Abstract
Background:
It is unclear whether traditional and genetic risk factors in middle age predict the onset of gout in older age.
Methods:
We studied the incidence of gout in older adults using the Atherosclerosis Risk in Communities study, a prospective U.S. population–based cohort of middle-aged adults enrolled between 1987 and 1989 with ongoing follow-up. A genetic urate score was formed from common urate-associated single nucleotide polymorphisms for eight genes. The adjusted hazard ratio and 95% confidence interval of incident gout by traditional and genetic risk factors in middle age were estimated using a Cox proportional hazards model.
Results:
The cumulative incidence from middle age to age 65 was 8.6% in men and 2.5% in women; by age 75 the cumulative incidence was 11.8% and 5.0%. In middle age, increased adiposity, beer intake, protein intake, smoking status, hypertension, diuretic use, and kidney function (but not sex) were associated with an increased gout risk in older age. In addition, a 100 µmol/L increase in genetic urate score was associated with a 3.29-fold (95% confidence interval: 1.63–6.63) increased gout risk in older age.
Conclusions:
These findings suggest that traditional and genetic risk factors in middle age may be useful for identifying those at risk of gout in older age.
Key Words: Gout, Urate genetics
Gout is the most common form of inflammatory arthritis and disproportionately affects adults over the age of 65 (1); there are 4.7 million older adults with gout in the United States (2). Although the prevalence is growing faster for older than younger adults (1,3,4), there is increasing awareness that the incidence and risk factors for gout differ between older and younger adults (5). No study has investigated the incidence of gout among older adults. Understanding the incidence of gout in older age and how the incidences differ from younger adults is an important and understudied area of gout epidemiology.
In older adults, traditional gout risk factors (6–11) may be present for years. However, it is unclear whether the presence of these traditional risk factors in middle age predicts gout onset in older age and whether there are sex differences in the incidence of gout in older adults. Additionally, genetic risk factors for increased urate concentrations (identified in cohorts of adults of all ages) may be predictive of gout onset in older adults (12). Yet, the balance between genetic and traditional risk in older adults is unclear.
To address the growing public health burden of gout in older adults, we have examined gout incidence and prevalence in older age and explored the ability of traditional and genetic risk factors in middle age to predict the onset of gout in older age, in the Atherosclerosis Risk in Communities (ARIC) study.
Materials and Methods
Study Design
ARIC is an ongoing, prospective U.S. population–based cohort, which enrolled 15,792 middle-aged (45–64 years) adults between 1987 and 1989. Participants were selected from four U.S. communities (Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland) and took part in examinations starting with a baseline visit (visit 1: 1987–1989), three near-term follow-up visits (visit 2: 1990–1992; visit 3: 1993–1995; visit 4: 1996–1998), and a 25-year follow-up (visit 5: 2011–2013). Participants were contacted annually as part of follow-up. Details of the ARIC cohort have been published elsewhere (13). Institutional Review Boards of the participating institutions approved the study protocols. All study participants provided written informed consent.
Assessment of Gout
Gout was defined on the basis of self-reported, physician-diagnosed gout and age of diagnosis at either visit 4 or the annual follow-up contact (2011–2012). Self-report of a physician diagnosis of gout has been reported to be a reliable and a sensitive measure of gout (14).
Study Population
The present study consisted of all ARIC participants who self-reported gout status and had available genetic data; only white participants were included because the genetic urate score (GUS) was derived from studies of white participants (12). We focused on three subsets: (i) prevalent gout through older adulthood (n = 9,526); (ii) incident gout in middle age (n = 7,997; onset between ages 45–64 and excluding gout onset prior to visit 1); and (iii) incident gout in older age (n = 6,765; onset at age 65 and older). Urate levels in older age (measured at visit 5) were assessed in a sample of 4,271 participants.
Urate Genetics
The GUS was calculated as published previously (12) and described in the Supplementary Material. The risk score included information on the single nucleotide polymorphism with the strongest association to serum urate for each of eight genomic loci (rs2078267 in SLC22A11, rs780093 in GCKR, rs1106766 in R3HDM2-INHBC region, rs675209 in RREB1, rs1967017 in PDZK1, rs13129697 in SLC2A9, rs2199936 in ABCG2 [r 2 > .9 with the functional variant rs2231142], and rs1165196 in SLC17A1) (12).
Traditional Gout Risk Factors
All risk factors were assessed at cohort entry using standard protocols (13,15) and represent gout risk factors in middle age (age 45–64). Body mass index (16) and blood pressure (17) were measured according to published methods. Hypertension was defined as measured systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg, or use of a medication to treat hypertension. Trained interviewers collected information on the medications used in the 2 weeks prior to the visit, including diuretic use.
Animal protein, organ meat, shellfish, and alcohol intake were assessed with a validated interviewer-administered semiquantitative food frequency questionnaire based on the validated Willett 61-item questionnaire (18,19).
Central laboratories performed analyses on baseline fasting specimens using conventional assays to obtain uric acid and creatinine values (among other specimens) at visit 1 (20). Uric acid was measured by the uricase method (21) and standardized across labs and visits. At visit 5, plasma urate level was measured using the enzymatic colorimetric method and this measure represents urate level in older age. To ensure comparability of the uric acid measures across all visits, uric acid levels were recalibrated to visit 5 based on a rerun of 200 frozen samples at each visit. Serum creatinine was measured using a modified kinetic Jaffé reaction. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (22,23).
Statistical Analysis
Gout prevalence and cumulative incidence in older age were estimated using a Kaplan–Meier approach with age as the time scale. Gout case event times were based on the reported age of physician diagnosis. Participants without gout were censored at their age at their last response to the gout query; therefore, the cumulative incidence estimates were all conditioned on survival to that age.
We estimated the hazard ratio (HR) and 95% confidence intervals (CIs) using Cox proportional hazards modeling with age as the time scale and Efron approximation for ties for traditional and genetic risk factors (24). Models were stratified by sex due to the potential for an interaction between sex and traditional as well as genetic risk factors. We tested whether there were sex-specific effects of GUS (per 100 µmol/L) and the eight individual single nucleotide polymorphisms forming the GUS using the Wald test of the interaction term in a model that included both men and women. Assumptions of the Cox proportional hazards model were confirmed by visual inspection of the complementary log–log plots in the sex-stratified models.
We tested whether GUS and individual urate-associated single nucleotide polymorphisms were associated with urate level in older adults using adjusted linear regression. All analyses were conducted using Stata SE, version 12.1. All reported p values are two-sided.
Results
Gout Prevalence
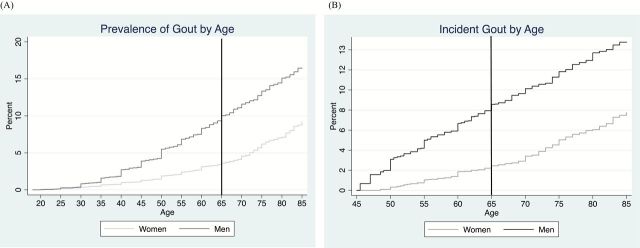
Of the 9,526 participants, 46.2% were male and the mean (SD) age at visit 1 was 54.0 (5.7). The prevalence of gout, regardless of age, was higher in men than in women (p < .001; Figure 1A); 31.9% of those with gout reported a physician diagnosis at age 65 or older. By age 65, 9.0% of men and 3.3% for women had a history of gout conditioned on survival; by 75 it was 13.3% and 6.2%, respectively (Figure 1A).
Figure 1.
Prevalence gout (A) and incidence of gout (B). The prevalence of gout (% of participants with a history of gout) across the age spectrum for participants of Atherosclerosis Risk in Communities was estimated using a Kaplan–Meier approach. All cumulative incidences (%) from visit 1 should be interpreted as the percentage of participants with newly diagnosed gout, conditioned on survival to that age.
Gout Incidence in Middle and Older Age
The cumulative incidence from middle age to age 65 was 8.6% in men and 2.5% in women conditioned on survival; by age 75 the cumulative incidence was 11.8% and 5.0%, respectively (Figure 1B).
The incidence of gout through middle and older age was greatest for those with the highest urate genetic risk (fourth quartile of GUS; Figure 2A). By age 65, the cumulative incidence from middle age to age 65 was 8.0% for those in the fourth quartile, 5.0% for those in the third quartile, 3.8% for those in the second quartile, and 3.3% for those in the first quartile conditioned on survival; the corresponding cumulative incidences by age 75 were 10.9%, 7.0%, 7.7%, and 5.9%, respectively.
Figure 2.
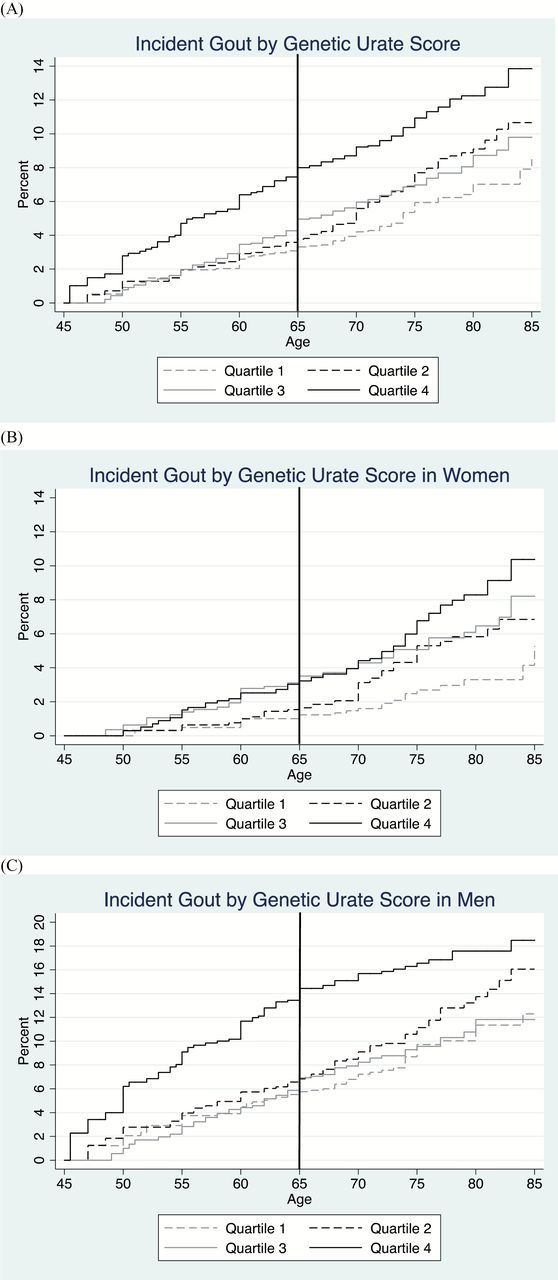
Cumulative incidence of gout by (A) quartile of genetic urate score stratified by sex (B and C). The cumulative incidence of gout from visit 1 for participants of Atherosclerosis Risk in Communities was estimated using a Kaplan–Meier approach. All cumulative incidences (%) from visit 1 should be interpreted as the percentage of participants with newly diagnosed gout, conditioned on survival to that age. The genetic urate score is measured in μmol/L. Quartile 1 of the genetic urate score ranges from −59.1 to −13.1; quartile 2: −13.2 to 0.3; quartile 3: 0.4 to 12.0; quartile 4: 12.1 to 60.8.
When stratified by sex, the genetic risk (fourth quartile of GUS) was evident through older age for men; for women, across the age range quartile 3 and quartile 4 of GUS have higher genetic risk (Figure 2B and C). From the figures, it appears that quartile 3 and quartile 4 in women confirm risk in middle age and all quartiles except the lowest are associated with risk in older age. The adjusted genetic risk of incident gout throughout middle and older age was present for men and women (Table 1); for every 100 µmol/L change in GUS, the risk of incident gout in middle and older age combined was 3.29 times greater (95% CI: 1.63–6.63) for men and 6.49 (95% CI: 2.76–15.26) for women even after controlling for important gout risk factors like kidney function (Table 1). Additionally, ABCG2 was associated with an increased risk of gout in men (HR = 1.96, 95% CI: 1.54–2.50) and women (HR = 1.60, 95% CI: 1.17–2.18). SLC2A9 was associated with a decreased risk of gout in men (HR = 0.76, 95% CI: 0.61–0.94) and women (HR = 0.60, 95% CI: 0.46–0.79; Table 1).
Table 1.
Risk of Incident Gout and Difference in Urate Level by Genetic Risk
| All Incident Gout Cases* | Incident Gout Cases Before Age 65* | Incident Gout Cases After Age 65* | Difference in Urate Level (mg/dL) for Older Adults* | |||||
|---|---|---|---|---|---|---|---|---|
| Men (n = 3,621) | Women (n = 4,376) | Men (n = 3,621) | Women (n = 4,376) | Men (n = 2,956) | Women (n = 3,809) | Men (n = 1,885) | Women (n = 2,386) | |
| Genetic urate score (100 µmol/L) | 3.29 | 6.49 | 6.65 | 13.30 | 1.53 | 4.78 | 0.52 | 1.41† |
| 1.63–6.63 | 2.76–15.26 | 2.45–18.06 | 2.98–59.33 | 0.57–4.14 | 1.68–13.63 | 0.17–0.86 | 1.09–1.73 | |
| Individual genes | ||||||||
| PDZK1 rs1967017 | 0.86 | 1.09 | 0.79 | 1.37‡ | 0.94 | 0.96 | −0.08 | −0.06 |
| 0.72–1.03 | 0.88–1.35 | 0.61–1.01 | 0.94–1.99 | 0.72–1.22 | 0.74–1.25 | −0.17–0.01 | −0.15–0.02 | |
| GCKR rs780093 | 0.76 | 0.95 | 0.72 | 0.93 | 0.80 | 0.97 | −0.06 | −0.05 |
| 0.64–0.91 | 0.77–1.18 | 0.56–0.92 | 0.64–1.35 | 0.62–1.03 | 0.74–1.26 | −0.15–0.04 | −0.14–0.03 | |
| SLC2A9 rs13129697 | 0.76 | 0.60 | 0.67 | 0.54 | 0.86 | 0.62 | −0.22 | −0.47† |
| 0.61–0.94 | 0.46–0.79 | 0.49–0.91 | 0.33–0.89 | 0.64–1.16 | 0.45–0.87 | −0.32 to −0.12 | −0.56 to −0.38 | |
| ABCG2 rs2199936 | 1.96 | 1.60 | 2.60 | 1.78 | 1.39 | 1.52 | 0.20 | 0.40‡ |
| 1.54–2.50 | 1.17–2.18 | 1.89–3.57 | 1.07–2.96 | 0.94–2.05 | 1.03–2.25 | 0.05–0.35 | 0.27–0.54 | |
| RREB1 rs675209 | 0.90 | 0.99 | 0.95 | 1.01 | 0.87 | 0.97 | −0.08 | −0.03 |
| 0.73–1.12 | 0.76–1.28 | 0.70–1.29 | 0.64–1.59 | 0.64–1.18 | 0.71–1.34 | −0.19–0.03 | −0.13–0.07 | |
| SLC17A1 rs1165196 | 1.09 | 0.97 | 1.20 | 0.87 | 0.99 | 1.02 | 0.10 | 0.12 |
| 0.91–1.31 | 0.78–1.20 | 0.93–1.54 | 0.61–1.26 | 0.76–1.28 | 0.78–1.33 | 0.01–0.19 | 0.04–0.20 | |
| SLC22A11 rs2078267 | 1.19 | 1.02 | 1.28 | 1.05 | 1.09 | 1.01 | 0.05 | 0.02 |
| 1.00–1.42 | 0.82–1.26 | 1.00–1.64 | 0.72–1.51 | 0.85–1.41 | 0.77–1.32 | −0.04–0.14 | −0.06–0.10 | |
| INHBC rs1106766 | 1.16 | 0.88 | 1.09 | 0.80 | 1.27 | 0.93 | 0.15 | 0.06 |
| 0.93–1.45 | 0.69–1.13 | 0.80–1.48 | 0.53–1.18 | 0.91–1.76 | 0.69–1.27 | 0.04–0.26 | −0.04–0.16 | |
Notes: *Adjusted for risk factors (Table 3).
† p for interaction with sex ≤ .001.
‡ p for interaction with sex < .05.
Urate Genetic Risk Factors and Incident Gout in Middle Age
GUS was associated with incident gout in men (HR = 6.65, 95% CI: 2.45–18.06) and women (HR = 13.30, 95% CI: 2.98–59.33) who developed gout before age 65 (Table 1). This association was much stronger than among those with a later gout onset after 65 years of age, consistent with the general observations that those genetically at highest risk often show early onset disease. ABCG2 was associated with an increased risk of gout during middle age for men (HR = 2.60, 95% CI: 1.89–3.57) and women (HR = 1.78, 95% CI: 1.07–2.96). SLC2A9 was associated with a decreased risk of gout in men (HR = 0.67, 95% CI: 0.49–0.91) and women (HR = 0.54, 95% CI: 0.33–0.89; Table 1).
Traditional and Genetic Risk Factors for Incident Gout in Older Adults
Of the 6,765 ARIC participants who were free of gout by age 65 and answered the gout query, 230 developed gout in older age (3.4%). In unadjusted analyses, traditional gout risk factors in middle age were associated with gout onset in older age (Table 2). However, there was no difference in age at baseline for those who developed gout in older age (p = .51), although this may be by design. Those who developed gout in older age had higher urate levels and a higher GUS score (Table 2).
Table 2.
Study Characteristics at Middle Age, by Incident Gout in Older Age
| No Gout (n = 6,535) | Incident Gout (n = 230) | |
|---|---|---|
| Mean (SD) or % | Mean (SD) or % | |
| Male sex | 43.4 | 52.2 |
| Age | 54.6 (5.8) | 54.8 (5.5) |
| Body mass index (kg/m2) | 26.6 (4.6) | 28.7 (5.3)* |
| Dietary risk factors | ||
| Protein (g/d) | 53.0 (23) | 57.0 (24)† |
| Organ meat (>2 servings/ mo) | 8.3 | 7.4 |
| Shellfish (>1 serving/wk) | 7.6 | 7.0 |
| Alcohol (drinks/d) | 0.66 (1.0) | 0.90 (1.1)† |
| Beer (drinks/wk) | 1.31 (4.3) | 2.10 (5.1)† |
| Wine (drinks/wk) | 0.93 (2.2) | 0.84 (2.2) |
| Liquor (drinks/wk) | 1.69 (3.8) | 2.43 (4.21)† |
| Smoking status | ||
| Current | 20.3 | 19.6† |
| Former | 35.1 | 44.8 |
| Never | 44.6 | 35.7 |
| Education (y) | ||
| <12 | 14.5 | 14.8 |
| 12–16 | 45.8 | 49.1 |
| 17–21 | 39.7 | 36.1 |
| Postmenopausal‡ | 68.8 | 77.3 |
| HRT use‡ | 22.8 | 29.1 |
| Coronary heart disease | 3.3 | 4.4 |
| Congestive heart failure | 2.7 | 4.8 |
| Hypertension | 23.2 | 40.9† |
| Diabetes | 6.6 | 9.6 |
| Categorical eGFR (mL/min/1.73 m2) | ||
| ≥90 | 52.6 | 43.0† |
| 60–89 | 45.4 | 52.2 |
| <60 | 2.0 | 4.8 |
| Diuretic use | 12.2 | 25.7† |
| Urate level (mg/dL) | 4.92 (1.4) | 6.26 (1.7)† |
| Genetic urate score (μmol/L) | −0.01 (0.2) | 0.02 (0.2)† |
Notes: Study characteristics were measured at baseline and thus, by study design are during middle age. eGFR = estimated glomerular filtration rate; HRT = hormone replacement therapy; SD = standard deviation.
*p ≤ .001.
† p < .05.
‡Among women.
The adjusted association between sex and incident gout in older age was not significant (HR = 1.29, 95% CI: 0.95–1.75; Table 3). Incident gout in older age was associated with increased adiposity (body mass index 30–34: HR = 1.86, 95% CI: 1.24–2.78; body mass index ≥ 35: HR = 3.90, 95% CI: 2.45–6.19), beer intake (per drink/wk: HR = 1.24, 95% CI: 1.06–1.46), protein intake (per 10g/d: HR = 1.05, 95% CI: 1.00–1.10), smoking status (current: HR = 1.70, 95% CI: 1.17–2.48 and former: HR = 1.43, 95% CI: 1.05–1.94), hypertension (HR = 1.46, 95% CI: 1.05–2.03), diuretic use (HR = 1.72, 95% CI: 1.18–2.49), and decreased kidney function (estimated glomerular filtration rate < 60: HR = 2.24, 95% CI: 1.19–4.21) in middle age. Results were similar when stratified by sex, although men with an estimated glomerular filtration rate < 60mL/min/1.73 m2 were at a greater increased gout risk than women with an estimated glomerular filtration rate < 60 (p for interaction = .008). There was no interaction of age and genetic score for men (p for interaction = .28) or for women (p for interaction = .84).
Table 3.
Relative Hazard (HR and 95% CI) of Incident Gout in Older Adults by Traditional and Genetic Risk Factors
| All Older Adults (n = 6,765) | Men (n = 2,956) | Women (n = 3,809) | |
|---|---|---|---|
| Male sex | 1.29 (0.95–1.75) | — | — |
| Genetic urate score (100 µmol/L) | 2.59 (1.26–5.31) | 1.53 (0.57–4.14) | 4.78 (1.68–13.63) |
| Body mass index (kg/m2) | |||
| <25 | Reference | Reference | Reference |
| 25–29 | 1.50 (1.07-2.12) | 1.26 (0.78-2.05) | 1.79 (1.11-2.89) |
| 30–34 | 1.86 (1.24–2.78) | 1.74 (0.98–3.09) | 1.85 (1.04–3.30) |
| ≥35 | 3.90 (2.45–6.19) | 3.71 (1.69–8.15) | 4.13 (2.30–7.43) |
| Protein (10g/d) | 1.05 (1.00–1.10) | 1.05 (0.98–1.12) | 1.05 (0.97–1.13) |
| Beer (drinks/wk) | 1.24 (1.06–1.46) | 1.25 (1.05–1.50) | 1.21 (0.81–1.80) |
| Smoking status | |||
| Current | 1.70 (1.17–2.48) | 1.57 (0.87–2.85) | 1.88 (1.15–3.09) |
| Former | 1.43 (1.05–1.94) | 1.54 (0.98–2.42) | 1.27 (0.81–2.00) |
| Never | Reference | Reference | Reference |
| Hypertension | 1.46 (1.05–2.03) | 1.33 (0.84–2.09) | 1.64 (1.02–2.64) |
| Diuretic use | 1.72 (1.18–2.49) | 1.58 (0.89–2.81) | 1.83 (1.12–2.98) |
| eGFR (mL/min/1.73 m2) | |||
| ≥90 | Reference | Reference | Reference |
| 60–89 | 1.10 (0.84–1.44) | 1.55 (1.05–2.31) | 0.77 (0.52–1.14) |
| <60 | 2.24 (1.19–4.21) | 3.92 (1.64–9.37) | 1.31 (0.51–3.33) |
| Postmenopausal | 1.18 (0.74–1.90) | ||
Notes: The associations between traditional and genetic risk factors and gout were estimated for all older adults as well as for men and women, separately. CI = confidence interval; eGFR = estimated glomerular filtration rate; HR = hazard ratio.
A 100 µmol/L increase in GUS was associated with a 2.59-fold (95% CI: 1.26–5.31) increased risk of gout in older age (Table 3). For men, there was limited evidence of genetic risk in older age (HR = 1.53, 95% CI: 0.57–4.14). For women, a 100 µmol/L change in GUS was associated with a 4.78-fold (95% CI: 1.68–13.63) increased risk of gout in older age after adjusting for other gout risk factors. The risk of gout by GUS was not statistically different between men and women (p for interaction = .14) although the point estimates appear to differ. ABCG2 was associated with an increased risk of gout during older age in women (HR = 1.52, 95% CI: 1.03–2.25) and SLC2A9 was associated with a decreased risk of gout in women (HR = 0.62, 95% CI: 0.45–0.87; Table 1).
Urate Genetics and Urate Levels in Older Adults
The mean urate level in older age was 5.73mg/dL (SD = 1.49); 5.39mg/dL (SD = 1.46) in women and 6.16mg/dL (SD = 1.42) in men. Every 100 µmol/L change in GUS was associated with 0.52mg/dL (95% CI: 0.17–0.86) higher urate levels in older age for men, after adjusting for other traditional risk factors; for women, this increase in GUS was associated with 1.41mg/dL (95% CI: 1.09–1.73) higher urate levels in older age (Table 1). These differences in the association between GUS and urate level were significantly higher in women than in men (p for interaction ≤ .001). ABCG2 was associated with an increased urate levels in older age for men (0.20, 95% CI: 0.05–0.35) and women (0.40, 95% CI: 0.27–0.54) and SLC2A9 was associated with a decreased urate levels in older age for men (−0.22, 95% CI: −0.32 to −0.12) and women (−0.47, 95% CI: −0.56 to −0.38; Table 1).
Discussion
In this U.S. population–based cohort of middle-aged adults who were followed through older adulthood, there was an increasing prevalence and incidence of gout for men and women as they aged; by age 75, 13.3% of men and 6.2% of women, conditioned on survival to age 75, had been diagnosed with gout. There was evidence that traditional risk factors, including modifiable risk factors like increased adiposity and dietary factors, in middle age are associated with the onset of gout in older age (up to 25 years later). These findings suggest that the pathway to gout in older age begins earlier in life. The genetic association was much stronger among those with gout onset in middle age than among those with gout onset in older age, consistent with the general observations that those genetically at highest risk often show early onset disease. For older women, there was a strong genetic association (GUS as well as ABCG2 and SLC2A9) with the urate level.
Cross-sectional studies have demonstrated that in the United States, gout disproportionately affects older adults (1) and the prevalence in older age has increased over the last decade (1,3). One previous study suggested that at gout onset, women are on average 10 years older than men (25). We were able to extend these findings by identifying which traditional and genetic risk factors in middle age associate with gout onset.
The risk of gout in older women is modified by decreasing levels of estrogen, a uricosuric hormone, after menopause (26,27). Increased urate levels in postmenopausal women are thought to be due to a lower fractional excretion rate of urate in the kidney that is influenced by decreased female hormones. In our study, we observed that—for both men and women—higher genetic risk showed a graded increase in the cumulative incidence and prevalence of gout. However, compared with men, women with a genetic risk had a twofold higher hazard of developing gout with onset at less than 65 years of age, whereas women at highest genetic risk with age of onset after 65 years of age had three times the risk. The differential effects of the urate genetics on urate could explain the stronger effects of the GUS on incident gout in women compared with men. Changes in the relative importance of transporters contributing to this score such as ABCG2, potentially as a result of altered hormone concentrations with age, may contribute to this observation. Our findings suggest that in older women, the ABCG2 risk variant is associated with higher urate levels than in older men.
The impact of genetics is still important in older adults, which means that the urate genetic risk factors are associated with gout at all ages. Although we were able to identify traditional risk factors in middle age that were associated with gout onset in older age, the GUS was a stronger risk factor (based on the point estimate). The magnitude of the association suggests that the genetic risk is a strong even through older age, potentially as strong as the modifiable risk factors. For those who are genetically at high risk, modification of other risk factors may have limited effects on the incidence of gout.
Several strengths and potential limitations of the present study deserve comment. This study is the first epidemiologic investigation of gout incidence, prevalence, and risk factors in older adults in a U.S. population–based setting. Our study contained detailed information on traditional risk factors as well as genetic risk factors. Although there have been both population-based and longitudinal studies of traditional risk factors and genetic risk factors, this is the first study to combine the two sets of risk factors to study gout in older adults. Our definition of gout did not require observation of monosodium urate crystals in joint fluid or fulfillment of the American College of Rheumatology criteria (28) for gout. However, in population-based cohort studies, synovial fluid analysis is logistically challenging and not ethical in asymptomatic participants. Our previous study suggests that self-reported gout is a reliable and valid measurement (14). The outcome of interest was reported at visit 4 and during the most recent annual follow-up call; therefore, the exclusion criterion with the greatest impact on sample size was the nonattendance to visit 4 or the annual follow-up, which may introduce selection bias. However, by design all participants in the analytic cohort had to survive to visit 4 or the annual follow-up; those that did not participate in the annual follow-up and were gout-free were censored at their visit 4 age; therefore, we are unable to perform a competing risk analysis. Furthermore, if there was loss to follow-up of those with more traditional risk factors in middle age, our ability to predict gout in older age may be attenuated. Also, the cumulative incidence of gout by age was conditioned on surviving to that age. This study confirms that the prevalence and incidence of gout continues to rise in older adults and further demonstrates that traditional and genetic risk factors in middle age predict the onset of gout in older adults. Physicians and health care professionals should be aware that gout is common during older age, that genetic risk of gout persists through older age, and that traditional risk factors that lead to gout in older adults may be present in middle age.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was jointly funded by the Arthritis National Research Foundation and the American Federation for Aging Research (to M.A.M.-D.). The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN26820 1100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). A.K. was supported by the Emmy Noether Programme (KO 3598/2-1) of the German Research Foundation.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
References
- 1. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi:10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi:10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 3. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687 e671. doi:10.1016/j.amjmed.2011.09.033 [DOI] [PubMed] [Google Scholar]
- 4. Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–1587. [PubMed] [Google Scholar]
- 5. De Leonardis F, Govoni M, Colina M, Bruschi M, Trotta F. Elderly-onset gout: a review. Rheumatol Int. 2007;28:1–6. [DOI] [PubMed] [Google Scholar]
- 6. Bhole V, de Vera M, Rahman MM, Krishnan E, Choi H. Epidemiology of gout in women: fifty-two-year followup of a prospective cohort. Arthritis Rheum. 2010;62:1069–1076. doi:10.1002/art.27338 [DOI] [PubMed] [Google Scholar]
- 7. Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742–748. [DOI] [PubMed] [Google Scholar]
- 8. Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–3007. [PubMed] [Google Scholar]
- 9. McAdams DeMarco MA, Maynard JW, Baer AN, Coresh J. Hypertension and the risk of incident gout in a population-based study: the Atherosclerosis Risk in Communities Cohort. J Clin Hypertens. 2012;14:675–679. doi:10.1111/j.1751-7176.2012.00674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–1281. [DOI] [PubMed] [Google Scholar]
- 11. Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–1103. [DOI] [PubMed] [Google Scholar]
- 12. Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi:10.1016/S0140-6736(08)61343-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 14. McAdams MA, Maynard JW, Baer AN, et al. Reliability and sensitivity of the self-report of physician-diagnosed gout in the campaign against cancer and heart disease and the atherosclerosis risk in the community cohorts. J Rheumatol. 2011;38:135–141. doi:10.3899/jrheum.100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson R, Chambless LE, Yang K, et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender ethnicity. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49:1441–1446. [DOI] [PubMed] [Google Scholar]
- 16. Idem. Operations Manual No. 2: Cohort Component Procedures, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 17. Idem. Operations Manual No. 11: Sitting Blood Pressure, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 18. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 19. Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T. Reliability of a food frequency questionnaire by ethnicity, gender, age, and education. Nutrit Res. 1996;16:735–745. [Google Scholar]
- 20. Center ARiCC. Operations Manual No. 10: Clinical Chemistry Determinations, Version 1.0. Chapel Hill, NC: University of North Carolina School of Public Health; 1987. [Google Scholar]
- 21. Iribarren C, Folsom AR, Eckfeldt JH, McGovern PG, Nieto FJ. Correlates of uric acid and its association with asymptomatic carotid atherosclerosis: the ARIC Study. Atherosclerosis Risk in Communities. Ann Epidemiol. 1996;6:331–340. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Int Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Astor BC, Arnett DK, Brown A, Coresh J. Association of kidney function and hemoglobin with left ventricular morphology among African Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2004;43:836–845. [DOI] [PubMed] [Google Scholar]
- 24. Hertz-Picciotto I, Rockhill B. Validity and efficiency of approximation methods for tied survival times in cox regression. Biometrics. 1977;53:1151–1156. [PubMed] [Google Scholar]
- 25. Harrold LR, Yood RA, Mikuls TR, et al. Sex differences in gout epidemiology: evaluation and treatment. Ann Rheum Dis. 2006;65:1368–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mikkelsen WM, Dodge HJ, Valkenburg H. The distribution of serum uric acid values in a population unselected as to gout or hyperuricemia: Tecumseh, Michigan 1959–1960. Am J Med. 1965;39:242–251. [DOI] [PubMed] [Google Scholar]
- 27. Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364:443–452. doi:10.1056/NEJMcp1001124 [DOI] [PubMed] [Google Scholar]
- 28. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.