Abstract
Background.
Mild parkinsonian signs have been documented in community-dwelling older adults without Parkinson’s disease. We estimated the proportion of older adults with parkinsonism and examined its association with adverse health outcomes and indices of brain pathology.
Methods.
Four parkinsonian signs were assessed with the motor portion of the Unified Parkinson’s Disease Rating Scale in 2,962 older adults who agreed to annual evaluation and brain autopsy. We used Cox proportional hazards models to examine the association of parkinsonism (two or more signs) and possible parkinsonism (one sign) with adverse health outcomes and regression models in 1,160 decedents to examine the association of parkinsonism and neuropathology.
Results.
At study entry about 25% (N = 776, 26.2%) had parkinsonism and 30% had possible parkinsonism (N = 885, 29.9%). Parkinsonism was strongly related to age. The frequency was 11.8% for people younger than 75 years, 29.1% for those aged 75–84 years, and 43.7% for those aged 85 years or older. Parkinsonism was associated with an increased hazard of death, of mild cognitive impairment, of Alzheimer’s disease and disability. Individuals with possible parkinsonism also had an increased risk for adverse health outcomes compared to individuals without parkinsonism. Postmortem indices of macroscopic and microscopic infarcts, arteriolosclerosis, and atherosclerosis were associated with parkinsonism proximate to death.
Conclusions.
Parkinsonism is common in older adults and is associated with an increased risk of adverse health outcomes and postmortem indices of brain pathology. Its association with age suggests that it will increase in our aging population.
Key Words: Parkinsonism, Aging, Neuropathology, Mortality, Disability.
Late-life motor impairment is common with older adults displaying a wide spectrum of impairments ranging from mild decreased muscle strength and bulk, impaired balance and reduced speed and dexterity to overt impairment with concomitant disability. Moreover, there has also been increasing recognition that mild parkinsonian signs including bradykinesia, tremor, rigidity, and parkinsonian gait are also common in older adults without Parkinson’s disease (PD) (1). Currently, there is no single scale that can be employed to capture all these aspects of impaired motor function. Efforts to document motor impairment in older individuals have been subsumed under several different constructs, often developed by different disciplines within the aging field and based on the assessments of different motor abilities (1–4). Thus, it may be necessary to employ a battery of motor constructs to more fully describe the extent of motor impairment in our aging population.
Prior approaches to quantifying parkinsonian signs and categorizing the presence of parkinsonism vary widely with most investigators focusing on signs other than gait and posture disturbance (5–8). In contrast, some prior studies have used continuous measures of parkinsonian signs rather than categorical measures of parkinsonism (9–11). Continuous measures have clear advantages for analytic purposes including greater precision and improved power to detect associations of small effect sizes. However, categorical measures typically have greater utility for clinical and public health issues, that is, does a person have parkinsonism and should a therapy be offered or studied, how common is parkinsonism and other motor impairments, and what is the magnitude of the public health burden (12).
Our prior work, which employed a continuous measure for parkinsonism, has shown that parkinsonian signs are associated with a wide range of adverse health outcomes and several indices of neuropathology (13–20). An important limitation of our prior work is that a continuous measure does not easily translate to clinical decision making or public health inferences. An effective index of parkinsonism requires an instrument that is reliable, easy to administer, and predicts outcomes of interest in older adults. Our prior work demonstrates that the modified motor portion of the Unified Parkinson’s Disease Rating Scale (mUPDRS) is reliable and easy to administer by nonphysicians (9). This work extends our prior work to create and validate a categorical measure of parkinsonism. In this study, we categorized parkinsonism based on the presence of two or more cardinal signs of parkinsonism (gait or posture disturbance, bradykinesia, rigidity, and tremor) in almost 3,000 older participants of the Religious Order Study (ROS) and Memory and Aging Project (MAP) (21,22). Further, we also categorize the presence of possible parkinsonism based on one parkinsonian sign thereby extending the categorization to a 3-level classification of parkinsonism. To validate this 3-level categorical measure, we first examined its association with adverse health outcomes in about 3,000 community-dwelling older adults. Next we examined its association with postmortem indices of age-related brain neuropathologies in 1,160 decedents.
Methods
Participants
Participants are from ROS and MAP, two ongoing studies of aging approved by the Institutional Review Board of Rush University Medical Center. Written informed consent for annual clinical examinations and an anatomical gift act for brain donation at the time of death were obtained from all study participants.
Both studies employ similar clinical and postmortem data collection by the same team of examiners allowing for combined analyses (21,22). Participation in the annual follow-up evaluations exceeds 90% and the autopsy rate exceeds 85%. At the time of these analyses (Supplementary Figure 1), 3,012 were enrolled in both studies (ROS 1,246; MAP 1,766) and 50 cases (1.7%) were missing an assessment of parkinsonism. These cases were younger but did not differ from the cases eligible for these analyses with respect to sex or education (results not shown). This left 2,962 participants who had completed their baseline clinical assessment of parkinsonism and were eligible for these analyses (ROS: N = 1,221; MAP: N = 1,741). Of those included in the baseline cross-sectional analyses of parkinsonian signs, there were 335 (ROS: 118; MAP: N = 217) who did not have follow-up data [246 died before follow-up or were not in the study long enough for follow-up and 89 (3.0%) were missing follow-up data] and were not included in the analyses of incident adverse health. Mean follow-up for all cases included in these analyses was 6.6 years (SD, 5.20 years).
Clinical Diagnoses and Adverse Health Outcomes
The annual uniform structured clinical evaluation includes a medical history and a neurologic examination that includes a modified version of the United Parkinson’s Disease Rating Scale (mUPDRS) (21,22). A diagnosis of PD was based on the history of the disease for which the participant was treated with levodopa.
Mortality: When an autopsy is obtained, date of death is known promptly. When no autopsy is obtained, we obtain information on date of death from an interview with a knowledgeable informant of searches of public databases as previously described (23).
Dementia or mild cognitive impairment was diagnosed in a three-step process. Nineteen cognitive tests were scored by a computer and summarized as a composite measure of global cognition (21,22). These data were reviewed by a neuropsychologist to diagnose cognitive impairment. Then participants were evaluated by a clinician who used all cognitive and clinical data to diagnose dementia status (21,22).
Disability was assessed annually via two self-report instruments. Basic activities of daily living were assessed using six items from the Katz scale (24). Mobility disability was assessed using the Rosow–Breslau scale, which assesses three walking performances (25).
Assessment and Classification of Parkinsonism
Assessment: Trained nurse clinicians administered the mUPDRS (9,10). There were 26 items which assessed 4 parkinsonian signs (Supplementary Table 1).
Classification: The categories of parkinsonism used for classification were based on the number of parkinsonian signs present. A sign was considered present if two or more of their respective items had at least a score of 1 indicating a mild abnormality (Supplementary Methods). A 2-level classification of parkinsonism was present if two or more of the four signs were documented. A 3-level classification of parkinsonism was also constructed by defining possible parkinsonism as the presence of one parkinsonian signs.
To quantify the degree of agreement for classification for both categorical measures, three nurses administered the mUPDRS scale to 70 participants twice within 3 weeks (9). A movement disorder specialist also examined the same cases once during the same time interval. The scores from these assessments were used to classify each case with both categorical measures.
We used interperson weighted kappa statistics to quantify the agreement for both categorical measures of each nurse with the movement disorder specialist and intraperson kappa of each nurse over time (26,27). For the 2-level measure, inter-rater kappas ranged from 0.66 to 0.73 and intra-rater kappas from 0.54 to 0.71, reflecting moderate to substantial agreement. For the 3-level measure, interperson weighted kappas ranged from 0.66 to 0.70, reflecting moderate to substantial agreement. Intra-rater weighted kappas were 0.48, 0.66, and 0.74, also reflecting moderate to substantial agreement.
For each participant included in these analyses, we also constructed an alternative categorical measure using a scoring approach employed by other investigators based on 10 UPDRS items (28).
Postmortem Indices
Brain removal, tissue sectioning and preservation, and a uniform gross and microscopic examination with quantification of postmortem indices followed a standard protocol (18–20). Postmortem examination (mean postmortem interval 8.6 hrs, SD = 7.73 hours) assessed seven common neuropathologies including: Alzheimer’s disease (AD) and Lewy Body Disease pathologies, nigral neuronal loss, chronic macroinfarcts and microinfarcts, atherosclerosis, and arteriolosclerosis as previously described (Supplementary Methods) (18–20).
Comorbidities and Other Covariates
Sex and years of education were recorded at the baseline interview. Age in years was computed from self-reported date of birth and clinical evaluation date. Seven chronic diseases were documented at baseline based on self-report of hypertension, diabetes, myocardial infarction, cancer, thyroid disease, head trauma, and stroke (22). Medications were inspected and coded using the Medi-Span system (Medi-Span, Inc.) (21,22)
Statistical Analyses
We examined bivariate associations of trichotomous parkinsonism with Spearman correlations. We employed a set of discrete-time Cox proportional hazards models to examine the association of dichotomous and trichotomous parkinsonism with incident adverse health outcomes. Cox models used continuous time when the outcome was mortality. In two additional sets of models, we examined the associations of parkinsonism and possible parkinsonism with adverse health outcomes by comparing the risks associated with combinations of terms indicating the three levels of parkinsonism. In further analyses, we used data from deceased subjects who had undergone autopsy, to examine the association of postmortem indices of neuropathology to trichotomous parkinsonism using logistic regression models. A priori level of statistical significance was 0.05. Models were examined graphically and analytically and assumptions were judged to be adequately met. Programming was done in SAS version 9.3 (SAS Institute Inc, Cary, NC) (29).
Results
Frequency of Parkinsonian Signs, Parkinsonism, and Possible Parkinsonism at Baseline
Parkinsonian Signs: There were 2,962 individuals included in these analyses followed on average for 7 years (mean 6.6 years; SD = 5.20 years) and their clinical characteristics at baseline are included in Table 1. At baseline, gait disturbance was the most common sign (N = 1,143, 35.6%) followed by postural and/or rest tremor (N = 642, 21.7%), bradykinesia (N = 588, 19.9%), and rigidity (N = 362, 12.2%).
Table 1.
Clinical Characteristics at Baseline
| Variable | All Mean (SD) or N (%) |
No Parkinsonism Mean (SD) or N (%) |
Possible Parkinsonism Mean (SD) or N (%) |
Parkinsonism Mean (SD) or N (%) |
|---|---|---|---|---|
| Cases | 2,962 | 1,301 (43.9%) | 885 (29.9%) | 776 (26.2%) |
| Demographics | ||||
| Age at Baseline (years) | 78.3 (7.82) | 75.1 (7.49) | 79.7 (7.21) | 81.9 (6.92) |
| Sex (female) | 2,138 (72.2%) | 995 (76.5%) | 614 (69.4%) | 529 (68.2%) |
| White, non-Hispanic | 2,608 (88.1%) | 1,083 (83.3%) | 795 (89.9%) | 730 (94.2%) |
| Education (years) | 16.0 (3.76) | 16.5 (3.80) | 15.8 (3.80) | 15.5 (3/88) |
| MMSE (score 30) | 27.7 (3.16) | 28.4 (2.31) | 27.6 (3.0) | 26.5 (4.09) |
| Global cognition | −0.01 ().65) | 0.20 (0.55) | −0.07 (0.63) | −0.30 (0.71) |
| Cognitive Diagnosis | ||||
| No cognitive impairment | 2,021 (68.7%) | 992 (76.8%) | 586 (66.6%) | 443 (57.4%) |
| Mild cognitive impairment | 740 (25.1%) | 269 (20.8%) | 241 (27.4%) | 230 (29.8%) |
| Dementia | 184 (6.2%) | 31 (2.4%) | 53 (6.0%) | 100 (12.9%) |
| Self-Report Conditions | ||||
| Hypertension | 1,442 (48.7%) | 595 (45.7%) | 443 (50.1%) | 404 (52.1%) |
| Diabetes | 363 (12.3%) | 127 (9.8%) | 126 (14.3%) | 110 (14.2%) |
| Myocardial infarction | 311 (10.5%) | 104 (8.0%) | 105 (11.9%) | 102 (13.2%) |
| Cancer | 895 (30.3%) | 392 (30.1%) | 261 (29.5%) | 242 (31.3%) |
| Thyroid disorder | 545 (18.4%) | 229 (17.6%) | 165 (18.7%) | 151 (19.5%) |
| Head trauma | 203 (6.9%) | 91 (7.0%) | 59 (6.7%) | 53 (6.9%) |
| Stroke | 235 (8.6%) | 53 (4.5%) | 83 (10.0%) | 99 (13.5%) |
| Parkinson’s disease | 67 (2.3%) | 14 (1.1.%) | 12 (1.4%) | 41 (5.3%) |
| ADL disability | 295 (10.0%) | 25 (1.9%) | 98 (11.1) | 172 (22.4%) |
| Mobility disability | 1,335 (45.3%) | 355 (27.3%) | 448 (51.0%) | 532 (69.5%) |
| Medications | ||||
| Neuroleptic | 39 (1.3%) | 9 (0.7%) | 13 (1.48%) | 17 (2.2%) |
| Thyroid replacement | 484 (16.3%) | 180 (13.8%) | 160 (18.1%) | 144 (18.6%) |
| Asthma medication | 215 (7.3%) | 87 (6.7%) | 77 (8.7%) | 51 (6.6%) |
| Antirdepressants | 378 (12.8%) | 120 (9.2%) | 110 (12.4%) | 148 (19.1%) |
Notes: ADL = activity of daily living; MMSE = mini-mental state examination.
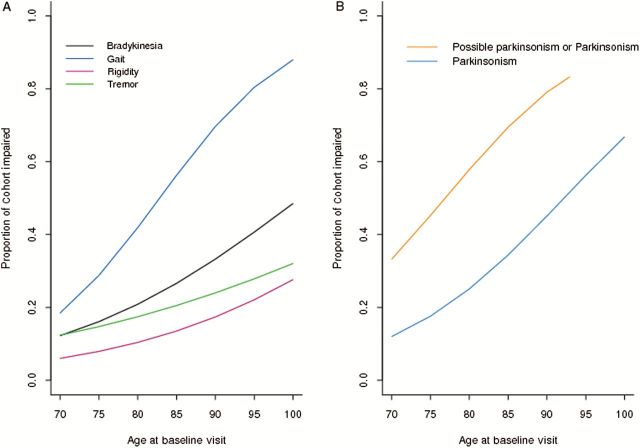
The proportion of individuals with each of the four parkinsonian signs increased with age at baseline (all p’s < .001; Supplementary Table 2); this increase was most prominent for parkinsonian gait (Table 2; Figure 1A). While parkinsonian gait and bradykinesia were similar in men and women, rigidity and tremor were more frequent in men (Table 2; Supplementary Table 2).
Table 2.
Age and Sex Specific Frequencies of Parkinsonian Signs, Parkinsonism, and Possible Parkinsonism
| Domain | All Ages N (%) |
Age Interval | ||
|---|---|---|---|---|
| <75 N (%) |
75–84 N (%) |
85+ N (%) |
||
| Cases | 2,962 | 1,005 (33.9) | 1,360 (45.9) | 597 (20.2) |
| Women | 2,138 (72.2) | 721 (71.7) | 979 (72.0) | 438 (73.4) |
| Men | 824 (27.8) | 284 (28.3) | 381 (28.0) | 159 (26.6) |
| Gait | 1,143 (38.6) | 169 (16.8) | 581 (42.7) | 393 (65.8) |
| Women | 846 (39.6) | 124 (17.2) | 430 (43.9) | 292 (66.7) |
| Men | 297 (36.0) | 45 (15.8) | 151 (39.3) | 101(63.5) |
| Rigidity | 362 (12.2) | 74 (7.4) | 175 (12.9) | 113 (18.9) |
| Women | 221 (10.3) | 43 (6.0) | 102 (10.4) | 76 (17.4) |
| Men | 141 (17.1) | 31 (10.9) | 73 (19.2) | 37 (23.3) |
| Bradykinesia | 588 (19.9) | 113 (11.2) | 293 (21.5) | 182 (30.5) |
| Women | 440 (20.6) | 87 (12.1) | 218 (22.3) | 135 (30.8) |
| Men | 148 (18.0) | 26 (9.2) | 75 (19.7) | 47 (29.6) |
| Postural/rest tremor | 668 (22.6) | 152 (15.1) | 360 (26.5) | 156 (26.1) |
| Women | 363 (17.0) | 62 (8.6) | 206 (21.0) | 95 (21.7) |
| Men | 305 (37.0) | 90 (31.7) | 154 (40.4) | 61 (38.4) |
| Possible parkinsonism | 885 (29.9) | 225 (22.4) | 456 (33.5) | 204 (34.2) |
| Women | 614 (28.7) | 141 (19.6) | 329 (33.6) | 144 (32.9) |
| Men | 271 (32.9) | 84 (29.6) | 127 (33.3) | 60 (37.7) |
| Parkinsonism | 776 (26.2) | 119 (11.8) | 396 (29.1) | 261 (43.7) |
| Women | 529 (24.7) | 76 (10.5) | 263 (26.9) | 190 (43.4) |
| Men | 247 (30.0) | 43 (15.1) | 133 (34.9) | 71 (44.7) |
Figure 1.
Parkinsonian signs, possible parkinsonism, and parkinsonism in older adult.
Parkinsonism and Possible Parkinsonism: At study entry about 25% (N = 776, 26.2%) showed parkinsonism [two signs: (N = 510, 17.2%), three signs: (N = 208, 7.0%), and four signs: (N = 58, 2.0%)]. Parkinsonian gait was the most common sign which occurred in combination with one or more of the other signs [716/776, 92.3%, (Supplementary Table 3)].
About 30% showed possible parkinsonism (N = 885, 29.9%) at study entry. Among these individuals, parkinsonian gait was also the most common isolated sign (gait: N = 447, 15.1%; postural/rest tremor: N = 259, 8.7%; bradykinesia: N = 113, 3.8%; rigidity: N = 66, 2.2%).
The proportion of individuals with possible parkinsonism or parkinsonism increased with age (Figure 1B) and more men were affected (Table 2; Supplementary Table 2). For example, odds of parkinsonism in persons aged 90 years at baseline is about twice that for 80-year-olds.
At baseline, almost 60% of individuals had parkinsonism or possible parkinsonism. This is similar to categorization of these same individuals using a prior published categorical measure based on 10 items of the UPDRS with which about 70% of these same individuals would be categorized as having mild parkinsonian signs (Supplementary Table 4) (28).
Parkinsonism and Adverse Health Outcomes
Prior work suggests that the presence of mild parkinsonian signs are associated with adverse health outcomes (17). We employed Cox proportional hazard models which included terms for age, sex, and education to examine the associations of dichotomous parkinsonism with adverse health outcomes. Dichotomous parkinsonism was associated with hazards of death and incident mild cognitive impairment, incident AD, and incident disability (Table 3, Series A). These associations were unchanged when we adjusted for seven chronic health conditions and cognitive function (Supplementary Table 5, Models A and G).
Table 3.
Parkinsonism and Adverse Health Outcomes
| Outcome | Events per 1,000 Person-Years |
Series A Dichotomous (No/Yes) |
Series B Trichotomous (None/Possible/Yes) |
Series C Term 1: Possible Versus None Term 2: Yes Versus None Term 3: Yes Versus Possible |
||
|---|---|---|---|---|---|---|
| Term 1 | Term 2 | Term 3 | ||||
| Mortality | 70.0 | 1.53 (1.36,1.72) |
1.53 1.36, 1.71) |
1.49 (1.30, 1.71) |
1.91 (1.66, 2.19) |
1.28 (1.13, 1.45) |
| AD | 37.7 | 1.74 (1.46, 2.08) |
1.67 (1.41, 1.99) |
1.28 (1.05, 1.568 |
1.91 (1.55, 2.34) |
1.49 (1.22, 1.58) |
| MCI | 76.4 | 1.61 (1.37,1.90) |
1.51 (1.30, 1.76) |
1.26 (1.07, 1.48) |
1.68 (1.42, 1.99) |
1.34 (1.13, 1.58) |
| Katz disability | 72.8 | 2.48 (2.14,2.86) |
2.15 (1.89, 2.46) |
1.47 (1.27, 1.70) |
2.59 (2.23, 3.02) |
1.77 (1.52, 2.05) |
| Mobility disability | 104.5 | 1.97 (1.60,2.41) |
1.59 (1.35, 1.88) |
1.29 (1.11, 1.49) |
1.77 (1.48, 2.12) |
1.38 (1.14, 1.66) |
Notes: We dichotomized four parkinsonian signs and constructed two categorical measures of parkinsonism. For dichotomous parkinsonism, NO parkinsonism was 0 or 1 parkinsonian sign; YES parkinsonism was 2 or more parkinsonian signs. For trichotomous parkinsonism: NONE was no parkinsonian signs, POSSIBLE was the presence of one parkinsonian sign; YES was the presence of two or more parkinsonian signs. Cases with dementia at baseline were excluded for the analysis of incident AD. Cases with MCI and dementia at baseline were excluded for analysis of incident MCI. Cases with Katz disability at baseline were excluded for the analysis of incident Katz disability. Cases with mobility disability at baseline were excluded for the analysis of incident mobility disability. The crude rate of incident adverse health outcomes is shown as events per 1,000 person-years of the number of persons without the outcome at baseline who developed the adverse health outcome during the study. Series A, B, and C show results from Cox proportional hazards models of time from baseline to first report of an adverse health outcome as a function of baseline parkinsonism. Each model also included terms to control for age, sex and education (not shown). Each cell shows the hazards ratio and 95% confidence interval for the parkinsonism predictor terms employed. In Series A and B, each cell shows the coefficient of a single term for parkinsonism (dichotomous parkinsonism – series A or trichotomous parkinsonism coded as 0 = None, 1 = possible and 2 = Definite – series B). In series C, we show hazard ratios estimated from models with two separate terms to code the three categories employed in the 3-level classification of parkinsonism. AD = Alzheimer’s disease; MCI = mild cognitive impairment.
Next we examined the associations of possible and probable parkinsonism with adverse health outcomes with no parkinsonism as the reference group. We repeated the analyses similar to those described above and found that this 3-level classification of parkinsonism was associated with adverse health outcomes, although some of the effect sizes were attenuated (Table 3, Series B). These associations were unchanged when we adjusted for seven chronic health conditions (Supplementary Table 5, Model A).
In sensitivity analyses, we excluded participants with a history of PD for which they had received levodopa (Table 1). Trichotomous parkinsonism remained associated with the adverse health outcomes examined above (Supplementary Table 5, Model B). Similar results were obtained when in addition to PD, we also excluded participants taking medications which may cause tremor or parkinsonism including neuroleptics, asthma medication, thyroid medication, or antidepressants (Table 1; Supplementary Table 5, Models C–F).
Next we repeated the approach described above including separate terms for possible parkinsonism and parkinsonism to examine the extent to which possible parkinsonism was associated with risk of adverse health outcomes compared to individuals without parkinsonism. In these analyses we found that individuals with possible parkinsonism as well as those with parkinsonism both had an increased risk of adverse health outcomes compared to individuals without parkinsonism (Table 3, Series C, Terms 1 and 2). In further analyses, we found that parkinsonism was associated with an increased hazard relative to possible parkinsonism for all adverse health outcomes (Table 3, Series C, Term 3).
Parkinsonism and Brain Pathology
There were 1,373 deaths after an average of nearly 7 years of follow-up (mean 6.4 years, SD = 4.58 years) with 1,187 autopsies (86.5%). At the time of these analyses, the uniform neuropathological examination was complete for 1,160 (ROS 613, MAP 547). Mean age at death was 88.3 years (SD = 6.65 years); 64.5% were women. At their last visit, about 10 months before death, more than 70% had either parkinsonism (46.8%, N = 543) or possible parkinsonism (24.5%, N = 284)).
Description of Neuropathologies: AD pathology was the single most common pathology (N = 731, 63.0%). One or more of the four vascular pathologies were present in more than 4/5(81.2%) of the cases [macroinfarcts (N = 406, 35.0%); microinfarcts (N = 332, 28.6%); arteriolosclerosis (N = 406, 35.4 %); and atherosclerosis (N = 454, 39.4 %)]. Nigral Lewy body pathology was present in more than 1/5 (N = 257, 22.2%) and moderate/severe nigral neuronal loss was present in more than 1/10 (N = 159, 13.7%) and nigral Lewy bodies together with moderate/severe nigral neuronal loss was present in 1/10 (N = 117, 10.0%).
Ordinal logistic regression models were employed to examine which neuropathologies were associated with parkinsonism. Each model included terms for one of the seven neuropathologies and controlled for age, sex, and education. Each of the four vascular pathologies was associated with parkinsonism but not AD pathology, Lewy body pathology, or nigral neuronal loss (Table 4, Models 1–7). Arteriolosclerosis and atherosclerosis showed independent associations with parkinsonism when all seven pathologies were included in a single model (Table 4, Model 8). These results were unchanged when we excluded cases with a history of PD proximate to death (Table 4)
Table 4.
Association of Brain Pathologies and Trichotomous Parkinsonism†
| Term | Models 1–7 | Model 8 |
|---|---|---|
| Macroinfarcts | 1.36 (1.08,1.72), p = .009 1.37 (1.08, 1.73), p = .009* |
1.14 (0.89,1.47), p = .307 1.15 (0.89,1.48), p = .290* |
| Microinfarcts | 1.28 (1.01,1.63), p = .044 1.28 (1.01,1.64), p = .045* |
1.17 (0.91,1.52), p = .219 1.17 (0.91,1.51), p = .229* |
| Arteriolosclerosis | 1.24 (1.11,1.39), p < .001 1.23 (1.09, 1.38), p < .001* |
1.16 (1.03,1.31), p = .017 1.14 (1.01,1.30), p = .035* |
| Atherosclerosis | 1.35 (1.18,1.55), p < .001 1.34 (1.17,1.54), p < .001* |
1.25 (1.08,1.45), p = .003 1.24 (1.07,1.44), p = .004* |
| Alzheimer’s disease pathology | 0.94 (0.72,1.22), p = .638 1.05 (0.88,1.26), p = .571* |
1.07 (0.89,1.28), p = .479 1.09 (0.91,1.30), p = .361* |
| Lewy bodies | 1.05 (0.88,1.25), p = .612 0.87 (0.67,1.15, p = .326* |
0.98 (0.71,1.34), p = .897 0.94 (0.69,1.29), p = .716* |
| Nigral neuronal loss | 1.01 (0.88,1.15), p = .939 0.92 (0.80, 1.06), p = .271* |
1.03 (0.88,1.21), p = .696 0.95 (0.80,1.12), p = .509* |
Notes: †Models 1–7 based on seven separate logistic regression models with the outcome trichotomous parkinsonism at the last visit proximate to death with terms for age, sex, and education (not shown) and each of the seven pathologies. The top row of each cell shows the association for a different pathology and parkinsonism proximate to death. Model 8 is based on a single model which included terms for age, sex, and education (not shown) and all seven pathologies together. Odds ratio (95% confidence interval and p value). We repeated each these eight models excluding participants (N = 43) with a history of PD and who had received levodopa. These results, identified with an ‘asterisk’ are shown in the bottom row of each cell.
Of the 543 decedents with parkinsonism, 310 cases had two of four parkinsonian signs present. We examined the six groups having different combinations of two parkinsonian signs; the frequency with which the postmortem findings were present did not vary across the six groups (all p values >.200). These findings were unchanged when we excluded cases with a history of PD (N = 9). (Supplementary Table 6).
Discussion
We created an index of parkinsonism, using the mUPDRS, a reliable instrument which is easy to administer by nonphysicians, to categorize parkinsonism in almost 3,000 older adults living in the community. About 25% showed parkinsonism at baseline which was strongly associated with age and affected more men. Possible parkinsonism (onesign) was more common than parkinsonism at baseline and affected 30%. Using this categorical measure, individuals with either possible parkinsonism or parkinsonism showed an increased risk of adverse health outcomes compared to those without parkinsonism after controlling for demographics, chronic health conditions, cognitive function and after we excluded cases with dementia, mild cognitive impairment, activity of daily living, and mobility disability as well as cases with a history of PD and several medications which can cause parkinsonian signs. In addition, parkinsonism was associated with age-related brain pathologies. These data suggest that parkinsonism is common in older adults and its presence identifies individuals at increased risk for adverse health outcomes and predicts brain pathology.
Several prior studies of parkinsonism have generally focused on assessments of nongait-related parkinsonian signs and dichotomized the presence or absence of these signs based on the assessment of 8–10 items from the UPDRS (5–8). The current study employed 26 items including both gait and nongait items from the UPDRS. Parkinsonian gait was the most frequent isolated signs as well as most common sign observed in combination with the other parkinsonian signs. These observations lend further support to prior studies that have reported the important contribution of parkinsonian gait to the adverse health outcomes associated with parkinsonism (13,18–20). Prior studies have reported that from 15% to 50% of older adults might show signs of parkinsonism which are associated with adverse health outcomes (1). This wide range is likely due in part to differences in the study populations and their ages as well as from methodological differences. For example, not all studies employed the same instruments to assess parkinsonism and even in those studies which employed the UPDRS, the number and specific items assessed varied (5–7,9,30). Furthermore, in contrast to prior studies that dichotomize the presence or absence of parkinsonian signs, the current study constructed a 3-level measure of parkinsonism. Thus, cases in the current study which were classified as possible parkinsoninsm would likely have been categorized as having mild parkinsonian signs by other studies (7). Nonetheless, despite these methodological differences, these signs are very common in older adults. Thus, given its association with age, the public health magnitude and challenge of parkinsonism is likely to grow in our aging population.
The increased risk of adverse health outcomes in individuals with possible parkinsonism in the current study suggests that there may be a dose–response to the biology underlying parkinsonism which may affect a much larger number of older individuals less severely. Thus, it is possible that even a single parkinsonian sign is sufficient to increase the risk of adverse health outcomes. These data suggest that the less severe burden of motor signs (ie, only one parkinsonian sign) in possible parkinsonism may be analogous to mild cognitive impairment, a less severe and an earlier stage of impaired cognition that may progress in some individuals to full blown dementia. Thus, possible parkinsonism may represent preclinical or premotor PD that may progress to more severe motor impairment or may manifest as mild motor signs associated with subclinical cerebrovascular disease pathology (31–36).
The current study extends prior studies by showing that parkinsonism is strongly associated with indices of all four cerebrovascular disease pathologies. These results contrast with prior studies that found that a continuous measure of parkinsonism was associated with PD, AD, and cerebrovascular disease pathologies. The effect size as well as the loss of statistical power associated with the use of a categorical measure and high prevalence of cerebrovascular pathologies is likely to explain the differences between the results of these studies. The current findings may have important clinical implications. For example, arteriolosclerosis cannot be assessed with conventional brain imaging and may offer an important new direction for preventing or decreasing the burden of parkinsonism in older adults. The prominence of arteriolosclerosis, which affected as many individuals as macroinfarcts, lends further support to the notion that small-vessel disease in old age may contribute to late-life motor impairment (18,34). These data underscore that the clinical phenotype of parkinsonism in older adults is not simply an early manifestation of PD but that like dementia, this phenotype is common and may derive from diverse causes. Further work is needed to develop more accurate clinical risk profiles, potentially including nonmotor functions along with neuroimaging, genetic, or biochemical biomarkers. Such efforts may increase the specificity for predicting distinct pathologies and etiologies (37).
There are several reasons to think that our study may have underestimated the contributions of common neuropathologies to parkinsonism in older adults. The postmortem indices for AD pathology were preferentially collected from traditional cognitive-related brain regions, while other motor-related regions rostral and caudal to the substantia nigra including brainstem, spinal cord, and muscle were not examined and are likely to make separate contributions to the severity of parkinsonism (19). Furthermore, other pathologies such as white matter loss were not measured. Further studies are needed to replicate our findings and to determine the full range of neuropathologies and other mechanisms which contribute to parkinsonism in old age.
There are several strengths to the study, including the community-based cohort with large numbers of women and men coming to autopsy following high rates of clinical follow-up and high autopsy rates. Uniform structured clinical procedures were used that included a detailed assessment of parkinsonian signs that has been widely used in other studies. There are a number of limitations too. The cohort is selected and replication of these findings in a more general population is needed. While the qualitative clinical assessment of parkinsonian signs employed in this study is a robust predictor of adverse health outcomes, these signs lack specificity as many different etiologies may contribute to their development. Further work is needed to develop quantitative measures that are more specific to characterize parkinsonism in older adults (38). Because there is no widespread routinely available biologic marker for PD, it is possible that some of cases identified with parkinsonism at baseline may have undiagnosed PD. However, the current analyses of postmortem data suggest that in contrast to PD which is uncommon in older adults affecting up to 5% by age 85 years (39), “parkinsonism” may affect 50% or more of adults aged 85 years or older and is more likely to be associated with non-PD neuropathology. This study was large since on an individual level the effect sizes are small. Nonetheless, from a public policy perspective given the extent of motor impairments in old age, even the modest effect sizes observed in the current study are likely to be important.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by the National Institute of Health (R01AG17917 and P30AG10161 to D.A.B., R01NS078009, R01AG040039 and R01AG043379 to A.S.B., and K08AG034290 to J.M.S.); the Caroline Weiss Law Fund for Research in Molecular Medicine, the Burroughs Wellcome Fund to J.M.S.; and the Illinois Department of Public Health, and the Borwell Endowment Fund to D.A.B.
Supplementary Material
Acknowledgments
We thank all the participants in the Religious Order Study and the Rush Memory and Aging Project. We also thank the staff of the Rush Alzheimer’s Disease Center.
References
- 1. Louis ED, Bennett DA. Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord. 2007;22:1681–1688. doi:10.1002/mds.21433 [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3. Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. [DOI] [PubMed] [Google Scholar]
- 4. Delmonico MJ, Harris TB, Lee J-S, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 5. Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43:2184–2188. [DOI] [PubMed] [Google Scholar]
- 6. Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol. 2004;61:1273–1276. [DOI] [PubMed] [Google Scholar]
- 7. Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62:297–302. doi:10.1001/archneur.62.2.297 [DOI] [PubMed] [Google Scholar]
- 8. Uemura Y, Wada-Isoe K, Nakashita S, Nakashima K. Mild parkinsonian signs in a community-dwelling elderly population sample in Japan. J Neurol Sci. 2011;304:61–66. doi:10.1016/j.jns.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 9. Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses’ ratings of parkinsonian signs with a modified Unified Parkinson’s Disease Rating Scale. Neurology. 1997;49:1580–1587. [DOI] [PubMed] [Google Scholar]
- 10. Bennett DA, Shannon KM, Beckett LA, Wilson RS. Dimensionality of parkinsonian signs in aging and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1999;54:M191–M196. [DOI] [PubMed] [Google Scholar]
- 11. Rosano C, Bennett DA, Newman AB, et al. Patterns of focal gray matter atrophy are associated with bradykinesia and gait disturbances in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:957–962. doi:10.1093/gerona/glr262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rose G. The Strategy of Preventive Medicine. Oxford: Oxford University Press, 1992. [Google Scholar]
- 13. Wilson RS, Schneider JA, Beckett LA, Evans DA, Bennett DA. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. [DOI] [PubMed] [Google Scholar]
- 14. Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539–544. [DOI] [PubMed] [Google Scholar]
- 15. Boyle PA, Wilson RS, Aggarwal NT, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology. 2005;65:1901–1906. [DOI] [PubMed] [Google Scholar]
- 16. Fleischman DA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Parkinsonian signs and functional disability in old age. Exp Aging Res. 2007;33:59–76. doi:10.1080/03610730601006370 [DOI] [PubMed] [Google Scholar]
- 17. Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the rush memory and aging project, a community-based cohort study. BMC Med. 2011;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buchman AS, Leurgans SE, Nag S, Bennett DA, Schneider JA. Cerebrovascular disease pathology and parkinsonian signs in old age. Stroke. 2011;42:3183–3189. doi:10.1161/strokeaha.111.623462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buchman AS, Nag S, Shulman JM, et al. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov Disord. 2012;27:1625–1631. doi:10.1002/mds.25142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchman AS, Shulman JM, Nag S, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71:258–266. doi:10.1002/ana.22588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the religious orders study. Curr Alzheimer Res. 2012;9:628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J Am Geriatr Soc. 2007;55:11–19. doi:10.1111/j.1532-5415.2006.01032.x [DOI] [PubMed] [Google Scholar]
- 24. Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 25. Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556–559. [DOI] [PubMed] [Google Scholar]
- 26. Kraemer HC. How many raters? Toward the most reliable diagnostic consensus. Stat Med. 1992;11:317–331. [DOI] [PubMed] [Google Scholar]
- 27. Kraemer HC. Extension of the kappa coefficient. Biometrics. 1980;36:207–216. [PubMed] [Google Scholar]
- 28. Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology. 2005;64:1157–1161. doi:10.1212/01.wnl.0000156157.97411.5e [DOI] [PubMed] [Google Scholar]
- 29. SAS/STAT® Software for Unix, Version (9.18) [computer program]. Cary, NC: SAS Institute, 2002–2003. [Google Scholar]
- 30. Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. [DOI] [PubMed] [Google Scholar]
- 31. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:263–269. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:280–292. doi:10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jack CR, Jr, Albert M, Knopman DS, et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement. 2011;7:256–262. doi:10.1016/j.jalz.2011.03.004 [Google Scholar]
- 34. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia. Stroke 2011;42:2672–2713. doi:10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siderowf A, Lang AE. Premotor Parkinson’s disease: concepts and definitions. Mov Disord. 2012;27:608–616. doi:10.1002/mds.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lerche S, Hobert M, Brockmann K, et al. Mild parkinsonian signs in the elderly–is there an association with PD? Crossectional findings in 992 individuals. PLoS One. 2014;9:e92878. doi:10.1371/journal.pone.0092878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berg D, Lang AE, Postuma RB, et al. Changing the research criteria for the diagnosis of Parkinson’s disease: obstacles and opportunities. Lancet Neurol. 2013;12:514–524. doi:10.1016/S1474-4422(13)70047-4 [DOI] [PubMed] [Google Scholar]
- 38. Buchman AS, Leurgans SE, Weiss A, et al. Associations between quantitative mobility measures derived from components of conventional mobility testing and Parkinsonian gait in older adults. PLoS One. 2014;9:e86262. doi:10.1371/journal.pone.0086262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol Mech Dis. 2011;6:193–222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.