As plant tissues dehydrate, water transport efficiency declines, a process typically attributed to air obstruction (embolism) in the xylem. Trifiló et al. (pages 5029–5039) dissect leaf hydraulic vulnerability and show that both xylem and living tissues may be important. If confirmed and clarified, an important role for outside-xylem hydraulic decline will change our understanding of how plants transport water and control biosphere carbon and water fluxes.
The study of leaf hydraulics has taken off exponentially in the past decade, after many decades of very sparse attention, especially compared with stem hydraulics. The obstacle was that leaves seemed impossibly complex. Whereas in stems, water flows through the xylem, in leaves water flows not only through the xylem within the complex venation network but also across living tissues outside the xylem, and evaporates somewhere in the leaf, before diffusing through the stomata. No part of that system was well understood.
The first step was to establish methods to reliably quantify the hydraulic conductance of the whole leaf (K leaf), defined as the flow rate divided by a given gradient in water potential (Ψ) from petiole to mesophyll (reviewed by Sack and Tyree, 2005). Subsequent experiments on leaves with severed veins allowed the dissection of leaf xylem hydraulic conductance (K x) from outside-xylem conductance (K ox) and showed that the xylem and outside-xylem compartments contribute strongly and similarly to total hydraulic resistance on average across species, with the relative contributions highly variable among species (reviewed by Sack and Holbrook, 2006).
Now each component of the leaf hydraulic system could be illuminated by creative and exciting research, with major breakthroughs on the role of the venation network (reviewed by Sack and Scoffoni, 2013), the properties of the living tissues, including aquaporins, which affect leaf water transport (Maurel et al., 2015), and the vapor phase transport pathways (Rockwell et al., 2014; Buckley, 2015). These studies further showed that K leaf is responsive to internal and external factors that affect both xylem and living tissues, such as temperature, light and water status. In this issue, Trifiló et al. (2016) extend the dissection approach to clarify the response of K leaf to dehydration.
Steep leaf response
The decline of K leaf with dehydration is known as ‘vulnerability’, by analogy with concepts established for stem hydraulics. Because water flows through the xylem under tension, it is susceptible to interruption by air, which can fill the xylem conduit and reduce the volume for water flow. The decline in stem conductivity with dehydration due to embolism often only occurs in very dehydrated stems. Debates surrounding the quantification of stem xylem embolism, its frequency during diurnal transpiration and drought, and its impact on hydraulic conductance are a testament to the importance of the phenomenon across scales in biology, as stem xylem embolism is now recognized as a primary determinant of species’ maximum heights, drought tolerances and ecological distributions.
Meanwhile, tens of studies in the past decade have shown steep hydraulic declines in leaves with dehydration before wilting or turgor loss, using multiple measurement approaches applied to different species (Box 1). This response has typically been attributed, albeit with little direct evidence, to embolism in the xylem. A widespread decline of K leaf under moderate dehydration would have major implications. Strong K leaf vulnerability relative to stems would drive stomatal responses under soil and atmospheric drought and thereby control carbon and water fluxes from leaves and canopies, with feedback on the climate system (Sperry et al., 2016).
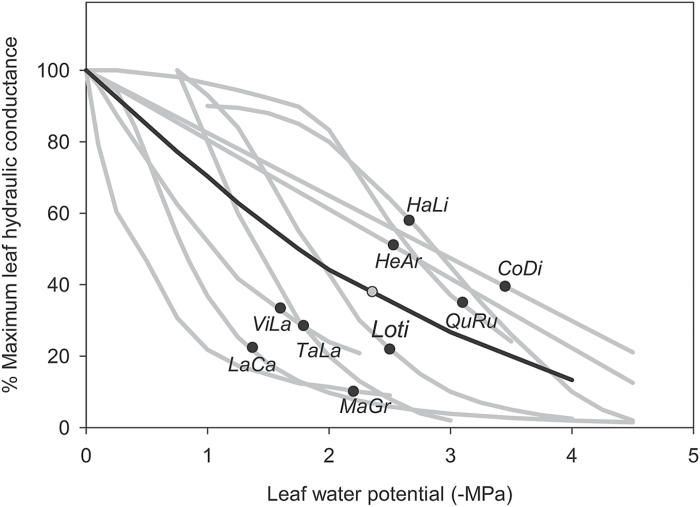
Box 1. Vulnerability of leaf hydraulic conductance (Kleaf) to dehydration, showing strong declines before turgor loss point
Kleaf vulnerability curves are shown for a sampling of nine diverse species (grey lines) ranging in drought tolerance. These were obtained as follows: Hakea lissosperma (HaLi), Lomatia tinctoria (LoTi) and Tasmania lanceolata (TaLa) from Blackman et al. (2010) – rehydration kinetics method; Comarostaphylis diversifolia (CoDi), Heteromeles arbutifolia (HeAr) and Lantana camara (LaCa) from Scoffoni et al. (2012) – evaporative flux method; and Magnolia grandiflora (MaGr), Quercus rubra (QuRu) and Vitis labrusca (ViLa) from Trifiló et al. (2016) – vacuum pump method. Turgor loss points are shown as black dots for each species. The average K leaf vulnerability curve is shown (black line) with the average turgor loss point (grey dot).
It is now imperative to determine what causes this steep leaf response, which can no longer be attributed a priori to xylem embolism. Trifiló et al. have applied rigorous refinements of previous methods to this problem. Importantly, they confirm the dramatic decline of K leaf before turgor loss point and, following Hernandez-Santana et al. (2016), demonstrate similar vulnerability using two different methods on the same species. They also used a recently developed vacuum method based on measuring leaves with severed minor veins to measure the leaf xylem hydraulic vulnerability (Scoffoni and Sack, 2015), and determined the outside-xylem hydraulic vulnerability by subtraction. They found strong hydraulic decline in both xylem and outside-xylem compartments, with both contributing to K leaf decline across four diverse species. They confirm that K ox vulnerability is associated with tissue shrinkage outside the xylem (Scoffoni et al., 2014), and speculate that this could protect the rest of the plant from embolism by driving a pre-emptive stomatal closure response to moderate dehydration.
Xylem and outside-xylem pathways
This research is especially significant because it clearly shows the potential roles of both xylem and outside-xylem pathways in controlling K leaf vulnerability and, by extension, whole-plant hydraulic conductance and productivity. These findings will change the way plant physiologists and ecologists think about water transport, with a major potential role for living tissues as well as xylem in control of the system.
This work raises the urgent need for additional work to confirm and extend the partitioning of K leaf vulnerability. Trifiló et al. showed that in two species, Aleurites moluccana and Vitis labrusca, K x and K ox both played important roles in K leaf decline, whereas in another two, Magnolia grandiflora and Quercus rubra, only K ox played a role. These findings, based on measurements of the rehydration of previously dehydrated leaves and of vacuum-driven water uptake under low irradiance, need confirmation on leaves transpiring under high irradiance as would be typical during photosynthesis. K leaf vulnerability depends on light, as water flow through living tissues is influenced by aquaporins that are responsive to both light and turgor (Maurel et al., 2015), and as tissues absorb light, vapor phase transport may drive a great deal of water movement from the mesophyll to the transpiring epidermis (Guyot et al., 2012; Rockwell et al., 2014; Buckley, 2015). Thus, as Trifiló et al. recognized, the role of K ox might be yet stronger under high irradiance.
Even more essentially, the method for determining K x decline and the inference that embolism is not the key driver of K leaf decline need to be validated, ideally with a visual method. A recently developed optical method (Brodribb et al., 2016) similarly showed that xylem embolism begins late in dehydration, but given that method’s low resolution at fine scales and its inability to scan within the entire tissue cross-section, it might not show all the emboli that occur in all the veins. To prove the conclusion that K ox decline can be the major driver of K leaf vulnerability, it will be necessary to apply microCT (X-ray microtomography) to determine potential embolism in veins for species that have also been analyzed for K leaf, K x and K ox decline.
The causes of K ox decline also need to be investigated. Given the complexity of K ox, these may relate to cell geometry changes during shrinkage, aquaporin activity and/or changes in the vapor phase transport. The application of models calibrated with anatomy will be an essential approach to determining the drivers of K ox decline. Finally, speculation that K ox decline may protect the xylem in the leaf and throughout the rest of the plant from tensions that induce embolism needs to be validated using models and measurements of whole-plant transport.
Shift in understanding
If K ox decline turns out to be the major driver of K leaf decline, this will shift our understanding of water transport in the whole plant, and thus in the entire soil–plant–atmosphere continuum. No longer will water flow through soil or dead xylem cells be considered the major control points – rather, the living tissues outside the xylem, including the stomata, leaf vein parenchyma and leaf mesophyll, may take a central position. The light and turgor responses of these cells within leaves would then be recognized as influencing water transport and stomatal sensitivity, drought tolerance and productivity at plant and landscape scale. Outside-xylem hydraulic conductance will need to be explicitly incorporated into new models of plant water use, ecohydrology, landscape and global fluxes, and climate. The dissection of leaf hydraulic pathways would thus shift our understanding of how living plant cells and tissues influence the biosphere.
References
- Blackman CJ, Brodribb TJ, Jordan GJ. 2010. Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytologist 188, 1113–1123. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Skelton RP, McAdam SAM, Bienaimé D, Lucani CJ, Marmottant P. 2016. Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytologist 209, 1403–1409. [DOI] [PubMed] [Google Scholar]
- Buckley TN. 2015. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant, Cell & Environment 38, 7–22. [DOI] [PubMed] [Google Scholar]
- Guyot G, Scoffoni C, Sack L. 2012. Combined impacts of irradiance and dehydration on leaf hydraulic conductance: insights into vulnerability and stomatal control. Plant, Cell & Environment 35, 857–871. [DOI] [PubMed] [Google Scholar]
- Hernandez-Santana V, Rodriguez-Dominguez CM, Fernandez JE, Diaz-Espejo A. 2016. Role of leaf hydraulic conductance in the regulation of stomatal conductance in almond and olive in response to water stress. Tree Physiology 36, 725–735. [DOI] [PubMed] [Google Scholar]
- Maurel C, Boursiac Y, Luu D-T, Santoni V, Shahzad Z, Verdoucq L. 2015. Aquaporins in plants. Physiological Reviews 95, 1321–1358. [DOI] [PubMed] [Google Scholar]
- Rockwell FE, Holbrook NM, Stroock AD. 2014. The competition between liquid and vapor transport in transpiring leaves. Plant Physiology 164, 1741–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C. 2013. Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytologist 198, 983–1000. [DOI] [PubMed] [Google Scholar]
- Sack L, Tyree MT. 2005. Leaf hydraulics and its implications in plant structure and function. In: Holbrook NM, Zweiniecki MA, eds. Vascular transport in plants. Oxford: Elsevier/Academic Press. [Google Scholar]
- Scoffoni C, McKown AD, Rawls M, Sack L. 2012. Dynamics of leaf hydraulic conductance with water status: quantification and analysis of species differences under steady-state. Journal of Experimental Botany 63, 643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoffoni C, Sack L. 2015. Are leaves “freewheeling”? Testing for a Wheeler-type effect in leaf xylem hydraulic decline. Plant, Cell & Environment 38, 534–543. [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Vuong C, Diep S, Cochard H, Sack L. 2014. Leaf shrinkage with dehydration: coordination with hydraulic vulnerability and drought tolerance. Plant Physiology 164, 1772–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry JS, Wang J, Wolfe B, Mackay DS, Anderegg WRL, McDowell NG, Pockman WT. 2016. Pragmatic hydraulic theory predicts stomatal responses to climatic water deficits. New Phytologist doi: 10.1111/nph.14059 [DOI] [PubMed] [Google Scholar]
- Trifiló P, Raimondo F, Savi T, Lo Gullo MA, Nardini A. 2016. The contribution of vascular and extra-vascular water pathways to drought-induced decline of leaf hydraulic conductance. Journal of Experimental Botany 67, 5029–5039. [DOI] [PubMed] [Google Scholar]