Abstract
Although the demographic revolution has produced hundreds of millions people aged 65 and older, a substantial segment of that population is not enjoying the benefits of extended healthspan. Many live with multiple chronic conditions and disabilities that erode the quality of life. The consequences are also costly for society. In the United States, the most costly 5% of Medicare beneficiaries account for approximately 50% of Medicare’s expenditures. This perspective summarizes a recent workshop on biomedical approaches to best extend healthspan as way to reduce age-related dysfunction and disability. We further specify the action items necessary to unite health professionals, scientists, and the society to partner around the exciting and palpable opportunities to extend healthspan.
Key Words: Healthspan, Longevity dividend, Models of care
Preamble: This viewpoint article synthesizes a 2-day workshop discussion in Tucson in April, 2014, on three general themes: Economics and Demographics of Aging; Models of Care and Care Delivery to Older Adults; and Biology of Aging; as well as on the specific action items we feel are pertinent today in order to systematically improve healthspan among current and future generations of older adults. This is not a review with a comprehensive overview of each of the topics but rather a distilled extract of our viewpoints on the above issues.
We live in a rapidly aging world and are experiencing an unprecedented rise in the number of older adults. The world’s population will experience a demographic “silver tsunami” in this century because of inevitable shifts in age structure—leading to a large increase in numbers of older adults and a concomitant increase in the prevalence of fatal and disabling chronic conditions (1). We make the case here that it may not only be possible to stem the tide of rising frailty and disability at the population level but simultaneously extend the period of healthy life at the level of individuals [referred to as a longevity dividend or geroscience (2) initiative (3)]. By 2030, those aged 65 or older will make up a quarter or more of the U.S. population and will number more than one billion across the globe by 2050 (4). At the same time, in the next 30 years, the number of Americans with chronic conditions will increase by nearly 40% and the number of noninstitutionalized, yet disabled, persons older than 65 years is expected to double to 40 million in the next 40 years (4). In addition, based on demographic trends, there will be fewer people to provide care for the rapidly growing and aging population (4). This demographic shift without a concomitant change in our approach to prevent or delay, treat and care for, the conditions and disabilities of later life, leaves us vulnerable to a large increase in the prevalence of age-related diseases, multimorbidities, disabilities, and functional impairments as populations age in the coming decades (Figure 1). These demographic trends carry enormous socioeconomic and health care implications (Figure 2).
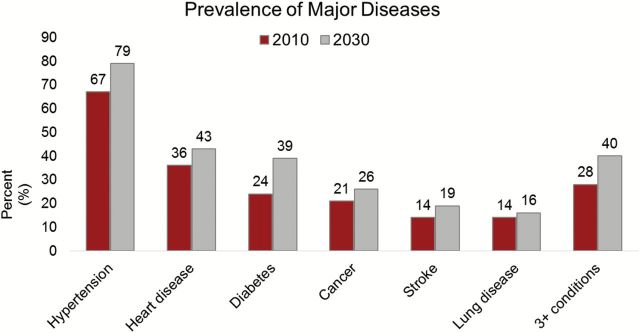
Figure 1.
Projections of disease in the population aged 65 and older, 2010–2030. The figure shows rates of self-reported disease (“Has a doctor ever told you . . . ”) based on projections using data the Health and Retirement Study and the National Health Interview Study.
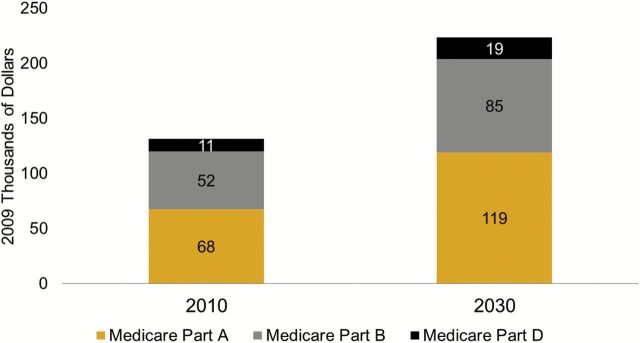
Figure 2.
Net present value of lifetime Medicare spending per person aged 65 in 2010 and 2030. Figure is based on projections from the Future Elderly Model using data from the Health and Retirement Study, the National Health Interview Study, and the Medicare Current Beneficiary Survey. Spending projections assume cost growth consistent with the targets set in the Affordable Care Act, that is, below Gross Domestic Product growth until 2019 and 1% above GDP growth from 2019 to 2030. Spending is discounted with a 3% rate from age 65 onward.
Other than a few progressive health care delivery models, the dominant health delivery structures remain disease centric and hospital based, incentivizing high volumes and high utilization (5,6) Therefore, although we have been aware of these demographic trends for a long time, the society remains poorly prepared for this demographic transition that carries enormous socioeconomic and health care implications (Figure 1).
Contrary to these pessimistic projections, we see every reason for hope that aging science (gerontologists) and health practice (geriatricians and other health professionals) can generate and implement interventions that extend healthy life. We already possess approaches that can maximize the benefits of population aging while minimizing the downside. These approaches include the following:
The widespread deployment of innovative population health-based clinical models of care targeting high cost older adults with advancing chronic conditions. These models combine short-term or longitudinal home-based interprofessional team care approaches with telemedicine/telemonitoring and prevention strategies to rapidly generate optimal health outcomes at reduced cost (7). (http://onlinelibrary.wiley.com/store/10.1111/jgs.12974/asset/jgs12974.pdf?v=1&t=icw4le4r&s=b1564bdbca20d5224a9acf7953927416dcd468bd). For example, Independence at Home, a current Center for Medicare and Medicaid Services demonstration model of home-based primary care (HBPC), reported average savings of $420 per beneficiary per month for more than 8,000 patients treated. (http://innovation.cms.gov/initiatives/independence-at-home/ and https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2015-Press-releases-items/2015-06-18.html).
The development of new preventive treatments and strategies that could simultaneously delay or ameliorate multiple age-related impairments in a single step. These derive from our increasing knowledge of the common biological mechanisms that drive both aging processes and facilitate age-related diseases. This represents a potential “silver bullet” that is capable of impacting not just one disease, or several diseases one-by-one, but rather, can strike at the root cause(s) of the aging processes and simultaneously delay or eliminate many diseases.
There is an urgent need to raise awareness and obtain political/societal action on the two above approaches. However, each approach remains somewhat limited to the primary communities that are testing them (geriatricians/health care professionals, and gerontologists, respectively). So far, despite a few notable efforts (8,9), neither of the two communities has been able to fully and effectively communicate the power and promise of health care outcomes and of longevity and healthspan research to one another, and even less so to the general public and the decision makers. However, momentum is building with recent publications (10–14) a campaign (http://healthspancampaign.org) and its movie (http://healthspancampaign.org/film/), several recent collaborative meetings, and an upcoming book (15) designed to cross-fertilize and develop the path forward to build a translational perspective and to develop communication strategies that bring together the aging research and clinical care communities.
To discuss these urgent issues and to chart the way forward, the University of Arizona Miraval Institute convened its inaugural think-tank symposium meeting in late April 2014, under the title “Preparing for an Aging World: Living Beyond 100” (Figure 3). The section summary from each of the three sessions of the workshop follows, concluding with an overall summary containing suggestions for immediate and future action (Figure 3).
Figure 3.
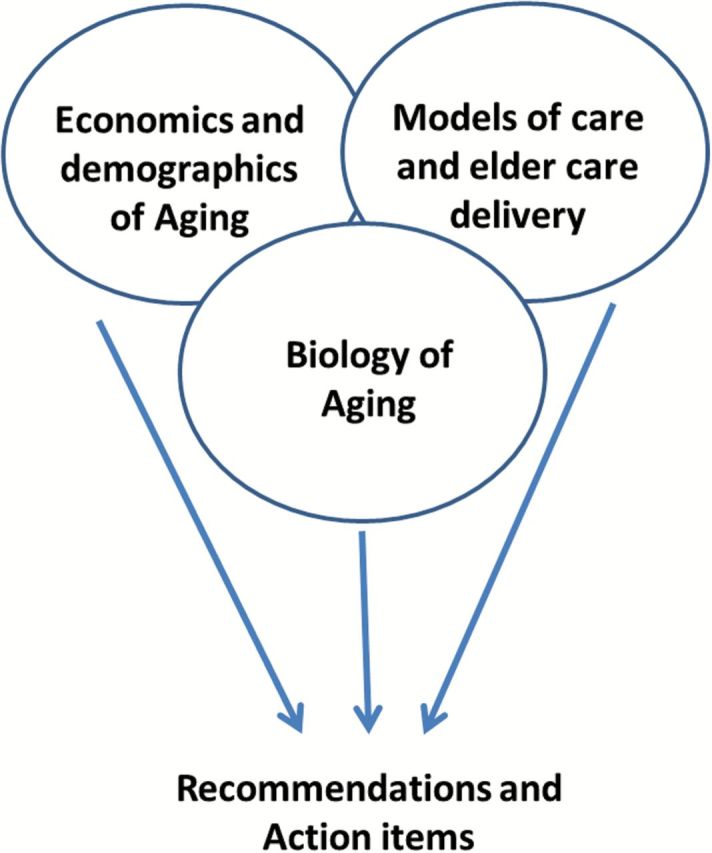
Themes and structure of the workshop.
Economics and Demographics of Aging
Life expectancy has been extended most everywhere. Many people are living well beyond the retirement age, and science and medicine continue to make rapid progress. Advanced age (>80 years of life) has become an achievable milestone for a growing segment of the population, and increases in lifespan (and healthspan) in model organisms may soon lead to translatable interventions in humans (15). The goal of aging science and health care, however, is not to extend life per se or make everyone live to 100, but rather, to provide people with an opportunity to live their lives in a state of good health (healthspan) for as long as possible. Experts in this session (Dana Goldman, S. Jay Olshansky, and Tom Perls) were tasked to provide a framework inclusive of the economics, demographics, and medical aspects of aging, to help us decide whether the current focus of medical research and investment should be shifted from the disease model to a delayed-aging model, building on the promise of public health, medical, and scientific interventions.
In considering changes in longevity and healthspan, the panel identified the following points:
Basic advances in public health and better nutrition, and more recently, improved behavioral risk factors (eg, reduced smoking) and treatments for specific diseases are responsible for most of the rise in life expectancy.
Unfortunately, increased disability rates and rates of multimorbidity are now accompanying increases in life expectancy in the United States, and likely all over the world—leaving healthy life span (healthspan) either declining or unchanged.
The rise of obesity suggests that older people may face even more health challenges in the future than cohorts reaching older ages today.
Although future gains in life expectancy may not come close to what was achieved in the 20th century, it is possible that aging science can have a dramatic positive influence on healthspan—leading to a compression of age-associated diseases and disability. Some of these gains may result from a marked increase in the prevalence of healthy behaviors.
There are other key findings about aging and longevity that can inform our understanding of aging demographics and economics. For example, as people age, they are less likely to fall victim to a single, isolated disease but instead die from multiple competing causes of death associated with aging or of age-related comorbidities. These conditions also create a frailty and disability profile. On the other hand, subgroups of the population have been shown to age more slowly and delay or escape such diseases as well as accompanying disabilities (16). Such compression for both diseases and disability is observed for people who currently survive to extreme old ages with their health largely intact (17). People living to 105+ years have been documented to exhibit substantial phenotypic homogeneity, suggesting the realistic opportunity to discover shared environmental and genetic determinants (18,19) of such exceptional survival.
A major question is whether and how much society should invest in an effort to postpone all fatal and disabling diseases simultaneously (extend healthspan) by delaying the biological processes of aging? In deciding, several related questions arise as follows:
What are the relative health and economic benefits/costs of delayed aging as compared with the disease intervention model?
Can we afford to continue with the disease intervention model given the large demographic shifts that are forthcoming and the anticipated diminishing returns from investments that treat diseases after they arise (15)? Or should we just continue to proactively treat fatal and disabling diseases?
Can society afford to invest in the science that would lead to accelerated development of interventions that extend healthy life?
Can society afford not to invest in the science that has the potential to have a transformative impact on health and health care spending in the coming decades?
To answer some of these questions, some of us (D.R.G. and S.J.O., with collaborators) used a well-validated model of future aging to predict medical spending, health conditions, functional status, and employment based on initial demographic and health conditions (4). Five scenarios were developed about the future course of mortality (projected to 2060) and compared along health and medical spending dimensions (4). The results demonstrate the following:
The number of people aged 65 and older in the United States is expected to more than double over the next 50 years, rising from 43 million in 2010 to 106 million by 2060. If delayed aging comes to pass, there would be just less than 7% (or about 7 million) more people aged 65 and older in the United States in 2060.
A significantly larger number of people who reach ages 65 and older between now and 2060 would be healthier, with lower per capita medical spending. However, an absolute increase in the number of people older than 65 years, though healthier, could still raise total costs.
A hypothetical small increase in the age of eligibility for national health insurance programs (Medicare in the United States) would fix this problem, but it is uncertain whether this or some other modification would be most appropriate to handle the larger, healthier older population, or the needs of segments of the population who may not benefit from delayed-aging interventions.
With regard to functional status, decades of improvement in the functional status of older Americans have halted since 2002 (20–23), suggesting that many of the historical drivers of better health in older adults will not continue.
Therefore, an unequivocal answer to the question of whether the current focus of medical research and investment should be shifted from the disease model to delayed aging depends on whether the potential gains can be realized and the adverse consequences allayed. It is clear that competing health risks limit the impact of major clinical breakthroughs for specific diseases—that is, making progress in one disease means another one will eventually emerge in its place. This universal problem of competing risks in aging populations makes research and investment to delay or slow aging highly valuable, given the evidence that delayed aging would yield notable reductions in most if not all fatal and disabling disease simultaneously.
Models of Care and Care Delivery for Older Adults
Although research to extend healthspan by delaying the biological processes of aging itself is the key long-term research goal, its translation into broadly applicable treatments in humans will not happen overnight. Until that translation is achieved, older adults will still suffer from multimorbidities, disabilities, and functional declines. Health care delivery systems have the potential to minimize disability and positively impact on healthspan in the short run, but have yet to meet that challenge. For example, the current U.S. health system is costly and inefficient and delivers suboptimal quality care, particularly for older adults with advancing chronic conditions. The United States spends nearly twice more per person on health care than other developed nations, as well as a higher percentage of Gross Domestic Product, and yet patients do not benefit from better outcomes. The same situation exists in other countries where fee-for-service model dominates, which are experiencing increasing costs and are on a path to repeat the U.S. health care crisis. This situation is unsustainable.
How can we directly improve health outcomes and extend healthspan? What are the most effective health care delivery interventions, and what are the barriers to their implementation? Experts in this session (D.C., M.J.F., and C.R.) were tasked to summarize the current situation and discuss future steps to achieve high value health care. P.C. was tasked to provide a perspective of growing old in an age of informatics and how information can impact both health choices and quality of life.
We first discussed the causes of our rapidly rising health care costs, which are primarily due to the following reasons:
a rapidly growing older population (24),
advances in medical technology that include expensive treatments and procedures (25,26),
a reimbursement structure based on the volume of care that incentivizes high utilization (5,6), and
an increase in unhealthy lifestyles, for example, smoking (27) and obesity (28).
The result is high utilization and high costs of care, which are not linked to outcomes. To transform fee-for-service system (U.S. health care being the most extreme example), incentives need to be aligned and linked to value, and tailored health care delivery models must be targeted to specific populations.
How can this be accomplished quickly? The highest cost group in the United States—the 5% of Medicare beneficiaries who, according to the Congressional Budget Office, account for approximately 50% of Medicare’s expenditures (http://cbo.gov/publication/16487)—should be initially targeted. They suffer from frequent acute exacerbations of their chronic conditions and have multiple costly emergency room visits and acute hospitalizations, about 30% of which are potentially avoidable (http://cbo.gov/publication/16487). Significant savings and improved health outcomes are possible if we bring the care of these frail, complex, and chronically ill patients into the home, a successful strategy clearly demonstrated by large, evidence-based programs, including
HBPC program that delivers team-based primary care in the home for individuals with advancing chronic conditions—as demonstrated in both U.S. VA (Veterans Administration), Medicare populations (29–31) and the Independence at Home Medicare Demonstration Project http://innovation.cms.gov/initiatives/independence-at-home/
Hospital at Home, which substitutes hospital care with equivalent services at home for selected conditions, such as heart failure and cellulitis—and yields better outcomes in terms of patient safety than hospital care, while saving 30%–60% of costs depending on the pre-existing costs in the system (7).
Unfortunately, in the fee-for-service reimbursement system, these models have not been easy to implement and have failed to reach the general U.S. population. However, the expanding number of integrated health systems, such as the VA, have opened the door to innovative, evidence-based financial models for our most frail and costly patients. Advances in information technology, and emerging home-based technology, have also been instrumental in helping to drive complex care safely into the home. By expanding access to high value, team-based home care medical programs, the system will be able to achieve financial sustainability—and free up resources to meet broader population health goals.
An inherent strategy of extending healthspan is to study and promote resilience in older adults. How can we understand resilience? Many who age will be hit by a number of adversities, such as the breakup of a marriage or chronic illnesses. We have all seen individuals who have become undone by life’s misfortunes or hard times, and we have seen others who seem to bounce back despite the odds, and although that is not limited to older adults, frailty, as defined by clinical criteria, correlates with poor health and survival outcomes [reviewed in (32,33)]. Several factors/behaviors influence resilience:
physical behaviors (diet, physical activity, and sleep),
social connectedness (staying engaged with relevant communities),
cognitive coping strategies (cognitive reframing, locus of control, dispositional optimism, and learning), and
pursuing a life of meaning and purpose.
Although we understand that resilience is critically important, we have a lot to learn about how to measure and effectively promote it. Careful studies are urgently needed in order to define the cellular and molecular basis of resilience and to provide mechanistic correlates that can be used to validate the efficacy of interventions to promote resilience in humans. Similarly, we are just beginning to examine and objectively characterize frailty, a geriatric syndrome characterized by limited physiologic reserves and a reduced ability to response to stressors. Frailty exists along the continuum of resilience, and is well known to geriatricians, but the cellular and molecular basis of frailty remains mysterious and insufficiently studied by basic scientists [reviewed in (34)]. Further investigations are necessary to provide us with a clearer course of action on both resilience and frailty, key contributors to healthspan.
We further considered the role of emerging technology, recognizing that it can potentially promote resilience and help older adults live healthier lives. Information technology affects all of us, with social media, communications technology, sensors, recording devices, decision support, and knowledge resources. It is pervasive, and it can help us live life well by helping us to shape our lives in response to information. We have been shaping our lives in response to information for millennia, but we will be able to do that better, and in surprising ways, as more information becomes available.
One can examine life as a sequence of choices that are conditioned on information. Because a choice today, large or small, can affect future outcomes and choices, life can be viewed as an enormous tree, each branch of which is a unique sequence of decisions and outcomes. If you made only one choice a day, and each choice led to only two outcomes, then in only a month the tree would contain more than a billion branches.
Computers can help us live our lives well in the face of complexity by allowing us to
Follow successful paths to extend our healthspan. A good way to plan pedestrian pathways is to plant grass and wait for a couple of years. The preferred pathways will show up as bare dirt. By analyzing the paths we each forge through life, there is much to be learned. We currently broadcast our lives on many social media channels, but this sort of information has never been correlated with health records, demographic and geographic information, and other sources. When it is, we will have high-resolution data about how people live their lives and we will be able to recommend statistically good paths and develop and test theories of the factors that affect healthspan. Search engine companies are doing this now with a focus on consumer behavior and outcomes. Advances in big data gathering and analytic techniques can help us to greatly broaden the focus to much bigger questions such as personalized and population health and human well-being.
Look ahead and recommend extended healthspan choices. Commercial vendors already use the paths followed by other people to suggest paths for you, such as when an online vendor recommends a sequence of purchases based on your prior purchases. Such algorithms exist that seek to maximize the future value of your decisions and can be applied to health choices affecting the healthspan and are already reasonably efficient if they are not required to look too far into your future.
These ideas come together elegantly in the age of information and big data. Algorithms that look into your future must know about you, or, rather, people who are so much like you that their health histories and health futures and yours are probably similar. In the past, we could only guess at the probabilities of outcomes of therapies, diets, and other life decisions, or pay the enormous costs of large-scale experimental trials. Today each of us is a source of data about how to live life well (or not-so-well) and each of us can help others live their lives better when their decisions are informed by our data. We might be approaching an era in which sharing one’s data is as much a public health responsibility as inoculation for communicable diseases. The risks to individuals are undeniable, but so are the benefits to society.
Overall, in preparing for an aging world, the health care needs of the frail elderly population can and must be met through the provision of high value home-based models of clinical care delivery targeted to the specific needs of the population. At the same time, we must learn more about and encourage activities that we know influence resilience: physical behaviors, social connectedness, cognitive reframing, and pursuing a life of meaning/purpose. And in the age of information and big data, we have the ability, and the responsibility, to maximize our chances of identifying successful paths to achieve the health outcomes that we hope to reach. By doing all these, we will gain significant health care savings and forge a strategy that allows us as a society to attain health and wellness for all our citizens.
The Biology of Aging: Is There a Magic Bullet?
The practical purpose of biogerontology is to understand the mechanisms regulating aging to extend healthspan and compress morbidity. The conceptualization of the relationship between aging and illness is important because it guides the decisions on what problems to research, how much funding to allocate to those problems, and how to use the results of such research (eg, in patient treatment strategies). Until recently, aging and age-related disease have been conceptualized as separate entities. As our knowledge of the mechanistic basis of aging grows, this distinction is becoming increasingly blurred.
Within this context, experts in this session (B.K.K., L.F., A.R., and J.N.-Ž.) explored the state of the art and asked whether interventions that extended lifespan also extend healthspan (either selectively or across the board). They also explored the extent to which these interventions, alone or in combination, could result in realistic treatments for age-associated conditions, disabilities and/or impairments.
Contemporary biology has identified an ever-increasing number of single-gene mutations, which produce large extensions in healthy lifespan in model organisms (35). Of these, mutants in the insulin/insulin-like growth factor signaling pathway (IIS mutants) are the most numerous and are among the best known (35).
The above single-gene mutants not only display significantly extended lifespans compared with wild-type controls, but, crucially, this effect is preserved across multiple species. Such mutants include a variety of mutations that affect the insulin receptor in selected tissues or global circulating insulin levels and insulin receptor substrates, all leading to decreased metabolic rates. In addition to being long lived, these mutants show decreased presentation of multiple markers of aging including the development of ulcerative dermatitis, bone thinning, increased memory T-cell number, and reduced motor coordination. Thus, interference in insulin signaling pathways delays multiple aspects of age-associated pathology and morbidity (some of which have historically been considered classical age-related diseases and others classified as natural aging changes. This looks akin to a ‘magic bullet’ but how does it work?
Other important pathways include, but are not limited to, controlling the rate of cellular biosynthesis via the mTOR pathway (36), maintenance of mitochondrial function (37), and modulation of metabolic stress (37) and proteostasis (38,39), although some of the above pathways can, and likely do, intersect and interact and may lie directly downstream from, dietary restriction (DR) (40,41), which itself is the longest known robust experimental intervention to increase lifespan and healthspan (42). All of the above point to the inseparable connection between aging and metabolism.
Along these lines, insulin signaling mutants show changes including reductions in protein biosynthesis and anabolic metabolism (42). Calorie or DR result in similar phenomena and may act via the same pathway alterations (42). DR can be studied in a wide variety of simple model organisms, laboratory rodents, and nonhuman primates, and it has been found in most of these to extend lifespan and/or improve many markers of health. DR in Rhesus monkeys protects against diabetes, cancer, cardiovascular disease, sarcopenia, presbycusis, and brain atrophy (43,44), and in humans, it causes multiple metabolic and molecular adaptations that protect against these age-associated diseases (45). A combination of reduced activity of the insulin/IGF1/mTOR pathway, higher insulin sensitivity, lower inflammation and oxidative stress damage, induction of chaperone and autophagy- mediated cell clearance, and elevated protein turnover and repair may be responsible at least in part for the DR-induced beneficial effects in preventing/delaying multiple age-associated diseases and in extending healthspan.
A conceptually exciting breakthrough occurred in 2009, when a study demonstrated for the first time that one can extend lifespan by pharmacologic intervention, by pharmacological inhibition of mTOR using the drug rapamycin. This treatment resulted in lifespan extension even when applied late in life in rodents (46), and depending on the study, resulted in healthspan benefits to a greater or lesser extent (46–48). Rapamycin is known to have significant side effects (such as insulin resistance, impaired acute immunity to infection even at low dose in older organisms (49,50), and testicular degeneration), which are not readily aligned with improved healthspan. However, some of these effects could be the result of interaction between the drug and other targets, including the closely related mTOR complex 2 (mTORC2) rather than the complex mTORC1 against which rapamycin is primarily active. More selective mTORC1 inhibitors as well as modified regimens of administration are in development, raising the possibility that the next generation of such “rapalogues” will have enhanced healthspan promoting effects compared with the parent molecule.
Although not perfect, rapamycin (Sirolimus) and everolimus are already in clinical use and may be good enough to be deployed in the context of some age-related diseases and syndromes for which no effective treatment currently exists. Mild cognitive impairment and pancreatic cancer were identified by the investigators as potentially suitable diseases for clinical trials in humans that could produce changes in prescribing practice and patient benefit with a timescale of months rather than decades (51,52). This is a profound step forward and suggests that significant improvements in the health of older people could be achieved at minimal costs (eg, a typical cost for rapamycin dosing is of the order of £10/$16 per patient per day). Moreover, metformin, another clinically approved and widely used drug that among other effects also inhibits mTOR, may have similar life-extending effects (53) and is even more affordable.
Alongside the idea that nutrient sensing and recycling play a role in aging is the observation that chronic inflammation increases with age. Overall, there is an increase in proinflammatory cytokines (most notably interleukin-6 (IL-6), but also in variable amounts C-reactive protein (CRP), IL-1b, tumor necrosis factor alpha (TNFα), IL-8, and others), and it is imperative to understand the basis of this increased inflammation.
There are a variety of mechanisms that may lead to increased levels of inflammation. The key and not mutually exclusive candidates include the following:
The accumulation of “trigger-happy” highly differentiated memory T cells (54) that have the propensity to produce proinflammatory cytokines [many of them responding to persistent reactivating infections such as the cytomegalovirus—CMV (54)].
Increased adipose tissue with high proinflammatory potential in older adults (55,56).
Decreased integrity of barriers, including the gut barrier, with changing microbiota that colonize the organism and that potentially translocate into the circulation, causing increased inflammation (57,58).
Accumulation of senescent (no longer division-competent) cells, believed to arise as one of the byproducts of anticancer defense (59). These cells do not die but undergo a variety of changes that are deleterious to the tissue in which they reside [such as calcification (60) or the production of proinflammatory cytokines (61)]. In young organisms, such senescent cells are usually cleared by the immune system, but this process declines with age. Because cell proliferation is tightly linked to nutrient availability, it makes sense that DR has been found to delay the appearance of senescent cells, and treatment of cell cultures with rapamycin delays cell cycle progression and the appearance of many of the deleterious features of senescence.
Of interest, aspirin leads to a modest lifespan extension effect in worms and mice (62,63). By contrast, Nonsteroidal anti-inflammatory drugs in humans are risk factors for hypertension and myocardial infarction, and much remains to be studied whether, and if yes, how, inflammation could be directly treated by these and other drugs in order to achieve the goal of extended healthspan. Perhaps one approach could be microbiome manipulation, because a growing body of work shows that microbiome manipulation can profoundly impact both health status and aging [reviewed in (64)]. Another potential intervention of interest has been polyphenolic resveratrol. Although this substance has not shown any effects on lifespan in the National Institute of Aging (NIA) intervention testing program (65), but it nonetheless improved the health and survival of mice on a high-calorie diet (66). In humans, resveratrol reduced proinflammation cytokines release from senescent fibroblasts in vitro (67). This reinforces the idea that better readouts of functional status beyond lifespan will probably be increasingly required as new potential interventions come on line. To that effect, the NIA Division of Aging Biology has recently (August, 2014) convened a workshop to address the measures of resilience and healthspan in animals that may correspond to those in humans.
Conclusions and a Call to Action
Aging, as a whole, remains a relatively ignored topic in the national and international debate. Advocacy and dissemination of information about aging and its impact on our world is fragmented in most countries (World Economic Forum, 2012), though there are recent notable efforts to improve that in the United States (The Healthspan Campaign) and United Kingdom (http://www.youtube.com/watch?v=2v34Dt6wBkE; http://www.theguardian.com/society/2014/jun/26/big-ageing-population-debate). Of great concern is the fact that there are insufficient national or international strategic efforts to prepare us for our rapidly aging world and the predicted surge of chronically ill and disabled older adults with their heavy cost burden and poor health outcomes. In this session, all participants, but specifically key discussants (R.G.A.F., D.P., and D.C.) were asked to consider, and answer, two key questions:
(i) What policy initiatives and health delivery changes are needed to promote high value health care and assure the development and integration of new knowledge about resilience and healthy lifestyles?
(ii) How can the promise of biology of aging research and of technology be harnessed to avert crisis, extend healthspan, and increase both individual and societal well-being?
We began the discussion with the following scenario: If the President of the United States and other leaders asked us what we required from them to advance the cause, how would we answer?
All agreed that additional federal funds must be appropriated for research on aging and healthspan, because current funding amounts to only about 0.003% of the total National Institutes of Health (NIH) budget, and to less than 1% of the NIA budget (3,68). Several current initiatives under the leadership of Drs. Felipe Sierra, Richard Hodes, Ron Kohanski, and their colleagues at the NIA were discussed. The Alliance for Aging Research has assisted the NIA in promoting the trans-NIH Geroscience Initiative Group. In October, 2013, this group met, and white papers were published [see the issue of J Gerontol A Biol Sci Med Sci 2014, 69(suppl 1)], critically addressing the interdisciplinary nature of aging research; a summary review of that meeting, with a progress assessment, was published recently by some of us [reviewed in (14)]. The extramural community is developing its own initiatives, most notably at the annual meetings of several aging research organizations including the Gerontological Society of America and the American Aging Association. Nongovernmental funding for basic research into the mechanisms of aging and its role as a driver of age-related diseases is critical. These organizations include the American Federation for Aging Research, the Glenn Foundation, and, until recently, the Ellison Medical Foundation.
Moreover, the critical importance of developing initiatives that cross-connect and integrate the aging-related clinical and research communities was reinforced. Close collaboration between physicians and other care providers and biogerontologists is essential to achieve a critical mass for effective advocacy for new models of health care and for the integration of basic biology of aging into preventive and treatment programs. We believe that these activities, which are currently underway, can lead to formulation, and signing, of legislation to implement a rational, comprehensive National Aging Research policy.
Despite enthusiasm for enhanced federal funding, however, we readily recognized that the costs would likely dwarf the NIA/NIH budgets. The expense of testing promising treatments in humans would overwhelm resources, given that a single Phase III clinical trial costs more than 1 billion. Therefore, the emerging consensus was that while an increase in research budgets should be sought from the Congress via the NIA/NIH, the government and its agencies alone will not be able to provide the full answer. To that effect, we identified several other opportunities. The pharmaceutical industry, including biotech companies, is showing increased interest (J. Craig Venter Institute; Calico, formed from former Google and Genentech leaders) and, along with philanthropy, is a clear potential partner
Even more attention was devoted to discussing partnerships with for-profit or nonprofit integrated health care delivery systems, which both are responsible for the cost and the delivery of health care for populations. Such systems are inherently interested in achieving the best outcomes (eg, high quality and safe care) at the lowest costs because they are responsible for the complete process of care of their populations. Overall, it was felt that such systems may be in position to seek out (“pull”) the relevant scientific discoveries, which was agreed to be the desired model to generate meaningful discovery. The other alternative, with the scientists marketing (“pushing”) their own discoveries to physicians and other care providers was felt not to be reliable or sufficiently targeted to the needs of the industry. In order to drive this partnership, a cost-share model was discussed where a percentage of the realized savings (eg, 20%–30% of it) would be directly reinvested into fundamental and translational research. In addition, a portion of the population served by the delivery system may be interested in taking part in research to promote healthy aging. In this financial model, individuals could be directly incentivized to participate in novel methods and treatments to foster health and wellness by lowering their premiums. This approach would fully align with the mission of most integrated health systems—to keep people healthier and reduce overall costs.
The Food and Drug Administration (FDA) was also mentioned as a target of action. Specifically, it would be very advantageous to potentially allow labels on approved drugs to identify that the drug is targeting the underlying mechanism of aging, leading to improvement for a named condition/indication (eg, diabetes, cardiovascular diseases). Such a change would bring keen interest from, and support by, private industry in a manner that does not exist at this moment. Industry would then have a greater incentive to develop interventions that may extend the healthspan. Given the potential of such drugs to phase out other current drugs on the market (eg, those treating individually the various chronic diseases of aging), one should not expect the pharmaceutical industry to enthusiastically embrace this approach upfront. However, if the door opens by a FDA ruling, market forces will likely prevail in favor of the most efficient and effective drugs. Some progress on that front is expected in the near future.
Philanthropy was mentioned as a key potential source of support as well, and two broad methods were discussed and debated. “Billionaire challenges” engage high-worth individuals and mobilizes them around an urgent socioeconomic and health/well-being issue. “Direct Crowdsourcing” uses social media for micro-fundraising by leveraging high internet traffic. There was unanimous agreement that this must be pursued at every level. Using the power of demographics and the economic imperative of extending healthspan is another major argument (4) that must remain at the center of our line of reasoning to drive investment in geroscience. Cautions were raised regarding the fact that many “snake oil salesman” pitches were made in the past and many are still being made nowadays. However, we felt that most of the aging research community is, for the first time, speaking with a single voice (eg, the video “Healthspan Imperative,” http://www.healthspancampaign.org/film/). Therefore, it will be critical to continue to appoint our best, most reputable and most articulate, scientists to inform and engage philanthropists, in addition to enlightening the public and lawmakers.
The list of action items from our discussion is summarized in Table 1.
Table 1.
Major Challenges Identified at the Workshop and Possible or Likely Solutions / Courses of Action in Resolving Them
| Problem/Challenge | Solution/Action |
|---|---|
| Low and declining (in real $) government support for research | (i) GSIG (NIH Geroscience Initiative Group) and GSIG-like initiatives—a multidisciplinary approach to aging research |
| (ii) Sustained science- medicine-economics–based advocacy | |
| (iii) National Aging Research legislation with renewed research investments | |
| (iv) International collaborations on care and treatment of older adults | |
| Enormous cost of clinical trials | (i) Partner with insurance- and-integrated health systems to test interventions and outcomes |
| (ii) Hold FDA hearings to allow drug labels to identify that a given drug is targeting the underlying mechanism of aging, leading to improvement for specific clinical condition(s) | |
| Disconnect between geriatricians and gerontologists over the tools and course of action | (i) Organize Action Panels at the interface of gerontology (AGE, GSA, AFAR) and geriatrics/ medical (AGS, ADGAP) organizations |
| Health care fragmented and inefficient | (i) Home care revolution for population health management |
| (ii) Interprofessional team approach | |
| (iii) Connect care and outcomes to biological and physiological measurements (biology of aging) | |
| Navigating diets, treatments, etc. too complex | Advanced informatics adapted to older adults to empower choices and find success stories of individuals with similar conditions and in similar situations |
The enormous challenges brought on by our rapidly aging world are absolutely critical to address and are coming to a head swiftly. It is imperative that we solve them. Some high impact solutions are already available. Others require vision, financing, and commitment. We must implement these solutions and conduct the research with vision, boldness, and perseverance and remove the barriers to their success. Time has never been more exciting for health professionals, scientists, and others to partner to extend healthspan, and continuous communication between them is overdue. May we be wise, as well as relentless, together, in successfully carrying out such partnerships.
Acknowledgments
The authors gratefully acknowledge the support from the University of Arizona College of Science and the Arizona Center on Aging for this conference and the support to authors individually from the Department of Health and Human Services/National Institutes of Health and a variety of foundation and philanthropic sources as follows: USPHS NIH grants R01 AG048021, R21045734, and N0100017 and the Bowman Medical Trust (J.N-Ž); XXX (D.P.G.); Defense Advanced Research Projects Agency (P.R.C.); The Piper Foundation Grant to the Arizona State University (D.C.); The Bakewell Foundation (L.F.); AG043080 and Ellison Medical Foundation Senior Scholar in Aging (B.K.K.); The Arizona Center on Aging (M.J.M.); The MacArthur Research Network on an Aging Society (S.J.O.); AG-023122 (T.P.); AG-024968 (D.P.); NIH RO1 AG045693 and P30 AG050911 (A.R.); USPHS grants R18 HS022763, U24 PCRC NINR, 1C1CMS331346-01-00, P30 AG044281 and grants from the Retirement Research Foundation (RRF), National Palliative Care Research Center and S. D. Bechtel, Jr. (C.R.); N0100017 (A.M.W.); Glenn Foundation For Medical Research (R.G.A.F.); and 1U1QHP287210100 HRSA, U48 DP 005002, Donald W. Reynolds Foundation and the Ann and Alden Hart Endowment (M.J.F.).
References
- 1. Group Avisory Council on Ageing Society. Global ageing population: Peril or promise? In: World Economic Forum, 2012. http://www.sjayolshansky.com/sjo/Background_files/WEF%20Book.pdf [Google Scholar]
- 2.https://www.nia.nih.gov/research/announcements/2013/06/nih-host-october-2013-geroscience-summit https://www.nia.nih.gov/research/announcements/2013/06/nih-host-october-2013-geroscience-summit
- 3. Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann N Y Acad Sci. 2007;1114:11–13. [DOI] [PubMed] [Google Scholar]
- 4. Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood). 2013;32:1698–1705. doi:10.1377/hlthaff.2013.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortese D. Integrated health systems. In Rouse WB, Cortese DA, ed. Engineering the System of Healthcare Delivery. Amsterdam: IOS Press; 2008:369–382. [Google Scholar]
- 6. O’Kane M, Corrigan J, Foote SM, et al. Crossroads in quality. Health Aff (Millwood). 2008;27:749–758. [DOI] [PubMed] [Google Scholar]
- 7. Cryer L, Shannon SB, Van Amsterdam M, Leff B. Costs for ‘hospital at home’ patients were 19 percent lower, with equal or better outcomes compared to similar inpatients. Health Aff (Millwood). 2012;31:1237–1243. doi:10.1377/hlthaff.2011.1132 [DOI] [PubMed] [Google Scholar]
- 8. Butler RN, Miller RA, Perry D, et al. New model of health promotion and disease prevention for the 21st century. BMJ. 2008;337:a399. doi:10.1136/bmj.a399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamerman D. Can biogerontologists and geriatricians unite to apply aging science to health care in the decade ahead? J Gerontol A Biol Sci Med Sci. 2010;65:1193–1197. doi:10.1093/gerona/glq117 [DOI] [PubMed] [Google Scholar]
- 10. Burch JB, Augustine AD, Frieden LA, et al. Advances in geroscience: impact on healthspan and chronic disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S1–S3. doi:10.1093/gerona/glu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howcroft TK, Campisi J, Louis GB, et al. The role of inflammation in age-related disease. Aging (Albany NY). 2013;5:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Seals DR, Melov S. Translational geroscience: emphasizing function to achieve optimal longevity. Aging (Albany NY). 2014;6:718–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. doi:10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 14. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi:10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olshansky SJ, Martin GM, Kirland JL. Aging: the longevity dividend. 2016.
- 16. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi:10.1093/gerona/glr223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sebastiani P, Solovieff N, Dewan AT, et al. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7:e29848. doi:10.1371/ journal.pone.0029848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sebastiani P, Sun FX, Andersen SL, et al. Families enriched for exceptional longevity also have increased health-span: findings from the long life family study. Front Public Health. 2013;1:38. doi:10.3389/fpubh.2013.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crimmins EM, Beltrán-Sánchez H. Mortality and morbidity trends: is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66:75–86. doi:10.1093/geronb/gbq088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakdawalla DN, Bhattacharya J, Goldman DP. Are the young becoming more disabled? Health Aff (Millwood). 2004;23:168–176. [DOI] [PubMed] [Google Scholar]
- 22. Bhattacharya J, Cutler DM, Goldman DP, et al. Disability forecasts and future Medicare costs. Front Health Policy Res. 2004;7:75–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hulsegge G, Picavet HS, Blokstra A, et al. Today’s adult generations are less healthy than their predecessors: generation shifts in metabolic risk factors: the Doetinchem Cohort Study. Eur J Prev Cardiol. 2014;21:1134–1144. doi:10.1177/2047487313485512 [DOI] [PubMed] [Google Scholar]
- 24. U.S. Department of Health and Human Services NIoH, National Institute of Aging. 90+ in the United States: 2006–2008. US Department of Commerce; 2010. [Google Scholar]
- 25. Meeker MG. Technology and system incentives as major drivers of cost. USA, Inc: A basic summary of America’s financial statements. Kleiner Perkins Caufield & Byer; 2011. [Google Scholar]
- 26. Shortell SM, Gillies R, Siddique J, et al. Improving chronic illness care: a longitudinal cohort analysis of large physician organizations. Med Care. 2009;47:932–939. doi:10.1097/MLR.0b013e31819a621a [DOI] [PubMed] [Google Scholar]
- 27. (U.S.) CfDCaP. Trends in Current Cigarette Smoking Among High School Students and Adults, United States, 1965–2011. [Google Scholar]
- 28. National Institute for Diabetes DaKD. Overweight and Obesity statistics. [Google Scholar]
- 29. Edes T, Kinosian B, Vuckovic NH, Nichols LO, Becker MM, Hossain M. Better access, quality, and cost for clinically complex veterans with home-based primary care. J Am Geriatr Soc. 2014;62:1954–1961. doi:10.1111/jgs.13030 [DOI] [PubMed] [Google Scholar]
- 30. Eric De Jonge K, Jamshed N, Gilden D, Kubisiak J, Bruce SR, Taler G. Effects of home-based primary care on medicare costs in high-risk elders. J Am Geriatr Soc. 2014;62:1825–1831. doi:10.1111/jgs.12974 [DOI] [PubMed] [Google Scholar]
- 31. Boling PA, Leff B. Comprehensive longitudinal health care in the home for high-cost beneficiaries: a critical strategy for population health management. J Am Geriatr Soc. 2014;62:1974–1976. doi:10.1111/jgs.13049 [DOI] [PubMed] [Google Scholar]
- 32. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 33. Rockwood K. What is frailty and how can it be conceptualized? J Intell Disabil Res. 2012;56:661–661. [Google Scholar]
- 34. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Žugich J. The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi:10.1016/j.exger.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 35. Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B Biol Sci. 2011;366:28–34. doi:10.1098/rstb.2010.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi:10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi:10.1038/nrd3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. [DOI] [PubMed] [Google Scholar]
- 39. Morimoto RI, Cuervo AM. Proteostasis and the aging proteome in health and disease. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S33–S38. doi:10.1093/gerona/glu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. [DOI] [PubMed] [Google Scholar]
- 41. Imai S. SIRT1 and caloric restriction: an insight into possible trade-offs between robustness and frailty. Curr Opin Clin Nutr Metab Care. 2009;12:350–356. doi:10.1097/MCO.0b013e32832c932d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi:10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi:10.1126/science.1173635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mattison JA, Roth GS, Beasley TM, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi:10.1038/nature11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi:10.1016/j.arr.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldberg EL, Romero-Aleshire MJ, Renkema KR, et al. Lifespan-extending caloric restriction or mTOR inhibition impair adaptive immunity of old mice by distinct mechanisms. Aging Cell. 2015;14:130–138. doi:10.1111/acel.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goldberg EL, Smithey MJ, Lutes LK, Uhrlaub JL, Nikolich-Žugich J. Immune memory-boosting dose of rapamycin impairs macrophage vesicle acidification and curtails glycolysis in effector CD8 cells, impairing defense against acute infections. J Immunol. 2014;193:757–763. doi:10.4049/ jimmunol.1400188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rozengurt E. Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front Physiol. 2014;5:357. doi:10.3389/fphys.2014.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yates SC, Zafar A, Hubbard P, et al. Dysfunction of the mTOR pathway is a risk factor for Alzheimer’s disease. Acta Neuropathol Commun. 2013;1:3. doi:10.1186/2051-5960-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi:10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sansoni P, Vescovini R, Fagnoni FF, et al. New advances in CMV and immunosenescence. Exp Gerontol. 2014;55:54–62. doi:10.1016/j.exger.2014.03.020 [DOI] [PubMed] [Google Scholar]
- 55. Grant R, Youm YH, Ravussin A, Dixit VD. Quantification of adipose tissue leukocytosis in obesity. Methods Mol Biol. 2013;1040:195–209. doi:10.1007/978-1-62703-523-1_15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Youm YH, Grant RW, McCabe LR, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18:519–532. doi:10.1016/j.cmet.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi:10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 58. Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci U S A. 2011;108(suppl 1):4586–4591. doi:10.1073/pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Campisi J. Between Scylla and Charybdis: p53 links tumor suppression and aging. Mech Ageing Dev. 2002;123:567–573. [DOI] [PubMed] [Google Scholar]
- 60. Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46:319–324. doi:10.1016/j.cyto.2009.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi:10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anisimov VN, Bartke A, Barzilai N, et al. The second international conference “genetics of aging and longevity”. Aging (Albany NY). 2012;4:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ayyadevara S, Bharill P, Dandapat A, et al. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid Redox Signal. 2013;18:481–490. doi:10.1089/ars.2011.4151 [DOI] [PubMed] [Google Scholar]
- 64. Guarente L. Aging research-where do we stand and where are we going? Cell. 2014;159:15–19. doi:10.1016/j.cell.2014.08.041 [DOI] [PubMed] [Google Scholar]
- 65. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi:10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Faragher RG, Burton DG, Majecha P, et al. Resveratrol, but not dihydroresveratrol, induces premature senescence in primary human fibroblasts. Age (Dordr). 2011;33:555–564. doi:10.1007/s11357-010-9201-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miller RA. Genetic approaches to the study of aging. J Am Geriatr Soc. 2005;53:S284–S286. [DOI] [PubMed] [Google Scholar]