Highlight:
PES, BOP1, and WDR12 (PeBoW) are plant ribosome biogenesis factors. PeBoW silencing in Arabidopsis causes immediate inhibition of leaf cell growth and proliferation through transcriptional modulation of cell-cycle genes and phytohormone-related genes.
Key words: Cell cycle genes, growth defects, kinematic analysis, nucleolar stress, phytohormones, ribosome biogenesis.
Abstract
The nucleolar protein pescadillo (PES) controls biogenesis of the 60S ribosomal subunit through functional interactions with Block of Proliferation 1 (BOP1) and WD Repeat Domain 12 (WDR12) in plants. In this study, we determined protein characteristics and in planta functions of BOP1 and WDR12, and characterized defects in plant cell growth and proliferation caused by a deficiency of PeBoW (PES-BOP1-WDR12) proteins. Dexamethasone-inducible RNAi of BOP1 and WDR12 caused developmental arrest and premature senescence in Arabidopsis, similar to the phenotype of PES RNAi. Both the N-terminal domain and WD40 repeats of BOP1 and WDR12 were critical for specific associations with 60S/80S ribosomes. In response to nucleolar stress or DNA damage, PeBoW proteins moved from the nucleolus to the nucleoplasm. Kinematic analyses of leaf growth revealed that depletion of PeBoW proteins led to dramatically suppressed cell proliferation, cell expansion, and epidermal pavement cell differentiation. A deficiency in PeBoW proteins resulted in reduced cyclin-dependent kinase Type A activity, causing reduced phosphorylation of histone H1 and retinoblastoma-related (RBR) protein. PeBoW silencing caused rapid transcriptional modulation of cell-cycle genes, including reduction of E2Fa and Cyclin D family genes, and induction of several KRP genes, accompanied by down-regulation of auxin-related genes and up-regulation of jasmonic acid-related genes. Taken together, these results suggest that the PeBoW proteins involved in ribosome biogenesis play a critical role in plant cell growth and survival, and their depletion leads to inhibition of cell-cycle progression, possibly modulated by phytohormone signaling.
Introduction
Ribosome biogenesis is a fundamental process that is tightly co-ordinated with cell growth and proliferation. Assembly of a functional ribosome is a complex, multi-step process with high demands in energy and resources (Henras et al., 2008; Kressler et al., 2010; Panse and Johnson, 2010; Karbstein, 2011; Woolford and Baserga, 2013). The mechanisms of rRNA processing and ribosome biogenesis have been elucidated in yeast, but not in higher eukaryotes including plants, due to their greater complexity. Orthologs of approximately 75% of yeast ribosome assembly factors have been identified in plants (Ebersberger et al., 2014), but only a small fraction of these factors have been functionally characterized (Pestov et al., 2001; Brown and Shaw, 2008; Horiguchi et al., 2012; Cho et al., 2013; Missbach et al., 2013; Weis et al., 2014; Jeon et al., 2015). Furthermore, it is largely unknown how plant ribosome biogenesis is regulated under fluctuating metabolic and environmental conditions.
Although the nucleolus is primarily associated with ribosome biogenesis, recent evidence suggests its involvement in diverse cellular processes, such as cell-cycle control, stress sensing and responses, DNA damage repair, pre-mRNA processing, and telomere metabolism (Boisvert et al., 2007; Shaw and Brown, 2012; Lam and Trinkle-Mulcahy, 2015). Perturbations in ribosome biogenesis or function in mammalian cells leads to nucleolar stress, which can cause cell-cycle arrest, senescence, and apoptosis through activation of the tumor suppressor p53 (Antoniali et al., 2014; Golomb et al., 2014; James et al., 2014). In this pathway, the ribosomal proteins RPL5 and RPL11 diffuse from the nucleolus to the nucleoplasm following nucleolar stress and bind to the E3 ubiquitin ligase MDM2, thereby blocking MDM2-mediated ubiquitination and degradation of p53, ultimately leading to cell-cycle arrest. However, nucleolar stress results in arrested cell proliferation in organisms lacking p53, such as yeast, Drosophila, and plants, suggesting a link between p53-independent mechanisms for nucleolar stress and the cell cycle (Donati et al., 2012; Cho et al., 2013; James et al., 2014).
Pescadillo (PES) is an evolutionarily conserved nucleolar protein that is essential for the viability of yeast and higher eukaryotes (Allende et al., 1996; Adams et al., 2002; Lerch-Gaggl et al., 2002). PES associates with Block of Proliferation 1 (BOP1) and WD Repeat Domain 12 (WDR12) to form the PeBoW complex in mammalian cells, and together they modulate pre-rRNA processing for the synthesis of 28S and 5.8S rRNAs, failure of which causes defective assembly of the 60S large ribosomal subunit (Strezoska et al., 2000; Lapik et al., 2004; Hölzel et al., 2005; Grimm et al., 2006; Rohrmoser et al., 2007). Yeast counterparts of these three proteins, Nop7/Yph1 (PES), Erb1 (BOP1), and Ytm1 (WDR12), form the Nop7 subcomplex in yeast pre-ribosomes, which are required to process 27S pre-rRNA into mature 25S and 5.8S rRNAs (Adams et al., 2002; Miles et al., 2005). Down-regulation or expression of a dominant negative mutant of PES, BOP1, or WDR12 results in cell-cycle arrest with altered expression of cell-cycle regulators, suggesting their function in cell proliferation control (Pestov et al., 2001; Hölzel et al., 2005; Li et al., 2009). Misregulation of PES and BOP1 has been linked to chromosomal instability and tumourigenesis (Killian et al., 2004; Li et al., 2009; Chung et al., 2011).
Our previous studies have shown that the PeBoW (PES-BOP1-WDR12) paradigm observed in mammals and yeast appears to be conserved in plants (Cho et al., 2013). Plant PES was found to interact with BOP1 and WDR12 in the nucleolus. Silencing of Arabidopsis PES via dexamethasone (DEX)-inducible RNAi delayed maturation of 25S rRNA and suppressed global translation, causing growth arrest and acute cell death. Virus-induced gene silencing of any of the PeBoW genes in Nicotiana benthamiana resulted in defective biogenesis of the 60S large ribosomal subunit. These results suggest that PES is essential to plant cell growth and survival by modulating ribosome biogenesis through a functional link with BOP1 and WDR12 (Cho et al., 2013). In this current study, we investigated further the protein characteristics of PES, BOP1, and WDR12, and analyzed phenotypes of BOP1- and WDR12-silenced Arabidopsis plants. In addition, we explored the molecular mechanisms of cell-cycle inhibition by disrupted ribosome biogenesis in PeBoW-deficient Arabidopsis plants.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana (ecotype Columbia-0) and Nicotiana benthamiana plants were grown in soil in a growth chamber at 22 °C under a 16-h light/8-h dark cycle. For growth on agar, Arabidopsis seeds were surface-sterilized and sown on Petri dishes containing MS medium [Murashige and Skoog salts (pH 5.7), 0.35% Phytagel (Sigma), and 2% sucrose] with ethanol (–DEX) or 10 µM DEX. For liquid culture, plant seedlings were grown in six-well plates containing 1ml of liquid medium [0.5 × Murashige and Skoog salts (pH 5.7) and 0.5% sucrose] at 23 °C and 100–120 μmol m−2 s−1 light intensity under a 16h light/8h dark cycle. At 7 d after sowing, seedlings were treated with ethanol or 20 μM DEX for 12h or 24h.
Generation of dexamethasone (DEX)-inducible BOP1 and WDR12 RNAi lines in Arabidopsis
For BOP1 RNAi, a 291-bp BOP1 cDNA fragment was PCR-amplified using BOP1-sense (F)/(R) primers containing XhoI and ClaI sites (5′-gctccacatgcggactttga-3′ and 5′-tcctggcaatttaagcttggg-3′) for the sense construct and BOP1-antisense (F)/(R) primers containing SpeI and BamHI sites (5′-gctccacatgcggactttga-3′ and 5′-tcctggcaatttaagcttggg-3′ for the antisense construct. For WDR12 RNAi, a 300-bp WDR12 cDNA fragment was PCR-amplified using WDR12-sense (F)/(R) primers containing XhoI and ClaI sites (5′-atggatatcgacggagaaga-3′ and 5′-tggtgtcacagcccttatgt-3′) for the sense construct and WDR12-antisense (F)/(R) primers containing SpeI and BamHI sites (5′-atggatatcgacggagaagatgtat-3′ and 5′-tggtgtcacagccct-3′) for the antisense construct. Using these constructs, DEX-inducible BOP1 and WDR12 RNAi transgenic Arabidopsis lines (ecotype Columbia-0) were generated by a floral dip method. After floral-dipping, seeds were harvested and sown on MS medium containing hygromycin (30mg l–1). Hygromycin-resistant primary T1 transformants were moved to soil to grow and set seeds. Seeds obtained from each T1 transformant were grown on hygromycin-containing medium to calculate the ratio of hygromycin-resistant to hygromycin-sensitive seedlings. Only the lines showing 3:1 segregation ratio were selected (T2 generation), and tested for gene-silencing phenotypes by germinating the seeds in ethanol- or DEX-containing medium. Dexamethasone (Sigma) was added to the medium to a final concentration of 10 μM in ethanol (0.033%) from 30mM stock solution. Five-to-eight independent T2 lines that showed strong silencing phenotypes on DEX-containing medium were selected for T3 propagation. Ten-to-sixteen plants of each selected independent T2 line were grown in soil to obtain seeds, and the seed batch that showed 100% hygromycin-resistance was selected as the homozygous T3 generation. For RNAi of BOP1 and WDR12, more than 30 independent T2 lines each were generated. Among the T2 lines tested, six independent BOP1 RNAi lines and five independent WDR12 RNAi lines exhibited strong growth retardation phenotypes when grown on DEX-containing medium, and were subsequently propagated for the T3 generation. Two independent BOP1 and WDR12 lines were finally selected, and their T3 and T4 homozygous seeds were used for the analyses throughout the study.
Agrobacterium-mediated transient expression
Agroinfiltration was carried out as described previously by Cho et al. (2013).
Subcellular localization using GFP fusion
Green fluorescent protein (GFP) fusion and confocal microscopy were performed as described previously by Cho et al. (2013). For drug treatment, GFP-fused PeBoW proteins were expressed in N. benthamiana leaves via agroinfiltration. After 36h, the leaves were treated with 20 μM mycophenolic acid (MPA), 5 μM Actinomycin D, and 0.03% methyl methane sulfate (MMS) by syringe infiltration. Epidermal cells of the leaves were then periodically observed directly by confocal microscopy.
Kinematic analysis of leaf growth
Kinematic analysis was performed as described previously by De Veylder et al. (2001) and Ahn et al. (2015).
RNA extraction from seedlings
PES, BOP1, and WDR12 RNAi seedlings were grown on MS media for 7 d and then transferred to medium containing 10 µM DEX. At 2 and 4 d after transfer, total RNA was isolated from equal fresh weights of seedlings using the IQeasyTM plus plant RNA extraction kit (iNtRON Biotechnology, Korea) according to the manufacturer’s instructions. The concentration and purity of RNAs were determined by NanoDrop 1000 (Thermo Scientific).
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR was performed using gene-specific primers as described previously by Cho et al. (2013). Supplementary Table S1 at JXB online lists the primers used in this study.
Immunoblotting
Immunoblotting was performed as described by Ahn et al. (2011) and Cho et al. (2013), using mouse monoclonal antibodies against the Flag tag (Sigma), GFP (Clontech), RPL10a (Santa Cruz Biotechnology), and histone H3 (Santa Cruz Biotechnology). The immunoblots were treated with horseradish peroxidase-conjugated goat anti-mouse IgG antibodies (Invitrogen), followed by signal detection using Imagequant LAS 4000 (GE Healthcare Life Sciences).
Co-immunoprecipitation
Co-immunoprecipitation was performed as described previously by Ahn et al. (2011) and Cho et al. (2013).
Measurement of cyclin-dependent kinase (CDK) activity
To purify glutathione S-transferase (GST)-fusion protein of the C-terminal domain of Arabidopsis RBR (GST-RBR-C), the RBR cDNA fragment corresponding to the amino acid residues 858–1014 was amplified by PCR and cloned into the pGEX-4T-1 vector (Amersham Bioscience). GST-RBR-C proteins were purified using Glutathione Excellose resin (Amersham Bioscience) following the manufacturer’s instructions. The p13Suc1-associated CDK activity was assayed as described by Boniotti and Gutierrez (2001) using histone H1 (Millipore) or the purified recombinant GST-RBR-C proteins as substrates.
Sucrose gradient sedimentation
Sucrose density gradient centrifugation was performed according to Cho et al. (2013).
Quantitative analysis of endogenous jasmonic acid and abscisic acid content
Frozen samples (0.15–0.17g) were grinded and phytohormones were extracted with 1ml of ethyl acetate (Lee et al., 2015). Ultra-performance liquid chromatography (ACQUITY®UPLC system, Waters Corp., Milford, MA, USA) coupled with a QTOF instrument (XEVO G2XS; Waters Corp.) was used for the analysis. The chromatographic separation was performed on an ACQUITY®UPLC BEH C18 column (100×2.1mm, i.d. 1.7 μm). The mobile phases consisted of solvent A (0.1% formic acid) and solvent B (acetonitrile). The gradient elution mode was programmed as follows: 20–25% B for 0.0–5.0min and 25–35% B for 5.0–10.0min. Mass spectrometry analysis was conducted in the negative ion mode with electrospray ionization (ESI).
Statistical analyses
Two-tailed Student’s t-tests were performed using the Minitab 16 program (Minitab Inc.; http://www.minitab.com) to investigate the statistical differences between the responses of the samples. Significant differences between controls and other samples are indicated as follows: *P≤0.05 and **P≤0.01.
Results
Characterization of dexamethasone (DEX)-inducible BOP1 and WDR12 RNAi lines
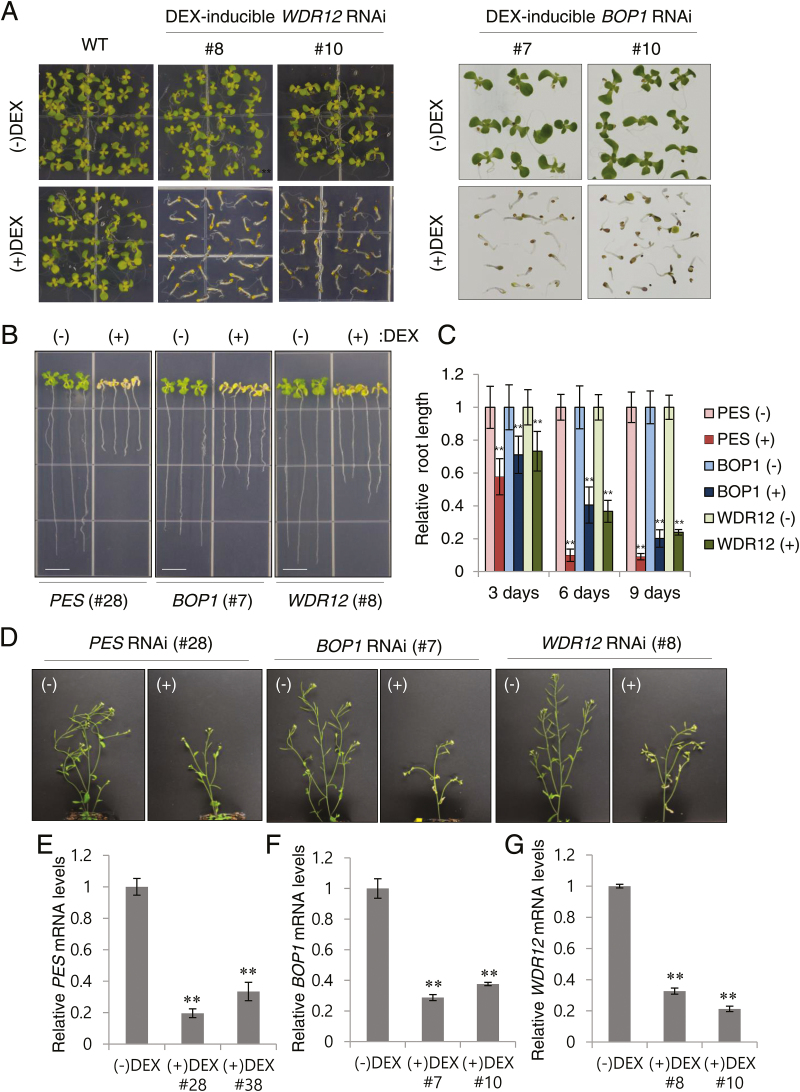
We generated DEX-inducible RNAi lines of BOP1 and WDR12 in Arabidopsis, in addition to the PES RNAi lines generated previously (Cho et al., 2013). Transgenic Arabidopsis plants (Col-0 ecotype) carried either a BOP1 RNAi construct that had an inverted repeat of a 291-bp BOP1 cDNA fragment or a WDR12 RNAi construct that had an inverted repeat of a 300-bp WDR12 cDNA fragment, both of which were under the control of a DEX-inducible transcription system (Aoyama and Chua, 1997). When sown on media containing 10 µM DEX, shoot growth of two independent PES (#28 and #38), BOP1 (#7 and #10) and WDR12 (#8 and #10) RNAi lines was immediately arrested following germination and plants died prematurely, mostly without forming true leaves, whereas seedlings grew normally on ethanol-containing medium (–DEX) (Fig. 1A and Supplementary Fig. S1; Cho et al., 2013). When these RNAi seedlings were grown on MS media for 7 d and then transferred to media containing 10 µM DEX for additional growth for 3–9 d, they exhibited precocious senescence of aerial tissues, anthocyanin accumulation, and retarded root growth compared with the control treatment (–DEX) (Fig. 1B, C; Supplementary Fig. S2A). These results suggest that PES, BOP1, and WDR12 play a critical role in early plant growth and development. When RNAi plants were grown in soil for 3 weeks and then sprayed with ethanol (–DEX) or 30 µM DEX for 5 d, DEX inhibited further development of inflorescences and induced senescence of flowers, cauline leaves, and siliques (Fig. 1D). The effect of RNAi on PES, BOP1, and WDR12 mRNA levels in the independent RNAi seedlings was determined by real-time quantitative RT-PCR using gene-specific primers (Fig. 1E–G; Supplementary Table S1). After transfer to media containing 10 µM DEX for additional growth for 3 d, seedlings of the corresponding RNAi lines exhibited significantly reduced PES, BOP1, and WDR12 transcript levels as compared to (–)DEX samples, suggesting RNAi-induced gene silencing. DEX treatment for 4 d, but not for 2 d, resulted in a decrease in total cellular 25S and 18S rRNAs in BOP1 (#7 and #10) and WDR12 (#8 and #10) RNAi lines, suggesting rRNA degradation (see Supplementary Fig. S3), as previously observed in PES RNAi lines (Cho et al., 2013).
Fig. 1.
Growth arrest and premature senescence phenotypes of BOP1- and WDR12-silenced plants. (A) Growth arrest phenotype of Arabidopsis dexamethasone (DEX)-inducible BOP1 (#7 and #10) and WDR12 (#8 and #10) RNAi lines. Seedlings were germinated on MS media that contained either ethanol (–DEX) or 10 μM DEX. (B) Retarded root growth and premature senescence of aerial tissues. DEX-inducible PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings were grown on MS media and then transferred to (–)DEX or (+)DEX media for vertical growth. Scale bars are 9mm. (C) Root length measurements in RNAi seedlings 3, 6, and 9 d after transfer to (–)DEX or (+)DEX media. Each data point represents the mean ± SD (n=20). Asterisks denote statistical significance as follows: *, P≤0.05; **, P≤0.01. (D) Premature senescence of the inflorescence in DEX-treated RNAi plants.
(E–G) Relative transcript levels in RNAi lines. Real-time quantitative RT-PCR analyses were performed on two independent PES (E), BOP1 (F), and WDR12 (G) RNAi lines. Transcript levels were quantified relative to (–)DEX samples using UBC10 mRNA levels as a control. Each value represents the mean ±SD of three replicates per experiment. (This figure is available in colour at JXB online.)
Subcellular localization of Arabidopsis BOP1, WDR12, and their mutants
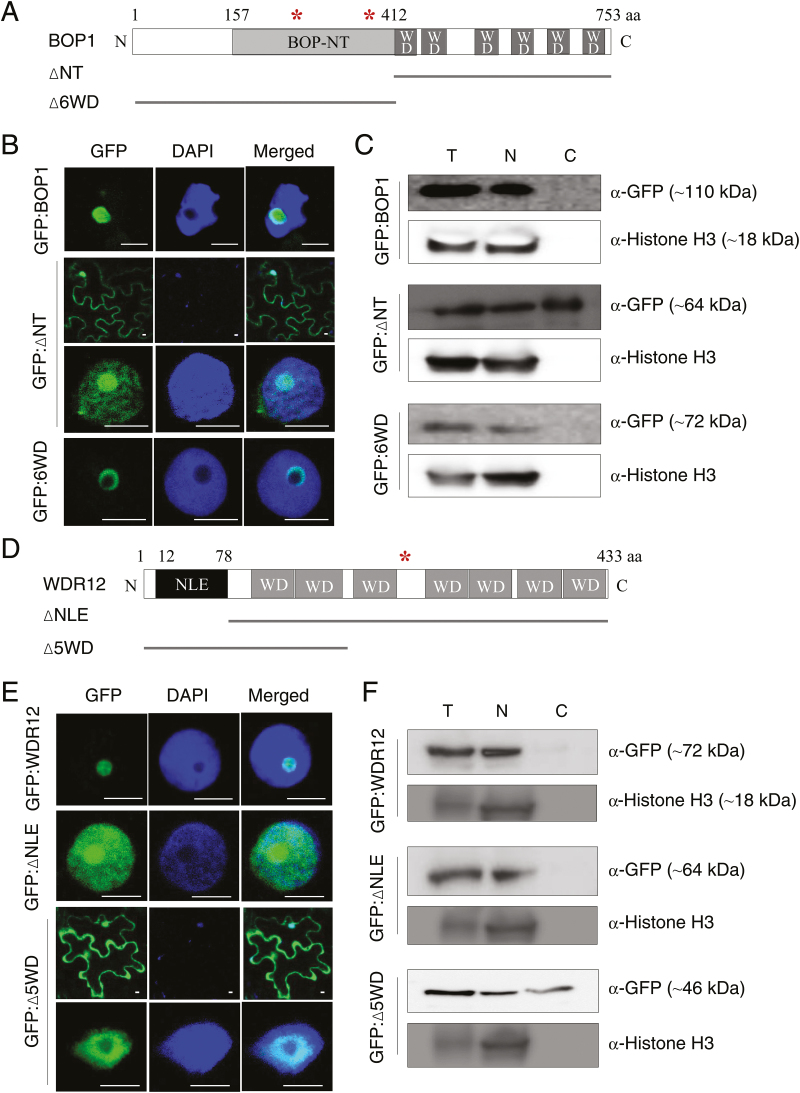
Previously, we have shown that BOP1 and WDR12 are mainly localized in the nucleolus (Cho et al., 2013). To determine which protein domains contribute to nucleolar localization, GFP fusion proteins of BOP1 and WDR12 deletion mutants were expressed in N. benthamiana leaves via agroinfiltration. BOP1 contains the BOP1 N-terminal domain (BOP-NT), six WD40 domains in the C-terminus, and two nuclear localization signals (marked with asterisks in Fig. 2A). Confocal laser scanning microscopy of leaf epidermal cells revealed that GFP:BOP1 predominantly localized in the nucleolus. However, deletion of the N-terminal region (∆NT), including the BOP-NT domain, resulted in BOP1 distribution in the nucleus and the cytosol, suggesting the importance of the N-terminal region for BOP1 nucleolar localization (Fig. 2B). When the C-terminal region containing six WD40 domains was deleted (∆6WD), GFP fluorescence was mostly detected in the nucleolar periphery (Fig. 2B). To confirm the subcellular localization of the BOP1 mutants, total (T), nuclear (N), and cytosolic (C) protein fractions were prepared from the agroinfiltrated N. benthamiana leaves, and subjected to immunoblot analyses using anti-GFP antibodies and anti-histone H3 antibodies as a nuclear marker (Fig. 2C). GFP:BOP1 and GFP:∆6WD were mostly associated with nuclear fractions, whereas GFP:∆NT was present in both nuclear and cytosolic fractions, consistent with the confocal data (Fig. 2B).
Fig. 2.
Subcellular localization of BOP1, WDR12, and their mutants. (A) Schematic of BOP1 and its deletion mutants (∆NT and ∆6WD). BOP-NT, BOP1 N-terminal domain; WD, WD40 domain. Nuclear localization signals (asterisks) are marked at amino acid residues 253 and 386. aa, amino acids. (B) Subcellular localization of BOP1 and its mutants using GFP fusion. The infiltrated leaves were briefly stained with DAPI to mark nuclei and examined by confocal laser scanning microscopy. Scale bars are 5 µm. (C) Subcellular fractionation. Nicotiana benthamiana leaf extracts expressing GFP fusion proteins of BOP1 and its mutants were fractionated and subjected to immunoblotting with anti-GFP antibodies. Total (T), nuclear (N), and cytosolic (C) fractions are indicated. Histone H3 was detected as a nuclear marker protein. The sizes of the protein bands are indicated. (D) Schematic of WDR12 and its deletion mutants (∆NLE and ∆5WD). NLE, Notchless-like domain; WD, WD40 domain. An asterisk at residue 246 indicates the nuclear localization signal. (E) Subcellular localization of WDR12 and its mutants using GFP fusion. Scale bars are 5 µm. (F) Leaf extracts expressing GFP fusion proteins of WDR12 and its mutants were fractionated and subjected to immunoblotting as described in (C). The sizes of the protein bands are indicated. (This figure is available in colour at JXB online.)
WDR12 contains the Notchless-like domain (NLE) at its N-terminus, followed by seven WD40 domains, and a nuclear localization signal at its center (Fig. 2D). In contrast to the predominant nucleolar localization of GFP:WDR12, GFP:∆NLE lacking the NLE domain was distributed throughout the nucleus (Fig. 2E). Green fluorescence of GFP:∆5WD lacking five C-terminal WD40 domains was observed in both the nucleus and the cytosol. The ∆7WD mutant that lacks all of the seven WD40 domains was not stably expressed in N. benthamiana leaves, regardless of the position of GFP tagging. These results suggest that both the NLE and C-terminal WD40 domains function in nucleolar localization of WDR12. Immunoblotting using anti-GFP antibodies detected GFP:∆NLE in the nuclear fraction, and GFP:∆5WD in both nuclear and cytosolic fractions (Fig. 2F), supporting the results of confocal microscopy (Fig. 2E).
In vivo interactions and ribosome association of the BOP1 and WDR12 mutants
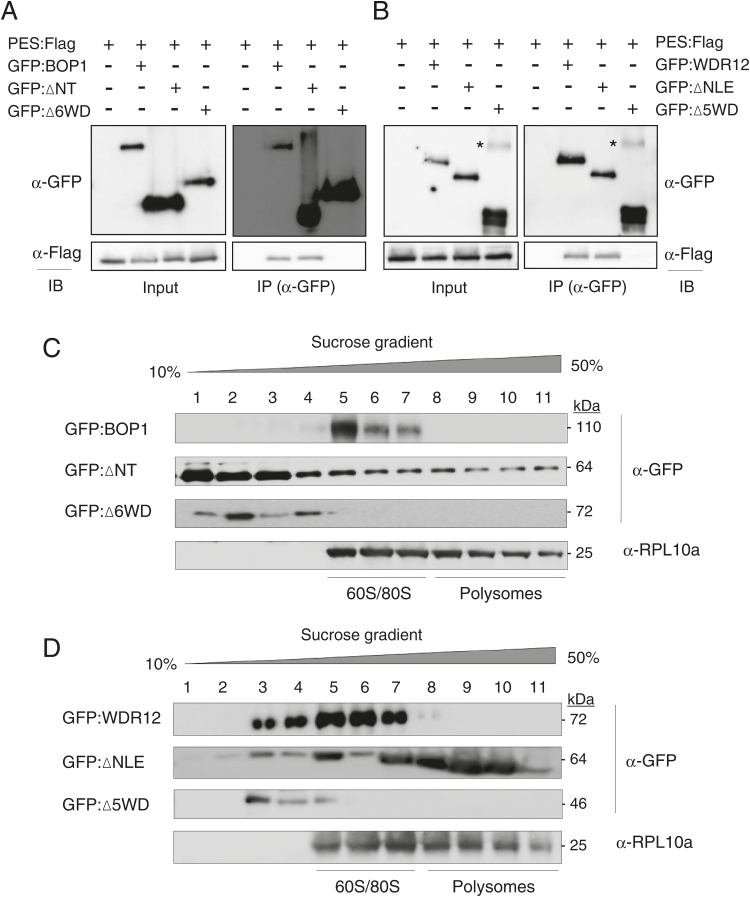
To demonstrate in vivo protein interactions between PES and deletion mutants of BOP1 and WDR12, co-immunoprecipitation was performed. Flag-fused PES (PES:Flag) was expressed together with GFP-fused BOP1, ∆NT, or ∆6WD in N. benthamiana leaves by agroinfiltration (Fig. 3A). Protein expression was confirmed by immunoblotting with anti-Flag and anti-GFP antibodies (input). GFP-fusion proteins were immunoprecipitated from cell extracts of the infiltrated leaves with anti-GFP antibodies. Immunoblotting was performed with anti-GFP antibodies to detect immunoprecipitated GFP-fusion proteins, and then with anti-Flag antibodies to detect PES:Flag as co-immunoprecipitants. GFP:BOP1 and GFP:∆NT were co-immunoprecipitated with PES:Flag, but co-immunoprecipitation did not take place when PES:Flag was expressed alone or co-expressed with GFP:∆6WD (Fig. 3A and Supplementary Fig. S4A). These results suggest that the six C-terminal WD domains are important for BOP1 interaction with PES (Fig. 3A). Similarly, GFP:WDR12 and GFP:∆NLE, but not GFP:∆5WD, were co-immunoprecipitated with PES:Flag, suggesting that the five C-terminal WD domains are important for WDR12 interaction with PES (Fig. 3B and Supplementary Fig. S4B).
Fig. 3.
Protein interactions and co-fractionation with ribosome subunits. (A) Co-immunoprecipitation of BOP1 with PES. Protein extracts were subjected to immunoprecipitation (IP) with anti-GFP antibody, and then co-immunoprecipitated PES:Flag was detected by immunoblotting (IB) with anti-Flag antibody. The asterisks indicate non-specific protein bands. (B) Co-immunoprecipitation of WDR12 with PES. Co-immunoprecipitation was performed as described in (A). (C) Co-fractionation of BOP1 and its mutants with ribosome subunits. After sedimentation of ribosomes through a sucrose density gradient, the fractions were subjected to immunoblotting with anti-GFP and anti-ribosomal protein L10a (RPL10a) antibodies. Lanes 1–11 indicate gradient fractions from top (10%) to bottom (50%). (D) Co-fractionation of WDR12 and its mutants with ribosome subunits. Sucrose density gradient centrifugation and immunoblotting were performed as described in (C).
To investigate which protein domains are important for the association of BOP1 and WDR12 with ribosomes, leaf cell extracts expressing GFP-fusion proteins of BOP1, WDR12, and their deletion mutants were fractionated on a 10–50% sucrose density gradient. After ultracentrifugation, fractions were collected, and immunoblot analysis was performed with anti-GFP antibodies (Fig. 3C, D). As a control, the same fractions were reacted with anti-RPL10a antibodies to detect the ribosomal protein L10a associated with 60S large ribosomal subunits, 80S monosomes, and polysomes. Full-length BOP1 was co-fractionated with the 60S/80S ribosome, whereas the ∆6WD mutant was detected in the lighter fractions of the gradient (Fig. 3C). ∆NT lacking the BOP1 N-terminus was detected in every fraction throughout the gradient, suggesting a lack of interaction specificity (Fig. 3C). WDR12 was mainly detected in fractions containing the 60S/80S ribosome and partially in the lighter fractions, whereas the ∆5WD mutant was detected only in the lighter fractions (Fig. 3D). ∆NLE was detected in most fractions of the gradient, lacking interaction specificity. Collectively, these results suggest that both the N-terminal domains and C-terminal WD40 domains are required for the specific association of BOP1 and WDR12 with 60S/80S ribosomes.
Translocation of PES, BOP1, and WDR12 into the nucleoplasm upon drug treatment
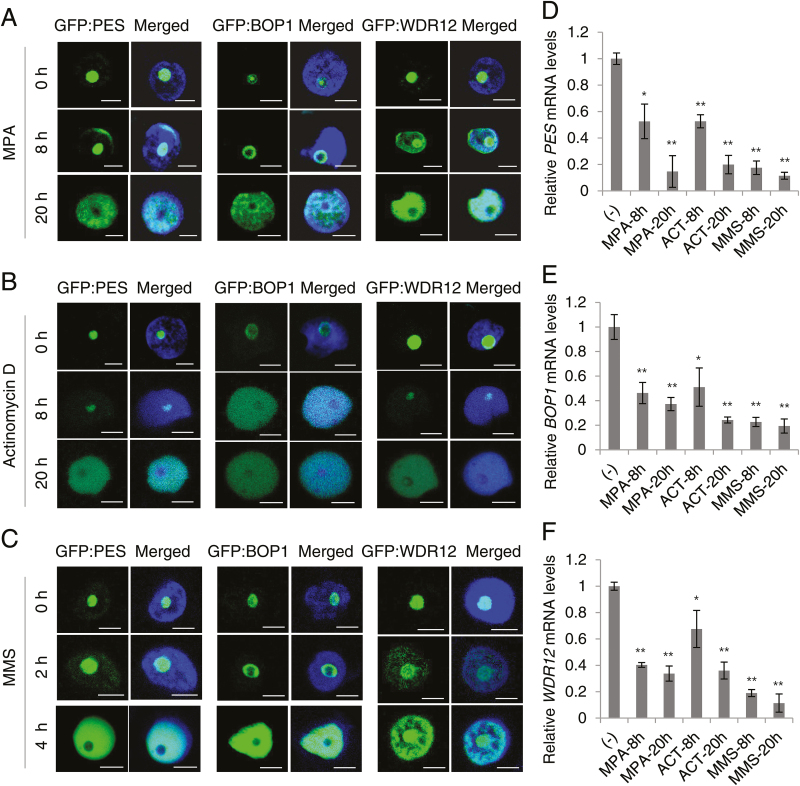
Treatment of animal and plant cells with a drug that depletes cellular guanine nucleotides or disrupts pre-rRNA synthesis leads to nucleolar disruption and an efflux of nucleolar proteins, such as nucleostemin, nucleolin, and nucleophosmin, into the nucleoplasm (Mayer and Grummt, 2005; Tsai and McKay, 2005; Boisvert et al., 2007; Jeon et al., 2015). We tested if nucleolar localization of PeBoW proteins is affected by these drugs. Nicotiana benthamiana leaves were agroinfiltrated to express GFP fusion proteins of PES, BOP1, and WDR12, and then treated with mycophenolic acid (MPA), actinomycin-D, or methyl methanesulfonate (MMS). MPA inhibits de novo synthesis of guanine nucleotides, subsequently disrupting rRNA synthesis and inducing nucleolar stress in human cells (Tsai and McKay, 2005; Huang et al., 2008). A low concentration of actinomycin-D specifically inhibits RNA polymerase I and blocks rRNA transcription (Tsai and McKay, 2005). MMS damages RNA as well as DNA by alkylation, and damaged rRNA molecules can lead to ribosomal dysfunction (Revenkova et al., 1999; Lundin et al., 2005). Confocal microscopy revealed that PES, BOP1, and WDR12 migrated from the nucleolus to the nucleoplasm 8 to 20h post-treatment with MPA or actinomycin-D (Fig. 4A, B). Translocation occurred even faster after MMS treatment, taking place within 4h (Fig. 4C). These results suggest that disrupted rRNA synthesis and nucleolar stress caused repartitioning of PeBoW proteins into the nucleoplasm. Real-time quantitative RT-PCR analyses revealed that transcript levels of PES, BOP1, and WDR12 all decreased upon drug treatment (Fig. 4D–F). Although the mechanism of protein translocation is unclear, efflux of biogenesis factors, such as PES, BOP1, and WDR12, may represent a cellular response to stresses by curtailing ribosome biogenesis, an extremely resource-demanding process.
Fig. 4.
Translocation of PeBoW proteins into the nucleoplasm in response to drug treatment. (A) Effects of mycophenolic acid (MPA). Nicotiana benthamiana leaves were agroinfiltrated with GFP fusion constructs and then treated with MPA (20 µM) for 0, 8, and 20h. Merged images with DAPI-stained nuclei are shown. Scale bars are 5 µm. (B) Effects of actinomycin-D (5 µM) after 0, 8, and 20h treatment. (C) Effects of methyl methanesulfonate (MMS; 0.03%) after 0, 2, and 4h treatment. (D–F) Real-time quantitative RT-PCR. Transcript levels of PES (D), BOP1 (E), and WDR12 (F) after drug treatment were compared with those prior to treatment (–). The UBC10 mRNA level was used as a control. (This figure is available in colour at JXB online.)
Kinematic analyses of leaf growth
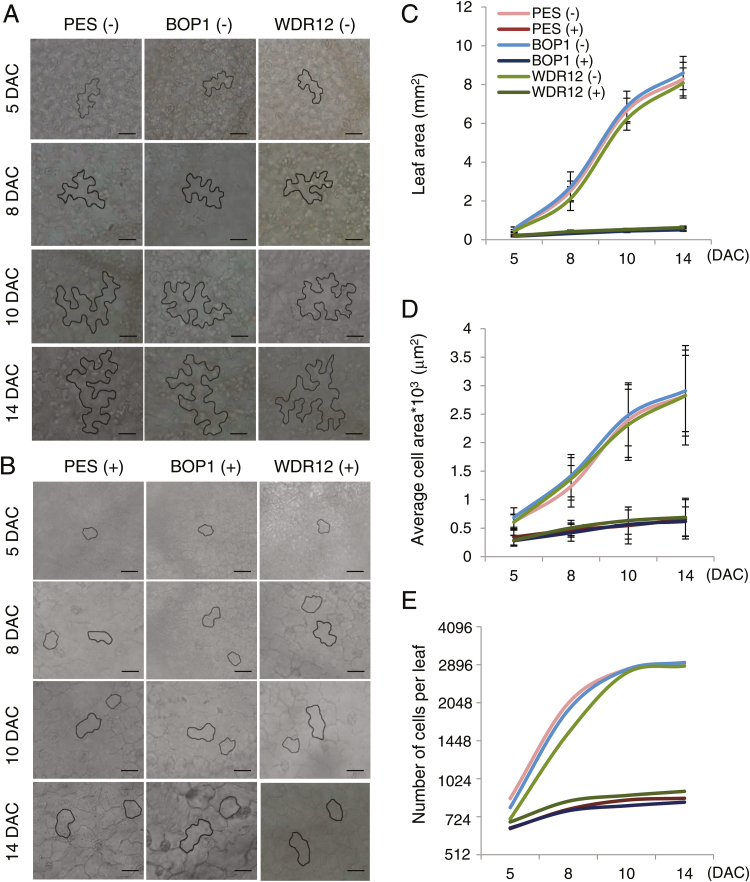
Cell division and cell expansion are two parameters of organ growth (Horiguchi et al., 2006; Anastasiou and Lenhard, 2007). To determine the effect of PeBoW depletion on cell division and expansion during leaf development, kinematic analyses were performed on the first true leaves of PES (#28), BOP1 (#7), and WDR12 (#8) RNAi plants (Fig. 5). After spraying with ethanol (–DEX) or 20 µM DEX, the first leaves of four-to-five plants were harvested at 5, 8, 10, and 14 d after cotyledon emergence (DAC) to assess leaf size and abaxial epidermal cell size, and to calculate the epidermal cell number using these two values (Fig. 5A, B). In DEX-treated plants, necrotic lesions developed in the first leaves after 14 DAC. The average leaf area and average epidermal cell area of ethanol-treated PES, BOP1, and WDR12 RNAi lines progressively increased up to 14 DAC, whereas those values barely increased in DEX-treated RNAi plants (Fig. 5C, D and Supplementary Table S2). There was a ~14.9-fold and ~4.4-fold difference in the average leaf area and epidermal cell area between ethanol- and DEX-treated samples at 14 DAC, respectively. The epidermal cell number per leaf increased rapidly during the early stages and then remained nearly constant from 10 DAC onward for ethanol-treated RNAi plants. The cell number only slightly increased in DEX-treated plants; the estimated cell number was ~3.4-fold lower than that of ethanol-treated plants at 14 DAC (Fig. 5E and Supplementary Table S2). These results suggest that PeBoW protein depletion strongly represses both leaf cell division and expansion. Interestingly, while the epidermal pavement cells of ethanol-treated plants had a fully differentiated puzzle-shaped structure, those of DEX-treated samples exhibited a much simpler structure with little lobe formation up to 14 DAC (Fig. 5A, B). DEX treatment also caused similar defects in leaf epidermal cell growth and morphology in PES (#38), BOP1 (#10), and WDR12 (#10) RNAi lines (see Supplementary Fig. S2B). Collectively, these results suggest that defective ribosome biogenesis caused by PeBoW protein depletion inhibited cell division, cell expansion, and pavement cell differentiation.
Fig. 5.
Kinematic analysis of leaf growth. PES (#28), BOP1 (#7), and WDR12 (#8) RNAi plants were grown in soil and sprayed with ethanol (–) or 20 µM DEX (+). The first leaves were collected from the plants at 5, 8, 10, and 14 d after cotyledon emergence (DAC).
(A, B) Representative abaxial epidermal cells from the leaves of RNAi seedlings sprayed with ethanol (A) or DEX (B). Individual cells are visualized by black outlines using ImageJ. Scale bars are 20 µm. (C) Average leaf area. (D) Average leaf epidermal cell area. (E) Calculated number of epidermal cells per leaf. (This figure is available in colour at JXB online.)
Reduced cyclin-dependent kinase activity in PeBoW-deficient plants
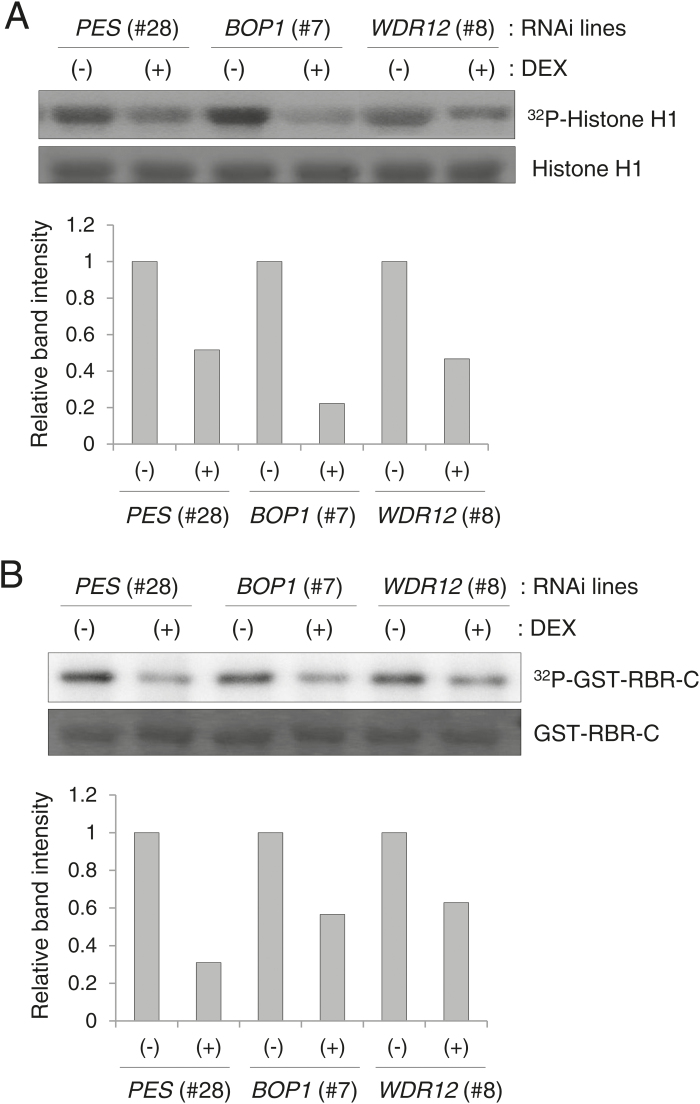
To investigate the molecular mechanisms of reduced cell division in PES (#28), BOP1 (#7), and WDR12 (#8) RNAi plants, we examined cyclin-dependent kinase (CDK) activity (Fig. 6). In plants, CDK Type A (CDKA), an authentic PSTAIRE CDK, plays a critical role at both the G1/S and G2/M phase transitions (Inzé and De Veylder, 2006; De Veylder et al., 2007). CDKA phosphorylates histone H1 and the C-terminal domain of retinoblastoma-related (RBR) protein in vitro as a substrate, and CDKA activity is positively correlated with the cell division rate (Boniotti and Gutierrez, 2001). Each RNAi line was grown for 7 d in MS media, and then transferred to MS media containing ethanol (–DEX) or 10 µM DEX for further growth for 3 d. Next, protein extracts prepared from the seedlings were incubated with p13SUC1 beads that bind to PSTAIRE CDKs with high affinity. The glutathione S-transferase (GST) fusion protein of the C-terminal domain of Arabidopsis RBR (GST-RBR-C) was purified from Escherichia coli. In vitro kinase assays were performed with the p13SUC1 beads and histone H1 (Fig. 6A) or GST-RBR-C (Fig. 6B) as a substrate. Quantification of the phosphoprotein band intensities using ImageJ (http://imagej.nih.gov/ij/) revealed that the levels of phosphorylated histone H1 and GST-RBR-C were significantly reduced in (+)DEX plants, compared with those in (–)DEX plants, suggesting reduced CDKA activity in PES-, BOP1-, and WDR12-deficient seedlings (Fig. 6A, B).
Fig. 6.
In vitro phosphorylation of histone H1 and the RBR C-terminus by CDKA. (A) Total CDKPSTAIRE was bound to p13Suc1-conjugated agarose beads, and in vitro kinase assays were performed using histone H1 as a substrate. After SDS-PAGE, the gel was dried and analyzed with a phosphorimager to detect 32P-labeled histone H1, while a duplicate gel was stained with Coomassie blue to show histone H1 proteins loaded in each lane (top). Band intensities of phosphorylated histone H1 in (+)DEX samples are compared with those in (–)DEX samples (bottom). (B) In vitro kinase assays were performed as described in (A) using the GST fusion protein of the C-terminal domain of RBR (GST-RBR-C) as a substrate (top). Relative band intensities of phosphorylated GST-RBR-C are shown (bottom).
Real-time quantitative RT-PCR analyses of cell cycle-related gene expression
The E2F-RBR pathway plays a critical role in cell-cycle progression by regulating the G1/S transition in plants (Inzé and De Veylder, 2006; De Veylder et al., 2007; Sablowski and Dornelas, 2014). RBR interacts with D cyclins (CycD) through a conserved LxCxE motif, and is phosphorylated by the CDKA/CycD complex depending on the cell-cycle phase (Boniotti and Gutierrez, 2001; Nakagami et al., 2002). In a hypophosphorylated form, RBR interacts with the E2F/DP complex to repress transcription of the E2F target genes required for S phase entry, DNA replication, cell-cycle progression, and chromatin dynamics (Egelkrout et al., 2002; Ramirez-Parra et al., 2003; Kuwabara and Gruissem, 2014). Plant E2Fs are activated following RBR phosphorylation and function as transcriptional regulators; E2Fa and E2Fb are transcriptional activators and positive regulators of the cell cycle, whereas E2Fc is a transcriptional repressor and suppressor of cell division (Inzé and De Veylder, 2006; De Veylder et al., 2007).
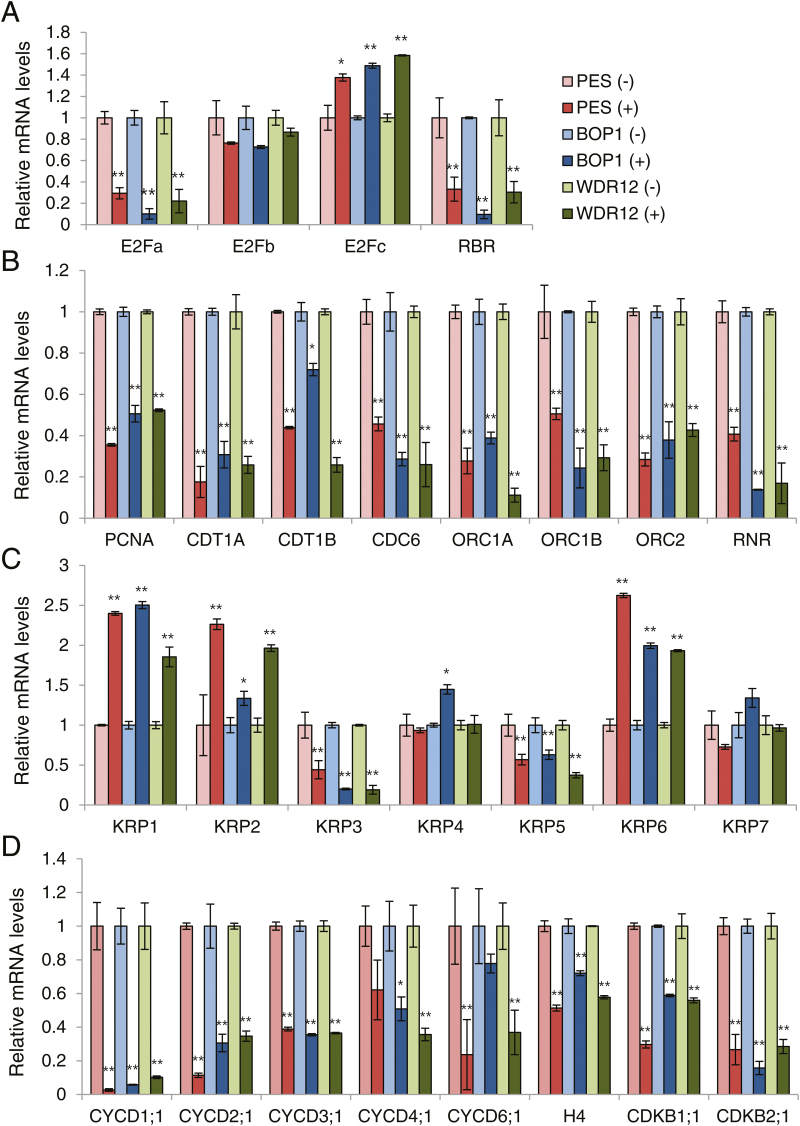
We examined cell cycle-related gene expression in the RNAi plants (Fig. 7). The PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings at 3 DAC in soil were sprayed with ethanol or 20 µM DEX for 5 d, and the first true leaves were harvested for real-time quantitative RT-PCR. Among the E2F-RBR pathway genes, transcript levels of E2Fa, E2Fb, and RBR were reduced, whereas E2Fc transcript levels were elevated in all RNAi lines following DEX treatment (Fig. 7A). Transcript levels of S phase-specific genes, including proliferating cell nuclear antigen (PCNA), chromatin licensing and DNA replication factor 1A (CDT1A), CDT1B, cell division cycle 6 (CDC6), origin recognition complex 1A (ORC1A), ORC1B, ORC2, and ribonucleotide reductase (RNR) were all significantly reduced in PES-, BOP1-, and WDR12-depleted leaves, suggesting reduced cell proliferation (Fig. 7B). Among seven Arabidopsis genes encoding Kip-related proteins (KRPs), which inhibit the activity of CDK/CycD complexes, KRP1, KRP2, and KRP6 were significantly up-regulated in DEX-treated samples, whereas expression of other KRP genes either decreased or remained constant (Fig. 7C). Furthermore, DEX treatment led to down-regulation of all of the CycD family genes tested (Fig. 7D). Transcript levels of histone H4, CDKB1;1, and CDKB2;1, all of which are expressed in actively dividing cells (Boudolf et al., 2004), were also reduced in the first leaves of the RNAi plants following DEX treatment (Fig. 7D). It is known that CDKA and the E2F-RBR pathway play important roles in cell-cycle progression from G1 to S phase in plant cells (Inzé and De Veylder, 2006; De Veylder et al., 2007; Sablowski and Dornelas, 2014). Reduced CDKA activity (Fig. 6) and the observed gene expression profiles suggest that impaired ribosome biogenesis caused by PeBoW deficiency inhibits the G1/S phase transition.
Fig. 7.
Expression of cell cycle-related genes. Real-time quantitative RT-PCR analyses were performed using the first leaves of the PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings sprayed with ethanol (–) or 20 µM DEX (+). Transcript levels are quantified relative to (–)DEX samples using UBC10 mRNA levels as a control. Data points represent means ±SD of three experiments. Asterisks denote statistical significance of the differences between the (–)DEX and (+)DEX samples: *, P≤0.05; **, P≤0.01. (A) E2F/RBR pathway genes. (B) S-phase genes. (C) KRP family genes. (D) CycD family, histone H4, and CDKB genes. (This figure is available in colour at JXB online.)
Expression of cell cycle-related genes during the early response to PeBoW silencing
To examine the early effects of PeBoW silencing on expression of cell-cycle genes, an Arabidopsis liquid culture system was employed (see Supplementary Fig. S5A). PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings were grown in liquid culture, and at 7 d after sowing (DAS), the seedlings were treated with ethanol (–DEX) or 20 µM DEX for 12h. Real-time quantitative RT-PCR suggested that 12-h DEX treatment caused significant silencing of their corresponding genes in the first leaves (Supplementary Fig. S5B). Silencing of the PeBoW genes subsequently caused visible changes in gene expression patterns of S-phase genes and cell cycle-related genes in the first leaves (Supplementary Fig. S6). Expression of E2Fa and all of the S phase-specific genes tested (PCNA, CDT1A, CDT1B, CDC6, ORC1A, ORC1B, ORC2, and RNR) was significantly reduced, while expression of KRP1 was up-regulated. Furthermore, histone H4, CDKB1;1, and CDKB2;1, as well as most of the CycD family genes tested except CYCD3;1, were down-regulated upon 12-h DEX treatment (Supplementary Fig. S6). Gene expression profiles after 24-h DEX treatment mostly mimicked the patterns observed after 12-h treatment, but the differences between (–)DEX and (+)DEX samples were more pronounced (Supplementary Fig. S7). Noticeably, 24-h DEX treatment caused down-regulation of E2Fb and E2Fc, and up-regulation of KRP7. CYCD3;1 transcript levels, which were up-regulated after 12-h DEX treatment, changed to the control levels after 24-h treatment. Thus, PeBoW silencing caused rapid transcriptional modification of the cell-cycle genes in the first leaves, the patterns of which suggest that nucleolar stress immediately inhibits cell cycle progression. Furthermore, several key cell-cycle modulators, such as E2Fb, E2Fc, and KRPs, exhibited different transcriptional modulation depending on the duration of DEX treatment and plant growth/culture conditions (Fig. 7, Supplementary Figs S6 and S7).
We next examined the early effect of PeBoW silencing on cell proliferation by analyzing the first leaves of PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings (7 DAS) grown in liquid culture, after treatment with ethanol (–DEX) or 20 µM DEX for 24h (see Supplementary Fig. S8). The average leaf area, abaxial epidermal cell area, and the estimated epidermal cell number all increased within the 24-h period in all of the ethanol-treated RNAi lines (–DEX). However, those values barely changed in DEX-treated samples, suggesting blocked cell division and expansion. Collectively, these results suggest that the nucleolar stress caused by PeBoW depletion induces rapid transcriptional changes of cell-cycle genes, leading to almost immediate arrest of cell proliferation and expansion in actively dividing leaf cells.
Expression of auxin- and jasmonic acid-related genes during the early response to PeBoW silencing
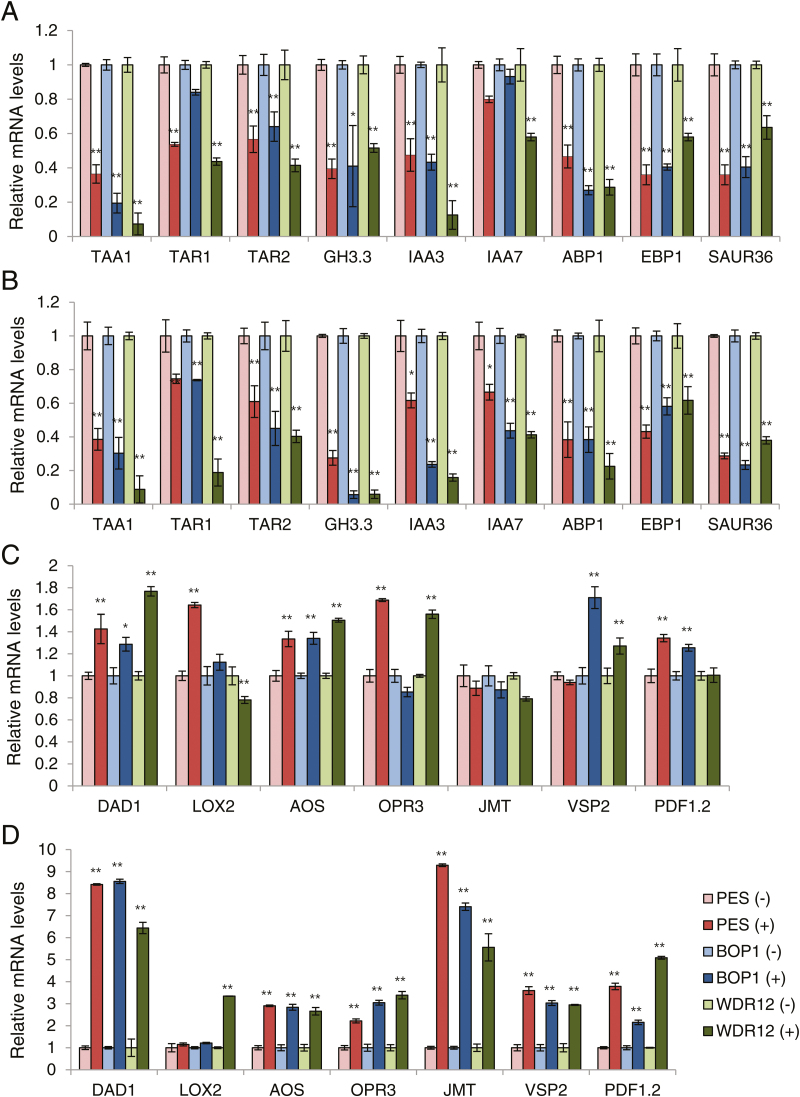
Since auxin is a major positive regulator of cell division and cell expansion in plants, we next tested whether transcriptional changes of auxin-related genes are involved in the early response to PeBoW silencing. PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings (7 DAS) grown in liquid culture were treated with ethanol (–DEX) or 20 µM DEX for 12h or 24h, and real-time quantitative RT-PCR was performed with RNA isolated from the first leaves. 12-h DEX treatment caused a visible reduction in transcript levels of auxin biosynthesis-related genes, TAA1 (tryptophan aminotransferase of Arabidopsis 1), TAR1 (tryptophan aminotransferase related 1) and TAR2, and those of auxin-responsive genes, GH3.3, IAA3 (indole-3-acetic acid protein 3), IAA7, EBP1 (ErbB3 binding protein 1), and SAUR36 (small auxin-up RNA 36) in all of the RNAi lines (Fig. 8A). Expression of ABP1 encoding a putative auxin receptor was also down-regulated after 12-h DEX treatment. 24-h DEX treatment mostly caused a similar reduction in transcript levels compared to 12-h treatment, while several genes, such as GH3.3 and IAA7, were further down-regulated (Fig. 8B). Thus, leaf cells’ early response to nucleolar stress includes rapid inhibition of auxin biosynthesis and signaling, which would negatively affect cell growth and proliferation.
Fig. 8.
Real-time quantitative RT-PCR analyses to determine transcript levels of auxin- and JA-related genes after 12- and 24-h DEX treatments. The PES (#28), BOP1 (#7), and WDR12 (#8) RNAi seedlings (7 DAS) grown in liquid culture were treated with ethanol (–) or 20 µM DEX (+) for 12h or 24h. The first leaves were collected for the analyses. Transcript levels were quantified relative to (–)DEX samples using UBC10 mRNA levels as a control. Each value represents the mean ±SD of three replicates per experiment. *, P≤0.05; **, P≤0.01. (A) Auxin-related genes after 12-h DEX treatment. (B) Auxin-related genes after 24-h DEX treatment. (C) JA-related genes after 12-h DEX treatment. (D) JA-related genes after 24-h DEX treatment. (This figure is available in colour at JXB online.)
Previous studies have suggested that the stress hormone jasmonic acid (JA) inhibits plant cell-cycle progression, repressing both cell division and expansion (Świa̧tek et al., 2002, 2004; Zhang and Turner, 2008; Noir et al., 2013; Wasternack and Hause, 2013). We tested if the nucleolar stress caused by PeBoW deficiency activates transcriptional changes of JA-related genes in the first leaves. 24-h DEX treatment resulted in significant transcriptional up-regulation of JA biosynthesis-related genes, DAD1 (defective in anther dehiscence 1), LOX2 (lipoxygenase 2), AOS (allene oxide synthase), OPR3 (12-oxo-phytodienoic acid reductase 3) and JMT (S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase), and JA-responsive genes, VSP2 (vegetative storage protein 2) and PDF1.2 (plant defensin 1.2), in all of the RNAi lines (Fig. 8D). DEX treatment for 12h caused only a mild up-regulation of most JA-related genes tested (Fig. 8C). Furthermore, the RNAi seedlings treated with 5 µM DEX for 6 d contained elevated endogenous JA contents, ~4.9 to ~15-fold higher than those of (–)DEX seedlings, consistent with the transcriptional up-regulation described above (see Supplementary Fig. S9). Cellular contents of another stress hormone abscisic acid were not significantly changed. Thus, nucleolar stress caused by PeBoW deficiency upregulated JA biosynthesis and signaling. Collectively, these results suggest an involvement of phytohormones, such as auxin and JA, in suppression of cell division and cell expansion in early stages of the nucleolar stress response in plants.
Discussion
In this study, we characterized further the protein structures and in planta functions of the nucleolar proteins PES, BOP1, and WDR12, and examined the effects of their deficiency on cell growth and proliferation. We identified protein domains of BOP1 and WDR12 that are required for nucleolar localization, protein interactions, and ribosome co-fractionation. Depletion of the PeBoW components involved in ribosome biogenesis caused reduced CDKA activity with hypophosphorylation of RBR, and rapid changes in gene expression profiles of cell cycle genes and auxin- and jasmonic acid-related genes, leading to immediate inhibition of leaf cell growth and proliferation.
Cell growth and cell proliferation are tightly linked, as cells cannot divide without reaching a certain cell mass. The key determining factor in cell growth is ribosome biogenesis, which, not surprisingly, is closely linked to cell-cycle regulation. Disruption of PeBoW functions result in rapid cell-cycle arrest in mammals. Expression of BOP1∆, a dominant-negative form of BOP1, in human cells almost immediately blocks rRNA processing (Pestov et al., 2001). The blocked 28S rRNA synthesis subsequently causes p53 activation, prohibiting cell-cycle progression through the G1/S checkpoint. In these cells, G1-specific CDK2 and CDK4 activities are down-regulated, the levels of CDK inhibitors p21Cip1 and p27Kip1 are elevated, and the cells lack hyperphosphorylated pRb (Pestov et al., 2001). Similarly, down-regulation of PES in human breast cancer cells inhibits cell-cycle progression during the G1/S transition, dramatically reducing cyclin D1 and up-regulating the CDK inhibitor p27Kip1 (Li et al., 2009). Conditional expression of a dominant negative mutant of WDR12 also induces rapid rRNA processing defects, followed by p53 activation and cell-cycle arrest (Hölzel et al., 2005). In this study, PeBoW deficiencies in plants caused hypophosphorylation of RBR, down-regulation of E2Fa and CycD transcripts, and up-regulation of several KRP gene transcripts encoding plant CDK inhibitors in the first leaves, suggesting cell-cycle inhibition at the G1/S transition (Figs 6, 7). In particular, transcriptional changes of those G1/S phase regulators occurred rapidly, after only 12h of PeBoW silencing, followed by almost immediate suppression of cell proliferation, suggesting that plant cells possess a highly sensitive detection mechanism for anomalies in ribosome biogenesis (see Supplementary Figs S6, S8). Ribosome biogenesis is a complex process that is particularly sensitive to diverse cellular stresses such as disturbed metabolism and unfavorable/toxic environmental conditions (Antoniali et al., 2014; Golomb et al., 2014). The inhibitory effect of nucleolar stress on cell-cycle progression, mostly occurring at the G1/S checkpoint, may represent an inherent surveillance mechanism that prevents DNA synthesis under less favorable metabolic conditions.
Analyses of short-term and longer-term effects of PeBoW silencing on gene expression revealed common and differential modulation of the cell-cycle genes, probably influenced by the duration of stress, plant developmental stages, and plant culture conditions. Down-regulation of E2Fa was commonly observed after 12h, 24h, and 5 d of DEX treatment under different growth conditions, and thus appears to be one of the key regulatory events against plant nucleolar stress (Fig. 7A and Supplementary Figs S6A, S7A). In contrast, expression of E2Fb and E2Fc fluctuated depending on the conditions of growth. Transcriptional activators E2Fa and E2Fb co-ordinately control cell division and endoreduplication, but display specific gene expression patterns and have distinct roles during cell-cycle progression (Magyar et al., 2005; Inzé and De Veylder, 2006; Sozzani et al., 2006; De Veylder et al., 2007; Sablowski and Dornelas, 2014). E2Fc functions as a repressor of cell division and inhibits expression of the S-phase genes, and plays a role in controlling the balance between cell proliferation and the switch to the endocycle program (del Pozo et al., 2002, 2006). The observed fluctuation of E2Fb or E2Fc transcript levels following PeBoW silencing may represent dynamic temporal regulation of E2Fb or E2Fc transcription in response to progressive nucleolar stress. In a similar fashion, up-regulation of CycD3;1 after 12-h DEX treatment may be an initial temporary response to PeBoW silencing (see Supplementary Fig. S6D), because CycD3;1 and E2Fa expression are correlated, and are known to act in a common pathway in controlling the G1/S transition (de Jager et al., 2009; Magyar et al., 2012). Since CYCD3;1 expression is induced by cytokinin (Riou-Khamlichi et al., 1999), there is a possibility that CycD3;1 was temporarily up-regulated in response to repressed auxin signaling in PeBoW-deficient plants.
We also observed that different KRP genes were up-regulated upon PeBoW silencing depending on the conditions; only KRP1 was induced after 12h DEX treatment, KRP1 and KRP7 after 24h treatment, and KRP1, KRP2, and KRP6 were induced after 5 d of DEX treatment (Fig. 7C and Supplementary Figs S6C, S7C). Thus, while different subsets of KRP genes responded to nucleolar stress under different conditions, KRP1 was commonly up-regulated, suggesting its leading role under the stress. KRP1 can move between leaf cells, and misexpression of KRP1 blocks both G1/S transition and entry into mitosis in a cell context-dependent manner, and induces cell death (Schnittger et al., 2003; Weinl et al., 2005). Based on recent analyses of single-to-quintuple mutants of Arabidopsis KRP genes, gradual changes in phenotypes from single- to higher-order mutants suggest that plant KRPs mostly function redundantly in a dose-dependent manner (Cheng et al., 2013). The finding that most of the E2F-regulated genes were up-regulated in Arabidopsis quintuple KRP mutants suggests that one mechanism of KRP function in cell division control is through regulation of E2Fs and E2F target genes (Cheng et al., 2013). Collectively, the variable transcriptional modulation of the key cell-cycle regulators in PeBoW-deficient plants under different conditions suggests plasticity of the plant response mechanisms against nucleolar stress.
In this study, nucleolar stress caused by PeBoW depletion induced rapid changes of gene expression in the first leaves that suggest down-regulation of auxin biosynthesis and signaling, and up-regulation of JA biosynthesis and signaling (Fig. 8). Simultaneously, epidermal cell proliferation in the first leaves was blocked (see Supplementary Fig. S8). Therefore, the opposite modulation of these phytohormone signals may act as an antimitogenic signal, inhibiting cell-cycle progression and causing rapid repression of cell proliferation in PeBoW-deficient plants. Auxin is a major regulator of plant growth and development, controlling both cell division and cell expansion. Auxin induces the expression of the core cell-cycle regulators, such as CycD, CDKA, and E2Fa, and reduces the expression of several KRP genes during shoot and root development, regulating the G1/S transition (Himanen et al., 2002; Braun et al., 2008; Perrot-Rechenmann, 2010). Auxin also increases E2Fb protein stability by modulating its proteolysis (Magyar et al., 2005). Auxin signaling pathways controlling the cell cycle may involve the auxin binding protein ABP1 and the AUX/IAA/SCFTIR1AFB pathways, which control the G1/S transition by acting on the CycD/RBR/E2F pathway (David et al., 2007; Tromas et al., 2009; Perrot-Rechenmann, 2010). We observed that ABP1 and several AUX/IAA genes were rapidly down-regulated upon PeBoW silencing, suggesting a possible involvement of the related pathways in cell-cycle repression during the early nucleolar stress response (Fig. 8A, B). Another phytohormone that seems to be associated with PeBoW deficiency is jasmonic acid (JA), based on the stimulated JA-related gene expression and the elevated JA contents (Fig. 8C, D and Supplementary Fig. S9). JA inhibits cell-cycle progression in synchronized tobacco BY-2 cells by blocking both G1/S and G2/M transitions (Świa̧tek et al., 2002). Methyl jasmonate suppresses Arabidopsis leaf growth by inhibiting cell proliferation and expansion, arresting leaf cells in the G1 phase (Noir et al., 2013). Treatment with coronatine, a high-affinity agonist of the JA receptor, also rapidly arrests Arabidopsis leaf growth, repressing the genes controlling cell division and expansion, such as D-type cyclins involved in the G1/S transition (Attaran et al., 2014). However, JA signaling components directly linked to the cell-cycle machinery are still unclear. Although not explored in this study, other phytohormones may also play a role in linking nucleolar status to cell-cycle control, either directly or indirectly through hormone cross-talk. Further studies would elucidate the signaling cascades linking nucleolar stress to the cell-cycle in plants.
Supplementary Data
Supplementary data are available at JXB online
Table S1. Primers used in this study.
Table S2. Data points for the kinematic analyses shown in Fig. 5.
Figure S1. Growth arrest phenotypes of PES-silenced plants.
Figure S2. Characterization of DEX-inducible PES (#38), BOP1 (#10), and WDR12 (#10) RNAi lines.
Figure S3. EtBr staining of total rRNA.
Figure S4. Unedited full images of Fig. 3A, B.
Figure S5. Gene silencing in RNAi seedlings grown in liquid culture.
Figure S6. Real-time quantitative RT-PCR analyses for the expression of cell cycle-related genes after 12-h DEX treatment.
Figure S7. Real-time quantitative RT-PCR analyses for the expression of cell cycle-related genes after 24-h DEX treatment.
Figure S8. Leaf cell division and expansion after 24-h DEX treatment.
Figure S9. Endogenous JA contents of the RNAi seedlings.
Acknowledgements
This research was supported by the Cooperative Research Program for Agriculture Science & Technology Development [Project numbers PJ01114701 (PMBC) and PJ01118901 (SSAC)] from the Rural Development Administration and the Mid-Career Researcher Program (No. 2014R1A2A1A11051690) from the National Research Foundation (NRF) of the Republic of Korea. Chang Sook Ahn was supported by the Research Fellow Scholarship (NRF-2013R1A1A2062026) from the NRF of the Republic of Korea.
References
- Adams CC, Jakovljevic J, Roman J, Harnpicharnchai P, Woolford JL., Jr 2002. Saccharomyces cerevisiae nucleolar protein Nop7p is necessary for biogenesis of 60S ribosomal subunits. RNA 8, 150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CS, Ahn H-K, Pai H-S. 2015. Overexpression of the PP2A regulatory subunit Tap46 leads to enhanced plant growth through stimulation of the TOR signaling pathway. Journal of Experimental Botany 66, 827–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn CS, Han JA, Lee H-S, Lee S, Pai H-S. 2011. The PP2A regulatory subunit Tap46, a component of the TOR signaling pathway, modulates growth and metabolism in plants. The Plant Cell 23, 185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Amsterdam A, Becker T, Kawakami K, Gaiano N, Hopkins N. 1996. Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Genes & Development 10, 3141–3155. [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Lenhard M. 2007. Growing up to one’s standard. Current Opinion in Plant Biology 10, 63–69. [DOI] [PubMed] [Google Scholar]
- Antoniali G, Lirussi L, Poletto M, Tell G. 2014. Emerging roles of the nucleolus in regulating the DNA damage response: the noncanonical DNA repair enzyme APE1/Ref-1 as a paradigmatical example. Antioxidants & Redox Signaling 20, 621–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Chua N-H. 1997. A glucocorticoid-mediated transcriptional induction system in transgenic plants. The Plant Journal 11, 605–612. [DOI] [PubMed] [Google Scholar]
- Attaran E, Major IT, Cruz JA, Rosa BA, Koo AJ, Chen J, Kramer DM, He SY, Howe GA. 2014. Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiology 165, 1302–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. 2007. The multifunctional nucleolus. Nature Reviews Molecular Cell Biology 8, 574–585. [DOI] [PubMed] [Google Scholar]
- Boniotti MB, Gutierrez C. 2001. A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. The Plant Journal 28, 341–350. [DOI] [PubMed] [Google Scholar]
- Boudolf V, Vlieghe K, Beemster GT, Magyar Z, Torres Acosta JA, Maes S, Van Der Schueren E, Inzé D, De Veylder L. 2004. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. The Plant Cell, 16, 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Wyrzykowska J, Muller P, David K, Couch D, Perrot-Rechenmann C, Fleming AJ. 2008. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. The Plant Cell 20, 2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Shaw PJ. 2008. The role of the plant nucleolus in pre-mRNA processing. Current Topics in Microbiology and Immunology 326, 291–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Cao L, Wang S, et al. 2013. Downregulation of multiple CDK inhibitor ICK/KRP genes upregulates the E2F pathway and increases cell proliferation, and organ and seed sizes in Arabidopsis. The Plant Journal 75, 642–655. [DOI] [PubMed] [Google Scholar]
- Cho HK, Ahn CS, Lee HS, Kim JK, Pai HS. 2013. Pescadillo plays an essential role in plant cell growth and survival by modulating ribosome biogenesis. The Plant Journal 76, 393–405. [DOI] [PubMed] [Google Scholar]
- Chung KY, Cheng IK, Ching AK, Chu JH, Lai PB, Wong N. 2011. Block of proliferation 1 (BOP1) plays an oncogenic role in hepatocellular carcinoma by promoting epithelial-to-mesenchymal transition. Hepatology 54, 307–318. [DOI] [PubMed] [Google Scholar]
- David KM, Couch D, Braun N, Brown S, Grosclaude J, Perrot-Rechenmann C. 2007. The auxin-binding protein 1 is essential for the control of cell cycle. The Plant Journal 50, 197–206. [DOI] [PubMed] [Google Scholar]
- de Jager SM, Scofield S, Huntley RP, Robinson AS, den Boer BG, Murray JA. 2009. Dissecting regulatory pathways of G1/S control in Arabidopsis: common and distinct targets of CYCD3;1, E2Fa and E2Fc. Plant Molecular Biology 71, 345–365. [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, et al. 2001. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. The Plant Cell 13, 1653–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Inzé D. 2007. The ins and outs of the plant cell cycle. Nature Reviews Molecular Cell Biology 8, 655–665. [DOI] [PubMed] [Google Scholar]
- del Pozo JC, Boniotti MB, Gutierrez C. 2002. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(SKP2) pathway in response to light. The Plant Cell 14, 3057–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C. 2006. The balance between cell division and endoreplicationdepends on E2Fc-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. The Plant Cell 18, 2224–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Montanaro L, Derenzini M. 2012. Ribosome biogenesis and control of cell proliferation: p53 is not alone. Cancer Research 72, 1602–1607. [DOI] [PubMed] [Google Scholar]
- Ebersberger I, Simm S, Leisegang MS, Schmitzberger P, Mirus O, von Haeseler A, Bohnsack MT, Schleiff E. 2014. The evolution of the ribosome biogenesis pathway from a yeast perspective. Nucleic Acids Research 42, 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelkrout EM, Mariconti L, Settlage SB, Cella R, Robertson D, Hanley-Bowdoin L. 2002. Two E2F elements regulate the proliferating cell nuclear antigen promoter differently during leaf development. The Plant Cell 14, 3225–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb L, Volarevic S, Oren M. 2014. p53 and ribosome biogenesis stress: the essentials. FEBS Letters 588, 2571–2579. [DOI] [PubMed] [Google Scholar]
- Grimm T, Hölzel M, Rohrmoser M, Harasim T, Malamoussi A, Gruber-Eber A, Kremmer E, Eick D. 2006. Dominant-negative Pes1 mutants inhibit ribosomal RNA processing and cell proliferation via incorporation into the PeBoW-complex. Nucleic Acids Research 34, 3030–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henras AK, Soudet J, Gérus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. 2008. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cellular and Molecular Life Sciences 65, 2334–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T. 2002. Auxin-mediated cell cycle activation during early lateral root initiation. The Plant Cell 14, 2339–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel M, Rohrmoser M, Schlee M, et al. 2005. Mammalian WDR12 is a novel member of the Pes1-BOP1 complex and is required for ribosome biogenesis and cell proliferation. Journal of Cell Biology 170, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Ferjani A, Fujikura U, Tsukaya H. 2006. Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. Journal of Plant Research 119, 37–42. [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Van Lijsebettens M, Candela H, Micol JL, Tsukaya H. 2012. Ribosomes and translation in plant developmental control. Plant Science 191–192, 24–34. [DOI] [PubMed] [Google Scholar]
- Huang M, Ji Y, Itahana K, Zhang Y, Mitchell B. 2008. Guanine nucleotide depletion inhibits pre-ribosomal RNA synthesis and causes nucleolar disruption. Leukemia Research 32, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzé D, De Veylder L. 2006. Cell cycle regulation in plant development. Annual Review of Genetics 40, 77–105. [DOI] [PubMed] [Google Scholar]
- James A, Wang Y, Raje H, Rosby R, DiMario P. 2014. Nucleolar stress with and without p53. Nucleus 5, 402–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y, Park YJ, Cho HK, Jung HJ, Ahn TK, Kang H, Pai H-S. 2015. The nucleolar GTPase nucleostemin-like 1 plays a role in plant growth and senescence by modulating ribosome biogenesis. Journal of Experimental Botany 66, 6297–6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbstein K. 2011. Inside the 40S ribosome assembly machinery. Current Opinion in Chemical Biology 15, 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian A, Le Meur N, Sesboüé R, Bourguignon J, Bougeard G, Gautherot J, Bastard C, Frébourg T, Flaman JM. 2004. Inactivation of the RRB1-Pescadillo pathway involved in ribosome biogenesis induces chromosomal instability. Oncogene 23, 8597–8602. [DOI] [PubMed] [Google Scholar]
- Kressler D, Hurt E, Bassler J. 2010. Driving ribosome assembly. Biochimica et Biophysica Acta 1803, 673–683. [DOI] [PubMed] [Google Scholar]
- Kuwabara A, Gruissem W. 2014. Arabidopsis RETINOBLASTOMA-RELATED and Polycomb group proteins: cooperation during plant cell differentiation and development. Journal of Experimental Botany 65, 2667–2676. [DOI] [PubMed] [Google Scholar]
- Lam YW, Trinkle-Mulcahy L. 2015. New insights into nucleolar structure and function. F1000Prime Reports 7, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapik YR, Fernandes CJ, Lau LF, Pestov DG. 2004. Physical and functional interaction between Pes1 and BOP1 in mammalian ribosome biogenesis. Molecular Cell 15, 17–29. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Park Y-J, Seo PJ, Kim J-H, Sim H-J, Kim S-G, Park C-M. 2015. Systemic immunity requires SnRK2.8-mediated nuclear import of NPR1 in Arabidopsis. The Plant Cell 27, 3425–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch-Gaggl A, Haque J, Li J, Ning G, Traktman P, Duncan SA. 2002. Pescadillo is essential for nucleolar assembly, ribosome biogenesis, and mammalian cell proliferation. Journal of Biological Chemistry 277, 45347–45355. [DOI] [PubMed] [Google Scholar]
- Li J, Yu L, Zhang H, Wu J, Yuan J, Li X, Li M. 2009. Down-regulation of pescadillo inhibits proliferation and tumorigenicity of breast cancer cells. Cancer Science 100, 2255–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin C, North M, Erixon K, Walters K, Jenssen D, Goldman AS, Helleday T. 2005. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Reserach 33, 3799–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, De Veylder L, Atanassova A, Bakó L, Inzé D, Bögre L. 2005. The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. The Plant Cell 17, 2527–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar Z, Horváth B, Khan S, Mohammed B, Henriques R, De Veylder L, Bakó L, Scheres B, Bögre L. 2012. Arabidopsis E2FA stimulates proliferation and endocycle separately through RBR-bound and RBR-free complexes. The EMBO Journal 31, 1480–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Grummt I. 2005. Cellular stress and nucleolar function. Cell Cycle 4, 1036–1038. [DOI] [PubMed] [Google Scholar]
- Miles TD, Jakovljevic J, Horsey EW, Harnpicharnchai P, Tang L, Woolford JL., Jr 2005. Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Molecular and Cellular Biology 25, 10419–10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missbach S, Weis BL, Martin R, Simm S, Bohnsack MT, Schleiff E. 2013. 40S ribosome biogenesis co-factors are essential for gametophyte and embryo development. PLoS ONE 28, e54084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Kawamura K, Sugisaka K, Sekine M, Shinmyo A. 2002. Phosphorylation of retinoblastoma-related protein by the cyclin D/cyclin-dependent kinase complex is activated at the G1/S-phase transition in tobacco. The Plant Cell 14, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, Shanahan H, Sugimoto K, Devoto A. 2013. Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiology 161, 1930–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse VG, Johnson AW. 2010. Maturation of eukaryotic ribosomes: acquisition of functionality. Trends in Biochemical Sciences 35, 260–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. 2010. Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspectives in Biology 2, a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestov DG, Stockelman MG, Strezoska Z, Lau LF. 2001. ERB1, the yeast homolog of mammalian BOP1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Research 29, 3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Parra E, Fründt C, Gutierrez C. 2003. A genome-wide identification of E2F regulated genes in Arabidopsis. The Plant Journal 33, 801–811. [DOI] [PubMed] [Google Scholar]
- Revenkova E, Masson J, Koncz C, Afsar K, Jakovleva L, Paszkowski J. 1999. Involvement of Arabidopsis thaliana ribosomal protein S27 in mRNA degradation triggered by genotoxic stress. The EMBO Journal 18, 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JA. 1999. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Rohrmoser M, Hölzel M, Grimm T, Malamoussi A, Harasim T, Orban M, Pfisterer I, Gruber-Eber A, Kremmer E, Eick D. 2007. Interdependence of Pes1, Bop1, and WDR12 controls nucleolar localization and assembly of the PeBoW complex required for maturation of the 60S ribosomal subunit. Molecular and Cellular Biology 27, 3682–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R, Dornelas MC. 2014. Interplay between cell growth and cell cycle in plants. Journal of Experimental Botany 65, 2703–2714. [DOI] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schöbinger U, Hülskamp M. 2003. Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. The Plant Cell 15, 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Brown J. 2012. Nucleoli: composition, function, and dynamics. Plant Physiology 158, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozzani R, Maggio C, Varotto S, Canova S, Bergounioux C, Albani D, Cella R. 2006. Interplay between Arabidopsis activating factors E2Fb and E2Fa in cell cycle progression and development. Plant Physiology 140, 1355–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strezoska Z, Pestov DG, Lau LF. 2000. BOP1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5. 8S RRNA processing and 60S ribosome biogenesis. Molecular and Cellular Biology 20, 5516–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świa̧tek A, Azmi A, Stals H, Inzé D, Van Onckelen H. 2004. Jasmonic acid prevents the accumulation of cyclin B1;1 and CDK-B in synchronized tobacco BY-2 cells. FEBS Letters 572, 118–122. [DOI] [PubMed] [Google Scholar]
- Świa̧tek A, Lenjou M, Van Bockstaele D, Inzé D, Van Onckelen H. 2002. Differential effect of jasmonic acid and abscisic acid on cell cycle progression in tobacco BY-2 cells. Plant Physiology 128, 201–211. [PMC free article] [PubMed] [Google Scholar]
- Tromas A, Braun N, Muller P, et al. 2009. The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE 4, e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RY, McKay RD. 2005. A multistep, GTP-driven mechanism controlling the dynamic cycling of nucleostemin. Journal of Cell Biology 168, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Hause B. 2013. Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinl C, Marquardt S, Kuijt SJ, Nowack MK, Jakoby MJ, Hülskamp M, Schnittger A. 2005. Novel functions of plant cyclin-dependent kinase inhibitors, ICK1/KRP1, can act non-cell-autonomously and inhibit entry into mitosis. The Plant Cell 17, 1704–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis BL, Missbach S, Marzi J, Bohnsack MT, Schleiff E. 2014. The 60S associated ribosome biogenesis factor LSG1-2 is required for 40S maturation in Arabidopsis thaliana. The Plant Journal 80, 1043–1056. [DOI] [PubMed] [Google Scholar]
- Woolford JL, Baserga SJ. 2013. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics 195, 643–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Turner JG. 2008. Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS ONE 3, e3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.