Abstract
Geropathology is the study of aging and age-related lesions and diseases in the form of whole necropsies/autopsies, surgical biopsies, histology, and molecular biomarkers. It encompasses multiple subspecialties of geriatrics, anatomic pathology, molecular pathology, clinical pathology, and gerontology. In order to increase the consistency and scope of communication in the histologic and molecular pathology assessment of tissues from preclinical and clinical aging studies, a Geropathology Research Network has been established consisting of pathologists and scientists with expertise in the comparative pathology of aging, the design of aging research studies, biostatistical methods for analysis of aging data, and bioinformatics for compiling and annotating large sets of data generated from aging studies. The network provides an environment to promote learning and exchange of scientific information and ideas for the aging research community through a series of symposia, the development of uniform ways of integrating pathology into aging studies, and the statistical analysis of pathology data. The efforts of the network are ultimately expected to lead to a refined set of sentinel biomarkers of molecular and anatomic pathology that could be incorporated into preclinical and clinical aging intervention studies to increase the relevance and productivity of these types of investigations.
Key Words: Geropathology, Aging, Geropathology Research Network, Molecular pathology, Histopathology, Healthy aging, Life-span, Health span.
Aging studies are lengthy and resource intensive; thus it is critical to maximize data from these types of investments (1). Historically, the gold standard for preclinical aging research has been life-span extension by some intervention such as genetic (2–4), dietary (5) or pharmaceutical (6,7). However, research in gerontology has suffered from the lack of well-documented pathological data to provide context and potential insight into the mechanism of action for life-span extension or the extension of the healthy, prefrail portion of life (health span) in aging animals. Pathology gives an insight into health by providing a more complete picture of the disease conditions present at a specific age as well as identifying background lesions that may significantly impact the health span of the animal (8). We are introducing the term “geropathology” to designate the study of aging and age-related lesions and diseases in the form of whole necropsies/autopsies, surgical biopsies, histology, and molecular biomarkers, which encompasses multiple subspecialties including geriatrics, anatomic pathology, molecular pathology, clinical pathology, and gerontology.
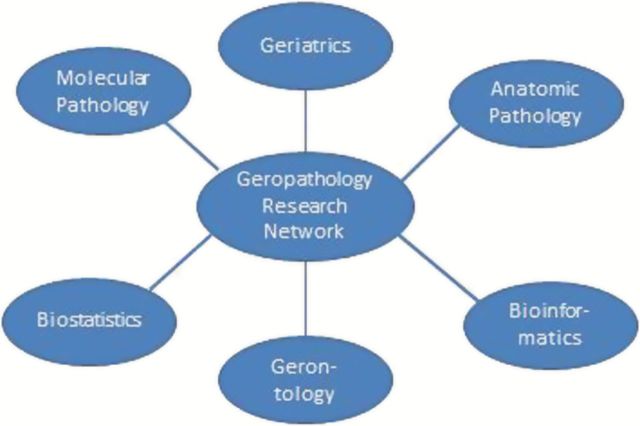
A long-term goal of geropathology is to establish consistent pathological evaluation as an essential component of studies involving aging animals and humans. The real challenge is the lack of specialized expertise and resources available to the aging research community (9,10). In an effort to meet this challenge, a Geropathology Research Network has been established to increase the consistency and communication of histologic and molecular pathology assessment of tissues from preclinical animal models. The hope is that this will inform future clinical trials on aging. The network consists of pathologists and scientists with expertise in the anatomic and molecular pathology of aging, the design of preclinical and clinical aging research studies, biostatistical methods of analysis of aging data, and bioinformatics for compiling and annotating large sets of data generated from aging studies (Figure 1).
Figure 1.
The Geropathology Research Network is driven by an interdisciplinary approach.
The Geropathology Research Network provides an environment to promote learning and exchange of scientific information and ideas for the aging research community through a series of symposia and network conferencing formats. The network is developing uniform ways of integrating pathology into life-span and health span studies, for example by providing consensus recommendations for standardizing the histological grading of lesions. Statistical analyses that integrate pathology data with longitudinal and cross-sectional life-span data and physiological function data will strengthen the relevance of findings generated in animal models of aging for translation to human studies. The Geropathology Research Network concept is significant because it will enhance the development and routine integration of pathology data into preclinical and clinical aging studies, encourage more pathologists and other scientists to specialize in the pathology of aging, and establish relevant standards to compare among species including humans. The efforts of the network are ultimately expected to lead to a refined set of sentinel biomarkers of molecular and anatomic pathology that could save on costs and increase the efficiency of conducting aging intervention studies.
Interdisciplinary Approach
The Geropathology Research Network is composed of an interdisciplinary team of scientists and pathologists committed to enhance the integration of the pathological assessment of tissues from aged animals and humans into hypothesis and/or translational driven aging research. It is imperative that pathology assessment be integrated into aging studies in an organized and uniform manner. Research scientists have the experience in study design and generation of data. They work with pathologists and statisticians on the experimental research design, which is influenced by a number of factors depending on the aims and objectives of the project (11,12). Standard protocols on necropsy and collection of tissues for comprehensive histologic examination in mice have been published (13–15) but must be adapted to aging research. Comprehensive histological examinations are critical to accurately diagnose morphological lesions, assess lesion severity, and provide a context for molecular, biochemical, and other physiological data. Microscopic pathological assessment of lesions in tissues from old animals and humans requires a highly trained person with appropriate experience. The most demanding requirements are for longitudinal life-span studies involving either the effect of a genetic modification or a therapeutic compound. A second common aging study design is the cross-sectional experiment where all animals in the cohort are evaluated at a specific age. Methods to distinguish between and characterize contributory and incidental lesions associated with aging and increased disease burden are critically needed. Incidental lesions are those generally thought not to contribute to death but may be relevant to less severe comorbidities or general frailty. Identification and weighting of probable cause of death and contributing factors to death in total disease burden need to be defined. Molecular markers associated with aging must be correlated with histologic lesion grade to better assess the utility of these markers for measuring health span.
The Geropathology Research Network includes statistical expertise in order to address the critical need for analyzing the data and correlating them with other endpoints such as echocardiographic data, cognition and memory, mobility, and grip strength. Consistent and well-defined lesion severity scoring offers a means for comparing across treatment groups or strains and interpreting outcomes (16). Organ-specific histological grading schemes for aging studies have been previously published and concepts such as “disease burden” and “contributing causes of morbundity” have been defined (17,18). Statisticians help validate the grading systems and enable translation to other species including humans.
The network includes expertise in bioinformatics because of the large amount of data that will be managed. For example, data from scored slide sets and molecular marker assays are entered into a searchable database. The Geropathology website http://depts.washington.edu/compmed/geropath/index.html will provide information on symposia and meetings and links to existing comprehensive data bases for pathology of comparative aging, including Pathbase, a database of mutant or genetically manipulated mice (19); the mouse pathology ontology system MPATH (20); the Center for Genomic Pathology; and the EuroPhenome and EMPReSS systems that provide access to data and procedures for mouse phenotyping driven by the European Mouse Phenotyping Consortium (21).
The interdisciplinary approach is strengthened by the formation and contribution of working groups focused on specific aspects of geropathology. The Molecular Pathology working group is prioritizing and developing protocols and assays for molecular biomarkers for senescence and other age-related processes using a variety of procedures (Figure 2). The Anatomic Pathology working group is adopting a standardized grading system for lesions in tissues from old animals, using the mouse as a prototype, that can be used to correlate with physiological function (Figure 3). One of the results will be a series of study set slides, as described below, that can be used as training tools for others with an interest in geropathology. A third working group is the translational group with the task of assessing the age-related relevance of molecular and histopathologic markers, and planning preclinical studies that incorporate these new or redefined sentinel probes.
Figure 2.
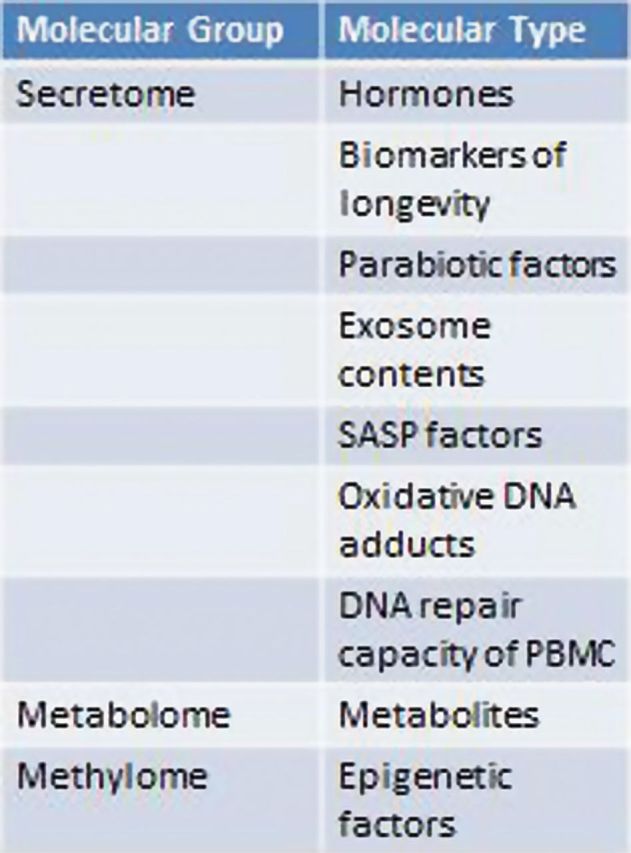
The Molecular Pathology Working group is charged with prioritizing the use of potential surrogate aging molecules.
Figure 3.
Initial objectives of the Anatomic Pathology Working group.
Training Environment
The Geropathology Research Network is developing sets of histology slides and molecular assay protocols to facilitate training in the pathology of aging animals, with mouse as the first species to be studied. These will generally be organized by research area, for example, cardiovascular aging, cancer, metabolic, skeletal muscle aging, neurodegeneration, tissue repair, and inflammation in tissues from old animals or progeria models. Histology sets will consist of glass slides and digital (virtual) slides that illustrate typical aging changes and system specific aging pathology in several common background strains. Slides will be drawn from archives of aging studies performed at various institutions (22). Slide sets will include graded examples of histologic severity scores for aging related pathology. The molecular assay protocols will consist of procedures to probe for markers of senescence, oxidative damage, autophagy, cell proliferation, apoptosis, mitochondrial dysfunction, angiogenesis, and hypoxia. The goal is to also develop comparable study sets for other species with varying life spans, with the rat, dog, and human given first priority. The network has future plans to work with scientists that use invertebrate models, such as worms and flies, for aging studies to help plan comparable study sets.
Symposium Series
A series of geropathology research symposia will provide a venue for presenting pathobiology of aging data and discussing challenges and opportunities in aging research. Discussions will also include developing standardized methods and protocols. The symposia will be targeted to scientists and pathologists with an interest in the pathobiology of aging. The symposia will also focus on recent research in comparative aging using a variety of experimental manipulations and discussing a number of organ systems and models. Symposia will build consensus recommendations on pathological analysis from specialists in the field of comparative aging and will incorporate interactive sessions. Participation by graduate students and pathology residents will be encouraged to foster the next generation of scientists and pathologists in aging research.
Translational Focus of the Network
An overarching objective of the Geropathology Research Network is to provide a means of translating histologic and molecular pathology data from preclinical studies to clinical intervention trials. Pathology assessment of aging cohorts has a number of advantages that can enhance other aging endpoints. First, it can validate and reveal the mechanism of action of life-span extension data by showing altered lesion burden. Second, it can validate a health span extension when no life-span extension is seen. Life-span studies in animals are very expensive and resource intensive, so if no life-span advantage is seen with a particular cohort, it may be that pathology scores will help identify some other valuable phenotype. For example, there may be a difference in the presence and/or severity of chronic inflammation which impacts quality of life rather than life span per se (Figure 4). Third, it is possible to determine the types and severity of lesions present and correlate the contributing causes of morbidity or mortality with different physiologic phenotypes (18). Fourth, it will help reveal novel information about the mechanisms of aging and age-related disease. For example, are aging lesions stochastic across a population or do they occur in a defined order?
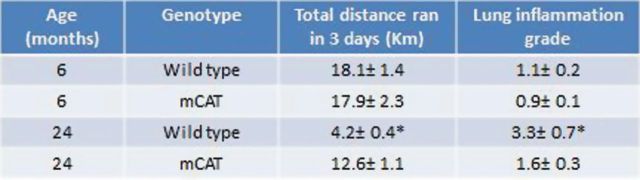
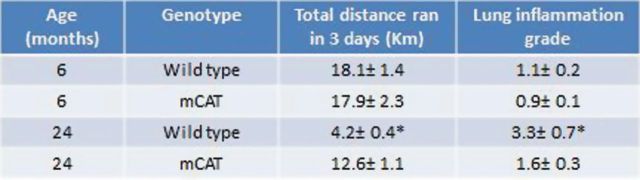
Figure 4.
Increased exercise endurance is associated with decreased lung inflammation in 24-month-old mice expressing mitochondrial catalase (mCAT) compared to 6-month-old mice expressing m/CAT. Exercise endurance was assessed by distance ran over a three day period. Inflammation grade was the average total score (from 1–4) for macrophage infiltration, perivascular and peribronchiolar lymphocytes, and intravascular leukocytes. N = 4–6/cohort, p ≤ .05 for 24 mo cohorts.
The Geropathology Research Network will develop consensus best practices in anatomic and molecular pathology protocols to allow for consistency and application to a variety of interventional studies. Comprehensive cross-sectional pathological assessment is not possible in human studies but biopsy specimens and serum collection are possible. Autopsy is rarely a part of human studies, so tissues for end of life pathology evaluation most likely will not be available. Therefore, animal models are key. Aging animal pathology data are critical in being able to identify organs or tissues that can be used as surrogate biomarkers for life span in human studies (23). For example, data generated from echocardiography to assess heart disease can be used to correlate with pathological findings in mouse studies (Figure 5), which then can be translated to human studies depending on the findings. The network will be in a position to determine how preclinical pathology data can help plan clinical longevity and health span studies. In addition, the focused effort by the Geropathology Research Network will help contribute to a better understanding of normal aging as related to daily diagnostic testing and clinical management of geriatric patients.
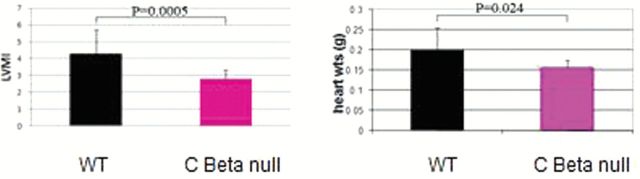
Figure 5.
Protein kinase A (PKA) Cβ null mice are resistant to age-induced cardiac hypertrophy, N = 12, 24–26 mo of age. Echocardiography revealed that aged PKA Cβ null mice had significantly lower LVMI (left ventricular mass index; determined by left ventricular mass standardized to tibia length) than WT littermates, suggesting cardiac dysfunction. Hearts were found to be almost 25% heavier than those of mutants suggesting heart weight could be an indicator of cardiac dysfunction.
Funding
This work is supported by National Institutes of Health grant R24 AG047115-01 to W.L.
Acknowledgments
We thank Drs. Jessica Snyder and Piper Treuting for their helpful suggestions on manuscript content.
References
- 1. Ladiges W , Ikeno Y , Liggitt D , Treuting PM.. Pathology is a critical aspect of preclinical aging studies. Pathobiol Aging Age Relat Dis . 2013. ; 3:1–4. doi:10.3402/pba.v3i0.22451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schriner SE , Linford NJ , Martin GM et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science . 2005. ; 308 : 1909 – 1911 . [DOI] [PubMed] [Google Scholar]
- 3. Liang H , Masoro EJ , Nelson JF , Strong R , McMahan CA , Richardson A.. Genetic mouse models of extended lifespan. Exp Gerontol . 2003. ; 38 : 1353 – 1364 . [DOI] [PubMed] [Google Scholar]
- 4. Amador-Noguez D , Yagi K , Venable S , Darlington G.. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell . 2004. ; 3 : 423 – 441 . [DOI] [PubMed] [Google Scholar]
- 5. Flurkey K , Astle CM , Harrison DE.. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci . 2010. ; 65 : 1275 – 1284 . doi:10.1093/gerona/glq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller RA , Harrison DE , Astle CM et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell . 2007. ; 6 : 565 – 575 . [DOI] [PubMed] [Google Scholar]
- 7. Nadon NL , Strong R , Miller RA et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordr) . 2008. ; 30 : 187 – 199 . doi:10.1007/s11357-008-9048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adissu HA , Estabel J , Sunter D et al. Histopathology reveals correlative and unique phenotypes in a high-throughput mouse phenotyping screen. Dis Model Mech . 2014. ; 7 : 515 – 524 . doi:10.1242/dmm.015263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schofield PN , Dubus P , Klein L et al. Pathology of the laboratory mouse: an International Workshop on Challenges for High Throughput Phenotyping. Toxicol Pathol . 2011. ; 39 : 559 – 562 . doi:10.1177/0192623311399789 [DOI] [PubMed] [Google Scholar]
- 10. Warren MV , Studley ML , Dubus P et al. An impending crisis in the provision of histopathology expertise for mouse functional genomics. J Pathol . 2009. ; 217 : 4 – 13 . doi:10.1002/path.2460 [DOI] [PubMed] [Google Scholar]
- 11. Ladiges W , Van Remmen H , Strong R et al. Lifespan extension in genetically modified mice. Aging Cell . 2009. ; 8 : 346 – 352 . doi:10.1111/j.1474-9726.2009.00491.x [DOI] [PubMed] [Google Scholar]
- 12. Sundberg JP , Berndt A , Sundberg BA et al. The mouse as a model for understanding chronic diseases of aging: the histopathologic basis of aging in inbred mice . Pathobiol Aging Age Relat Dis . 2011. ; 1–9 . doi:10.3402/pba.v1i0.7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ruehl-Fehlert C , Kittel B , Morawietz G et al. Revised guides for organ sampling and trimming in rats and mice–part 1. Exp Toxicol Pathol . 2003. ; 55 : 91 – 106 . [PubMed] [Google Scholar]
- 14. Kittel B , Ruehl-Fehlert C , Morawietz G et al. Revised guides for organ sampling and trimming in rats and mice–part 2. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol . 2004. ; 55 : 413 – 431 . [DOI] [PubMed] [Google Scholar]
- 15. Morawietz G , Ruehl-Fehlert C , Kittel B et al. Revised guides for organ sampling and trimming in rats and mice–part 3. A joint publication of the RITA and NACAD groups. Exp Toxicol Pathol . 2004. ; 55 : 433 – 449 . [DOI] [PubMed] [Google Scholar]
- 16. Gibson-Corley KN , Olivier AK , Meyerholz DK.. Principles for valid histopathologic scoring in research. Vet Pathol . 2013. ; 50 : 1007 – 1015 . doi:10.1177/0300985813485099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ikeno Y , Bronson RT , Hubbard GB , Lee S , Bartke A.. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci . 2003. ; 58 : 291 – 296 . [DOI] [PubMed] [Google Scholar]
- 18. Treuting PM , Linford NJ , Knoblaugh SE et al. Reduction of age-associated pathology in old mice by overexpression of catalase in mitochondria. J Gerontol A Biol Sci Med Sci . 2008. ; 63 : 813 – 822 . [DOI] [PubMed] [Google Scholar]
- 19. Schofield PN , Gruenberger M , Sundberg JP.. Pathbase and the MPATH ontology. Community resources for mouse histopathology. Vet Pathol . 2010. ; 47 : 1016 – 1020 . doi:10.1177/0300985810374845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schofield PN , Sundberg JP , Sundberg BA , McKerlie C , Gkoutos GV.. The mouse pathology ontology, MPATH; structure and applications. J Biomed Semantics . 2013. ; 4 : 18–34 . doi:10.1186/2041-1480-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallon AM , Blake A , Hancock JM.. EuroPhenome and EMPReSS: online mouse phenotyping resource. Nucleic Acids Res . 2008. ; 36 : D715 – D718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pettan-Brewer C , Treuting PM.. Practical pathology of aging mice. Pathobiol Aging Age Relat Dis . 2011. ; 1:1–16 . doi:10.3402/pba.v1i0.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scudamore CL. Integrating pathology into human disease modelling–how to eat the elephant. Dis Model Mech . 2014. ; 7 : 495 – 497 . doi:10.1242/dmm.016394 [DOI] [PMC free article] [PubMed] [Google Scholar]