ABSTRACT
Mechanisms that maintain proliferation and delay cell differentiation in the intestinal crypt are not yet fully understood. We have previously shown the implication of histone methylation in the regulation of enterocytic differentiation. In this study, we investigated the role of histone deacetylation as an important epigenetic mechanism that controls proliferation and differentiation of intestinal cells using the histone deacetylase inhibitor suberanilohydroxamic acid (SAHA) on the proliferation and differentiation of human and mouse intestinal cells. Treatment of newly confluent Caco‐2/15 cells with SAHA resulted in growth arrest, increased histone acetylation and up‐regulation of the expression of intestine‐specific genes such as those encoding sucrase‐isomaltase, villin and the ion exchanger SLC26A3. Although SAHA has been recently used in clinical trials for cancer treatment, its effect on normal intestinal cells has not been documented. Analyses of small and large intestines of mice treated with SAHA revealed a repression of crypt cell proliferation and a higher expression of sucrase‐isomaltase in both segments compared to control mice. Expression of SLC26A3 was also significantly up‐regulated in the colons of mice after SAHA administration. Finally, SAHA was also found to strongly inhibit normal human intestinal crypt cell proliferation in vitro. These results demonstrate the important implication of epigenetic mechanisms such as histone acetylation/deacetylation in the regulation of normal intestinal cell fate and proliferation. J. Cell. Biochem. 116: 2695–2708, 2015. © 2015 The Authors. Journal of Cellular Biochemistry published by Wiley Periodicals, Inc.
Keywords: INTESTINAL CELLS, HDAC INHIBITION, SAHA, PROLIFERATION, DIFFERENTIATION
The crypt‐villus axis of the small intestine is a functional unit characterized by constant cell renewal. This process involves rapid and continuous proliferation and migration of the stem cell population located in the lower part of the crypt, to extrusion of differentiated specialized senescent cells at the tip of the villus [Bjerknes and Cheng, 2005; Scoville et al., 2008]. Detailed characteristics of this dynamic system have been extensively studied over the last three decades and based on these studies, the crypt has been identified as a proliferating unit containing three compartments: the stem cells, the transient amplifying (TA) zone and the terminally differentiating cells which are located in the lower, middle, and upper third of the crypt, respectively [Cheng and Leblond, 1974; Li and Clevers, 2010]. Intestinal cell determination is associated with cell movement from the stem cell zone to the TA zone. Migrating cells have the potential to generate differentiated cell types of the secretory (goblet cells, enteroendocrine cells, and Paneth cells) or absorptive lineages. Terminal differentiation of absorptive or secretory cells, except for Paneth cell precursors that complete their differentiation at the crypt base, occurs in the upper third of the crypt [van der Flier and Clevers, 2009], ensuring the formation of fully functional specialized intestinal epithelial cell types.
Differentiation of each lineage, which derives from a common stem cell precursor [Cheng and Leblond, 1974], involves various intermediate progenitor cells and occurs above the stem cell zone [van der Flier and Clevers, 2009]. Progenitor cells should first exit the stem cell zone for specific commitment before undertaking up‐ or downward migration [Cheng and Leblond, 1974; Bjerknes and Cheng, 2005; van der Flier and Clevers, 2009]. In the TA zone, secretory cells rapidly differentiate while terminal differentiation of absorptive cells is hindered due to multiple cell divisions. Notably, absorptive cell progenitors divide approximately four times before undergoing terminal differentiation while secretory cell progenitors divide only once or twice, explaining why the majority of cells on the villi are absorptive cells [Bjerknes and Cheng, 2005].
Although studies on crypt functional organization in man have shown the expression of enterocyte markers such as aminopeptidase N and dipeptidylpeptidase IV in both crypt and villus cells and the precursor forms of sucrase‐isomaltase (SI) by the immature cells of the middle crypt [Beaulieu et al., 1989], terminal differentiation of absorptive cells is only achieved in the upper third of the crypts [Beaulieu, 1997; Benoit et al., 2010]. However, mechanisms by which terminal differentiation is repressed in the TA zone have not yet been fully characterized.
In the TA zone, epithelial cells express the transcription factors CDX2 and HNF1α which are responsible for absorptive cell differentiation [Benoit et al., 2010]. The question rising is what prevents absorptive cells from immediately undertaking terminal differentiation in response to these factors. One possibility would be suppressive epigenetic mechanisms such as histone methylation/deacetylation [Tou et al., 2004]. Our recent results have shown epigenetic control of gene expression via polycomb group proteins (PcG) consisting of the repression of absorptive cell lineage differentiation in the TA compartment which involves PRC2‐mediated histone H3 lysine‐27 trimethylation (H3K27me3) [Benoit et al., 2012]. These findings prompted us to investigate the involvement of other epigenetic mechanisms in the regulation of enterocyte proliferation and differentiation.
In the current work, we investigate the role of histone acetylation as another important epigenetic mechanism that may control proliferation and differentiation of intestinal cells. Histone deacetylases (HDACs) are important mediators of deacetylation of lysine residues within DNA bound core histones. Histone deacetylation increases the interaction of DNA with nucleosomes and suppresses gene expression by limiting the access of RNA polymerase, transcription factors, and regulatory elements to the DNA promoter [Marks et al., 2000]. HDACs are important regulators of cell proliferation and transformation and are over‐expressed in different malignancies [Glozak and Seto, 2007] such as colorectal cancer [Mariadason, 2008]. Acetylation of histones neutralizes the histone charge and induces a more relaxed chromatin conformation resulting in the exposure of the DNA promoter to the transcriptional machinery and subsequent expression of genes which are usually involved in cellular differentiation [Marks et al., 2000]. Based on their homology with yeast HDACs, human HDACs are classified into 4 classes, HDACI, HDACII, HDACIII, and HDACIV: class I includes HDAC1, ‐2, ‐3, ‐8; class II includes HDAC4, ‐5, ‐7, and ‐9; HDAC6 and ‐10 constitute class III and HDAC11 is classified as IV [Marks, 2007]. Class I and II HDACs have important implications in intestinal physiology and are over‐expressed in colon tumors [Mariadason, 2008]. The activities of these HDACs are associated with cellular proliferation and repression of gene expression. HDAC inhibitors are compounds that suppress the activity of class I or II or both classes of HDACs. For example, butyrate, a product of bacterial fermentation present in the lumen of the colon in millimolar concentrations, has been used as an HDAC inhibitor in colorectal cancer cell lines such as Caco‐2 where it can reduce Caco‐2 cell proliferation and induce expression of some differentiation genes such as DPP4 and alkaline phosphatase [Mariadason et al., 2000], although butyrate failed to promote the terminal differentiation of Caco‐2 cells which is achieved by the expression of intestine‐specific terminal differentiation genes such as SI [Beaulieu, 1997; Benoit et al., 2010]. Suberanilohydroxamic acid (SAHA) can inhibit all eight human class I and II HDACs [Marks, 2007] and has been recently used in phase I and II clinical trials for the treatment of refractory cutaneous T‐cell lymphoma (CTCL) and multiple myeloma [Duvic et al., 2007; Richardson et al., 2008]. SAHA has been used as an anticancer agent in monotherapy and in combination therapy in association with other agents such as fluorouracil in colorectal cancer [Marks, 2007]. However, its effect on normal intestinal cells has not been characterized.
In the present study, using SAHA as a broad HDAC inhibitor, we demonstrate for the first time a significant up‐regulation of the expression of terminal differentiation genes such as SI and SLC26A3 in intestinal cells both in vitro and in vivo. Furthermore, SAHA negatively controls growth of proliferative Caco‐2/15 and normal HIEC cells and suppresses cell proliferation in mouse intestinal crypts. These results provide new insights into the molecular mechanism(s) regulating proliferation and differentiation of intestinal cells during crypt‐villus axis organization.
MATERIALS AND METHODS
CELL CULTURE
The Caco‐2/15 cell line, a relevant model of human intestinal differentiation, was a gift from Dr. A. Quaroni (Cornell University, Ithaca, NY). Culture conditions, characterization of the Caco‐2/15 cell line and counting cell numbers have been described previously [Beaulieu and Quaroni, 1991; Vachon and Beaulieu, 1992; Benoit et al., 2010]. The Human Intestinal Epithelial Crypt‐like (HIEC) cell line is a normal non‐transformed and non‐immortalized intestinal crypt cell line [Beaulieu and Ménard, 2012]. HIEC cells were cultured according to protocols established in our laboratory [Benoit et al., 2010; Guezguez et al., 2014]. Briefly, Caco‐2/15 and HIEC cells were grown in 100 mm or 6 well plastic dishes in DMEM containing 10% FBS for Caco‐2/15 cells or Opti‐MEM containing 4% FBS for HIEC cells both supplemented with 2 mM glutamax and 10 mM HEPES. Media were changed every 48 h or everyday as indicated. Cell viability was routinely assessed using trypan blue.
HDAC INHIBITOR TREATMENTS
Caco‐2/15 cell cultures were treated at the time they reached confluence (95‐100% confluence) for a period of 4 days with 0, 2, 5, 7.5, and 10 µM of the HDAC inhibitor SAHA. DMSO was used as control. In some experiments, subconfluent (∼50% confluence) and 4 day post‐confluent Caco‐2/15 cells were also treated for a period of 4 days with 5 and 10 μM SAHA or DMSO as control. Thirty day post‐confluent Caco‐2/15 cells were also used as positive control for fully differentiated cells. HIEC cells were grown to >90% confluence with 10 µM SAHA for a period of 48 h. For treated cells, the medium and inhibitor or DMSO were renewed daily. Total RNA and protein were extracted from SAHA‐treated and control cells at various stages of confluence.
ANTIBODIES
Antibodies used in this study were: mouse anti‐actin #MAB1501 (WB:1/50000) (EMD Millipore, Etobicoke, ON, CA), mouse anti‐SI monoclonal antibody (HSI‐14) produced and characterized as described previously [Beaulieu et al., 1989] (WB: 1/10), rabbit anti‐hyperacetylated histone H4 # 06‐946 (WB: 1/2000) (EMD Millipore, Etobicoke, ON, CA), goat anti‐mouse sucrase‐isomaltase # sc‐27603 (WB: 1/1000, IF: 1/200) (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti‐SLC26A3 # HPA036055 (IF: 1/100) (Sigma–Aldrich, Oakville, ON, CA), mouse anti‐BrdU # 11810740001 (IF: 1:50) (Roche Diagnostics, Mississauga, ON, CA).
MICE
Balb/c mice at the time of experiments were 3 weeks of age (20 g). Mice were housed and fed a normal diet and animal experimentation was performed according to a protocol approved by the Animal Ethical Committee of the Université de Sherbrooke. Mice were injected intraperitoneally with SAHA (100 mg/kg/day) or DMSO alone for 2 days based on preliminary results showing that alterations in SI expression were already detected after 48 h of treatment while no other changes such as diarrhea or obvious change in appetite were noted. At the end of the treatment period, the mice were euthanized and whole intestinal samples were harvested for each animal as follows: (a) proximal ileum, first half of proximal colon and distal colon used for protein extracts and Western blot (WB) analyses, (b) distal ileum, second half of the proximal colon and distal colon used for RNA extractions and quantitative polymerase chain reaction (qPCR), and (c) mid‐ileum and mid‐colon for paraffin embedding for immunofluorescence and bromodeoxyuridine (BrdU) localization assay. We did not observe any gross morphological changes in intestinal samples or other organs.
WESTERN BLOT ANALYSIS
After dissection, intestinal samples (ileum and proximal colon) were immediately snap frozen in liquid nitrogen and homogenized in 1X Laemmli buffer with a tissue blender. Cells were scraped in 1X Laemmli buffer as previously described [Seltana et al., 2013]. After sonication, samples were centrifuged and supernatants were stored at −80°C until use. Proteins were separated under denaturing and reducing conditions on 12% SDS‐PAGE (15% for acetylated histone H4) gels and transferred onto nitrocellulose membranes. After blocking in 5% skim milk, membranes were probed with the primary antibody overnight at 4°C, except for SI which was incubated at room temperature, followed by incubation with horseradish peroxidase‐conjugated (GE Healthcare, Baie d′Urfe, QC, CA) or AlexaFluor 488 (Invitrogen, Burlington, ON, CA) secondary antibodies. Signals were detected as previously described using the Immobilon Western kit (Millipore) for histone H4 and SI [Seltana et al., 2013] and Molecular Imager FX (BioRad) for β‐actin.
RNA EXTRACTION AND qPCR
Total RNA from Caco‐2/15 cells or intestinal tissues was extracted with RiboZol reagent according to the manufacturer's instructions (Amresco, OH), quality tested (RIN > 9.0) and reverse‐transcribed to cDNA using Omniscript (Qiagen) as described previously [Dydensborg et al., 2006] according to MIQE guidelines [Bustin et al., 2010]. cDNAs were amplified by qPCR for expressions of SI, villin (VIL1), cingulin (CGN), dipeptidylpeptidaseIV (DPP4), ion exchanger SLC26A3, and p21WAF1(CDKN1A) in human samples and mouse sucrase‐isomaltase and ion exchanger Slc26a3 transcripts (Sis and Slc26a3) as described previously [Dydensborg et al., 2006; Tremblay et al., 2011; Seltana et al., 2013]. Quantitative PCR monitored with Brilliant II SYBR QPCR Low ROX Master Mix (Agilent, Mississauga, ON) was performed on a Mx3000P QPCR System (Stratagene, Mississauga, ON). Relative expression of human genes normalized to RPLP0 expression, a validated reference gene for human intestinal cells [Dydensborg et al., 2006] or mouse genes normalized to a pool of three reference genes (Rplp0, B2m and Gusb) [Wang et al., 2010], was evaluated according to the Pfaffl method [Pfaffl, 2001]. Monitoring of the expression of these reference genes vs known amounts of input RNA revealed that none of them were affected by SAHA treatment. All primers used in the study are listed in Table I.
Table I.
Primers Used in This Study
| Gene symbol | Sense primer | Antisense primer | Accession no. |
|---|---|---|---|
| SI | 5′‐GAGGACACTGGCTTGGAGAC‐3′ | 5′‐ATCCAGCGGGTACAGAGATG‐3′ | NM_001041 |
| SLC26A3 | 5′‐GCAGCTAGTGTGGCATTTCA‐3′ | 5′‐TCCGCCTAAAGAAACCAATG‐3′ | NM_000111 |
| RPLPO | 5′‐GCAATGTTGCCAGTGTCTG‐3′_ | 5′‐GCCTTGACCTTTTCAGCAA‐3′_ | BC019014 |
| CDKN1A | 5′‐GGAAGACCATGTGGACCTGT‐3′ | 5′‐TAGGGCTTCCTCTTGGAGAA‐3′ | NM_00389.4 |
| VIL1 | 5′‐GGCCAGCCAAGATGAAATTA‐3′ | 5′‐CTCAAAGGCCTTGGTGTTGT‐3′ | NM_007127.2 |
| CGN | 5′‐GCTCCTGTTAGCTCGTGGTC‐3′ | 5'‐GAAAAGGCTCAGTTGGCTTG‐3′ | NM_020770.2 |
| DPP4 | 5′‐AAGTGGCGTGTTCAAGTGTG‐3′ | 5′‐CAGGGCTTTGGAGATCTGAG‐3′ | NM_001935.3 |
| Sis | 5′‐GGGGAAAAGGAACAACCAGT‐3′ | 5′‐CCAGCTGATTTGTATTGGTTCA‐3′ | NM_001081137.1 |
| Slc26a3 | 5′‐GACAAACTTGCTCGGTGTGA‐3′ | 5′‐TGAGAATCCTTCCGAATTGT‐3′ | NM_021353.3 |
| B2m | 5′‐CTGACCGGCCTGTATGCTAT‐3′ | 5′‐CAGTCTCAGTGGGGGTGAAT‐3′ | NM_009735.3 |
| Rplp0 | 5′‐TGCCACACTCCATCATCAAT‐3′ | 5′‐CGAAGAGACCGAATCCCATA‐3′ | NM_007475.5 |
| Gusb | 5′‐TCGGAGAGCTCATCTGGAAT‐3′ | 5′‐AGAACGTGAACGGTCTGCTT‐3′ | NM_010368.1 |
IMMUNOFLUORESCENCE
Intestinal samples were fixed in 4% paraformaldehyde, embedded in paraffin and 5 µm tissue sections were prepared. Tissues were deparaffinized with xylene and rehydrated in decreasing ethanol concentrations. Antigen retrieval was performed by boiling slides in 10 mM citric acid for 10 min followed by a blocking step in 2% BSA for 1 h. The goat anti‐mSI and mouse anti‐SLC26a3 primary antibodies were detected with anti‐goat or anti‐mouse Alexa Fluor 488‐conjugated secondary antibodies. Evans blue was used for counterstaining and nuclei were stained with DAPI. Negative controls consisted of slides incubated with only the secondary antibody. Slides were observed with a Leica DM‐RXA microscope and images were acquired using the MetaMorph imaging system (Universal Imaging, West Chester, PA).
IN VIVO AND IN VITRO BrdU ASSAYS
BrdU incorporation assays were performed by intraperitoneal injection of 10 ml/kg of bromodeoxyuridine (10mM) 45 min before euthanization of mice. Immunofluorescence was performed according to the In Situ Cell Proliferation Kit FLUOS® (Roche Diagnostics, Mississauga, ON, CA) protocol by incubating fixed tissues (see above) for 1 h at 37°C with a mouse BrdU antibody. The number of BrdU positive nuclei per crypt was measured by counting positive signals for at least 30 crypts for each sample. The BrdU assay on HIEC cells treated with SAHA for 48 h was performed essentially as described elsewhere [Guezguez et al., 2014]. Cells were incubated 4 h with normal medium containing BrdU before being stained with anti‐BrdU antibody and DAPI.
CASPASE‐3 FLUOROMETRIC ASSAY
The caspase‐3 fluorometric assay was performed on Caco‐2/15 and HIEC cells as described previously [Beausejour et al., 2012]. The specific fluorogenic substrate used was acetyl Asp‐Glu‐Val‐Asp 7‐amido‐4‐trifluoromethyl‐coumarin (Ac‐DEVD‐AFC; Enzo Life Sciences, Farmingdale, NY) resulting in the release of the fluorescent 7‐amino‐4‐trifluoromethyl‐coumarin (AFC) catalysis product. At day 0, Caco‐2/15, or HIEC cells were treated with DMSO or 10 µM SAHA for 4 days and then were harvested and read with a Victor X5 Multimode Plate Reader (PerkinElmer, Waltham, MA). Caspase‐3 activity was expressed as μM AFC/mg of protein.
STATISTICAL ANALYSIS
All in cellulo experiments were performed at least 3 times and representative results are reported. For in vivo studies, specimens from a minimum of 4 mice per condition were used. Statistical analysis of the experiments was done as previously described [Seltana et al., 2013] with the student‐two‐tailed t‐test (one‐way ANOVA) and differences at P ˂ 0.05 were considered significant. Data are expressed as mean ± SEM. All authors had access to the study data and had reviewed and approved the final manuscript.
RESULTS
SAHA RESTORED HISTONE ACETYLATION AND INHIBITED GROWTH OF Caco‐2/15 CELLS
HDAC inhibitors including SAHA can increase the level of histone acetylation of treated cell lines [Marks et al., 2000]. Therefore, the effect of SAHA was assessed on the histone acetylation status of the Caco‐2/15 cell line which is a model for studying enterocytic differentiation of intestinal absorptive cells [Beaulieu and Quaroni, 1991; Vachon and Beaulieu, 1992]. As shown in Figure 1A, as compared with the control which had no detectable effect on the histone H4 acetylation level (AcH4) of Caco‐2/15 after 96 h, treatment with increasing concentrations of SAHA showed a significant accumulation of AcH4 which gradually increased with higher concentrations reaching a maximum AcH4 level after treatment with 10 µM SAHA.
Figure 1.
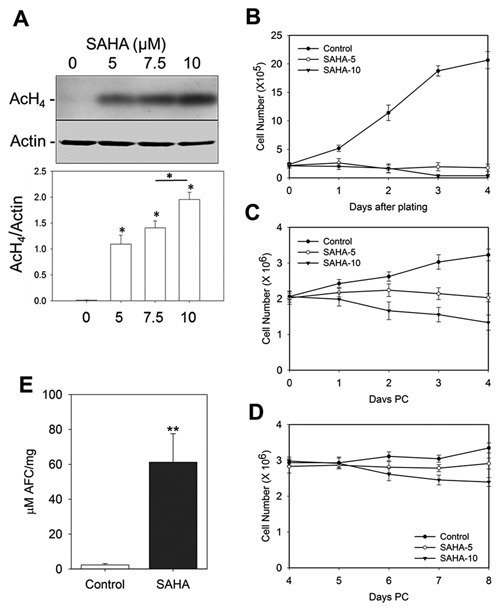
Effect of SAHA on AcH4, proliferation and apoptosis of Caco‐2/15 cells. SAHA induces histone acetylation, negatively regulates proliferation and promotes apoptosis of Caco‐2/15 cells. (A) Newly confluent Caco‐2/15 cells were treated with the indicated concentrations of SAHA for 4 days. Total cell protein was isolated from the Caco‐2/15 cells and 25 µg protein lysate was migrated on a 15% SDS‐PAGE gel followed by Western blot analysis and quantified as described in the Materials and Methods. Actin was used as the loading control. A sharp increase in the relative levels of AcH4 in cells treated with various concentrations of SAHA compared to the control was observed. (B–D) SAHA or DMSO alone (control) was added to highly proliferative sub‐confluent (B), newly confluent (C) and 4 day post‐confluent (D) Caco‐2/15 cells for a period of 4 days. Proliferation assays performed during the time of treatment clearly show the effect of SAHA on cell growth is highly pronounced on sub‐confluent Caco‐2/15 cells. However, post‐confluent cells, in a time‐dependent manner, are less influenced by the anti‐proliferative effects of SAHA. (E) Caspase‐3 activity assay to evaluate apoptosis of newly confluent cells treated 4 days with 10 μM of SAHA vs control. Data represent the mean ± SEM from three independent experiments. * P ˂ 0.05; ** P = 0.02. PC: Post‐confluence.
SAHA is known to trigger growth arrest and/or apoptosis of transformed cells including colorectal cancer cells [Hsi et al., 2004; Marks, 2004]. To validate the effects of SAHA on Caco‐2/15 cells, cells from different stages of confluence were treated with 5 or 10 µM SAHA (Fig. 1B–D). When proliferating sub‐confluent Caco‐2/15 cells were treated with SAHA, there was nearly a complete inhibition of cell proliferation after 3 days (Fig. 1B). However, the degree to which SAHA restrained cell proliferation decreased as Caco‐2/15 cells became confluent. When SAHA was added to newly confluent Caco‐2/15 cells (day 0) and the treatment was continued until 4 days post‐confluence, a time‐ and dose‐dependent reduction of cell proliferation was observed (Fig. 1C). Notably, 10 µM SAHA had a pronounced negative regulatory effect on the viability of both sub‐ and newly confluent Caco‐2/15 cells. This negative regulation is consistent with the effect reported for SAHA on actively proliferating cells [Hsi et al., 2004; Marks, 2004]. Interestingly, differentiating Caco‐2/15 cells at 8 days post‐confluence displayed more resistance to SAHA growth inhibition and cell death (Fig. 1D). These results show that the effects of SAHA significantly diminish with acquisition of quiescence in post‐confluent Caco‐2/15 cells. To further characterize the effect of SAHA on Caco‐2/15, cell apoptosis was evaluated using a caspase‐3 activity assay. Consistent with previous reports on colorectal cancer cells [Wilson et al., 2006], SAHA induced apoptosis in newly confluent Caco‐2/15 cells treated for 4 days (Fig. 1E).
HDAC INHIBITION INDUCES SELECTIVE GENE EXPRESSION IN Caco‐2/15 CELLS
HDAC inhibitors including SAHA have selective regulatory effects on the transcription patterns of a restricted set (∼2%) of cellular genes [Van Lint et al., 1996]. Therefore, we decided to analyze transcriptional expression (mRNA) of a panel of genes responsible for proliferation, differentiation, and polarization of intestinal cells in response to SAHA. Having found that 10 µM SAHA had the maximum effect on the histone acetylation levels of Caco‐2/15 cells, this concentration was chosen for treatment of newly confluent cells. p21 cyclin‐dependent kinase inhibitor (CDKN1A) is among the genes which are up‐regulated in the presence of HDAC inhibitors [Gui et al., 2004]. Therefore, the effect of SAHA on CDKN1A mRNA levels was examined by qPCR analysis. Newly confluent Caco‐2/15 cells cultured with SAHA for 4 days displayed an increase in CDKN1A expression up to 30‐fold compared to control cells (Fig. 2A). The over‐expression of this transcript which encodes an inhibitor of cyclin‐dependent kinases [Xiong et al., 1993] can explain in part the observed decrease in proliferation of Caco‐2/15 cells in the presence of SAHA (Fig. 1C). To characterize the effect of SAHA on intestine‐specific gene expression, transcript levels of some well‐known intestinal cell terminal differentiation markers were analyzed by qPCR. As expected, SAHA treatment during 4 days of post‐confluent culture induced selective expression of differentiated intestinal cell markers (Fig. 2B–D). For the first time, we show that mRNA levels for the Cl/HCO3 exchanger protein SLC26A3 [Talbot and Lytle, 2010] was significantly increased in Caco‐2/15 cells in response to HDAC inhibition (Fig. 2B). In addition, expression of the VIL1 transcript was significantly increased in response to SAHA treatment (Fig. 2C). These results are in agreement with our previous finding that expression of differentiation and polarization markers could be coupled events in newly differentiating Caco‐2/15 cells [Seltana et al., 2013]. However, expression of other markers associated with cellular differentiation such as CGN (Fig. 2D) and DPP4 (data not shown) were not modulated by HDAC inhibition, consistent with the selective regulatory effect of SAHA on specific genes.
Figure 2.
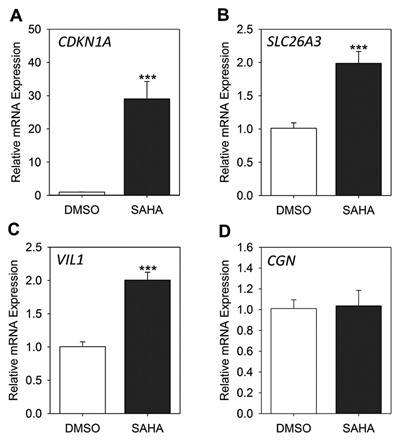
Effect of SAHA on gene expression of Caco‐2/15 cells. Newly confluent Caco‐2/15 cells were treated with 10 µM SAHA or DMSO alone for 4 days. The mRNA levels of expression of CDKN1A (A), SLC26A3 (B), VIL1 (C) and CGN (D) were determined by qPCR. Data represent the mean ± SEM from four independent experiments. ***P ˂ 0.005 SAHA versus control.
SAHA REGULATES EXPRESSION OF THE ENTEROCYTE‐SPECIFIC GENE SI
The mechanism(s) that trigger differentiation and enterocyte‐specific gene expression in intestinal absorptive cells have not been fully characterized. It is known that enterocytic differentiation of intestinal cells is associated with robust expression of the SI gene [Beaulieu and Quaroni, 1991]. SI is a terminal differentiation specific marker which is up‐regulated during crypt‐to‐villus cell organization [Benoit et al., 2012] and post‐confluent Caco‐2/15 cell differentiation [Beaulieu and Quaroni, 1991]. To assess the effect of SAHA on the differentiation of Caco‐2/15 cells, we determined the levels of SI expression at various stages of post‐confluence in Caco‐2/15 cells treated with the HDAC inhibitor. As shown in Figure 3A, in the presence of SAHA, there is a dose‐dependent up‐regulation of SI transcript expression in post‐confluent Caco‐2/15 cells (P ˂ 0.005). This regulatory effect is consistent with the increased levels of histone acetylation in SAHA‐treated cells (Fig. 1A); in the presence of 10 µM SAHA, the observed maximum degree of histone acetylation coincides with a dramatic (over 50‐fold) over‐expression of SI mRNA (Fig. 3A). To verify if the observed induction of SI mRNA expression resulted in increased protein levels, we examined protein expression in SAHA‐treated and control cell cultures by Western blot analysis. Figure 3B illustrates a dose‐dependent increase of SI protein expression in cells incubated with different SAHA concentrations for four days post‐confluence. Consistent with the qPCR results, the highest level of SI expression was observed when Caco‐2/15 cells were cultured with 10 µM SAHA. The magnitude of the SAHA effect, however, significantly decreased in spontaneously differentiating 8 day post‐confluent Caco‐2/15 cells. In these cells, SAHA induced only a 1.6‐fold increase in SI mRNA expression (P ˂ 0.005) (Fig. 3C), confirming previous findings on the resistance of post‐confluent differentiating Caco‐2/15 cells to butyrate‐induced cell differentiation [Mariadason et al., 2001]. To characterize the relevance of these findings to the physiology of Caco‐2/15 cells, we compared the levels of SI expression in SAHA‐treated cells with that of differentiating post‐confluent cells. In control cells, the levels of SI mRNA in 4 and 8 day post‐confluent cells were comparable to those found in cells cultured under standard conditions (Fig. 3C), confirming that DMSO has no significant effect on SI expression in Caco‐2/15 cultures. Interestingly, the level of SI mRNA accumulation in 4 day post‐confluent cells treated with SAHA was comparable to the level of SI expression in differentiated Caco‐2/15 cells maintained at confluence for up to 30 days (Fig. 3C). These results indicate that HDAC inhibition with SAHA can induce an early differentiation program in Caco‐2/15 cells.
Figure 3.
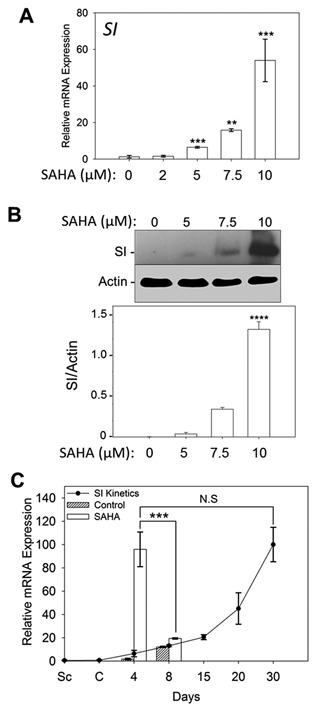
SI transcript and protein levels increase in response to SAHA. SAHA treatment significantly increases mRNA and protein levels of SI in confluent Caco‐2/15 cells. (A,B) Dose‐dependent up‐regulation of SI mRNA levels in newly confluent cells treated 4 days with SAHA (A) and representative Western blot and densitometric analyses showing corresponding protein up‐regulation (B). (C) Slight increase in SI transcript expression in 8 day post‐confluent Caco‐2/15 cells shows that as cells proceed with differentiation, they become more resistant to the effect of SAHA. However, the level of SI mRNA expression in newly confluent Caco‐2/15 cells treated for 4 days with 10 µM SAHA was found to be significantly higher than that of 8 day post‐confluent cells and comparable to that of fully differentiated cells (30 day post‐confluence). Data represent the mean ± SEM from four independent experiments. **P ˂ 0.02; ***P ˂ 0.005; ****P ˂ 0.0005 versus controls except for 4 d versus 8 d comparison in C. N.S: non‐significant.
SAHA PROMOTES AcH4 AND SUPPRESSES PROLIFERATION OF INTESTINAL CELLS IN VIVO
The in vitro effects of SAHA on histone acetylation were confirmed in vivo by determining the levels of AcH4 in mouse intestines. A significant up‐regulation of AcH4 in the intestinal samples of mice that received SAHA was observed in comparison with control animals (Fig. 4A). Effects of HDAC inhibitors on the proliferation of transformed cell lines and in vivo tumor growth have been extensively investigated. SAHA has well‐known anti‐proliferative activity in various in vivo models of cancer [Marks, 2007]. For example, intraperitoneal administration of SAHA (100 mg/kg/day) significantly suppresses the growth of CWR22 human prostate xenograft tumors [Butler et al., 2000]. However, the physiological consequence of HDAC inhibition by SAHA in the context of normal proliferative intestinal cells has never been determined. Based on our finding that SAHA treatment decreases the proliferative capacity of Caco‐2/15 cells, we assessed the proliferation of mouse intestinal crypt cells by determining the level of BrdU incorporation into ileal and colonic crypts after a 48 h SAHA treatment. Compared to control mice, the number of BrdU‐labelled cells in the crypt compartment of mice injected with SAHA decreased in both the ileum (Fig. 4B and D) and colon (Fig. 4C and E). Quantitation of BrdU‐positive cells showed a significant decrease of labelled cells in both the ileum (P ˂ 0.005) and colon (P ˂ 0.05) (Fig. 4F and G) of mice injected with SAHA.
Figure 4.
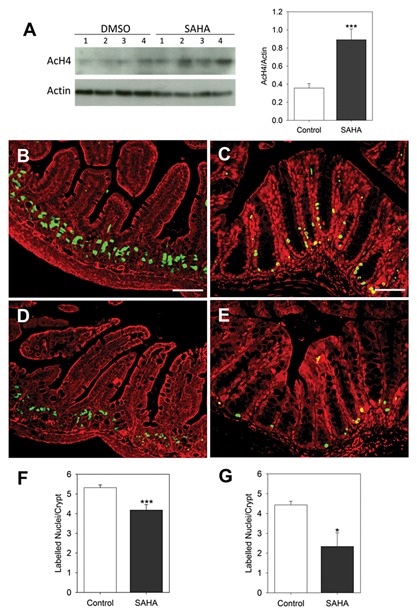
In vivo SAHA treatment leads to decreased proliferation of intestinal cells. (A) Western blot for detection of AcH4 in 4 specimens of mouse ileum treated with either SAHA or DMSO alone and corresponding densitometry analysis. (B–G) 45 min before euthanization, 21 day old control and SAHA‐treated mice were injected with BrdU then ileum and mid‐colon tissue sections were prepared for immunofluorescence. Representative illustrations of BrdU positive nuclei in crypt are provided for ileum (B,D) and colon (C,E) samples obtained from mice treated with DMSO alone (B–C) or SAHA (D‐E) for 48 h. Average numbers of BrdU positive nuclei per crypt were calculated for ileum (F) and colon (G). Results are representative of mean ± SEM from 4 to 5 mice for each treatment. 30 crypts were counted for each sample. Green: BrdU positive nuclei, Red: Evans blue staining. Scale bar = 50 µm. *P ˂ 0.05, *** P ˂ 0.005.
SAHA REPRESSES PROLIFERATION OF NORMAL HUMAN INTESTINAL CRYPT HIEC CELLS
To confirm the above in vivo results in a context relevant to human physiology, we examined the effects of SAHA on the proliferation of HIEC cells, a human normal non‐transformed and non‐ immortalized intestinal crypt cell line. Consistent with our in vivo results, incubation of HIEC cells with SAHA (48 h) resulted in a very significant decrease (P < 0.0005) in the proliferative capacity of the cell line (Fig. 5A). In order to determine if SAHA had any pro‐apoptotic effects on HIEC cells, the caspase‐3 activity assay was performed and the results showed that even after 4 days of treatment SAHA had no significant effect on this parameter for normal cells (Fig. 5B).
Figure 5.
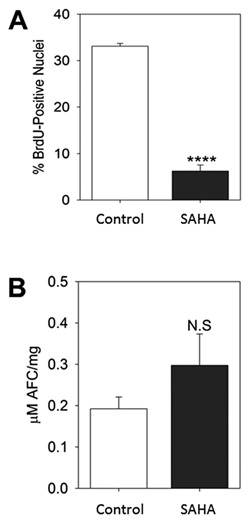
SAHA represses proliferation of normal intestinal HIEC cells. To assess proliferation (A), HIEC cells were treated with SAHA or DMSO alone for 48 h followed by BrdU assay. Data are represented as the percentage of BrdU positive nuclei relative to the total number of nuclei stained with DAPI. To determine apoptosis (B), caspase‐3 activity was assessed on newly confluent HIEC cells treated 4 days with DMSO alone (Control) or SAHA. In both sets of analyses, data are expressed as the mean ± SEM from three independent experiments. ****P ˂ 0.0005. N.S.: Non‐significant.
SAHA INDUCES IN VIVO DIFFERENTIATION OF INTESTINAL CELLS
Finding that SAHA affects intestine‐specific gene expression in Caco‐2/15 cells prompted us to use a mouse model to determine if these results could be reproduced in vivo. Indeed, the effect of SAHA on the physiology of normal cells in vivo has never been directly assessed. One of the aims of the current work was therefore to investigate the potential link between SAHA‐induced histone acetylation and the differentiation of normal intestinal cells. In this regard, mice were injected intraperitoneally with SAHA (100 mg/kg/day) or DMSO alone (control mice) daily for two days. Samples of ileum and colon were processed for qPCR, Western blot and immunofluorescence to analyze the expression of terminal differentiation genes such as mouse Sis and Slc26a3. qPCR analysis confirmed the increased expression pattern of Sis in the SAHA‐treated mouse ileum (P ˂ 0.05) and colon (P ˂ 0.05) compared to control mice (Fig. 6A and B). To determine if up‐regulation of Sis mRNA was translated into increased protein levels, we performed Western blot analysis to determine SIS protein expression in SAHA treated and control mouse intestines. As expected, a significant increase in protein expression was detected in the ileum and colon of mice treated with SAHA compared to controls (Fig. 6C–F). To further confirm and extend these results, we performed indirect immunofluorescence on the ileum of control and SAHA‐treated mice, using the same anti‐SIS antibody employed for Western blot experiments. The specificity of the antibody for the detection of SIS was confirmed (Fig. 6G). As compared to control intestines (Fig. 6H), an apparent increase in SIS expression was consistently noted in the apical membrane of the ileal villi compartments of mice treated with SAHA (Fig. 6I). Interestingly, in our experiments we regularly observed extension of some of the SIS signal to the lower villus and upper third of the crypt structure (Fig. 6I arrow, and 6 K) in a region where SIS expression was not detected in control mice (Fig. 6H and J). These results clearly indicate that HDAC inhibition has a positive regulatory effect on SIS expression and therefore, cellular differentiation along the crypt‐villus axis.
Figure 6.
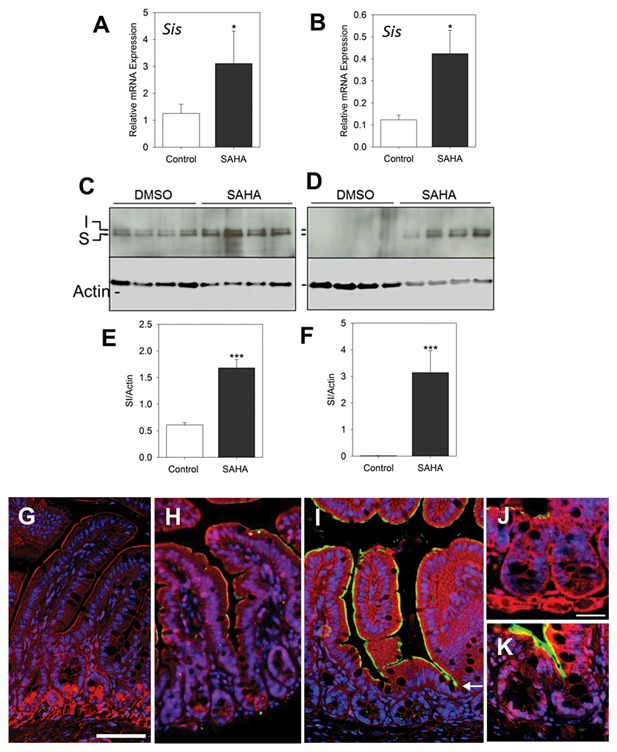
In vivo HDAC inhibition with SAHA induces differentiation of enterocytes. Mice were treated with DMSO alone or SAHA for 48 h and colon and ileum samples were extracted for qPCR, Western blot and immunofluorescence, to evaluate expression levels of the enterocyte terminal differentiation gene SI. (A,B) qPCR on ileum (A) and proximal colon (B) samples shows an up‐regulation of SI mRNA in mice receiving SAHA; n = 5, *P ˂ 0.05. (C–F) Western blot analysis showing increased expression of SI protein in mouse ileum (C) and proximal colon (D) and corresponding densitometric analysis for ileum (E) and proximal colon (F) samples. Data are expressed as the mean ± SEM of intestinal samples obtained form 4 to 5 mice for each condition. ***P ˂ 0.005. (G–K) Representative immunofluorescence staining pictures for the detection of SI in the ileum. Negative staining control (G) confirming the lack of non‐specific staining. A relatively weak but specific staining for SI detection (green) was observed at the luminal domain of villus cells of control mice that received DMSO alone (H) while an increase in the intensity of SI signal was consistently observed in samples of mice that received SAHA (I). SI staining was also found in the opening of the crypts in SAHA treated specimens (arrow in I, K), a phenomenon not seen in controls (H,J). Blue: DAPI to label nuclei, Red: Evans blue counterstaining. Scale bar = 50 μm for G–I and 25 μ for J and K.
To determine the effect of HDAC inhibition on colonic cell differentiation, we chose to characterize the expression of Slc26a3 as a marker for mature functional colonocytes. qPCR analysis revealed a more than 2‐fold increase in Slc26a3 expression in the mouse colon in response to HDAC inhibition with SAHA (P ˂ 0.005) (Fig. 7A). Indirect immunofluorescence was performed to study the localization and protein expression level of SLC26A3 in the mid‐colons of mice. Control slides incubated with only the secondary antibody showed no staining (Fig. 7B). As compared to control colons where a moderate staining for SLC26A3 was observed along the apical membrane of surface colonocytes (Fig. 7C), staining intensity of SLC26A3 was significantly higher in the apical surface of differentiated colonocytes of mice having received SAHA (Fig. 7D). In addition, the SLC26A3 fluorescent signal extends to the middle of the crypt compartment in the mouse colon in response to HDAC inhibition (Fig. 7D and F), a phenomenon which is not observed in control mice (Fig. 7C and E). These results indicate that histone acetylation can trigger an early colonocyte differentiation program manifested by the up‐regulation of SLC26A3 expression in the middle of colonic crypts.
Figure 7.
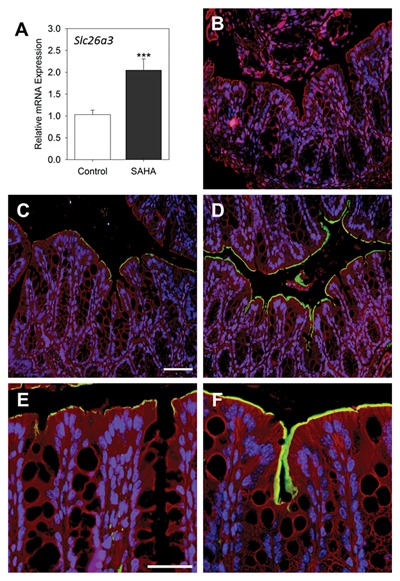
In vivo HDAC inhibition with SAHA induces differentiation of colonocytes. Mice were treated with SAHA for 48 h and colon and ileum samples were extracted for qPCR and immunofluorescence to evaluate expression levels of the enterocyte terminal differentiation gene Slc26a3. (A) mRNA expression of Slc26a3 in control and SAHA‐treated mice was determined by qPCR. Data are expressed as the mean ± SEM of colon samples obtained from 4 to 5 mice for each condition. *** P ˂ 0.005. (B–F) Representative immunofluorescence staining of the detection of SLC26A3 in the colon. Negative staining control (B) confirming the lack of non‐specific staining. Immunofluorescence on mid‐colon sections of mice for the detection of SLC26A3 (green) in control (C,E) and corresponding SAHA‐treated mouse colon (D,F) showing strong staining in luminal domain of both surface and upper gland epithelial cells. Blue: DAPI to label nuclei, Red: Evans blue counterstaining. Scale bar = 50 μm for C,D and 25 μ for E,F.
DISCUSSION
Failure of the intestinal epithelium to maintain a constant renewal based on coordinated dynamic balance between proliferation and differentiation can result in disease conditions such as cancer. Therefore, a detailed characterization of these mechanisms is essential to better comprehend intestinal physiology under normal and cancer conditions. We have recently shown that cooperation between transcription factors such as HNF1α and CDX2 is involved in down‐regulation of proliferation and induction of differentiation in intestinal cells [Escaffit et al., 2006; Benoit et al., 2010]. In contrast, epigenetic control of gene expression via PcG results in the repression of absorptive lineage differentiation in the TA compartment which involves PRC2‐mediated H3K27me3 [Benoit et al., 2012]. There is accumulating evidence of the regulatory role of HDACs in proliferation and differentiation of colon cancer cells in vitro and intestinal tumorigenesis in vivo [Mariadason, 2008]. However, there is little information regarding the implication of HDACs and histone acetylation in the normal physiology of intestinal cells. Using SAHA as a well‐known HDAC inhibitor, in the current work, we characterize the importance of histone acetylation as another important epigenetic modification in the regulation of proliferation and differentiation of intestinal cells.
HDAC enzymes are expressed in the proliferating crypt compartment of the small intestine and colon, consistent with their role in the maintenance of cell proliferation [Dokmanovic et al., 2007; Mariadason, 2008]. These enzymes are over‐expressed in many transformed cell types including colorectal cancer [Glozak and Seto, 2007] while treatment of colorectal cancer cells with the pan‐HDAC inhibitor SAHA induces apoptosis and proliferation arrest [Wilson et al., 2006]. We show herein that SAHA simultaneously induces histone acetylation and represses proliferation of Caco‐2/15 cultures, possibly through an induction of expression of genes involved in cell cycle control such as CDKN1A. Although known as an inhibitor of cell cycle [Xiong et al., 1993], CDKN1A has been reported to be expressed in differentiating post‐confluent intestinal Caco‐2/15 and tsFHI cells [Evers et al., 1996; Quaroni and Beaulieu, 1997]. It has also been found to be indispensable for apoptosis in breast cancer cells treated with an HDAC inhibitor [Chopin et al., 2004]. In agreement with these studies, our results propose mechanistic links between histone acetylation, CDKN1A expression, proliferation arrest, intestine‐specific gene expression and apoptosis in Caco‐2/15 cells. The exact mechanism(s) underlying the divergent patterns of the pre‐ and post‐confluent Caco‐2/15 cell response to the pro‐differentiation and anti‐proliferative effects of HDAC inhibitors needs further investigation but is reminiscent of the effects reported with butyrate on Caco‐2 cells [Mariadason et al., 2001]. Nevertheless, the fact that SAHA treatment of HIEC cells induced about an 80% reduction in the proliferative potential of cells without apparent apoptotic effect indicates that HDAC inhibition specifically alters normal undifferentiated crypt cell proliferation.
These data obtained on experimental cell models are confirmed by in vivo studies demonstrating that treatment of mice with therapeutic doses of SAHA results in a significant decline in cell proliferation in the crypt compartment while the in situ terminal deoxynucleotidyl transferase mediated dUTP nick‐end labeling assay [Beausejour et al., 2012] confirmed the lack of apoptosis resulting from the SAHA treatment. Our work is the first to demonstrate in vivo, the inhibitory effects of SAHA on the proliferation of normal dividing intestinal crypt cells, in agreement with the results observed in vitro using HIEC. A recent report suggests that conditional genetic depletion of two class I HDACs (HDAC1 and HDAC2) results in increased proliferation and migration of intestinal cells in knock‐out mice [Turgeon et al., 2013]. However, mice deficient in class I HDAC have been shown to have reduced cell number and intestinal mucosal thickness [Zimmermann et al., 2007], consistent with our data that HDAC inhibition results in proliferation defects in the mouse intestine. One possibility for this discrepancy could be the compensation of HDAC1 and HDAC2 depletion by other HDACs such as HDAC3 and HDAC8 which have strong positive regulatory effects on cell growth and cell cycle progression [Witt et al., 2009]. For example, HDAC3 which is a potent inducer of cellular proliferation is maximally expressed in proliferating crypt cells of the normal intestine [Wilson et al., 2006]. The advantage of using SAHA to suppress HDAC activities is its global inhibitory effect on all class I (HDACs 1,2,3 and 8) and II (HDACs 4, 5, 7, and 9) HDACs concurrently [Marks, 2004].
Differentiation of enterocytes from proliferating cells is a complex process including significant reprogramming of gene expression. While in the TA zone, progenitors of enterocytes undergo division cycles before completing a terminal differentiation program, which is associated with the expression of SI. The level of Caco‐2/15 cell confluence used in our study recapitulates to some extent the state of enterocytes in their last dividing cycles before starting to undertake a differentiation program. The switch from proliferative state to differentiated cell is associated with cooperation between complex cellular and molecular mechanisms. We have already shown that transcription factors (HNF1α and CDX2) and epigenetic modifications such as histone methylation play important roles in the terminal differentiation of intestinal cells [Escaffit et al., 2006; Benoit et al., 2010; Benoit et al., 2012]. In this study, we demonstrate a direct correlation between histone acetylation and expression of the intestine‐specific terminal differentiation marker SI at both transcript and protein levels in post‐confluent Caco‐2/15 cells as well as in the mouse intestine. The effects are quantitatively significant as demonstrated by SI mRNA levels in 4 day post‐confluent cells treated with SAHA reaching comparable levels as those found in Caco‐2/15 cells after 30 days of post‐confluence culture. These results are confirmed in vivo by showing an intense ileal SIS signal which extends down to the top of the intestinal crypts of mice treated with SAHA. In fact, acetylated histones have been shown to occupy the SIS promoter as cells start to undertake a terminal differentiation program during transition from crypt to villus axis [Suzuki et al., 2008]. These results identify histone deacetylation as a major suppressor of SI gene expression and terminal differentiation of enterocytes in the TA zone. The effect of SAHA on the gene expression of Caco‐2/15 cells is quite selective and is not limited to SI expression. In addition to SI, the up‐regulated expression of other enterocyte‐specific terminal differentiation and polarization markers such as SLC26A3, also known as Down Regulated in Adenoma (DRA), and VIL1, respectively, was observed after exposing cells to SAHA. A previous study has proposed a functional relationship between the cell polarity marker E‐cadherin and the Na,K‐ATPase ion transporter [Rajasekaran et al., 2001]. Consistent with this study and our recent work [Seltana et al., 2013], we show here that differentiation and polarization can be coupled events and are co‐regulated at the chromatin level. We also monitored the expression of other markers associated with intestinal cell differentiation such as CGN, DPP4, HNF1A, and CDX2, and in agreement with the selective regulatory effects of SAHA on specific genes [Van Lint et al., 1996], none of them was modulated by HDAC inhibition. Knockdown of SUZ12 protein as a component of the PRC2 complex caused early expression of a subset of differentiation markers such as SI and alkaline phosphatase but not the pro‐differentiation factors HNF1α and CDX2 [Benoit et al., 2012]. This suggests that HDACs and PcG proteins, through different mechanisms, share similar functions in parallel to controlling or hindering differentiation of enterocytes in the TA zone. It is noteworthy to state that our laboratory has recently found another mechanism that controls differentiation of Caco‐2/15 cells. Inhibition of Src family kinases accelerated the overall differentiation program manifested by up‐regulation of the expression of more than 10 genes including SI, CDH1, VIL1, HNF1A, and CDX2, and a reduction in the activity of PRC2‐related H3K27me3 [Seltana et al., 2013]. The main distinction between the inhibition of the activity of Src and HDACs is that SAHA has no effect on the expression of the transcription factors HNF1A and CDX2. Nevertheless, the significant effects of SAHA on SI and SLC26A3 expression reveal the unsuspected role of HDACs and histone acetylation in the regulation of enterocyte‐specific terminal differentiation.
To study the in vivo effects of SAHA on the differentiation of intestinal cells we chose to determine the levels of SIS and SLC26A3 expression as markers of ileal enterocyte differentiation [Beaulieu and Quaroni, 1991] and colonocyte maturation [Talbot and Lytle, 2010], respectively. This is the first study to characterize the impact of therapeutic doses of SAHA on normal intestinal cells. We show by three independent approaches (IF, WB, and qPCR) that SAHA treatment promotes an up‐regulation of both SIS and SLC26A3 in the mouse ileum and colon, respectively. Interestingly, immunofluorescence experiments characterize a specific expression of both SIS and SLC26A3 in the apical membrane of the glandular epithelium of both intestinal segments suggesting that SAHA promotes precocious epithelial differentiation in crypts. This observation is consistent with previous results from us and other groups proposing that epigenetic modifications can be important determinants of terminal differentiation of progenitor enterocytes in the TA compartment [Suzuki et al., 2008; Benoit et al., 2012]. Indeed, methylation of histone H3 with the PRC2 complex (H3K27me3) [Benoit et al., 2012] and other histone methylation patterns [Suzuki et al., 2008] can suppress differentiation of enterocytes in the TA zone, allowing progenitor cells to undergo multiple rounds of division before undertaking a differentiation program. The results presented herein suggest that HDAC activity can also repress differentiation of progenitor cells while maintaining cellular proliferation. In this context, it is interesting to note that replacement of methyl groups by acetyl groups on histones has been associated with the transition of a cell from the proliferative to differentiated state in various systems [Suzuki et al., 2008; Ong and Corces, 2011; Buchi et al., 2014].
Considering that SLC26A3/DRA expression is suppressed in colon cancer and has been suggested to work as a tumor suppressor gene [Gorbatenko et al., 2014], the data showing an up‐regulation of this molecule in the normal colon by SAHA is intriguing. SLC26A3/DRA can negatively regulate proliferation of intestinal crypt cells while Slc26a3/DRA‐deficient mice exhibit an expansion of the colonic crypt proliferative zone [Schweinfest et al., 2006]. Our results showing that SLC26A3/DRA up‐regulation in response to SAHA treatment coincides with a significant inhibition of crypt cell proliferation are consistent with these studies. In this context, it is pertinent to note that in 2006, SAHA was approved by the FDA for the treatment of refractory cutaneous T cell lymphoma (CTCL) in patients with progressive solid and hematologic malignancies. Some of the most common adverse side effects of SAHA have been reported to be related to gastrointestinal symptoms such as nausea, vomiting or diarrhea [Duvic et al., 2007]. Based on our data, we can speculate that these effects are due to the inhibition of crypt cell proliferation affecting the overall renewal of the intestinal epithelium.
In conclusion, the results of the present study identify a novel mechanism involved in the regulation of intestinal cell proliferation and differentiation. Indeed, in addition to our previous studies characterizing regulatory pathways of intestinal cell differentiation and proliferation [Benoit et al., 2010; Benoit et al., 2012; Seltana et al., 2013], the current study shows the important impact of HDACs on the proliferation and differentiation of normal and transformed intestinal cells. In fact, HDAC inhibition by SAHA is associated with the repression of cell proliferation and induction of intestine‐specific gene expression.
ACKNOWLEDGMENTS
The authors thank Elizabeth Herring for reviewing the manuscript. This work was supported by Grant MOP 123415 from the Canadian Institute of Health Research (JFB). JFB is the recipient of the Canada Research Chair of Intestinal Physiopathology. JFB and PHV are members of the Fonds de la recherche du Québec/Santé‐funded Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke.
REFERENCES
- Beaulieu JF. 1997. Recent work with migration/patterns of expression: cell matrix interactions in human intestinal cell differentiation In: Halter F, Winton D, Wright NA, editors. The Gut as a Model in Cell and Molecular Biology. Dordrecht: Kluwer Academic Publishers; pp 165–179. [Google Scholar]
- Beaulieu JF, Ménard D. 2012. Isolation, characterization, and culture of normal human intestinal crypt and villus cells. Methods Mol Biol (Clifton, N.J.) 806:157–173. [DOI] [PubMed] [Google Scholar]
- Beaulieu JF, Nichols B, Quaroni A. 1989. Posttranslational regulation of sucrase‐isomaltase expression in intestinal crypt and villus cells. J Biol Chem 264:20000–20011. [PubMed] [Google Scholar]
- Beaulieu JF, Quaroni A. 1991. Clonal analysis of sucrase‐isomaltase expression in the human colon adenocarcinoma Caco‐2 cells. Biochem J 280(Pt 3):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beausejour M, Noel D, Thibodeau S, Bouchard V, Harnois C, Beaulieu JF, Demers MJ, Vachon PH. 2012. Integrin/Fak/Src‐mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: Selective engagement and roles of PI3‐K isoform complexes. Apoptosis 17:566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit YD, Lepage MB, Khalfaoui T, Tremblay E, Basora N, Carrier JC, Gudas LJ, Beaulieu JF. 2012. Polycomb repressive complex 2 impedes intestinal cell terminal differentiation. J Cell Sci 125:3454–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit YD, Pare F, Francoeur C, Jean D, Tremblay E, Boudreau F, Escaffit F, Beaulieu JF. 2010. Cooperation between HNF‐1alpha, Cdx2, and GATA‐4 in initiating an enterocytic differentiation program in a normal human intestinal epithelial progenitor cell line. Am J Physiol Gastrointest Liver Physiol 298:G504–G517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. 2005. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289:G381–G387. [DOI] [PubMed] [Google Scholar]
- Buchi F, Masala E, Rossi A, Valencia A, Spinelli E, Sanna A, Gozzini A, Santini V. 2014. Redistribution of H3K27me3 and acetylated histone H4 upon exposure to azacitidine and decitabine results in de‐repression of the AML1/ETO target gene IL3. Epigenetics 9:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S. 2010. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence‐based quantitative real‐time PCR experiments. BMC Mol Biol 11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon‐Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM. 2000. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60:5165–5170. [PubMed] [Google Scholar]
- Cheng H, Leblond CP. 1974. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141:537–561. [DOI] [PubMed] [Google Scholar]
- Chopin V, Toillon RA, Jouy N, Le Bourhis X. 2004. P21(WAF1/CIP1) is dispensable for G1 arrest, but indispensable for apoptosis induced by sodium butyrate in MCF‐7 breast cancer cells. Oncogene 23:21–29. [DOI] [PubMed] [Google Scholar]
- Dokmanovic M, Clarke C, Marks PA. 2007. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989. [DOI] [PubMed] [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. 2007. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T‐cell lymphoma (CTCL). Blood 109:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dydensborg AB, Herring E, Auclair J, Tremblay E, Beaulieu JF. 2006. Normalizing genes for quantitative RT‐PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am J Physiol Gastrointest Liver Physiol 290:G1067–G1074. [DOI] [PubMed] [Google Scholar]
- Escaffit F, Pare F, Gauthier R, Rivard N, Boudreau F, Beaulieu JF. 2006. Cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem Biophys Res Commun 342:66–72. [DOI] [PubMed] [Google Scholar]
- Evers BM, Ko TC, Li J, Thompson EA. 1996. Cell cycle protein suppression and p21 induction in differentiating Caco‐2 cells. Am J Physiol 271:G722–G727. [DOI] [PubMed] [Google Scholar]
- Glozak MA, Seto E. 2007. Histone deacetylases and cancer. Oncogene 26:5420–5432. [DOI] [PubMed] [Google Scholar]
- Gorbatenko A, Olesen CW, Boedtkjer E, Pedersen SF. 2014. Regulation and roles of bicarbonate transporters in cancer. Front Physiol 5:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guezguez A, Pare F, Benoit YD, Basora N, Beaulieu JF. 2014. Modulation of stemness in a human normal intestinal epithelial crypt cell line by activation of the WNT signaling pathway. Exp Cell Res 322:355–364. [DOI] [PubMed] [Google Scholar]
- Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. 2004. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter‐associated proteins, including HD AC1. Proc Natl Acad Sci USA 101:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi LC, Xi X, Lotan R, Shureiqi I, Lippman SM. 2004. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces apoptosis via induction of 15‐lipoxygenase‐1 in colorectal cancer cells. Cancer Res 64:8778–8781. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. 2010. Coexistence of quiescent and active adult stem cells in mammals. Science 327:542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariadason JM. 2008. HDACs and HDAC inhibitors in colon cancer. Epigenetics 3:28–37. [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Rickard KL, Barkla DH, Augenlicht LH, Gibson PR. 2000. Divergent phenotypic patterns and commitment to apoptosis of Caco‐2 cells during spontaneous and butyrate‐induced differentiation. J Cell Physiol 183:347–354. [DOI] [PubMed] [Google Scholar]
- Mariadason JM, Velcich A, Wilson AJ, Augenlicht LH, Gibson PR. 2001. Resistance to butyrate‐induced cell differentiation and apoptosis during spontaneous Caco‐2 cell differentiation. Gastroenterology 120:889–899. [DOI] [PubMed] [Google Scholar]
- Marks PA. 2004. The mechanism of the anti‐tumor activity of the histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA). Cell Cycle 3:534–535. [DOI] [PubMed] [Google Scholar]
- Marks PA. 2007. Discovery and development of SAHA as an anticancer agent. Oncogene 26:1351–1356. [DOI] [PubMed] [Google Scholar]
- Marks PA, Richon VM, Rifkind RA. 2000. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst 92:1210–1216. [DOI] [PubMed] [Google Scholar]
- Ong CT, Corces VG. 2011. Enhancer function: new insights into the regulation of tissue‐specific gene expression. Nat Rev Genet 12:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A, Beaulieu JF. 1997. Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology 113:1198–1213. [DOI] [PubMed] [Google Scholar]
- Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr. , Bander NH, Peralta Soler A, Rajasekaran AK. 2001. Na,K‐ATPase beta‐subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell 12:279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P, Mitsiades C, Colson K, Reilly E, McBride L, Chiao J, Sun L, Ricker J, Rizvi S, Oerth C, Atkins B, Fearen I, Anderson K, Siegel D. 2008. Phase I trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) in patients with advanced multiple myeloma. Leuk Lymphoma 49:502–507. [DOI] [PubMed] [Google Scholar]
- Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. 2006. Slc26a3 (dra)‐deficient mice display chloride‐losing diarrhea, enhanced colonic proliferation, and distinct up‐regulation of ion transporters in the colon. J Biol Chem 281:37962–37971. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. 2008. Current view: Intestinal stem cells and signaling. Gastroenterology 134:849–864. [DOI] [PubMed] [Google Scholar]
- Seltana A, Guezguez A, Lepage M, Basora N, Beaulieu JF. 2013. Src family kinase inhibitor PP2 accelerates differentiation in human intestinal epithelial cells. Biochem Biophys Res Commun 430:1195–1200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mochizuki K, Goda T. 2008. Histone H3 modifications and Cdx‐2 binding to the sucrase‐isomaltase (SI) gene is involved in induction of the gene in the transition from the crypt to villus in the small intestine of rats. Biochem Biophys Res Commun 369:788–793. [DOI] [PubMed] [Google Scholar]
- Talbot C, Lytle C. 2010. Segregation of Na/H exchanger‐3 and Cl/HCO3 exchanger SLC26A3 (DRA) in rodent cecum and colon. Am J Physiol Gastrointest Liver Physiol 299:G358–G367. [DOI] [PubMed] [Google Scholar]
- Tou L, Liu Q, Shivdasani RA. 2004. Regulation of mammalian epithelial differentiation and intestine development by class I histone deacetylases. Mol Cell Biol 24:3132–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay E, Ferretti E, Babakissa C, Seidman EG, Levy E, Menard D, Beaulieu JF. 2011. Gene‐expression profile analysis in the mid‐gestation human intestine discloses greater functional immaturity of the colon as compared with the ileum. J Pediatr Gastroenterol Nutr 52:670–678. [DOI] [PubMed] [Google Scholar]
- Turgeon N, Blais M, Gagne JM, Tardif V, Boudreau F, Perreault N, Asselin C. 2013. HDAC1 and HDAC2 restrain the intestinal inflammatory response by regulating intestinal epithelial cell differentiation. PLoS One 8:e73785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH, Beaulieu JF. 1992. Transient mosaic patterns of morphological and functional differentiation in the Caco‐2 cell line. Gastroenterology 103:414–423. [DOI] [PubMed] [Google Scholar]
- van der Flier LG, Clevers H. 2009. Stem cells, self‐renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260. [DOI] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Verdin E. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5:245–253. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang J, Liu D, Su Y. 2010. Normalizing genes for real‐time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal Biochem 399:211–217. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Byun DS, Popova N, Murray LB, L'Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. 2006. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281:13548–13558. [DOI] [PubMed] [Google Scholar]
- Witt O, Deubzer HE, Milde T, Oehme I. 2009. HDAC family: What are the cancer relevant targets?. Cancer Lett 277:8–21. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. 1993. P21 is a universal inhibitor of cyclin kinases. Nature 366:701–704. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, Floss T, Wurst W, Minucci S, Gottlicher M. 2007. Reduced body size and decreased intestinal tumor rates in HDAC2‐mutant mice. Cancer Res 67:9047–9054. [DOI] [PubMed] [Google Scholar]


