Abstract
Purpose
To determine the association between vision‐related quality of life (VRQOL) and levels of visual function loss in the Early Manifest Glaucoma Trial (EMGT).
Methods
Two hundred and fifty‐five patients were included in the EMGT between 1993 and 1997 and followed regularly by ophthalmic examinations. A Swedish translation of the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ‐25) was self‐administered at several follow‐up visits until 2014. We analysed the association between Rasch‐calibrated NEI VFQ‐25 scores and visual function in the best eye at the final follow‐up visit.
Results
Ninety‐one per cent (233/255) of all participants completed the NEI VFQ‐25 at least once. In univariate logistic regression analysis, NEI VFQ‐25 scores were modestly associated with visual acuity (VA) (r 2 = 0.330, p < 0.001), visual field index (VFI) (r 2 = 0.200, p < 0.001) and perimetric mean deviation (MD) (r 2 = 0.193, p < 0.001). In multivariate analysis, VA and VFI together accounted for approximately 40% (r 2 = 0.380) of the NEI VFQ‐25 scores. NEI VFQ‐25 scores were significantly higher for patients with no visual impairment (mean 73 ± 22) than for visually impaired patients (mean 31 ± 15, p < 0.001). VFI worse than 50% or MD worse than −18 dB was significantly associated with low VRQOL scores (p < 0.001).
Conclusions
Our results support the widespread, albeit arbitrary, use of a better‐eye visual field of <50% as an important threshold for a significant reduction in VRQOL.
Keywords: blindness, low vision, open‐angle glaucoma, vision‐related quality of life, visual impairment
Introduction
According to the current European Glaucoma Society Guidelines, the goal of glaucoma treatment is to preserve the patient's vision‐related quality of life (VRQOL). In clinical practice, measurements of the visual field (VF) are performed to evaluate the severity and progression of glaucoma. However, it is not immediately clear how glaucomatous VF loss and visual acuity (VA) reflect the impact of the disease or patients’ perceptions of disability due to glaucoma.
The relationship between visual function and self‐reported quality of life in patients with glaucoma has been addressed in an increasing number of papers, and the National Eye Institute Visual Function Questionnaire 25 (NEI VFQ‐25) is the most widely used VRQOL instrument (Mangione et al. 2001). However, many studies have found only a modest correlation between NEI VFQ‐25 scores and VF status in patients with glaucoma (Gutierrez et al. 1997; Parrish et al. 1997; Mills et al. 2001; Sumi et al. 2003; Hyman et al. 2005; McKean‐Cowdin et al. 2008; van Gestel et al. 2010), and, importantly, all of those investigations used the original scoring system published and recommended by the developers of the instrument, which has been criticized by a number of researchers for not producing interval‐leveled estimates of VRQOL. This problem can be addressed by applying modern psychometric methods such as Rasch analysis (Massof 2007; Pesudovs et al. 2010). Rasch analysis has been used to evaluate NEI VFQ‐25 data in low‐vision populations with various ocular diseases (Massof & Fletcher 2001; Marella et al. 2010; Pesudovs et al. 2010), but information is limited regarding the usefulness of this instrument in populations with a wider range of glaucoma damage (Medeiros et al. 2015). Hence, further studies are needed to determine at what stages of functional damage the NEI VFQ‐25 can reveal the impact of glaucoma on VRQOL (Nassiri et al. 2013).
The Early Manifest Glaucoma Trial (EMGT) is a prospective randomized clinical trial in which patients with newly detected early manifest glaucoma were included from 1993 to 1997 and continued with prospective standardized follow‐up visits including standard automated perimetry (SAP) and VA measurements until the end of December 2013. VRQOL was assessed over time using a validated Swedish translation of the NEI VFQ‐25, which was administered for the last time in 2013. The EMGT cohort comprised only patients with early glaucoma at the beginning of the trial but included patients with all stages of the disease by the end of the trial in 2013. Therefore, we used data from the final follow‐up visit to analyse the association between visual function and VRQOL in patients with glaucoma that had been followed prospectively for up to 20 years. More precisely, we conducted this study using data on a cohort of patients that had initially been diagnosed with early glaucoma and were followed for up to 20 years with these specific aims: (i) to report the frequency of visual impairment, (ii) to evaluate the VRQOL and (iii) to analyse the association between VRQOL and visual function.
Methods
This study was approved by both the Ethical Review Board of Lund University, Lund, Sweden, and the Committee on Research Involving Human Subjects of the State University of New York, Stony Brook, New York, and all patients provided written informed consent. The EMGT is registered as clinicaltrials.gov Identifier: NCT00000132 (registration date: September 23, 1999).
The design of the EMGT has been described in detail previously (Leske et al. 1999; Heijl et al. 2002). Briefly, the EMGT enrolled 255 patients with newly diagnosed and untreated open‐angle glaucoma (including primary open‐angle and exfoliation glaucoma) involving early to moderate VF loss. Eligible patients had at least one eye with repeatable glaucoma‐related VF defects demonstrated by the Humphrey 30‐2 full threshold test. Included patients were randomized to treatment or to no initial treatment and prospective follow‐up according to a standard protocol. Each follow‐up visit included evaluation of the VF by SAP, measurement of the best‐corrected VA using Snellen decimal charts and a general ophthalmic examination.
Vision‐related quality of life over time was also evaluated in the EMGT using the NEI VFQ‐25. The original English version of this instrument was translated into Swedish and validated by the EMGT investigators (Hyman et al. 2005). The NEI VFQ‐25 was self‐administered for the first time at a median of 3 years after randomization and later on average every second year until the end of the trial in 2013.
Only patients that had completed the NEI VFQ‐25 at least once in the EMGT were eligible for this study, and we analysed data consisting of responses from the final NEI VFQ‐25 and the last available VF and VA measurements. The term ‘visual impairment’ was used to include both low vision and blindness based on the best‐corrected VA and/or VF status of the better eye according to the World Health Organization (WHO) criteria (low vision VA < 0.3 and/or central VF < 20°; blind VA < 0.05 and/or central VF < 10°). As specified in the WHO criteria, we classified patients as follows: (i) blind in both eyes, (ii) low vision in both eyes, (iii) one blind eye or (iv) one eye with low vision.
NEI VFQ‐25 scoring
The NEI VFQ‐25 consists of 25 items representing 11 subscales and one single item rating general health (Table 1). Twenty‐three of the items have five or six response options (the sixth stating ‘stopped doing for other reason/not interested in doing’).
Table 1.
National Eye Institute Visual Function Questionnaire 25 – items and subscales
| No. | Item | Subscale | Response categories (n) | Missing data (%) | Floor effect (%) | Ceiling effect (%) |
|---|---|---|---|---|---|---|
| 1 | General health | General health | Quality (6) | 2.2 (5/233) | 17.1 (39/228) | 7.0 (16/228) |
| 2 | General vision | General vision | Quality (6) | 1.3 (3/233) | 0.4 (1/230) | 7.7 (18/230) |
| 3 | Worry about eyesight | Mental health | Frequency (5) | 0.9 (2/233) | 2.6 (6/231) | 20.8 (48/231) |
| 4 | Pain around eyes | Ocular pain | Quality (5) | 0.9 (2/233) | 0 (0/231) | 28.9 (136/231) |
| 5 | Reading normal print | Near vision | Difficulty (5) | 2.6 (6/233)a | 8.8 (20/227) | 33.0 (75/227) |
| 6 | Seeing well up close | Near vision | Difficulty (5) | 8.6 (20/233)a | 8.9 (19/213) | 28.6 (61/213) |
| 7 | Finding objects on crowded shelf | Near vision | Difficulty (5) | 5.6 (13/233)a | 2.7 (6/220) | 45.0 (99/220) |
| 8 | Reading street signs | Distance vision | Difficulty (5) | 5.6 (13/233)a | 5.0 (11/220) | 50.5 (111(220) |
| 9 | Going down stairs at night | Distance vision | Difficulty (5) | 10.3 (24/233)a | 4.3 (9/209) | 23.0 (48/209) |
| 10 | Seeing objects off to side | Peripheral vision | Difficulty (5) | 3.4 (8/233)a | 1.8 (4/225) | 38.2 (86/225) |
| 11 | Seeing how people react | Social function | Difficulty (5) | 4.7 (11/233)a | 2.3 (5/222) | 55.0 (122/222) |
| 12 | Difficulty matching clothes | Colour vision | Difficulty (5) | 8.2 (19/233)a | 2.8 (6/214) | 65.0 (139/214) |
| 13 | Visiting others | Social function | Difficulty (5) | 12.0 (28/233)a | 4.4 (9/205) | 55.6 (114/205) |
| 14 | Going out to movies/plays | Distance vision | Difficulty (5) | 29.6 (69/233)a | 12.2 (20/164) | 48.8 (80/164) |
| 15 | Driving in daylight | Driving | Difficulty (5) | 77.3 (180/233) | 0 (0/53) | 90.6 (48/53) |
| 16 | Driving in difficult conditions | Driving | Difficulty (5) | 79.4 (185/233)a | 10.4 (5/48) | 14.6 (7/48) |
| 17 | Accomplish less | Role limitations | Frequency (5) | 3.0 (7/233) | 12.0 (27/226) | 45.1 (102/226) |
| 18 | Limited endurance | Role limitations | Frequency (5) | 4.3 (10/233) | 9.9 (22/223) | 41.7 (93/223) |
| 19 | Amount of time in pain | Ocular pain | Frequency (5) | 4.3 (10/233) | 0.5 (1/223) | 64.6 (144/223) |
| 20 | Stay home most of the time | Dependency | Agreement (5) | 3.0 (7/233) | 5.3 (12/226) | 73.9 (167/226) |
| 21 | Frustrated | Mental health | Agreement (5) | 2.6 (6/233) | 7.1 (16/227) | 64.3 (146/227) |
| 22 | No control | Mental health | Agreement (5) | 2.6 (6/233) | 8.8 (20/227) | 59.0 (134/227) |
| 23 | Rely too much on others’ words | Dependency | Agreement (5) | 2.6 (6/233) | 7.5 (17/227) | 70.9 (161/227) |
| 24 | Need much help from others | Dependency | Agreement (5) | 2.6 (6/233) | 8.8 (20/227) | 70.5 (160/227) |
| 25 | Embarrassment | Mental health | Agreement (5) | 3.0 (7/233) | 2.2 (5/226) | 76.1 (172/227) |
Items in gray indicate excluded before Rasch analysis.
A sixth option (stopped doing for other reasons/not interested in doing) was considered as missing data.
Response categories concern quality of an item (questions 1, 2 and 4), difficulty of an activity (questions 5–16), frequency of a problem (questions 3 and 17–19) and agreement with a problem (questions 20–25). The questions about general health and general vision, and the two items concerning ocular pain were excluded from our analysis, because they are not related to visual ability (Massof 2007; Marella et al. 2010). We also excluded all items regarding driving due to extensive missing data (Table 1) (Pesudovs et al. 2007). Thereafter, we recoded the numerical responses to the NEI VFQ‐25 and assigned each response a score from 0 (most negative) to 4 (most positive), with the sixth response category coded as missing data. If necessary, category responses were reversed for Rasch analysis so that the polarity would be the same for all included items.
Rasch analysis of NEI VFQ‐25 scores
Winsteps software version 3.81.0 (Winsteps, Chicago, IL, USA) was used to perform Rasch analysis using three Andrich rating‐scale models (Andrich 1978), one for each of the NEI VFQ‐25 rating scales included in our analysis. This approach has been described elsewhere (Massof & Fletcher 2001). We chose to use Rasch analysis, because it enabled us to estimate interval‐scaled visual ability scores for glaucoma patients from the ordinal ratings of items in the NEI VFQ‐25 (Massof 2002). Rasch analysis was performed to calculate measures of the observed visual ability of each participant (person measure) and the visual ability required for each item (item measure). The unit of those estimated measures is called a logit (log‐odds unit), which is calculated as the log‐odds ratio of the probability that a participant will select a particular rating category in an item over 1 minus the same probability. The logit values place patients according to their abilities and items according to their difficulties on the same linear interval scale (i.e. logit scale).
Initially, we investigated the evidence of disordered thresholds for each type of rating scale. Disordered thresholds indicate that participants had difficulties in discriminating between response levels for an item, but this problem can often be solved by combining adjacent response categories. The initial fit of the 19 included NEI VFQ‐25 items to the Rasch model revealed disordered thresholds for the rating scale asking about agreement with a problem ranging from completely true to not true at all. The probability of choosing the response categories 1 or 3 was not higher at any point on the logit scale compared to selecting any of the other categories. Accordingly, we collapsed the response categories 0 and 3 with the adjacent categories 1 and 4, respectively, and thereafter, response category 2 was no longer the most probable category at any point on the logit scale. Category 2 represents a neutral response and thus cannot logically be combined with an adjacent response category, and therefore, we coded response category 2 answers as missing data. Hence, the final rating scale was dichotomous, with two response categories: 0 (true) and 1 (not true).
When response category performance was deemed satisfactory, item and person measures were calculated. We determined the fit of the data to the Rasch model (i.e. whether each item measured a single underlying construct; in the NEI VFQ‐25 ‘visual ability’) by calculating fit statistics and evaluating unidimensionality. We chose to report infit (information‐weighted fit) statistics, primarily because they are relatively insensitive to distortion from outliers (Pesudovs et al. 2010). Infit mean square (MNSQ) values are expected to be 1, and values between 0.7 and 1.3 are considered productive for measurement (Wright & Linacre 1994). Items with infit values outside this range were excluded from all analyses. After combining response categories, four items had infit values >1.3 and thus did not fit the model, and we removed those items iteratively as follows: less‐control‐over‐what‐is‐done, do‐things‐that‐embarrass‐self, stay‐home‐most‐of‐time and worry‐about‐eyesight.
Unidimensionality was tested by principal component analysis (PCA) of the residuals (difference between observed and expected responses). In such assessment, data are considered unidimensional if the variance explained by the principal component exceeds 60% (Smith 2002). In addition, the contrast to the principal component should not provide a significant explanation for the remaining variance (e.g. <2.0 Eigenvalue units) (Smith 2002). The PCA of the residuals of the remaining 15 items revealed multidimensionality, and the principal component accounted for 67.8% of the observed variance, which was very close to the 68.8% expected by the model. The first contrast accounted for 2.4 Eigenvalue units and the second contrast for 1.8 Eigenvalue units. Five items loaded positively onto the first contrast and belonged to the role difficulties (two items), mental health (one item) and dependency (two items) subscales. Ten items loaded positively onto the second contrast: eight of those belonged to the visual functioning subscales (near vision, distance vision, colour vision and peripheral vision), and the other two belonged to the social functioning subscale. Based on the results of the PCA, we formed two separate scales: in one, the visual functioning items were combined with the two social functioning items; in the other, the five items belonging to social and emotional functioning were combined (Table 2). All items in the two separate scales showed acceptable fit to the model, and PCA of the residuals of both subscales revealed unidimensionality (Table 2).
Table 2.
Rasch analysis fit statistics of the two scales
| Scale | Items in scale (n) | Misfitting items (n) | Person separation index | Person separation reliability | Mean ± SE Person measure (logits) | Mean ± SD Item measure (logits) | Principal component analysis (Eigenvalues) |
|---|---|---|---|---|---|---|---|
| Visual functioning | 10 | 0 | 2.45 | 0.86 | 1.95 ± 0.72 | 0.00 ± 0.11 | 1.7 |
| Socio‐emotional | 5 | 0 | 1.60 | 0.72 | 2.37 ± 1.34 | 0.00 ± 0.12 | 1.7 |
We tested differential item functioning (DIF), which occurs when subgroups of individuals within the sample respond differently to an item. Presence of DIF leads to incorrect estimates of the logit for an item (Massof 2011). We defined DIF according to the scale proposed by Pesudovs et al. (2010): differences <0.5 logits = DIF‐free or at least DIF‐trivial; differences between 0.5 and 1.0 logits = minimal DIF (probably unimportant); and differences >1.0 logits = notable DIF. Differential item functioning was tested for age (≤83 versus ≥84 years), gender, glaucoma stage (≤−6.75 versus ≥−6.76 dB in better eye) and general health. Responses to the NEI VFQ‐25 question about general health were used to divide participants into those rating their general health as at least ‘good’ and those indicating worse subjective general health. Three items of the visual functioning subscale showed minimal DIF: going‐down‐steps‐stairs‐at‐night demonstrated by gender (women 0.58, p = 0.0073), matching‐clothes by glaucoma stage (worse stage 0.53, p = 0.0355) and reading‐print by general health (worse general health 0.51, p = 0.0106). No item had to be excluded due to large DIF (>1.0 logits).
Finally, the overall performance of the model was evaluated using person separation indices (e.g. reliability coefficient) and targeting. The reliability coefficient can be used to determine the number of statistically distinct levels of person ability that an instrument can discriminate. For results to be included, we required a reliability coefficient of ≥0.8, which means that the instrument can distinguish at least three different levels of person ability (Massof 2011). Targeting evaluates the suitability of the difficulty of the items on the instrument in relation to the person ability of the sample and is considered good if the difference between mean scores for items and persons is <1 logits (Pesudovs et al. 2010). We did not specify a formal criterion for minimal targeting, because we did not intend to change the NEI VFQ‐25, but rather to analyse results obtained with the instrument in its existing form.
The difference between the mean person measure and the mean item measure was >1.0 logits for both subscales (Table 2), indicating that the items were not optimally matched to the sample. Person separation indices were acceptable for the visual functioning subscale but were not satisfactory for the ‘socio‐emotional’ subscale (Table 2). Therefore, our final Rasch model contained only the 10 items belonging to the visual functioning model (Table 3).
Table 3.
Fit statistics of the National Eye Institute Visual Function Questionnaire 25 items included in the final model
| No. | Itema | Measure (logits) | SE | Infit MNSQ | Outfit MNSQ |
|---|---|---|---|---|---|
| 9 | Going down stairs at night | 1.10 | 0.11 | 1.08 | 1.19 |
| 6 | Seeing well up close | 0.80 | 0.10 | 0.92 | 0.95 |
| 5 | Reading normal print | 0.57 | 0.10 | 0.93 | 0.91 |
| 10 | Seeing objects off to side | 0.32 | 0.10 | 1.04 | 1.11 |
| 14 | Going out to movies/plays | 0.30 | 0.13 | 1.12 | 0.94 |
| 8 | Reading street signs | −0.13 | 0.12 | 1.16 | 1.05 |
| 7 | Finding objects on crowded shelf | −0.18 | 0.11 | 0.85 | 0.79 |
| 13 | Visiting others | −0.70 | 0.12 | 0.89 | 0.74 |
| 11 | Seeing how people react | −0.74 | 0.11 | 0.90 | 0.84 |
| 12 | Difficulty matching clothes | −1.33 | 0.14 | 1.12 | 1.08 |
MNSQ = mean square.
The most difficult item is at the top and the least difficult item at the bottom of the table.
Statistical analysis
To facilitate comparison with other studies, we used linear transformation to rescale Rasch estimates of person measures to positive values (hereafter designated as Rasch‐calibrated NEI VFQ‐25 scores), with 0 as the lowest possible visual ability. Rasch‐calibrated NEI VFQ‐25 scores calculated from the final model were used in all analyses.
One‐way anova and Bonferroni tests were performed to evaluate potential differences in VRQOL between the various levels of visual impairment. Uni‐ and multivariate logistic regression analyses were performed to evaluate the association between the Rasch‐calibrated NEI VFQ‐25 scores and VF status at final follow‐up visit defined by the mean deviation (MD), visual field index (VFI) and VA in the better eye, and gender, age and presence of cataract. All factors showing a statistically significant association in univariate analysis were subsequently included into the multivariate model. The data were analysed using spss version 22 (SPSS Inc., Chicago, IL, USA). A significance level of p < 0.05 was used in all analyses.
Results
In all, 91.4% (233) of the 255 patients with glaucoma originally included in the EMGT completed at least one NEI VFQ‐25 questionnaire. Also, 109 patients (42.8%) were still under follow‐up in December 2013, and 89.9% (98) of those 109 completed the questionnaire for the last time during 2013. Mean time between the final NEI VFQ‐25 and the end of the trial (i.e. December 31, 2013, or death) was 18.5 ±22.7 months. The follow‐up time after randomization was 15 ±4 years on average and was more than 10 years for over 80% of patients.
At the final follow‐up visit, the patients were on average aged 83.3 ± 5.5 years (median 84, range 62–95 years), and 68% (158/233) were women. Mean MD values for the better and worse eye were −8.0 ± 6.7 dB (median −6.8, range −29.02 to 1.92) and −14.3 ± 7.9 dB (median −13.7, range −30.65 to 0.98), respectively. Better‐eye mean VFI was 79 ± 21% (median 84%, range 4–100%), and worse‐eye mean VFI was 56 ± 27% (median 58%, range 0–100%). Visual acuity ranged from 0.03 to 1.58 in the better eye (mean VA 0.72 ± 0.28) and from no light perception to 1.38 in the worse eye (mean VA 0.54 ± 0.28).
At the last visit, 3% (8/233) of the patients were blind, 6% (13/233) had low vision, 9% (22/233) had one blind eye, and 10% (24/233) had one eye with low vision. Glaucoma was the major cause of blindness and low vision in 88% (7/8) and 62% (8/13), respectively. Age‐related macular degeneration was the other main cause of visual impairment [occurring in 29% (5/21) of visually impaired patients: in one of eight blind patients and in four of 13 reduced‐vision patients].
Rasch‐calibrated NEI VFQ‐25 scores could be calculated for 231 (99.1%) of 233 patients using the visual functioning model; calculation was not possible for the remaining 2, because those patients answered stopped doing this for other reason/not interested in doing on all items included in the final model. The mean linear transformed person measure was 66 ± 22 (median 67, range 0–104).
Rasch‐calibrated NEI VFQ‐25 scores were moderately correlated with patients’ visual function at the last visit, showing the highest correlation with VA (r 2 = 0.330, p < 0.001). Correlation with Rasch‐calibrated NEI VFQ‐25 scores was slightly stronger for better‐eye VFI (r 2 = 0.200, p < 0.001) than for better‐eye MD (r 2 = 0.193 p < 0.001) and was weak for both age (r 2 = 0.039, p = 0.003) and gender (r 2 = 0.033, p = 0.006). No significant correlation was found between Rasch‐calibrated NEI VFQ‐25 scores and cataract status (r 2 = 0.001, p = 0.609). We chose to include VFI in the multivariate model, because, compared to MD, it was better correlated with the person measures in univariate analysis. In multivariate regression analysis, only VA and VFI were significantly associated with Rasch‐calibrated NEI VFQ‐25 scores (Table 4). Better‐eye VA and better‐eye VFI accounted for nearly 40% (r 2 = 0.380) of the Rasch‐calibrated NEI VFQ‐25 scores.
Table 4.
Correlation coefficients from multivariate regression analysis with Rasch‐estimated person measures
| Reference | Adjusted r 2 | p‐value | |
|---|---|---|---|
| Model summary | 0.388 | <0.001 | |
| Age | Per year | 0.903 | |
| Gender | Male | 0.115 | |
| VFIa in eye with better VF | Per % | <0.001 | |
| VA in eye with better VA | Per 0.1 unit on Snellen scale | <0.001 |
VA = visual acuity, VF = visual field.
Mean deviation value was not included in the multivariate model, because the visual field index (VFI) showed higher correlation with Rasch person measures in univariate analysis.
Rasch‐calibrated NEI VFQ‐25 scores for patients with and those without visual impairment were 31 ± 15 and 69 ± 20, respectively (p < 0.001). Figure 1 shows the Rasch‐calibrated NEI VFQ‐25 scores for patients with reduced vision or blindness in one or both eyes. In Bonferroni tests, differences in VRQOL between directly adjacent visual impairment groups did not reach statistical significance (all p > 0.05), but the difference between patients with one blind eye and those with reduced vision in the better eye did reach borderline significance (p = 0.076).
Figure 1.
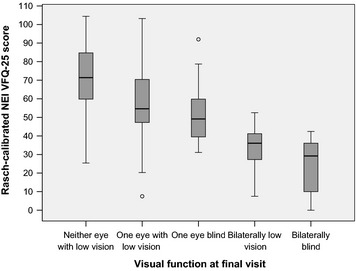
Rasch‐calibrated NEI VFQ‐25 scores in relation to visual function at final visit. Patients with low vision or blindness in one eye had significantly lower NEI VFQ‐25 scores compared to patients without impaired vision in either eye. NEI VFQ‐25, National Eye Institute Visual Function Questionnaire 25.
Rasch‐calibrated NEI VFQ‐25 scores included maximum values (i.e. good VRQOL) for patients with a VFI of ≥50% (or MD ≥ −18 dB) in the better eye, but the maximum Rasch‐calibrated NEI VFQ‐25 scores never exceeded 70 in patients with a VFI < 50% (or MD < −18 dB) in the better eye (Fig. 2). Scores differed significantly between patients with better‐eye VFI values of <50% compared to those with VFI ≥ 50% (mean scores 39 ± 21 versus 68 ± 21, p < 0.001), or with better‐eye MD values of <−18 dB compared to those with ≥−18 dB (mean scores 39 ± 21 versus 68 ± 21, p < 0.001).
Figure 2.
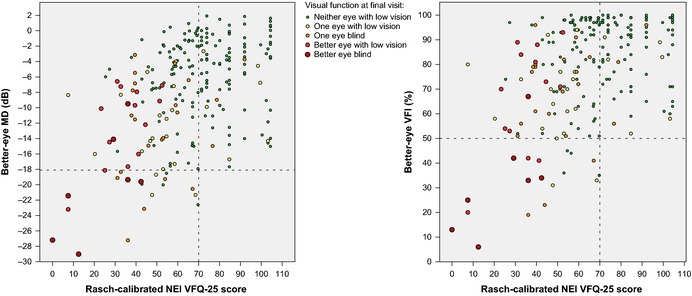
Correlation of Rasch‐calibrated NEI VFQ‐25 scores with MD and VFI. Data are shown as better‐eye MD in dB (left) and better‐eye VFI in % (right) divided by visual impairment level according to the World Health Organization criteria. The VRQOL scores for patients with a better‐eye VFI of <50% (or MD < −18 dB) never exceeded 70. dB, decibel; MD, mean deviation; NEI VFQ‐25, National Eye Institute Visual Function Questionnaire 25; VFI, visual field index; VRQOL, vision‐related quality of life.
Discussion
The current study is the first investigation to analyse VRQOL in patients with glaucoma who had been followed an average of 15 years after diagnosis, which represents a period that is longer than the average number of years that patients with glaucoma live following diagnosis (Quigley & Vitale 1997; Broman et al. 2008; Peters et al. 2013). At the last follow‐up visit, 9% of the patients were visually impaired, and these subjects rated their VRQOL significantly lower than those with maintained visual function. Significantly worse VRQOL was also reported by patients with impaired vision in one eye compared to those with no impairment in either eye. However, the relationship between VF loss and VRQOL was weak. Correlation with VRQOL was marginally higher for better‐eye VFI than for better‐eye MD, but was stronger for better‐eye VA than for VF status. In multivariate analysis, better‐eye VA and better‐eye VFI together explained nearly 40% of the Rasch‐calibrated NEI VFQ‐25 scores. Interestingly, age was only weakly correlated with VRQOL, and the effect of age was no longer significant in multivariate analysis. Cataract in the better eye had no impact on VRQOL. Our results indicate that a better‐eye VFI of 50% or a better‐eye MD of 18 dB is an important threshold for VRQOL in patients with glaucoma.
The strengths of this study include its prospective design, which ensured very little missing data. In addition, the long follow‐up time gave information on patients with the entire range of glaucoma severity, even though the patients initially enrolled in the EMGT had relatively early disease. Another advantage of our analysis is that we used a Rasch model to analyse the results of the NEI VFQ‐25 in order to limit the shortcomings of the original published scoring system.
Our investigation also had some limitations. The study population included only prospectively followed patients, and hence, it was not possible to determine if and to what extent patient satisfaction with the care setting might have impacted the results. The NEI VFQ‐25 was never administered to 22 (8.6%) of the 255 patients enrolled into the EMGT. However, we believe it is unlikely that VRQOL would have been rated differently by this subgroup compared to the patients that were included in our analysis, because there were no statistically significant differences in baseline characteristics between these subgroups (Hyman et al. 2005).
Similar to previously published results, we found a significant, albeit moderate, relationship between VF loss in the better eye and VRQOL (Gutierrez et al. 1997; Parrish et al. 1997; Mills et al. 2001; Sumi et al. 2003; Hyman et al. 2005; McKean‐Cowdin et al. 2008; van Gestel et al. 2010). Our finding that correlation with the NEI VFQ‐25 scores was stronger for VFI than for MD agrees with results reported by Sawada et al. (2011). The association with VRQOL was weaker for VF loss in the worse eye than for VF loss in the better eye (results not shown), which also concurs with other studies (van Gestel et al. 2010; Sawada et al. 2011; Okamoto et al. 2014). Better‐eye VA showed the strongest correlation with VRQOL, and better‐eye VA and better‐eye VFI together explained nearly 40% of the Rasch‐calibrated NEI VFQ‐25 scores. Nevertheless, there was still 60% unexplained variation in the outcomes, indicating that there are additional factors that influence the perception of VRQOL in patients with glaucoma. One such factor might be that the various locations or types of VF defects have different effects on VRQOL (van Gestel et al. 2010; Tabrett & Latham 2012; Murata et al. 2013). The rate of progression of the disease could be another factor influencing VRQOL (Lisboa et al. 2013; Medeiros et al. 2015), allowing patients with slower progression to develop compensatory mechanisms that enable them to cope better with visual function losses.
Some authors advocate using binocular VF results to evaluate VRQOL, because monocular measures might overestimate VF loss (Asaoka et al. 2011), and patients with overlapping VF loss might experience more difficulties in everyday life (Coleman 2007). However, the results of other cross‐sectional studies indicate that evaluating the VF of the better eye provides information similar to data obtained by binocular assessment (Kulkarni et al. 2012; Saunders et al. 2012) and that the association between VRQOL and better‐eye MD is similar to the association between VRQOL and binocular VF (McKean‐Cowdin et al. 2008; Ramulu 2009; van Gestel et al. 2010; Arora et al. 2013; Okamoto et al. 2014). In the Collaborative Initial Glaucoma Treatment Study, Musch et al. (2006) found that patients with glaucoma had better VRQOL after cataract surgery. In our sample, cataract in the better eye was not correlated with VRQOL. However, we did not evaluate differences in VRQOL before and after surgery.
Our results confirm the earlier observation that the NEI VFQ‐25 is a multidimensional instrument measuring two aspects of visual ability (Massof & Fletcher 2001; Marella et al. 2010; Pesudovs et al. 2010). The first scale, which has been designated the visual functioning scale by Marella et al. (2010), contains all items from the near vision, distance vision, colour vision, peripheral vision and social functioning subscales. The second scale has been called a socio‐emotional scale (Marella et al. 2010), and it includes the mental health, dependency and role difficulty subscales of the NEI VFQ‐25. The reliability of the socio‐emotional subscale was not acceptable in our study, and hence, we chose not to include data from this subscale in our analyses. There are two explanations for the unsatisfactory reliability: first, we found distorted response category thresholds in the rating scale associated with most of the socio‐emotional items; second, we had to eliminate four items from the socio‐emotional subscale due to misfit to the model. Both these aspects decreased the possibility to distinguish different ability groups by their responses to the socio‐emotional subscale.
In our sample, patients’ ability was not optimally matched to the item difficulty of the NEI VFQ‐25. This might be explained by the relatively high number of EMGT patients that still had good visual function at the last follow‐up visit, even if the cohort included patients with a wide range of glaucoma severity stages. Similar targeting problems have previously been observed in an evaluation of VRQOL in a cataract population (Pesudovs et al. 2010). Other studies have reported much better targeting when the NEI VFQ‐25 was administered to low‐vision populations (Massof & Fletcher 2001; Dougherty & Bullimore 2010; Marella et al. 2010).
Varma et al. (2006) demonstrated lower VRQOL in Los Angeles Latino Eye Study participants with visual impairment (defined by VA alone), and Gutierrez et al. (1997) found a significant relationship between Advanced Glaucoma Intervention Study scores and NEI VFQ‐25 outcomes. In our investigation, Rasch‐calibrated NEI VFQ‐25 scores were significantly lower for visually impaired glaucoma patients than for those with better visual function. Poor visual function in one eye only also substantially reduced the reported VRQOL, and, not surprisingly, the negative impact on VRQOL was greater when both eyes were impaired.
Many patients with VF loss of <50% (e.g. VFI 50% or MD −18 dB) in the better eye rated their VRQOL at a level similar to that reported by patients with no VF loss in the better eye. However, in patients with VF loss of >50% in the better eye, the Rasch‐calibrated NEI VFQ‐25 score never exceeded 70 (Fig. 2). Also, the patients with VF loss of >50% had significantly lower VRQOL that was comparable to that noted in patients with low vision in the better eye.
In conclusion, we found a statistically significant, although modest, correlation between VRQOL measured by the NEI VFQ‐25 and better‐eye VF loss. Our results support the arbitrary, but widely used, limit of a better‐eye VF loss of <50% as an important threshold for severe functional impairment.
This study was supported by the Swedish Research Council, grant K2011‐63X‐10426‐19‐3, and grants U10EY10260 and U10EY10261 from the National Eye Institute, Bethesda, Maryland; the Herman Järnhardt Foundation; the Foundation for Visually Impaired in Former Malmöhus County; and Skåne County Council's Research and Development Foundation. The sponsor or funding organization had no role in the design or conduct of this research.
References
- Andrich D (1978): A rating scale formulation for ordered response categories. Psychometrika 43: 561–573. [Google Scholar]
- Arora KS, Boland MV, Friedman DS, Jefferys JL, West SK & Ramulu PY (2013): The relationship between better‐eye and integrated visual field mean deviation and visual disability. Ophthalmology 120: 2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka R, Crabb DP, Yamashita T, Russell RA, Wang YX & Garway‐Heath DF (2011): Patients have two eyes!: binocular versus better eye visual field indices. Invest Ophthalmol Vis Sci 52: 7007–7011. [DOI] [PubMed] [Google Scholar]
- Broman AT, Quigley HA, West SK et al. (2008): Estimating the rate of progressive visual field damage in those with open‐angle glaucoma, from cross‐sectional data. Invest Ophthalmol Vis Sci 49: 66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AL (2007): Sources of binocular suprathreshold visual field loss in a cohort of older women being followed for risk of falls (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 105: 312–329. [PMC free article] [PubMed] [Google Scholar]
- Dougherty BE & Bullimore MA (2010): Comparison of scoring approaches for the NEI VFQ‐25 in low vision. Optom Vis Sci 87: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gestel A, Webers CAB, Beckers HJM, van Dongen M, Severens JL, Hendrikse F & Schouten J (2010): The relationship between visual field loss in glaucoma and health‐related quality‐of‐life. Eye 24: 1759–1769. [DOI] [PubMed] [Google Scholar]
- Gutierrez P, Wilson MR, Johnson C et al. (1997): Influence of glaucomatous visual field loss on health‐related quality of life. Arch Ophthalmol 115: 777–784. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L & Hussein M (2002): Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- Hyman LG, Komaroff E, Heijl A, Bengtsson B & Leske MC (2005): Treatment and vision‐related quality of life in the early manifest glaucoma trial. Ophthalmology 112: 1505–1513. [DOI] [PubMed] [Google Scholar]
- Kulkarni KM, Mayer JR, Lorenzana LL, Myers JS & Spaeth GL (2012): Visual field staging systems in glaucoma and the activities of daily living. Am J Ophthalmol 154: 445–451 e443. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L & Bengtsson B (1999): Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 106: 2144–2153. [DOI] [PubMed] [Google Scholar]
- Lisboa R, Chun YS, Zangwill LM, Weinreb RN, Rosen PN, Liebmann JM, Girkin CA & Medeiros FA (2013): Association between rates of binocular visual field loss and vision‐related quality of life in patients with glaucoma. JAMA Ophthalmol 131: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S & Hays RD (2001): Development of the 25‐item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol 119: 1050–1058. [DOI] [PubMed] [Google Scholar]
- Marella M, Pesudovs K, Keeffe JE, O'Connor PM, Rees G & Lamoureux EL (2010): The psychometric validity of the NEI VFQ‐25 for use in a low‐vision population. Invest Ophthalmol Vis Sci 51: 2878–2884. [DOI] [PubMed] [Google Scholar]
- Massof RW (2002): The measurement of vision disability. Optom Vis Sci 79: 516–552. [DOI] [PubMed] [Google Scholar]
- Massof RW (2007): An interval‐scaled scoring algorithm for visual function questionnaires. Optom Vis Sci 84: 689–704. [DOI] [PubMed] [Google Scholar]
- Massof RW (2011): Understanding Rasch and item response theory models: applications to the estimation and validation of interval latent trait measures from responses to rating scale questionnaires. Ophthalmic Epidemiol 18: 1–19. [DOI] [PubMed] [Google Scholar]
- Massof RW & Fletcher DC (2001): Evaluation of the NEI visual functioning questionnaire as an interval measure of visual ability in low vision. Vision Res 41: 397–413. [DOI] [PubMed] [Google Scholar]
- McKean‐Cowdin R, Wang Y, Wu J, Azen SP, Varma R & Los Angeles Latino Eye Study Group (2008): Impact of visual field loss on health‐related quality of life in glaucoma – The Los Angeles Latino Eye Study. Ophthalmology 115: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros FA, Gracitelli CP, Boer ER, Weinreb RN, Zangwill LM & Rosen PN (2015): Longitudinal changes in quality of life and rates of progressive visual field loss in glaucoma patients. Ophthalmology 122: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RP, Janz NK, Wren PA, Guire KE & Cigts Study Grp (2001): Correlation of visual field with quality‐of‐life measures at diagnosis in the Collaborative Initial Glaucoma Treatment Study (CIGTS). J Glaucoma 10: 192–198. [DOI] [PubMed] [Google Scholar]
- Murata H, Hirasawa H, Aoyama Y, Sugisaki K, Araie M, Mayama C, Aihara M & Asaoka R (2013): Identifying areas of the visual field important for quality of life in patients with glaucoma. PLoS ONE 8: e58695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch DC, Gillespie BW, Niziol LM, Janz NK, Wren PA, Rockwood EJ, Lichter PR & Collaborative Initial Glaucoma Treatment Study G (2006): Cataract extraction in the collaborative initial glaucoma treatment study: incidence, risk factors, and the effect of cataract progression and extraction on clinical and quality‐of‐life outcomes. Arch Ophthalmol 124: 1694–1700. [DOI] [PubMed] [Google Scholar]
- Nassiri N, Mehravaran S, Nouri‐Mahdavi K & Coleman AL (2013): National Eye Institute Visual Function Questionnaire: usefulness in glaucoma. Optom Vis Sci 90: 745–753. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Sugisaki K, Murata H, Hirasawa H, Mayama C & Asaoka R (2014): Impact of better and worse eye damage on quality of life in advanced glaucoma. Sci Rep 4: 4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish RK, Gedde SJ, Scott IU, Feuer WJ, Schiffman JC, Mangione CM & MontenegroPiniella A (1997): Visual function and quality of life among patients with glaucoma. Arch Ophthalmol 115: 1447–1455. [DOI] [PubMed] [Google Scholar]
- Pesudovs K, Burr JM, Harley C & Elliott DB (2007): The development, assessment, and selection of questionnaires. Optom Vis Sci 84: 663–674. [DOI] [PubMed] [Google Scholar]
- Pesudovs K, Gothwal VK, Wright T & Lamoureux EL (2010): Remediating serious flaws in the National Eye Institute Visual Function Questionnaire. J Cataract Refract Surg 36: 718–732. [DOI] [PubMed] [Google Scholar]
- Peters D, Bengtsson B & Heijl A (2013): Lifetime risk of blindness in open‐angle glaucoma. Am J Ophthalmol 156: 724–730. [DOI] [PubMed] [Google Scholar]
- Quigley HA & Vitale S (1997): Models of open‐angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci 38: 83–91. [PubMed] [Google Scholar]
- Ramulu P (2009): Glaucoma and disability: which tasks are affected, and at what stage of disease? Curr Opin Ophthalmol 20: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LJ, Russell RA & Crabb DP (2012): Practical landmarks for visual field disability in glaucoma. Br J Ophthalmol 96: 1185–1189. [DOI] [PubMed] [Google Scholar]
- Sawada H, Fukuchi T & Abe H (2011): Evaluation of the relationship between quality of vision and the visual function index in Japanese glaucoma patients. Graefes Arch Clin Exp Ophthalmol 249: 1721–1727. [DOI] [PubMed] [Google Scholar]
- Smith EV Jr (2002): Detecting and evaluating the impact of multidimensionality using item fit statistics and principal component analysis of residuals. J Appl Meas 3: 205–231. [PubMed] [Google Scholar]
- Sumi I, Shirato S, Matsumoto S & Araie M (2003): The relationship between visual disability and visual field in patients with glaucoma. Ophthalmology 110: 332–339. [DOI] [PubMed] [Google Scholar]
- Tabrett DR & Latham K (2012): Important areas of the central binocular visual field for daily functioning in the visually impaired. Ophthalmic Physiol Opt 32: 156–163. [DOI] [PubMed] [Google Scholar]
- Varma R, Wu J, Chong K, Azen SP & Hays RD (2006): Impact of severity and bilaterality of visual impairment on health‐related quality of life. Ophthalmology 113: 1846–1853. [DOI] [PubMed] [Google Scholar]
- Wright B & Linacre J (1994): Reasonable mean‐square fit values. Rasch Meas Trans 8: 370 Available at: http://www.rasch.org/rmt/rmt83b.htm (accessed 8 May 2015). [Google Scholar]


