6) in 2015. In the field experiments at Hokkaido University, seeds were sown in paper pots in a plastic greenhouse (cross A) or in the ILD field (cross B), and 10 d later the seedlings were transplanted into the field. Sowing dates were 1 June in 2012, 10 June in 2013, 29 May in 2014, and 22 May in 2015. The date of the first flower appearance (R1; Fehr et al., 1971) was recorded individually and expressed as the number of days after sowing (DAS).
DNA marker analysis
Simple sequence repeat (SSR) markers developed by Song et al. (2004, 2010), Hisano et al. (2007), and Kong et al. (2010), and those developed in this study based on the Williams 82 genomic sequence (Schmutz et al., 2010; Gmax v. 2.0, https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Gmax) were used for association analysis and linkage mapping. Primers for SSRs developed in this study (SSR-J1 to J5) are listed in Supplementary Table S1 at JXB online. DNA extraction and SSR marker analysis were performed as described previously (Kong et al., 2014; Zhao et al., 2016). In addition, DNA markers (FT5a-Pro-indel and FT5a-3′ UTR-indel) were developed to detect insertion/deletions (indels) in the promoter region and the 3′ untranslated region (UTR) of FT5a; the amplified products were separated in 1% or 2% agarose gel, stained with ethidium bromide, and visualized under UV light.
Association and QTL analyses
A linkage map for RILs homozygous for e1-nl (cross A) constructed by Yamaguchi et al. (2014) was used for QTL analysis; it contained 127 markers and covered 2023 cM. We added six SSRs to fine-map the QTL for flowering time. In cross B, we first tested associations of 100 SSR markers with flowering time by one-way analysis of variance for selected early-flowering and late-flowering F2 plants, and then constructed linkage maps for SSR markers significantly associated with flowering time using the whole population. The construction of linkage maps and QTL analyses were performed with JoinMap 4.1 (Van Ooijen, 2011) and MapQTL ver.5 (Van Ooijen, 2004), respectively.
Fine-mapping
We genotyped seven SSR markers flanking the GMES5027 in the progeny of RILs #46 (n=96) and #64 (n=27) of cross A, and detected four recombinant plants. Based on the segregation patterns in the progeny, we estimated the genotypes at the QTL for four recombinants and 10 non-recombinant control plants, and compared them with graphical genotypes constructed by using SSR markers. BLAST was used to search for homology.
Sequence analysis
The FT5a genomic region (4858bp) from 3.0kb upstream from the start codon to the end of the 3′ UTR was sequenced for the four parents. PCR was performed with ExTaq polymerase (TaKaRa) with total DNA as a template. The amplified fragments were ligated into the pGEM T-Easy vector (Promega), and cloned into E. coli JM109 Competent Cells (TaKaRa). Purified plasmids were used as templates for forward and reverse sequencing reactions by using a BigDye Terminator v. 3.1 Cycle Sequencing kit, and sequenced with an ABI PRISM 3100 Avant Genetic Analyzer (both from Applied Biosystems) in accordance with the manufacturer’s instructions. Plant cis-acting regulatory DNA elements (PLACE; Higo et al., 1999) analysis and DNA pattern search (http://www.geneinfinity.org/sms/sms_DNApatterns.html) were carried out to detect possible cis-elements in the FT5a genomic region. Sequencing primers are listed in Supplementary Table S1. FT5a genomic sequences of TH, 1532-1, H-e3, and J02 can be found in the GenBank/EMBL/DDBJ data libraries under the accession numbers LC128590, LC128591, LC128592, and LC128593, respectively.
Expression analysis
NILs for the ef and lf alleles at the QTL detected were grown in a greenhouse at an average temperature of 24 °C; natural daylength (<12h in November and December) was extended to 20h by using incandescent lamps. Fully developed trifoliate leaves were sampled individually at Zeitgeber time 3 at 15, 25, and 35 d after emergence, immediately frozen in liquid N2, and stored at −80 °C. Total RNA was isolated from frozen leaves by using TRIzol Reagent (Invitrogen). DNase I (TaKaRa) was used to remove genomic DNA. cDNA was synthesized from 1 µg of total RNA using an oligo (dT) 20 primer or a random primer cocktail (TaKaRa). Transcript levels of FT5a, FT2a, and E1 were determined by quantitative real-time PCR (qRT-PCR). Each qRT-PCR mixture (20 µl) contained 0.05 µl of the cDNA synthesis reaction, 5 µl of 1.2 µM primer premix, and 10 µl SYBR Premix ExTaq Perfect Real Time (TaKaRa). A CFX96 Real-Time System (Bio-Rad) was used. The PCR cycling conditions were 95 °C for 3min followed by 40 cycles of 95 °C for 10s, 58 °C for 30s, 72 °C for 20s, and 78 °C for 2s. Fluorescence was quantified before and after the incubation at 78 °C to monitor the formation of primer dimers. The mRNA for β-tubulin was used as an internal control. A reaction mixture without reverse transcriptase was also used as a control to confirm the absence of genomic DNA contamination. For each transcript, amplification of a single DNA fragment was confirmed by melting curve analysis and gel electrophoresis of the PCR products. Averages and standard errors of relative expression levels were calculated from qRT-PCR results for three independent plants. Primers used in expression analyses are listed in Supplementary Table S2.
RACE analysis
3′ RACE was performed to determine the 3′ UTR sequences of the ef and lf alleles using the SMARTer RACE cDNA Amplification Kit (Clontech). The primer 5′-GCCCTAGGGTTACTGTTGGTGGTGAA-3′ was designed based on the Williams 82 sequence. The cDNAs from NILs for ef and lf alleles were used as templates, and the amplified products were cloned and sequenced.
Single-nucleotide polymorphism (SNP) calling
SNPs were called from the re-sequencing data of 302 worldwide cultivated and wild soybean collections (Zhou et al., 2015) and 137 early-maturing landraces and improved cultivars developed in northeast China (Liu et al., unpublished data). Paired-end re-sequencing reads were mapped to the Williams 82 soybean reference genome (Gmax_275_Wm82.a2.v1; Schmutz et al., 2010) with the Burrows–Wheeler Aligner software (v0.7.10) (Li and Durbin, 2009) using the default parameters. The SAMtools software (v0.1.19) (Li et al., 2009) was used to convert mapping results into BAM (binary alignment/map) format and then to sort the BAM files by the chromosomal position of the SNP. Duplicated reads were filtered with the Picard package (v1.90) (http://broadinstitute.github.io/picard). The GATK software (v3.0-0-g6bad1c6) (McKenna et al., 2010) was used to realign the reads around indels and produce a realigned BAM file for each accession as follows: the RealignerTargetCreator tool was used to identify regions where realignment was needed, and then the IndelRealigner tool was used to realign these regions. SNPs were called at a population level with SAMtools. SNPs with quality scores <40 were discarded. Haplotype networks were constructed using SNPs with frequencies of rare variants of 4% or more.
Results
Segregation of flowering time
Frequency distributions of flowering time in the segregating populations of the two crosses are presented in Fig. 1. The RILs (F5) of cross A exhibited a broad distribution from 47 to 84 DAS. The observed variation was mostly accounted for by the E1 genotypes; RILs homozygous for e1-nl flowered at 47 to 60 DAS, whereas those homozygous for E1 flowered on average 20 d later. The flowering times of F6 plants were significantly correlated (r=0.84, P<0.001) with those of their parents (F5), indicating that flowering times in RILs were stable between 2010 and 2011.
Fig. 1.
Segregation of flowering time in the progeny of two crosses, Toyoharuka (TH) × 1532-1 (Cross A) and Harosoy near-isogenic lines for e3 (H-e3) × Jiagedaqi-02 (J02) (Cross B). Frequency distributions of days to flowering (DTF) are presented for the whole population (closed bars), plants or lines homozygous for the ef allele (open bars), and those homozygous for the lf allele (grey bars) at qDTF-J. In cross A, recombinant inbred lines (F5) were classified into lines homozygous for e1-nl and E1 alleles. Flowering time was recorded as the date of the appearance of the first flower (R1). Arrows indicate the average flowering times of parents and lines with the ef or lf alleles. The numbers near the arrows indicate the mean values of flowering time in each genotypic class (ef or lf). DAS, days after sowing.
The segregation of flowering time in cross B was evaluated under ILD conditions. The F2 and F6 RIL populations showed a continuous distribution, with flowering times intermediate between those of the parents, J02 and H-e3. The frequency of plants that flowered at almost the same time as H-e3 was 16% in F2 and 10% in F6, and was lower than expected from monogenic inheritance (25% in F2 and approximately 50% in F6), suggesting that at least two genes contribute to the difference in photoperiod sensitivities between H-e3 and J02.
QTL analysis for flowering time
Yamaguchi et al. (2014) identified a QTL for days to flowering (DTF) in linkage group J (Chromosome16, here tentatively designated as qDTF-J1) in 99 RILs of cross A homozygous for e1-nl. To map qDTF-J1 more precisely, we added two SSR markers (SSR-J1 and SSR-J2) and recalculated the logarithm (base 10) of odds (LOD) scores (Fig. 2). The highest LOD scores (21.7 and 14.9) were detected at GMES5027, the marker that was also detected in both F5 (2012) and F6 (2013) populations by Yamaguchi et al. (2014). This QTL accounted for 55% of the total phenotypic variance in flowering time in F5 and for 36% in F6. The additive effect of the TH allele was 2.4 d in the F5 population and 1.4 d in the F6 population.
Fig. 2.
Logarithm (base 10) of odds (LOD) score plots of qDTF-J1 and qDTF-J2, the QTLs for flowering time in linkage group J (Chr. 16), in Toyoharuka (TH) × 1532-1 (Cross A) and Harosoy near-isogenic line for e3 (H-e3) × Jiagedaqi-02 (J02) (Cross B), respectively.
To detect QTLs for flowering time in cross B, we tested the association between marker genotypes and flowering times for selected plants, eight early-flowering and eight late-flowering. Of the 100 SSR markers tested, eight markers showed significant associations, and linkage maps of their flanking regions were constructed for QTL analysis. By using linkage maps covering 408 cM, the major QTL was detected in linkage group J (Chr. 16), which had the highest LOD score, 4.9 (Fig. 2), and two minor QTLs were detected in linkage groups G (Chr. 18) and O (Chr. 10), which had LOD scores of 2.7 and 1.9, respectively (Supplementary Fig. S1). The QTL in linkage group J (tentatively designated as qDTF-J2) accounted for 27% of the total phenotypic variance in the F2 population. The tagging marker Sat_339 was located near GMES5027 (Hwang et al., 2009), indicating that qDTF-J2 may be identical to or located close to qDTF-J1. qDTF-J2 was further confirmed in the F6 RIL population; the LOD score was 11.9 (Fig. 2), and it accounted for 51% of the total variance in flowering time. The additive effect of the H-e3 allele was 4.9 d in the F2 population and 4.1 d in the F6 population.
Fine-mapping of qDTF-J1
To narrow down the genomic position of qDTF-J1, we genotyped seven SSR markers flanking GMES5027 in the progeny of RILs #46 (n=96) and #64 (n=27), and detected four recombinant plants. Based on the segregation patterns in the progeny, we estimated the genotypes at qDTF-J1 for four recombinants and ten non-recombinant control plants, and compared them with graphical genotypes constructed by using SSR markers (Fig. 3). The recombinant plant #46-3, which was homozygous for the 1532-1 allele in the region from SSR-J3 to GMES1870 but heterozygous in the region from GMES5027 to SSR-J2, flowered as early as plant #46-8-35, which was homozygous for the 1532-1 allele in the whole region. Plant #46-8-21, which was homozygous for the TH allele in the region from SSR-J3 to SSR-J5 but heterozygous in the region from SSR-FT3a to SSR-J2, segregated for flowering time similar to the heterozygous plant #46-8-12. The recombinant plant #64-8, which was heterozygous in the region from SSR-J3 to SSR-J4 but homozygous for the 1532-1 allele in the region from SSR-J5 to SSR-J2, flowered as early as plants #64-4 and #64-22, which were homozygous for the 1532-1 allele. Plant #64-21, which was heterozygous in the region from SSR-J3 to SSR-J5 but homozygous for the 1532-1 allele in the region from SSR-FT3a to SSR-J2, segregated similar to heterozygous plants #64-15 and #64-16. Based on these results, we delimited the QTL to a 107-kb region between SSR-J5 and SSR-FT3a. According to the Williams 82 genome sequence (Schmutz et al., 2010), nine genes are annotated in this region: four genes for apyrase proteins (Glyma.16G043300, Glyma.16G043400, Glyma.16G043500, Glyma.16G043700) and one gene each for tetratricopeptide repeat-like superfamily protein (Glyma.16G043600), an aquaporin-like superfamily protein (Glyma.16G043800), a transmembrane protein of unknown function with a DUF106 domain (Glyma.16G043900), FT5a (Glyma.16G044100), and an unannotated protein (Glyma.16G044000) (Table 1). BLAST searching revealed that Glyma.16G044000 had 86% amino acid sequence identity with Glycine soja NAD(P)H-quinone oxidoreductase subunit O (European Nucleotide Archive accession number KHN09611). According to the analysis of transcriptional networks that contribute to floral initiation under inductive SD (Wong et al., 2013), the expression of all of the annotated genes except Glyma.16G044100 (FT5a) was not up-regulated, whereas FT5a expression was up-regulated shortly after SD induction. Because FT5a is a functional FT ortholog and promotes flowering of soybean under non-inductive conditions when ectopically expressed (Nan et al., 2014; Guo et al., 2015), FT5a was the most likely candidate for qDTF-J1. FT5a is linked in tandem to FT3a (Kong et al., 2010). The genotypes at SSR-FT3a, which targets the SSR in the first intron of FT3a (Kong et al., 2010), were inconsistent with the estimated genotype at qDTF-J1 (Plant #64-21).
Fig. 3.
Fine-mapping of qDTF-J1 and annotated genes in the delimited genomic region. Four recombinants, two (#46-3 and #46-8-21) from RIL #46 and two (#64-8 and #64-21) from RIL #64, and ten non-recombinant control plants were genotyped at seven SSRs. The genotype at qDTF-J1 was estimated by progeny testing (right panel). The segregation of flowering time (DAS, days after sowing) is indicated in a box-plot format with ranges (horizontal lines), interquartile ranges (boxes), and medians (vertical lines). Closed bars, regions homozygous for the ef allele; open bars, homozygous for the lf allele; hatched bars, heterozygous; chequered bars, regions where recombinations occurred. Nine open reading frames (arrows) are predicted in a genomic region of 107-kb delimited by SSR-J5 and SSR-FT3a.
Table 1.
Annotation of the genes in the delimited genomic region for qDTF-J1 by fine-mapping.
| Gmax 2.0 primary protein ID | Annotation |
|---|---|
| Glyma.16G043300 | Apyrase 2 (ATAPY2, APY2) |
| Glyma.16G043400 | Apyrase 2 (ATAPY2, APY2) |
| Glyma.16G043500 | Apyrase 2 (ATAPY2, APY2) |
| Glyma.16G043600 | Tetratricopeptide repeat like superfamily protein |
| Glyma.16G043700 | Apyrase 2 (ATAPY2, APY2) |
| Glyma.16G043800 | Aquaporin-like superfamily protein (SIP1;2) |
| Glyma.16G043900 | Protein of unknown function DUF106, transmembrane |
| Glyma.16G044000 | Not annotated |
| Glyma.16G044100 | Phosphatidylethanolamine-binding protein (FT, PEBP) |
Sequence analysis of FT5a
We sequenced the genomic region of FT5a (4858bp) in the parents of the two crosses from 3.0kb upstream from the start codon to the end of the 3′ UTR. In all four parents, the sequences of coding regions were identical to that of Williams 82. There were a total of 17 polymorphisms among the four parents: eight SNPs (#1, 2, 3, 4, 6, 7, 9, and 11), one indel (#12), and four SSRs (#5, 8, 10, and 13) in the promoter region; two SNPs (#14 and 15) in the third intron; and two indels (#16 and 17) in the 3′ UTR (Fig. 4). The two indels (15bp and 49bp) in the 3′ UTR were confirmed by 3′ RACE. Because of these two deletions, the 3′ UTR of FT5a from 1532-1 (472bp) was 64bp shorter than that from TH.
Fig. 4.
DNA polymorphisms in the FT5a genomic region among four soybean cultivars and the Williams 82 reference sequence. Gray boxes, UTR; closed boxes, exons. Black bars indicate the promoter and introns. TH, Toyoharuka; J02, Jiagedaqi-02; W82, Williams 82; H-e3, near-isogenic line of Harosoy for e3. This figure is available in color at JXB online.
1532-1 and J02 shared the same polymorphisms except for one SNP (#2) in the promoter. In contrast, TH and H-e3 differed from 1532-1 and J02 by the same eight SNPs, three indels, and four SSRs. The Williams 82 sequence (Glyma.16G044100) differed from those of TH and H-e3 in one and two SNPs, respectively. Although fine-mapping was performed only for qDTF-J1, qDTF-J2 is most likely identical to qDTF-J1, because both QTLs were mapped at adjacent SSRs, and the DNA polymorphisms in the candidate gene were common between the two crosses. The two QTLs are renamed hereafter as qDTF-J with the early-flowering (ef) and late-flowering (lf) alleles.
Confirmation of the effect of qDTF-J on flowering time
We confirmed the association between the genotype at qDTF-J and flowering time by using the progeny of RILs heterozygous for qDTF-J and derived NILs for the ef and lf alleles. A DNA marker to detect the indel in the FT5a promoter was used to genotype qDTF-J. In the progeny of four e1-nl-RILs heterozygous at qDTF-J in cross A (#46, 47, 64, and 97), plants homozygous for the ef allele from 1532-1 flowered on average 2.3 to 4.1 d earlier than those homozygous for the lf allele from TH in the field condition (Table 2). Differences in average flowering times between plants homozygous for the marker genotypes were statistically significant for all RILs except #47. The association was further confirmed in the progeny (F8) of three heterozygous plants selected from RILs #47 and #97. qDTF-J thus had a stable (although a relatively small) effect on flowering time. In all segregating families (F7 and F8), average flowering times of heterozygous plants were almost the same or slightly later than those of plants homozygous for the ef allele, but earlier than those of plants homozygous for the lf allele, suggesting that the ef allele at qDTF-J behaved as a dominant or partially dominant allele.
Table 2.
Associated segregation of genotypes at a DNA marker FT5a-Pro-indel with flowering time in field conditions.
| Family | No. of plants | Flowering time (mean ± SD) | One-way ANOVA | |||
|---|---|---|---|---|---|---|
| AA | AB | BB | F-value | P | ||
| F7 (2013) | ||||||
| L46 | 21 | 42.3±1.0 | 40.3±1.5 | 39.9±1.3 | 4.1 | 0.034 |
| L47 | 24 | 41.3±1.3 | 39.3±2.0 | 39.0±1.0 | 3.5 | 0.052 |
| L64 | 27 | 40.8±0.7 | 38.8±1.3 | 36.7±1.6 | 18.6 | <0.001 |
| L97 | 28 | 43.1±1.3 | 41.2±1.3 | 39.0±2.2 | 11.4 | <0.001 |
| F8 (2014) | ||||||
| L47-8 | 67 | 48.5±1.8 | 44.4±1.5 | 43.4±0.8 | 62.1 | <0.001 |
| L97-2 | 36 | 47.3±1.6 | 44.1±0.9 | 43.5±0.8 | 31.4 | <0.001 |
| L97-5 | 35 | 46.2±2.6 | 43.8±1.0 | 43.3±0.7 | 10.1 | <0.001 |
A and B indicate alleles from Toyoharuka and 1532-1, respectively.
Flowering time indicates number of days after sowing
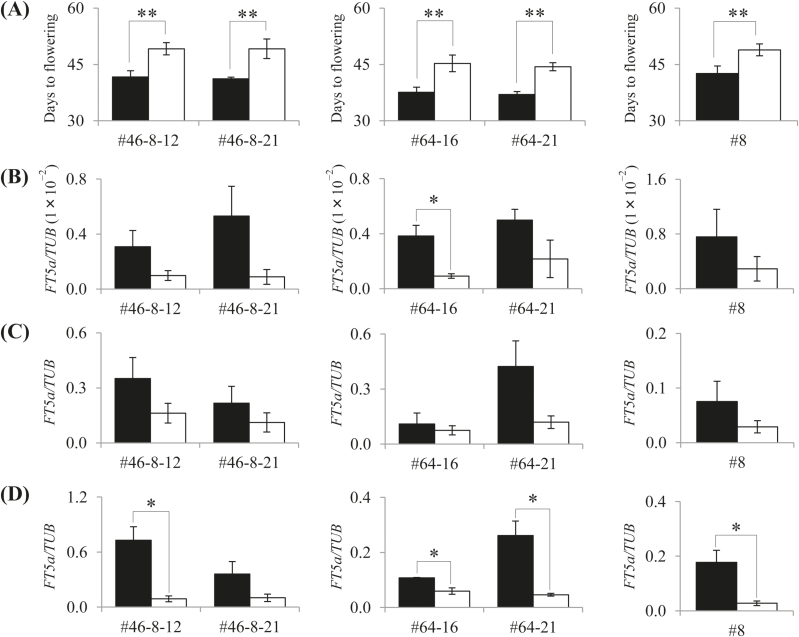
The flowering time in the LD condition was also significantly different (P<0.001) between the ef and lf alleles in all sets of NILs tested in cross A: the NILs for ef flowered 8 to 10 d earlier than those for lf (Fig. 5A). In the progeny of RIL #8 from cross B, plants homozygous for the ef allele from J02 flowered on average 8 d earlier than plants homozygous for the lf allele from H-e3 (Fig. 5A).
Fig. 5.
Flowering time and FT5a expression in near-isogenic lines for the ef (closed bars) and lf (open bars) alleles at qDTF-J. (A) Days to flowering after emergence (DAE) under LD conditions (20h light/4h dark). Error bars show standard deviation (n=10). (B–D) Relative levels of FT5a mRNA at (B) 15 DAE, (C) 25 DAE, and (D) 35 DAE. Values are given relative to β-tubulin (TUB) transcript levels. Error bars show standard error of the mean of three biological replicates (independent plants). * P<0.05 and ** P<0.001 (Student’s t test).
Association between flowering time and FT5a expression levels
To determine whether the difference in flowering times between plants carrying different alleles at qDTF-J could be explained by different FT5a transcript levels, we compared the FT5a transcript levels between the NILs. The expression levels of FT5a were higher in NILs for the ef allele than in those for the lf allele at all three time points tested (Fig. 5B–D). Some of these differences were statistically significant, in particular at 35 d after emergence. Therefore, early flowering in the NILs was associated with higher expression levels of FT5a. We also analyzed the expression of eight annotated genes other than FT5a in the 107-kb genomic region. As expected, their expression patterns varied among the three sets of NILs (Supplementary Fig. S2)
We also assayed the FT2a transcript abundance in NILs for the ef and lf alleles to evaluate whether it was associated with the difference in flowering times. FT2a expression was very low in the NILs developed from cross A (Supplementary Fig. S3) because both parents had the e9 allele, in which SORE-1 inserted in the first intron attenuates FT2a expression (Zhao et al., 2016). Low FT2a expression was also found in NILs developed from cross B; the low expression was probably due to E1 expression (Supplementary Fig. S3). There was no difference in FT2a expression levels between NILs for the ef and lf alleles, suggesting that the difference in FT5a expression most likely contributed to the differences in flowering times between NILs.
PLACE analysis and DNA pattern search for cis elements
We compared the distribution of cis elements in the FT5a genomic region between the ef and lf alleles with a focus on the known cis elements in Arabidopsis FT recognized by various transcriptional factors, such as the CCAAT box for nuclear factor Y (NF-Y), CO-responsive elements CORE1 and CORE2, the CArG box for MADS-box proteins, the E box for cryptochrome 2-cryptochrome-interacting basic helix-loop-helix (CIB) complex, and the Dof-binding site (AAAG) for CYCLING DOF FACTOR (CDF) (Andrés and Coupland, 2012; Song et al., 2015). By using PLACE (Higo et al., 1999) and DNA pattern search, we found that these cis elements were similar in both alleles, except for three indels. An insertion of 41bp (#12; Fig. 4) in the promoter of the ef allele contained one CArG box and two Dof elements, whereas two insertions in the lf allele (#16 and 17) contained three Dof elements. In the ef allele, deletion #16 generated a novel E box. In addition, the insertion in the ef allele contained an I box core motif (GATAA), a sequence conserved upstream of light-regulated genes, which activates their transcription in response to light signals (Terzaghi and Cashmore, 1995; Martínez-Hernández et al., 2002; López-Ochoa et al., 2007). The difference in cis elements in the indels may be responsible for the differences between the alleles in FT5a expression levels.
DNA marker analysis and SNP calling
Genetic materials with intermediate combinations of DNA polymorphisms between the ef and lf alleles may be useful to determine which polymorphisms are responsible for different expression levels. We first surveyed the 41-bp insertion in the promoter and two deletions (15bp and 49bp) in the 3′ UTR in 50 early-maturing accessions (Xu et al., 2013). Ten accessions had the same combination of polymorphisms as the ef allele; there were no accessions with the insertion only or the deletions only (Table S3). We then carried out SNP calling from re-sequencing data of 439 soybean accessions. A total of 114 polymorphisms, which included 94 SNPs, seven indels of <10bp, and 13 SSRs, were detected. Using 13 SNPs with frequencies of rare variants of 4% or more in the cultivated soybean population, we identified 22 haplotypes in the 377 cultivated accessions and seven haplotypes in the 62 wild accessions (Fig. 6). The ef alleles from 1532-1 and J02 corresponded to haplotype Hap3, whereas the lf alleles corresponded to haplotypes Hap13 in TH and Hap14 in H-e3. Hap13 (frequency, 46%) and Hap14 (40%) were most common, whereas Hap3 (4%) was rarely observed; all of the Hap3 accessions originated in northern Japan and northern China. Haplotypes Hap17 to Hap25 differed from Hap13 and Hap14 in SNPs in the 3′ UTR. Interestingly, there were almost no intermediate haplotypes between Hap3 (ef) and Hap13 and Hap14 (lf) in the cultivated soybean; only five accessions shared one or two of seven diagnostic SNPs of the ef allele. There were no polymorphisms in the promoter among 13 haplotypes including the two most common haplotypes. Wild soybean shared four haplotypes, Hap3, Hap5, Hap7, and Hap11, with cultivated soybean, but the haplotypes most common in cultivated accessions (Hap13 and Hap14) were not found in wild accessions. Taken together, these data show that the ef allele is a rare haplotype distinct from the haplotypes most common in the cultivated soybean population; it is also present in the wild soybean population.
Fig. 6.
Haplotypes identified with 13 SNPs detected in the FT5a genomic region in 439 soybean accessions. Blanks mean the same nucleotide as in Williams 82. TH, Toyoharuka; J02, Jiagedaqi-02; W82, Williams 82; H-e3, near-isogenic line of Harosoy for e3. This figure is available in color at JXB online.
Because the three indels between the ef and lf alleles could not be identified with certainty in the re-sequencing data, we used DNA markers to analyze eight northeastern Chinese accessions with Hap3 or Hap5 for the presence of these indels. Seven accessions with Hap3 had the same combination of indels as the ef allele, whereas the accession with Hap5 had the same combination as the lf allele.
We also compared flowering times between accessions carrying the ef or lf alleles among 50 early-flowering accessions with known maturity genotypes (Xu et al., 2013). The accessions with the ef allele flowered earlier than those with the lf allele among the accessions with the maturity genotype of e1/e2/e3/E4 or e1-as/e2/e3/E4, although only one accession had the ef allele among those with each of the two genotypes (Supplementary Table S4). A further study with more accessions is needed to confirm the association detected in each of the two genotypic classes.
qDTF-J controls flowering time in the E1 genetic background
The abundance of the FT5a transcript depends on the genotype at the E1 locus (Xu et al., 2015). To determine whether the effect of qDTF-J on flowering time depends on the genotype at the E1 locus, we assessed the association between the genotype at qDTF-J and flowering time in the E1-RIL population of cross A. The ranges of flowering time were similar (spanning ~13 d) in the e1-nl-RIL and E1-RIL F5 populations (Fig. 1). In both populations, RILs homozygous for the ef allele flowered earlier (on average by 4 d) than those homozygous for the lf allele; the difference in average flowering time between the FT5a genotypes was highly significant (P<0.001) in both E1 genotypes. A similar effect of the FT5a alleles was also detected in cross B (Fig. 1). Therefore, qDTF-J controlled flowering time independently of the E1 genotype.
Discussion
FT5a is a candidate for qDTF-J
In this study, we describe molecular dissection of a QTL for flowering time under LD conditions, which was detected in linkage group J (Chr. 16) in two independent crosses between early-maturing soybean cultivars. Fine-mapping delimited the QTL to a 107-kb region that contained nine annotated genes, including FT5a, a soybean ortholog of FT. Sequencing of the FT5a genomic region revealed that, despite the identical coding sequences, parents carrying the ef allele at the QTL differed by a total of 15 DNA polymorphisms from parents carrying the lf allele. In both crosses, FT5a expression levels were higher in NILs for the ef allele than in NILs for the lf allele. Taken together, the data indicate that the QTLs detected in the two crosses were identical, and different expression of FT5a was the cause of the difference in flowering time.
FT2a and FT5a appear to play major roles as florigens and redundantly initiate flowering in soybean (Kong et al., 2010; Fan et al., 2014; Guo et al., 2015). The overexpression of FT2a and FT5a driven by the 35S promoter promotes flowering of soybean plants under non-inductive conditions (Sun et al., 2011; Nan et al., 2014; Guo et al., 2015). Recently, FT2a was determined to be identical to the maturity gene E9 (Zhao et al., 2016). The DNA polymorphisms detected in this study in the FT5a genomic region probably contribute to the natural variation of flowering time in soybean by affecting FT5a transcript levels.
Various transcription factors bind to the cis elements of FT to control its expression in Arabidopsis (reviewed by Andrés and Coupland, 2012; Song et al., 2015). In Arabidopsis, the zinc-finger transcriptional regulator CO integrates the circadian rhythm and light signals to directly interact with the FT promoter and activate FT transcription (Putterill et al., 1995; Imaizumi et al., 2003). It directly binds to CORE1 and CORE2, which have the consensus sequence TGTG(N2-3)ATG (Tiwari et al., 2010). The NF-Y complex interacts with CO and binds to the distal enhancer element of FT (CCAAT) to recruit CO to proximal cis regulatory elements in the FT promoter (Cao et al., 2014). Furthermore, MADS-box proteins, such as FLOWERING LOCUS C (FLC) and SHORT VEGETATIVE PHASE (SVP), bind to the CArG boxes in the promoter and introns of FT (Helliwell et al., 2006; Lee et al., 2007; Li et al., 2008; Deng et al., 2011; Tao et al., 2012; Balanzà et al., 2014; Mateos et al., 2015). CDFs and CIBs bind to the Dof element and the E box, respectively (Song et al., 2015). We found one CArG and two Dof elements in the 41-bp insertion in the promoter of the ef allele and three Dof elements in two insertions in the lf allele. Additionally, the insertion in the ef allele contained an I box, and an E box was generated by a 49-bp deletion (#17) in 3′ UTR.
Different levels of FT5a mRNA might also be explained by differences in its stability caused by polymorphisms in the 3′ UTR, which may affect miRNA-binding sites or mRNA conformation (DeRidder et al., 2012). In plants, most experimentally verified miRNA target sites are located in coding regions, with only a few in 5′ UTRs or 3′ UTRs, or in non-coding RNAs (Allen et al., 2005; Addo-Quaye et al., 2008; German et al., 2008). We surveyed the miRNA target sites in the 3′ UTR of FT5a by using the TAPIR program (Bonnet et al., 2010), but could not detect any target sites in the two qDTF-J alleles. Further studies are needed to determine not only whether the cis elements detected in the insertions in the promoter and 3′ UTR have any effect on FT5a expression but also whether deletions in the 3′ UTR influence FT5a mRNA stability.
Molecular diversity of FT5a and the origin of the ef allele
We found that the ef allele corresponded to a rare haplotype (Hap3) that was distinct from the common haplotypes (Hap13 and Hap14) corresponding to the lf alleles from TH and H-e3; the accessions with Hap3 had the same combination of three indels as the ef allele. Because the accession with Hap5, which differed by two SNPs from Hap3, had the same combination of indels as the lf allele, the 41-bp insertion in the promoter and 15-bp and 49-bp deletions in the 3′ UTR may be characteristics of Hap3.
Because of its low frequency, the ef allele may not have been a major contributor to the natural variation of flowering time in cultivated soybean. Rather, it may have adaptive significance in some environments, in particular at high latitudes where early-flowering genotypes are desirable. The distribution of SNPs was discontinuous between Hap3 (ef allele) and common haplotypes (lf allele), suggesting that the ef allele did not originate from the lf allele via the accumulation of mutations. Because Hap3 was also observed in the wild soybean population, the ef allele might have been introgressed from wild soybean during domestication and/or subsequent genetic diversification.
qDTF-J controls flowering time independently of the PHYA–E1 pathway
The E1 locus produces the most marked effect on the flowering time of soybean (Bernard, 1971; Upadhyay et al., 1994; Cober et al., 2001; Watanabe et al., 2004; Tsubokura et al., 2014). It has multiple alleles, including the functional E1 allele; a leaky e1-as allele (traditionally designated e1); and dysfunctional alleles, such as e1-nl, e1-fs, and e1-b3a (Xia et al., 2012; Zhai et al., 2015). Although E1 overexpression strongly inhibits the expression of both FT2a and FT5a (Xia et al., 2012), E1 alleles regulate the expression of FT2a and FT5a differently. The E1 and e1-as alleles inhibit FT2a expression similarly, whereas the inhibitory effect of the E1 allele on FT5a expression is stronger than those of e1-as and e1-nl, suggesting that the E1 locus controls FT5a in a more direct way than it controls FT2a (Kong et al., 2010; Xu et al., 2015).
The effects of qDTF-J on flowering time, however, were detected in the genetic backgrounds of both e1-nl and E1 (Fig. 1). This is in sharp contrast to the effect of e9, a leaky FT2a allele, which is expressed at low levels: the effect of e9 on flowering is detectable only in the e1-nl background and not in the E1 background (Kong et al., 2014; Lu et al., 2016). The DNA polymorphisms that determine the difference in expression between the ef and lf alleles may not be the same as those involved in the PHYA–E1 pathway, a major controller of flowering in soybean (Xu et al., 2015).
The effect of qDTF-J was also detected in the progeny of the cross between H-e3 and J02, both of which have the e1-as allele. J02 has weak photoperiod sensitivity to ILD, although it has the same genotypes at the maturity loci E1–E4 as the Harosoy NIL for e3; the latter is photoperiod-sensitive under ILD (Xu et al., 2013). This weak sensitivity of J02 is controlled by qDTF-J and at least two minor QTLs (Fig. 2 and Supplementary Fig. S1). Therefore, elevated FT5a expression may be one of the genetic factors that promote flowering under FR-enriched LD conditions. The NIL for the ef allele at qDTF-J developed from the cross between H-e3 and J02 had higher FT5a expression than that for the lf allele, although both NILs had similar E1 transcript abundances (Supplementary Fig. S3). Therefore, the level of FT5a expression was not directly correlated with that of E1 expression, consistent with the finding that the DNA polymorphisms that increase FT5a expression may not affect the cis elements involved in the PHYA–E1 pathway.
Genetic factors activating FT2a and FT5a expression under inductive conditions for flowering remain poorly understood. The soybean CO orthologs GmCOL1a/GmCOL1b and GmCOL2a/GmCOL2b can fully complement the late-flowering phenotype of the Arabidopsis co-1 mutant, suggesting that they are potential inducers of flowering in soybean (Wu et al., 2014). However, GmCOL1a and GmCOL1b inhibit flowering under LD conditions (Cao et al., 2015). This is similar to the rice (Oryza sativa) Heading date 1 (Hd1) protein, an ortholog of CO, which activates the expression of Hd3a, an ortholog of FT, under SD conditions, but suppresses Hd3a expression under LD conditions (Doi et al., 2004). Further studies are needed to determine the transcription factor(s) that activate(s) the expression of both FT2a and FT5a under inductive conditions of flowering to improve our understanding of the molecular mechanisms of flowering in soybean. DNA polymorphisms between the ef and lf alleles detected in this study would be useful for identifying such transcriptional activator(s).
Supplementary Data
Supplementary data are available at JXB online.
Table S1. Primers for DNA markers and sequencing.
Table S2. Primers for quantitative RT-PCR.
Table S3. Presence or absence of three indels in the promoter and 3′ UTR of FT5a in early-maturing soybean accessions.
Table S4. Variation of flowering time in accessions with the ef or lf alleles in different multi-locus genotypes at E1, E2, E3 and E4.
Fig. S1. Two minor QTLs for reduced photoperiod sensitivity of Jiagedaqi-02.
Fig. S2. Relative levels of Glyma.16G043400 to Glyma.16G044000 mRNA at 35 DAE in near-isogenic lines for the ef and lf alleles at qDTF-J.
Fig. S3. Relative levels of FT2a and E1 mRNA in near isogenic lines for the ef and lf alleles at qDTF-J.
Acknowledgments
We thank E. R. Cober (Agriculture and Agri-Food Canada, Canada) for supplying seeds of the cv Harosoy isoline. This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (SFC1003) to T. Yamada; by the Natural Science Foundation of China (31430065, 31371643, and 31571686) and the Strategic Action Plan for Science and Technology Innovation of the Chinese Academy of Sciences (XDA08030108) to B. Liu and F. Kong.
References
- Addo-Quaye C, Eshoo TW, Bartel DP, Axtell MJ. 2008. Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Current Biology 18, 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Carrington JC. 2005. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121, 207–221. [DOI] [PubMed] [Google Scholar]
- Andrés F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues. Nature Reviews Genetics 13, 627–639. [DOI] [PubMed] [Google Scholar]
- Balanzà V, Martínez-Fernández I, Ferrándiz C. 2014. Sequential action of FRUITFULL as a modulator of the activity of the floral regulators SVP and SOC1. Journal of Experimental Botany 65, 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard RL. 1971. Two genes for time of flowering in soybeans. Crop Science 11, 242–244. [Google Scholar]
- Bonnet E, He Y, Billiau K, van de Peer Y. 2010. TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26, 1566–1568. [DOI] [PubMed] [Google Scholar]
- Cao D, Li Y, Lu S, et al. 2015. GmCOL1a and GmCOL1b function as flowering repressors in soybean under long-day conditions. Plant Cell Physiology 56, 2409–2422. [DOI] [PubMed] [Google Scholar]
- Cao S, Kumimoto RW, Gnesutta N, Calogero AM, Mantovani R, Holt BF., 3rd 2014. A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis. The Plant Cell 26, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A, Pantalone VR, Ustun A, Allen FL, Landau-Ellis D, Trigiano RN, Gresshoff PM. 2003. Quantitative trait loci for agronomic and seed quality traits in an F2 and F4:6 soybean population. Euphytica 129, 387–393. [Google Scholar]
- Cheng L, Wang Y, Zhang C, et al. 2011. Genetic analysis and QTL detection of reproductive period and post-flowering photoperiod responses in soybean. Theoretical and Applied Genetics 123, 421–429. [DOI] [PubMed] [Google Scholar]
- Cober ER, Stewart DW, Voldeng HD. 2001. Photoperiod and temperature responses in early-maturing, near-isogenic soybean lines. Crop Science 41, 721–727. [Google Scholar]
- Deng W, Ying H, Helliwell CA, Taylor JM, Peacock WJ, Dennis ES. 2011. FLOWERING LOCUS C (FLC) regulates development pathways throughout the life cycle of Arabidopsis. Proceedings of the National Academy of Sciences, USA 108, 6680–6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRidder BP, Shybut ME, Dyle MC, Kremling KA, Shapiro MB. 2012. Changes at the 3′-untranslated region stabilize Rubisco activase transcript levels during heat stress in Arabidopsis. Planta 236, 463–476. [DOI] [PubMed] [Google Scholar]
- Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A. 2004. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes and Development 18, 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C, Hu R, Zhang X, Wang X, Zhang W, Zhang Q, Ma J, Fu YF. 2014. Conserved CO-FT regulons contribute to the photoperiod flowering control in soybean. BMC Plant Biology 14, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr WR, Caviness CE, Burmood DT, Pennington JS. 1971. Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Science 11, 929–931. [Google Scholar]
- Funatsuki H, Kawaguchi K, Matsuba S, Sato Y, Ishimoto M. 2005. Mapping of QTL associated with chilling tolerance during reproductive growth in soybean. Theoretical and Applied Genetics 111, 851–861. [DOI] [PubMed] [Google Scholar]
- German MA, Pillay M, Jeong DH, et al. 2008. Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nature Biotechnology 26, 941–946. [DOI] [PubMed] [Google Scholar]
- Githiri SM, Yang D, Khan NA, Xu D, Komatsuda T, Takahashi R. 2007. QTL analysis of low temperature induced browning in soybean seed coats. Journal of Heredity 98, 360–366. [DOI] [PubMed] [Google Scholar]
- Guo G, Xu K, Zhang X, et al. 2015. Extensive analysis of GmFTL and GmCOL expression in northern soybean cultivars in field conditions. PLoS ONE 10, e0136601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, Peacock WJ, Dennis ES. 2006. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. The Plant Journal 46, 183–192. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Research 27, 297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano H, Sato S, Isobe S, et al. 2007. Characterization of the soybean genome using EST-derived microsatellite markers. DNA Research 14, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Tepperman JM, Quail PH. 2000. GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 97, 9789–9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang TY, Sayama T, Takahashi M, et al. 2009. High-density integrated linkage map based on SSR markers in soybean. DNA Research 16, 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Tran HG, Swartz TE, Briggs WR, Kay SA. 2003. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426, 302–306. [DOI] [PubMed] [Google Scholar]
- Keim P, Diers BW, Olson TC, Shoemaker RC. 1990. RFLP mapping in soybean: association between marker loci and variation in quantitative traits. Genetics 126, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, Githiri SM, Benitez ER, Abe J, Kawasaki S, Hayashi T, Takahashi R. 2008. QTL analysis of cleistogamy in soybean. Theoretical and Applied Genetics 117, 479–487. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Okuda S, Takahashi M, Matsunaga R, Nakazawa Y. 2007. Quantitative trait loci mapping of pubescence density and flowering time of insect-resistant soybean (Glycine max L. Merr.). Genetics and Molecular Breeding 30, 635–639. [Google Scholar]
- Kong F, Liu B, Xia Z, et al. 2010. Two coordinately regulated homologs of FLOWERING LOCUS T are involved in the control of photoperiodic flowering in soybean. Plant Physiology 154, 1220–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Nan H, Cao D, et al. 2014. A new dominant gene E9 conditions early flowering and maturity in soybean. Crop Science 154, 1220–1231. [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. 2007. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes and Development 15, 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Bailey MA, Mian MA, Shipe ER, Ashley DA, Parrott WA, Hussey RS, Boerma HR. 1996. Identification of quantitative trait loci for plant height, lodging, and maturity in a soybean population segregating for growth habit. Theoretical and Applied Genetics 92, 516–523. [DOI] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. 2008. A repressor complex governs the integration of flowering signals in Arabidopsis. Developmental Cell 15, 110–120. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, et al. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Abe J. 2010. QTL mapping for photoperiod insensitivity of a Japanese soybean landrace Sakamotowase. Journal of Heredity 101, 251–256. [DOI] [PubMed] [Google Scholar]
- Liu B, Fujita T, Yan ZH, Sakamoto S, Xu D, Abe J. 2007. QTL mapping of domestication-related traits in soybean (Glycine max). Annals of Botany 100, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Kanazawa A, Matsumura H, Takahashi R, Harada K, Abe J. 2008. Genetic redundancy in soybean photoresponses associated with duplication of the phytochrome A gene. Genetics 180, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Kim MY, Kang YJ, Van K, Lee YH, Srinives P, Yuan DL, Lee SH. 2011. QTL identification of flowering time at three different latitudes reveals homeologous genomic regions that control flowering in soybean. Theoretical and Applied Genetics 123, 545–553. [DOI] [PubMed] [Google Scholar]
- López-Ochoa L, Acevedo-Hernández G, Martínez-Hernández A, Argüello-Astorga G, Herrera-Estrella L. 2007. Structural relationships between diverse cis-acting elements are critical for the functional properties of a rbcS minimal light regulatory unit. Journal of Experimental Botany 58, 4397–4406. [DOI] [PubMed] [Google Scholar]
- Lu S, Li Y, Wang J, et al. 2016. Identification of additional QTLs for flowering time by removing the effect of the maturity gene E1 in soybean. Journal of Integrative Agriculture 15, 42–49. [Google Scholar]
- Mansur LM, Lark KG, Kross H, Oliveira A. 1993. Interval mapping of quantitative trait loci for reproductive, morphological, and seed traits of soybean (Glycine max L.) Theoretical and Applied Genetics 86, 907–913. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernández A, López-Ochoa L, Argüello-Astorga G, Herrera-Estrella L. 2002. Functional properties and regulatory complexity of a minimal RBCS light-responsive unit activated by phytochrome, cryptochrome, and plastid signals. Plant Physiology 128, 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos JL, Madrigal P, Tsuda K, Rawat V, Richter R, Romera-Branchat M, Fornara F, Schneeberger K, Krajewski P, Coupland G. 2015. Combinatorial activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C define distinct modes of flowering regulation in Arabidopsis. Genome Biology 16, 3 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, et al. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Research 20, 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan H, Cao D, Zhang D, Li Y, Lu S, Tang L, Yuan X, Liu B, Kong F. 2014. GmFT2a and GmFT5a redundantly and differentially regulate flowering through interaction with and upregulation of the bZIP transcription factor GmFDL19 in soybean. PLoS ONE 9, e97669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orf JH, Chase K, Jarvik T, Mansur LM, Cregan PB, Adler FR, Lark KG. 1999. Genetics of soybean agronomic traits. I. Comparison of three related recombinant inbred populations. Crop Science 39, 1642–1651. [Google Scholar]
- Pooprompan P, Wasee S, Toojinda T, Abe J, Chanpran S, Srinives P. 2006. Molecular marker analysis of days of flowering in vegetable soybean (Glycine max (L.) Merrill). Kasetsart Journal 40, 573–581. [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. 1995. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857. [DOI] [PubMed] [Google Scholar]
- Saindon G, Voldeng HD, Beversdorf WD, Buzzell RI. 1989. Genetic control of long daylength response in soybean. Crop Science 29, 1436–1439. [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. 2007. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, et al. 2010. Genome sequence of the palaeopolyploid soybean. Nature 463, 178–183. [DOI] [PubMed] [Google Scholar]
- Song QJ, Jia GF, Zhu YL, Grant D, Nelson RT, Hwang EY, Hyten DL, Cregan PB. 2010. Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Science 50, 1950–1960. [Google Scholar]
- Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, Specht JE, Cregan PB. 2004. A new integrated genetic linkage map of the soybean. Theoretical and Applied Genetics 109, 122–128. [DOI] [PubMed] [Google Scholar]
- Song YH, Ito S, Imaizumi T. 2013. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends in Plant Science 18, 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. 2015. Photoperiodic flowering: time measurement mechanisms in leaves. Annual Review of Plant Biology 66, 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Jia Z, Cao D, Jiang B, Wu C, Hou W, Liu Y, Fei Z, Zhao D, Han T. 2011. GmFT2a, a soybean homolog of FLOWERING LOCUS T, is involved in flowering transition and maintenance. PLoS ONE 6, e29238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Shen L, Liu C, Liu L, Yan Y, Yu H. 2012. Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. The Plant Journal 70, 549–561. [DOI] [PubMed] [Google Scholar]
- Tasma IM, Lorenzen L, Green D, Shoemaker R. 2001. Mapping genetic loci for flowering time, maturity, and photoperiod insensitivity in soybean. Molecular Breeding 8, 25–35. [Google Scholar]
- Terzaghi WB, Cashmore AR. 1995. Light-regulated transcription. Annual Review of Plant Physiology and Plant Molecular Biology 46, 445–474. [Google Scholar]
- Tiwari SB, Shen Y, Chang HC, et al. 2010. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytologist 187, 57–66. [DOI] [PubMed] [Google Scholar]
- Tsubokura Y, Watanabe S, Xia Z, Kanamori H, Yamagata H, Kaga A, Katayose Y, Abe J, Ishimoto M, Harada K. 2014. Natural variation in the genes responsible for maturity loci E1, E2, E3 and E4 in soybean. Annals of Botany 113, 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay AP, Summerfield RH, Ellis RH, Roberts EH, Qi A. 1994. Variation in the durations of the photoperiod-sensitive and photoperiod-insensitive phases of development to flowering among eight maturity isolines of soyabean [Glycine max (L.) Merrill]. Annals of Botany 74, 97–101. [DOI] [PubMed] [Google Scholar]
- Van Ooijen JW. 2004. MapQTL® 5, Software for the mapping of quantitative trait loci in experimental populations. Wageningen, Netherlands: Kyazma BV. [Google Scholar]
- Van Ooijen JW. 2011. Multipoint maximum likelihood mapping in a full-sib family of an outbreeding species. Genetics Research 93, 343–349. [DOI] [PubMed] [Google Scholar]
- Wang D, Graef GL, Procopiuk AM, Diers BW. 2004. Identification of putative QTL that underlie yield in interspecific soybean backcross populations. Theoretical and Applied Genetics 108, 458–467. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Harada K, Abe J. 2012. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breeding Science 61, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Hideshima R, Xia Z, et al. 2009. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics 182, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Tajuddin T, Yamanaka N, Hayashi M, Harada K. 2004. Analysis of QTLs for reproductive development and seed quality traits in soybean using recombinant inbred lines. Breeding Science 54, 399–407. [Google Scholar]
- Watanabe S, Xia Z, Hideshima R, Tsubokura Y, Sato S, Harada K. 2011. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics 188, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CE, Singh MB, Bhalla PL. 2013. The dynamics of soybean leaf and shoot apical meristem transcriptome undergoing floral initiation process. PLoS ONE 8, e65319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Price BW, Haider W, Seufferheld G, Nelson R, Hanzawa Y. 2014. Functional and evolutionary characterization of the CONSTANS gene family in short-day photoperiodic flowering in soybean. PLoS ONE 9, e85754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Watanabe S, Yamada T, et al. 2012. Positional cloning and characterization reveal the molecular basis for soybean maturity locus E1 that regulates photoperiodic flowering. Proceedings of the National Academy of Sciences, USA 109, E2155– E2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Xu Z, Liu B, et al. 2013. Genetic variation in four maturity genes affects photoperiod insensitivity and PHYA-regulated post-flowering responses of soybean. BMC Plant Biology 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yamagishi N, Zhao C, et al. 2015. The soybean-specific maturity gene E1 family of floral repressors controls night-break responses through down-regulation of FLOWERING LOCUS T orthologs. Plant Physiology 168, 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Sayama T, Sasama H, Yamazaki H, Miyoshi T, Tanaka Y, Ishimoto M. 2014. Mapping of quantitative trait loci associated with terminal raceme length in soybean. Crop Science 54, 2461–2468. [Google Scholar]
- Yamanaka N, Ninomiya S, Hoshi M, Tsubokura Y, Yano M, Nagamura Y, Sasaki T, Harada K. 2001. An informative linkage map of soybean reveals QTLs for flowering time, leaflet morphology and regions of segregation distortion. DNA Research 8, 61–72. [DOI] [PubMed] [Google Scholar]
- Zhai H, Lü S, Wu H, et al. 2015. Diurnal expression pattern, allelic variation, and association analysis reveal functional features of the E1 gene in control of photoperiodic flowering in soybean. PLoS ONE 10, e0135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WK, Wang YJ, Luo GZ, Zhang JS, He CY, Wu XL, Gai JY, Chen SY. 2004. QTL mapping of ten agronomic traits on the soybean (Glycine max L. Merr.) genetic map and their association with EST markers. Theoretical and Applied Genetics 108, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Zhao C, Takeshima R, Zhu J, et al. 2016. A recessive allele for delayed flowering at the soybean maturity locus E9 is a leaky allele of FT2a, a FLOWERING LOCUS T ortholog. BMC Plant Biology 16, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Jiang Y, Wang Z, et al. 2015. Resequencing 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nature Biotechnology 33, 408–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.