Abstract
Local field potentials (LFPs) and spikes (SPKs) sampled at the thalamocortical recipient layers represent the inputs from the thalamus and outputs to other layers. Previous studies have shown that SPK‐constructed receptive fields (RFSPK) of cortical neurons are much smaller than LFP‐constructed RFs (RFLFP). The difference in cortical RFLFP and RFSPK is therefore a plausible indication of local networking. The presence of a boarder RFLFP appears due to contamination, to some degree, from remote sites. Our studies of the mouse primary auditory cortex show that the best frequencies and minimum thresholds of RFSPK and RFLFP were similar. We also observed that the RFLFP area was only slightly larger than the RFSPK area, a very different finding from previous reports. The bandwidth of RFLFP was slightly broader than that of RFSPK at all levels. These data do not support the explanation that bioelectrical signals from distant sites impact on cortical LFP through volume conduction. That the cortical LFP represents a local event is further supported by comparisons of RFSPK and RFLFP after cortical inhibition by muscimol and cortical disinhibition by bicuculine. We conclude that the difference between RFSPK (output of cortical neurons) and RFLFP (input of cortical neurons) results from intracortical processing, including cortical lateral inhibition and excitation.
Keywords: auditory cortex, local field potential, mouse, neuronal receptive field, spike, systems neuroscience
Introduction
Neurons in deep layer III and layer IV of the primary sensory cortex are primarily driven by thalamocortical inputs (Kyriazi & Simons, 1993; Miller et al., 2001; Hirsch, 2003; Metherate et al., 2005; Barkat et al., 2011; Hackett et al., 2011). The output of cortical neurons is not simply the summation of thalamocortical inputs; it is shaped by the networking of cortical local circuitry (Eggermont, 1996; Noreña & Eggermont, 2002; Kenet et al., 2003; Fiser et al., 2004; Kaur et al., 2004; Metherate et al., 2004; Kurt et al., 2007; Berens et al., 2008; Rasch et al., 2009; Liu & Galindo‐Leon, 2010; Happel et al., 2010; Moeller et al., 2010; Eggermont et al., 2011). Acquiring new insights into the workings of local cortical networks continues to challenge neuroscientists.
Auditory information processing at the recipient layers of the sensory cortex has been studied by comparing cortical local field potentials (LFPs) that represent the inputs of cortical neurons and the spike activities (SPKs) that represent the outputs of cortical neurons (Lorente de No, 1947; Eccles, 1951; Eggermont, 1996; Chrobak & Buzsáki, 1998; Buzsáki & Draguhn, 2004; Metherate et al., 2004; Lakatos et al., 2005; Mormann et al., 2005; Canolty et al., 2006; Einevoll et al., 2007; Katzner et al., 2009; Kelly et al., 2010; Denker et al., 2010, 2011; Eggermont et al., 2011). In animals such as monkeys and cats, the LFP‐constructed receptive field (RFLFP) and the SPK‐constructed receptive field (RFSPK) of auditory cortical neurons are mostly similar in their best frequencies (BFs) but show large differences in their spectral ranges. The bandwidth of RFLFP can be much greater than that of RFSPK, particularly at high sound levels (Eggermont, 1996, 1998; Noreña & Eggermont, 2002; Kaur et al., 2004; Machens et al., 2004; Metherate et al., 2004; Noreña et al., 2008; Kajikawa & Schroeder, 2011; Gaucher et al., 2012). The sharper RFSPK, i.e. the outputs of cortical neurons, signifies the manipulation or processing of auditory information in cortical circuitry. Although cortical processing is complex, cortical inhibition mediated by γ‐aminobutyric acid receptor A (GABAAR) is believed to significantly influence the outputs of cortical neurons (Muller & Scheich, 1988; Hirsch et al., 1998; Wang et al., 2000, 2002; Foeller et al., 2001; Noreña & Eggermont, 2002; Wehr & Zador, 2005; Eggermont et al., 2011; de Cheveigné et al., 2013). A long‐standing and critical issue is whether the broader RFLFP can be partly attributed to signals from remote sites due to volume conduction (Kajikawa & Schroeder, 2011).
We addressed this issue by examining the RFLFP and RFSPK of the mouse primary auditory cortex. We found that the RFLFP was only slightly broader than RFSPK and both RFSPK and RFLFP were only slightly broadened by microiontophoresis of the GABAAR antagonist bicuculine. Using paired recordings with ~100 μm separation, we discovered that microiontophoresis of the GABAAR agonist muscimol resulted in the complete elimination of activity in one site but not the other.
Materials and methods
Thirty‐seven female C57 mice 4–5 weeks old and weighing 15.5–21 g were used in our study. All procedures were in accordance with the Canadian Council on Animal Care and approved by the Health Science Animal Care Committee at the University of Calgary (Protocol number M10029).
Animal preparation
All experiments were performed under anesthesia. Before surgery, the mouse was anesthetized using an intraperitoneal (i.p.) injection of ketamine and xylazine; the initial dose was 85 and 15 mg/kg, respectively. The anesthetic level was examined about every 40 min by stimulation of the mouse tail. If the mouse showed any response to tail stimulation, an additional dose of ketamine (17 mg/kg, i.p.) and xylazine (3 mg/kg, i.p.) was administered. The mouse was mounted on a custom‐made head holder that was placed in a soundproof chamber. The mouse head was immobilized by clamping the palate and nasal bone. After clipping the scalp hair with scissors, the skull was exposed by making a midline incision and removing the connective tissue and muscle. The position of the mouse head was adjusted to align the bregma and lambda at one horizontal level. A dental drill was used to create an opening of ~3 mm on the skull and the left primary auditory cortex was exposed. Throughout the surgery and experiments, the mouse body temperature was maintained at 37 °C using a feedback‐controlled heating pad.
Acoustic stimulation
Pure tone bursts of 20 ms duration and 5 ms rising/decay time were used for acoustic stimulation. Digital sinusoid wave bursts were generated and converted to analog wave bursts by using a real‐time processor (RP2; Tucker‐Davis Tech. Inc., Gainesville, FL, USA). The analog signal was sent to a condenser loud speaker via a digitally controlled attenuator (PA5; Tucker‐Davis) and a power amplifier. A flat frequency–response curve was sustained by using output signals from the RP2 that ranged from 9.2 to 20 V. The frequency and amplitude of tone bursts were either manually or automatically varied using Brainware software (Tucker‐Davis). The condenser loud speaker was positioned 35 cm from and 45° right of the mouse right ear. The speaker output was calibrated using a Larson–Davis condenser microphone (Model 2520) and a microphone preamplifier (Model 2200C) and the tone intensity (at mouse right ear) was expressed as decibels sound pressure level (dB SPL, re. 20 μPa). Tone bursts were delivered to the mouse at a rate of 1 Hz when cortical responses to identical tone bursts were examined or at a rate of 4 Hz when cortical responses to a series of tone bursts with various frequencies and amplitudes were examined. For RF construction, a frequency–amplitude (FA) scan was used. For each FA scan, tone bursts were randomly varied from 3 to 40 kHz in frequency and from 0 to −100 dB in amplitude. A total of 798 FA blocks were used. An identical tone burst was presented ten times per FA block.
Recording of SPKs and LFPs in the left primary auditory cortex
A tungsten electrode or a multibarrel glass electrode with a carbon‐fiber in the central tubing was used for recording neural activities in the primary auditory cortex. The impedance of the recording electrodes was approximately 1–2 MΩ. The multibarrel electrode was also used for drug microiontophoresis to the recording site (i.e. recorded neurons) as described below. The recording electrode was connected to a 16‐channel preamplifier (PA16) and amplifier (RA16; both from Tucker‐Davis). Bioelectrical signals were digitized in PA16 and then transferred to RA16 using an optical fiber. In RA16, the signals were fed to two recording channels; one was amplified 10 000 times and filtered with a bandpass of 0.3–10 kHz for SPK recording and the other was amplified 1000 times and filtered with a bandpass of 1–200 Hz for LFP recording. The electrode was oriented perpendicularly to the auditory cortical surface for penetration. We typically made 5–8 electrode penetrations in each animal to confirm the placement of the electrode within the primary auditory cortex. Optimal neuronal responses to tone stimulation were usually recorded at a depth of about 300–600 μm below the brain surface (i.e. at layers III–IV). The original tracings from two recording channels were saved in DAM files using BrainWare data acquisition software (Tucker‐Davis) for offline data processing.
Microniontophoretic injection of saline, muscimol and BMI
The multibarrel glass electrode with a tip diameter of 15–20 μm consisted of six glass tubes. Five glass barrels surrounded a central tube that contained a carbon‐fiber connected to the PA16 preamplifer of the recording system (see above). Three of the surrounding barrels were filled with isotonic saline solution (0.9% NaCl, pH 7.0), one for grounding, one for balancing and one for saline injection. The remaining barrels were filled with either muscimol (a GABAAR agonist, 9 mm, pH 6.47) or bicuculline methiodiode (BMI, a GABAAR antagonist, 10 mm, pH 3.0). The barrels were connected to the Neuro Phore System (BH‐2; Harvard Apparatus, Inc., Holliston, MA, USA) for microiontophoretic injection of drugs. The injection current was +50 nA for muscimol, +50 nA for BMI and +50 nA for saline. A retention current of −30 nA for muscimol and −25 nA for BMI was applied to each drug‐filled barrel during non‐injection periods. Drug injections began after control data (RFs) were sampled and continued until RF data under drug impact were completed.
Data processing and statistical analysis
The original bioelectrical tracings (SPKs and LFPs) from the saved DAM files were analysed and processed by our custom‐made software (SoundCode). Neuronal responses to the tone bursts and RFs obtained from the SPK and LFP data were compared.
SPK was defined as the action potential in which the amplitude was larger than a voltage‐trigger level that was 1.2 times larger than the largest noise amplitude. We set this level to make it comparable with our LFP data (see below). We compared the RFSPK using trigger voltage levels 1.2 and 2 times higher than the noise level. Excluding the clearly lower SPK numbers, the RF areas and bandwidths at the 1.2 times trigger level were similar to those at the 2 times trigger level. Our calculation utilized 3 times the standard deviation of the noise amplitude. Due to the stable noise level and large sample size, our calculated trigger level was lower than 1.2 times. Additionally, we examined the action potentials and field excitatory postsynaptic potentials (fEPSPs) of single cortical neurons using loose patch clamp recordings (impedance of the electrode tip was 15 MΩ). The RFs constructed on these action potentials and fEPSPs were mostly identical (data not shown). SPKs were sorted or isolated using a computer‐based spike‐sorting technique, i.e. using nine parameters of the SPK waveform: peak, peak time, peak duration, valley, valley time, valley duration, peak–valley amplitude, peak raising slope and peak–valley slope (Suga et al., 1997; Yan & Ehret, 2002; Yan & Zhang, 2005; Luo et al., 2008). Rasters and peri‐stimulus time histograms (PSTs) or PST cumulative histograms (PSTCs) were plotted to display the responses of cortical neurons to tone stimuli. The binwidth of PSTs and PSTCs was 1 ms. The auditory response magnitude of cortical neurons was the SPK number, i.e. the sum of all action potentials of cortical neurons in response to ten identical tone stimuli within a time frame of 10–60 ms from the onset of the tone bursts. The response latency was the time interval from the tone onset to the intersection point of the baseline to the rising slope of the PSTC.
LFPs were averaged waveforms based on ten tracings in response to ten identical tone stimuli. Cortical LFPs typically consisted of a negative‐going wave followed by a positive‐going wave. In this study, LFP amplitude and latency were measured based on the negative‐going wave as the negative‐going wave largely represents the assembly of thalamocortical fEPSPs. LFP amplitude was the microvolt difference from the baseline to the peak of the negative‐going wave within a time frame of 10–60 ms. LFP latency was the time interval from the tone onset to the intersection point of the baseline and slope of the negative‐going wave.
RFs were visualized by plotting SPKs (raster, PSTs or PSTCs) and LFPs (averaged tracings) as a function of the tone frequencies and amplitudes (FA scan). RF area was defined by the response threshold curve of cortical neurons to single tone frequencies as described below.
For SPK data, the criteria for determining a threshold to a given frequency were the dB SPLs for which cortical neurons showed at least one spike more than the spike number without tone presentation. In addition, the spike numbers to both 5‐ and 10‐dB levels below threshold needed to be lower than or equal to that without tone presentation (Suga et al., 1997; Yan & Ehret, 2002; Yan & Zhang, 2005; Luo et al., 2008). Based on these criteria, the thresholds to all frequencies were digitally analysed. The curve connecting the thresholds to all frequencies represented the frequency‐threshold tuning curve, i.e. RFSPK.
For LFP data, the criteria for determining a threshold to a given frequency were the dB SPLs to which LFP amplitude was larger than 1.2 times the voltage fluctuation without tone presentation. In addition, the LFP amplitudes to both 5‐ and 10‐dB levels below threshold needed to be lower than or equal to 1.2 times the voltage fluctuation without tone presentation. The curve connecting the thresholds to all frequencies represented the frequency‐threshold tuning curve, i.e. RFLFP.
The RF was quantified with multiple parameters: BF, minimum threshold (MT), spike number (SPK)/amplitude (LFP) and latency at 10–20 dB above the MT, bandwidths (octaves) at 10, 30, 50 and 70 dB above the MT (BW10, BW30, BW50 and BW70) and RF areas below 30 and 50 dB SPL (RF30 and RF50). The BF was the frequency (kHz) to which cortical neurons showed the lowest response threshold. The MT was the tone amplitude (dB SPL) to which cortical neurons showed the lowest response threshold across all frequencies. The BWs were the difference in octaves between the high‐ and low‐frequency boundaries of RF measured at 10, 30, 50 and 70 dB above the MT. RF30 and RF50 were the number of frequency‐amplitude blocks within RF below 30 and 50 dB SPL.
RFSPK and RFLFP were compared based on these RF parameters. All data were expressed as mean ± SD. A Mann–Whitney U test was used to examine the significance of latency data differences. A paired t‐test was used to examine the significance of other data differences between RFSPK and RFLFP and the changes in RFSPK and RFLFP before and after drug application. A P value of less than 0.05 was considered statistically significant.
Results
In total, multi‐unit responses at 51 auditory cortex recording sites were sampled from 37 mice, and the RFSPK and RFLFP were compared. Of this group, multi‐unit responses at 15 auditory cortex recording sites were recorded using the multibarrel electrode, seven for examining the muscimol (GABAAR agonist) effects and eight for examining the BMI (GABAAR antagonist) effects on the RFSPK and RFLFP of cortical neurons.
Examples of RFSPK and RFLFP recorded with the same electrode
Figure 1 shows an example of RFSPK and RFLFP sampled simultaneously using the same tungsten electrode. Tone‐evoked spikes, as denoted by red dots that align because of relatively steady response latencies, exhibit a clear response area (i.e. RFSPK). Obvious spontaneous spikes and spike bursts appeared outside of the RFSPK area and their occurrences varied randomly in time. Tone‐evoked LFPs (negative blue waveforms) superimposed on spikes are clear in all frequency–amplitude blocks within the RFSPK area. At the boundary of the RFSPK area, some blocks show negative‐going waves with rare tone‐evoked spikes. Outside the RFSPK area, it is clear that spontaneous spikes and/or spike bursts were not accompanied by LFPs. The BFs based on both RFSPK and RFLFP were 17 kHz. The MT of RFSPK was 30 dB SPL and that of RFLFP was 25 dB SPL, 5 dB lower than RFSPK MT.
Figure 1.
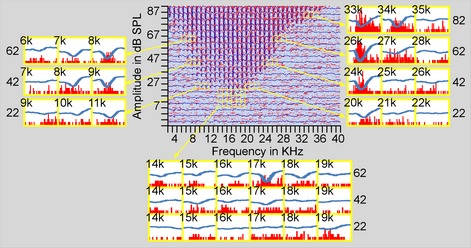
Examples of the responses of auditory cortex neurons to tones of various frequencies and amplitudes. The central panel shows the raster of SPKs and averaged tracings of LFPs that are superimposed. The superimposed PSTs and LFTs from the MT boundaries are shown in the lower panel while those around 10, 30, 50 and 70 dB above MT are shown in the left and right panels.
The RF boundary region of this sample is further illustrated by the PSTs that overlapped with LFPs around the MT, both sides of the RF at 10, 30 and 50 dB above the MT, and the high‐frequency side at 70 dB above the MT. Around the MT area (Fig. 1, lower panel), tone‐evoked spikes are visualized at the block 17 kHz/30 dB SPL (17/30 block) but not at the 17/25 and 17/20 blocks. Tone‐evoked LFPs can be identified at the 17/30 and 17/25 blocks. At blocks 14/30, 15/30, 16/30, 18/30, 15/25, 16/25 and 19/20, spikes that were spontaneous or evoked by tone stimuli cannot be visually confirmed. Based on our criteria, these blocks were not included in the RFSPK area. At these blocks, however, tone‐evoked LFPs are identifiable. The remaining blocks in the lower panel show neither tone‐evoked spikes nor LFPs. At the low‐frequency side of RFSPK area (Fig. 1, left panel), tone‐evoked spikes and LFPs appear at blocks 7/75, 6/75, 8/55 and 7/55. Tone‐evoked LFPs also appear at blocks 5/75, 11/35 and 10/35 in which tone‐evoked spikes are unclear. At the high‐frequency side (Fig. 1, right panel), LFPs appear only at the blocks in which tone‐evoked spikes are clear.
These examples demonstrate a similarity in the size of RFSPK and RFLFP. The RFLFP was slightly larger than the RFSPK, particularly at lower tone levels.
Similar BFs and MTs of RFSPK and RFLFP
The RFSPK BFs of our samples ranged from 10 to 25 kHz (17.31 ± 3.43 kHz), which was not significantly different (n = 51, P > 0.05) from the RFLFP BFs that ranged from 9 to 24 kHz (17.02 ± 3.36 kHz). The BFs measured based on RFSPK and RFLFP were highly correlated (slope = 0.88, r = 0.81, P < 0.001, Fig. 2A1). The RFLFP BFs could be either higher or lower than the RFSPK BFs but the difference was < 5 kHz. Of 51 samples, 23 (45.09%) neurons had identical BFs, 19 (37.25%) had a 1‐kHz difference, five (9.8%) had a 2‐kHz difference and four (7.8%) had a > 2‐kHz difference between RFSPK and RFLFP (Fig. 2A2). The RFSPK MTs ranged from −0.76 to 32.1 dB SPL, 13.70 ± 6.67 dB SPL, which was significantly higher than the FRLFP MTs that ranged from −0.76 to 31.7 dB SPL, 11.88 ± 6.63 dB SPL (n = 51, P < 0.001). The MTs of RFSPK and RFLFP were also correlated (slope = 0.96, r = 0.88, P < 0.001, Fig. 2B1). The RFLFP MTs were either equal to (31 neurons, 60.78%) or ~5 dB lower than (20 neurons, 39.22%) the RFSPK MTs (Fig. 2B2). The BFs and MTs of the sampled neurons fell within the central range of C57 mouse hearing.
Figure 2.
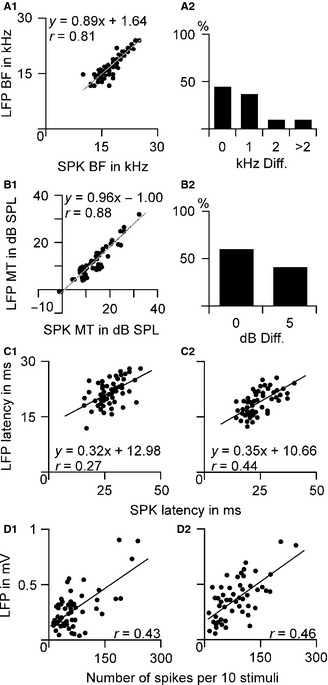
Comparisons of RFSPK and RFLFP. The BFs (A1) and MTs (B1) of RFSPK and RFLFP were highly correlated and their differences were small, a few kHz difference in BFs (A2) and <5 dB in MTs (B2). Their response latencies and magnitudes at 10 dB (C1 and D1) and 20 dB (C2 and D2) above the MT were also correlated.
Correlations of latency and magnitude of spikes and LFPs in response to tone stimuli
In most cases, response latencies measured from SPK data were longer than those from LFP data. On average, SPK latencies were 27.17 ± 5.78 ms at 10 dB above the MT and 23 .75 ± 6.32 ms at 20 dB above the MT. LFP latencies were 21.59 ± 3.55 ms at 10 dB above the MT and 18.29 ± 3.32 ms at 20 dB above the MT. SPK latencies were significantly longer than LFP latencies at both 10 dB (n = 51, P < 0.001) and 20 dB (n = 51, P < 0.001) above the MT. The average differences in latencies between SPK and LFP were 6.47 ± 4.03 ms at 10 dB and 5.46 ± 4.44 ms at 20 dB above the MT. As shown in Fig. 2C, the SPK and LFP latencies were linearly correlated to each other at both 10 dB (Fig. 2C1, r = 0.27, P = 0.055) and 20 dB (Fig. 2C2, r = 0.44, P < 0.01) above the MT.
The response magnitudes were expressed by SPK number and LFP amplitude in response to BF tones at 10 and 20 dB above the MT. SPK numbers were 70.71 ± 54.31 in response to tones at 10 dB above MT and 84.08 ± 51.64 at 20 dB above MT (n = 51). LFP amplitudes were 281.49 ± 194.53 μV in response to tones at 10 dB above MT and 364.84 ± 188.11 μV at 20 dB above MT (n = 51). SPK response magnitudes were linearly correlated to the LFP ones at both tone levels 10 dB (Fig. 2D1, r = 0.43, P < 0.01) and 20 dB (Fig. 2D2, r = 0.46, P < 0.001) above the MT.
Correlations of bandwidths and areas of RFSPK and RFLFP
The shapes of RFSPK and RFLFP were similar; RFLFP appeared slightly larger than RFSPK as shown in Fig. 1. We compared the frequency BWs of RFSPK and RFLFP at 10, 30, 50 and 70 dB above the MT. All BWs of RFSPK were narrower than those of RFLFP. The statistically significant narrowing was found at lower tone levels only (Fig. 3A, open and filled columns), i.e. BW10 (P < 0.001) and BW30 (P < 0.05). As many MTs of RFLFP were lower than those of RFSPK, we used RFSPK MT as a reference point to measure and compare the BWs of RFSPK and RFLFP. The RFLFP BWs measured at 10, 30, 50 and 70 dB above the RFSPK MTs were broader than those above RFLFP MTs (Fig. 3, filled and hatched columns). Compared with the RFSPK BWs, RFLFP BWs referred to RFSPK MTs were significantly broader at all four levels (Fig. 3, open and hatched columns), i.e. BW10 (P < 0.001), BW30 (P < 0.001), BW50 (P < 0.001) and BW70 (P < 0.05).
Figure 3.
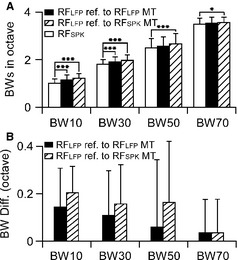
Comparisons of RFSPK and RFLFP BWs. The BW increased (A) and BW differences gradually decreased (B) in response to the increases in tone level. *P < 0.05, ***P < 0.001.
The RFLFP BWs were commonly several kilohertz wider than RFSPK BWs (Fig. 3B). At higher tone levels, RFLFP BWs (such as BW50 and BW70) were not different from RFSPK BWs in many neurons. When RFLFP BWs were referred to RFLFP MTs, the differences of RFLFP and RFSPK were 0.14 ± 0.17 octaves (oct) for BW10, 0.11 ± 0.19 oct for BW30, 0.06 ± 0.28 oct for BW50 and 0.03 ± 0.11 oct for BW70 (n = 51, Fig. 3B, filled column). When RFLFP BWs were referred to RFSPK MTs, the differences of RFLFP and RFSPK were 0.21 ± 0.12 oct for BW10, 0.16 ± 0.17 oct for BW30, 0.17 ± 0.25 oct for BW50 and 0.03 ± 0.11 oct for BW70 (n = 51, Fig. 3B, hatched column). The differences in BWs between RFSPK and RFLFP were negatively related to the tone level.
We further analysed the areas between RFSPK and RFLFP below 30 dB SPL (RF30) and 50 dB SPL (RF50). As expected based on the differences in MTs and BWs, the RF30 and RF50 of RFSPK were smaller than those of RFLFP. RF30 was 57.15 ± 21.53 kHz × 5 dB for RFSPK and 69.96 ± 24.64 kHz × 5 dB for RFLFP; these results were significantly different (n = 51, P < 0.001) and linearly correlated (Fig. 4A). The RF50 of RFSPK, 142.54 ± 34.05 kHz × 5 dB, was also significantly smaller than that of RFLFP, 162.37 ± 37.69 kHz × 5 dB (n = 51, P < 0.001), and also linearly correlated (Fig. 4B). On average, the RF30 of RFSPK was 17.25 ± 12.18% smaller than that of RFLFP and RF50 was 11.63 ± 6.23% smaller.
Figure 4.
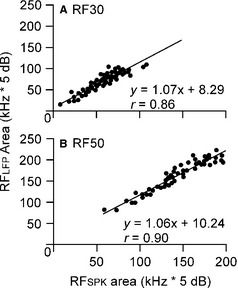
Comparisons of RFSPK and RFLFP areas below 30 dB SPL (A) and 50 dB SPL (B).
Effects of cortical inhibition at the muscimol microiontophoretic site and the remote site
We next examined the effects of microiontophoretic injection of muscimol on auditory responses of cortical neurons recorded by a multibarrel electrode (injection site) and a tungsten electrode placed about ~100 μm apart from each other (non‐injection site). Auditory responses were induced by a tone at the BF and 20 dB above the MT of the auditory cortex neurons recorded by the multibarrel electrode. A pair of examples is shown in Fig. 5; both auditory cortex sites recorded by the multibarrel and tungsten electrodes tuned to 16 kHz and had similar RFs. At 20 dB above the MT, the microiontophoresis of muscimol largely reduced the response magnitudes (both SPK and LFP) recorded by the multibarrel electrode but only slightly reduced those recorded by the tungsten electrode (Fig. 5A1–2 and B1–2). Neuronal responses returned 41 min after cessation of the muscimol application (Fig. 5A3 and B3). In total, seven pairs of samples were recorded in seven mice. Of seven samples recorded with the multibarrel electrodes, the auditory responses (SPK and LFP) of three samples were completely eliminated and those of four neurons were largely reduced as shown in Fig. 5A1. The auditory responses of seven samples recorded by the tungsten electrode (about 100 μm from the site of the muscimol application) were slightly reduced. The changes in SPK number and LFP amplitude were synchronous.
Figure 5.
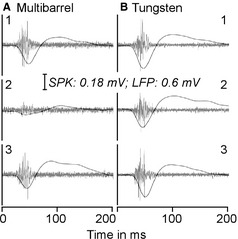
An example of the effect of muscimol microiontophoresis on the auditory responses of auditory cortex neurons recorded with a multibarrel electrode (A) and a tungsten electrode (B) separated by about 100 μm in placement. Neurons recorded by the two electrodes showed vigorous responses to tones at BF and 20 dB above the MT (A1 and B1). Muscimol application to the neurons in A drastically reduced the auditory responses of the neurons in A (A2) but only slightly reduced the auditory responses of the neurons in B (B2). The changes in both SPK and LFP were closely related (A1–2 and B1–2). The auditory responses recovered 26 min after the cessation of muscimol microiontophoresis are also shown (A3 and B3).
For the seven samples recorded by the multibarrel electrode, muscimol application reduced their SPK number from 81.43 ± 17.39 to 15.57 ± 15.50 (a 80.89% decrease, P < 0.001) and their LFP amplitude from 404.14 ± 180.86 to 54.43 ± 60.68 μV (an 81.60% decrease, P < 0.005). For the seven samples recorded by the tungsten electrode, muscimol application reduced their SPK number from 97.29 ± 61.58 to 68.29 ± 48.12 (a 34.85% decrease, P < 0.01) and their LFP amplitude from 429.43 ± 266.43 to 260.86 ± 153.02 μV (a 37.01% decrease, P < 0.05). In three samples recorded by the multibarrel electrode, their auditory responses were completely eliminated by muscimol. The extent of the decreased auditory responses in their counterparts recorded by the tungsten electrode was small, 40.39% for SPK and 37.40% for LFP. In terms of percentage changes, there were no differences between SPK numbers and LFP amplitudes. The percentage decreases in both SPK and LFP were significantly larger in samples recorded by the multibarrel electrode than in those recorded by the tungsten electrode (n = 7, P < 0.005 for SPK and P < 0.05 for LFP).
Effects of cortical BMI on cortical RFSPK and RFLFP
The microiontophoresis of BMI in the vicinity of the electrode tip disinhibited the recorded neurons. Sampled multi‐units showed obvious increases in response magnitudes including SPK number and LFP magnitude while slightly broadening their RF BWs. The changes induced by BMI application were very similar in RFSPK and RFLFP (Fig. 6). We obtained eight samples from eight mice. BMI microiontophoresis increased SPK number by 165.52 ± 129.20%, from 74.12 ± 28.25 to 182.25 ± 67.55 (P < 0.001) while broadening the overall BW (sum of BW10–50) by only 11.42 ± 10.35%, from 1.78 ± 0.67 to 2.03 ± 0.76 oct (P < 0.01). Similar results were seen with the LFP data. BMI microiontophoresis increased LFP magnitude by 160.36 ± 130.89%, from 372.25 ± 210.64 to 759.38 ± 190.27 μV (P < 0.001) while broadening the overall BW by only 10.09 ± 11.14%, from 1.89 ± 0.64 to 2.07 ± 0.71 oct (P < 0.01). No statistical differences were found in the percentage changes in both the response magnitudes and BWs between RFSPK and RFLFP (P > 0.05).
Figure 6.
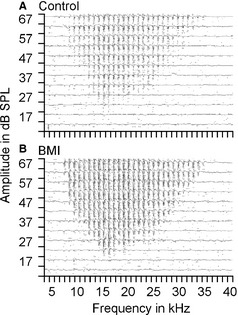
Examples of the responses of auditory cortex neurons to tones of various frequencies and amplitudes before (A) and after (B) microiontophoresis of BMI. The raster of SPK (dots) and superimposed averaged tracings of LFP (traces) are shown. BMI application slightly enlarged the excitatory response area but greatly increased the response magnitude.
Discussion
Extracellular field potentials in the brain are directly associated with proximate neural activities. The composition of the bioelectrical signals obtained through extracellular recording with a high‐impedance electrode originates from various neural processes (Cole, 1968; Freeman & Stone, 1969; Martin, 1976; Mitzdorf, 1985; Borg‐Graham et al., 1998; Noreña & Eggermont, 2002; Metherate et al., 2004; Gieselmann & Thiele, 2008; Kajikawa & Schroeder, 2011; de Cheveigné et al., 2013). Two important neural components, LFP and SPK, have been used for the investigation of neural functions at the systems level. LFP is a low‐frequency voltage fluctuation (less than ~300 Hz) that is believed to mostly represent the integrated EPSPs (Gustafsson, 1984; Mitzdorf, 1985; Buzsáki & Kandel, 1998; Schroeder et al., 1998; Galván et al., 2001; Logothetis, 2003; Nunez, 2006; Kurt et al., 2007; Berens et al., 2008; Eggermont et al., 2011; Lindén et al., 2011). Extracelluar signals sorted by different frequency filter settings help to differentiate the input (LFP) and output (SPK) of cortical neurons, providing a unique tool to probe the dynamics of cortical local circuitry (Lorente de No, 1947; Eccles, 1951; Eggermont, 1996; Chrobak & Buzsáki, 1998; Buzsáki & Draguhn, 2004; Metherate et al., 2004; Lakatos et al., 2005; Mormann et al., 2005; Canolty et al., 2006; Einevoll et al., 2007; Katzner et al., 2009; Denker et al., 2010, 2011; Kelly et al., 2010; Eggermont et al., 2011).
Anatomical investigations using the rabbit model have demonstrated that a single thalamocortical neuron is capable of innervating a large cortical area. In other words, a single cortical neuron may receive inputs from many thalamocortical neurons (De Venecia & McMullen, 1994). Theoretically, the RFs or spectral ranges of cortical neurons should be much broader than those of thalamocortical neurons. It is understood that the spectral range measured on neuronal spikes in the cat auditory cortex is not dramatically broader than that in the auditory thalamus (Calford et al., 1983; Miller et al., 2001, 2002). The misalignment of anatomical and physiological findings is reconciled by the comparison of cortical SPK and LFP RFs in various species including cats, guinea pigs and monkeys. These studies confirm that the RFs or frequency tunings of cortical neurons constructed on LFPs are much broader than those constructed on SPKs (monkey visual cortex: Buracas et al., 1998; cat cortex: Miller et al., 2002; Noreña & Eggermont, 2002; rat cortex: Wehr & Zador, 2005). The findings suggest that the output of cortical neurons are extensively shaped by intracortical neural circuits, i.e. the inhibitory processing through GABAARs (Hirsch et al., 1998; Muller & Scheich, 1988; Wang et al., 2000, 2002; Foeller et al., 2001; Noreña & Eggermont, 2002; Wehr & Zador, 2005; Eggermont et al., 2011; de Cheveigné et al., 2013).
In this study, we compared the RFSPK and RFLFP recorded by the same electrode in the primary auditory cortex of mouse. We have shown that the shapes of the RFSPK and RFLFP were similar (Fig. 1); the BFs, MTs, BWs and RF sizes of the RFSPK and RFLFP were highly correlated (Figs 2, 3, 4). The RFSPK BF could be higher, lower or equal to RFLFP BF from neuron to neuron but there were no significant differences between them. In contrast, the RFSPK MTs were never lower than RFLFP MTs; RFSPK MTs were significantly higher than RFLFP MTs. For the most part, the RFSPK BWs were narrower than RFLFP BWs. This close correspondence between RFSPK and RFLFP could be a specific feature of the mouse auditory cortex.
Our findings in mice are generally in line with previous findings with some notable differences. First, the difference in spectral ranges between RFSPK and RFLFP are much smaller than those observed in large animals. The RFLFP BW can be two to three times the RFSPK BW in cats (Noreña & Eggermont, 2002), guinea pigs (Gaucher et al., 2012; de Cheveigné et al., 2013) and monkeys (Kajikawa & Schroeder, 2011). Our data revealed that the RFLFP BW was only about 10% wider than the RFSPK BW (Fig. 3B) in C57 mice; this 10% could be more significant due to the measurement on a linear scale. This difference is particularly interesting because the absolute area of the auditory cortex is smaller but the range of spectral representation is wider in the mouse. Secondly, the differences in RF size and spectral range appeared to depend on sound levels. The higher the tone level, the smaller the differences of BWs were between RFSPK and RFLFP (Fig. 3B). Statistically significant data were reported at BW10 and BW30 only (Fig. 3). This level‐dependence was also indicated by the comparative results at RF30 and RF50. RFSPK size was 17.25 ± 12.18% smaller than RFLFP size below 30 dB SPL while RFSPK size was 11.63 ± 6.23% smaller than RFLFP size below 50 dB SPL.
Cortical LFP is considered a focal bioelectrical signature of neuronal activity that is closely correlated to functional magnetic resonance imaging and electroencephalography signals. Despite being an ‘ancient’ technique, measuring the LFP remains a valuable and widely used tool for investigating the neural mechanisms of cortical sensory processing and behaviors (Kreiman et al., 2006; Liu & Newsome, 2006). Stimulus‐evoked cortical LFP is understood to represent the assembly of EPSPs of cortical recipient layers. The broadness of RFLFP area to RFSPK area represents neuronal subthreshold activities.
A problematic issue is that the cortical region contributing to the LFP appears large in scale, varying from hundreds of microns to a few millimeters depending on the approach (Destexhe et al., 1999; Kreiman et al., 2006; Katzner et al., 2009; Xing et al., 2009). An important dispute remains the origin of the LFP, specifically if the cortical LFP is contaminated by unrelated electrical signals from a remote region (Katzner et al., 2009; Eggermont et al., 2011; Kajikawa & Schroeder, 2011; Lindén et al., 2011; Gaucher et al., 2012). The key issue here is not the size of the cortical region but whether the cortical LFP component from its more remote origins is the result of neural conduction or volume conduction. If it is the result of neural conduction, the remote component of LFP is actually a local event and a feature of neural integration of the recorded neurons. If it is the result of volume conduction, the remote component is probably a contaminated recording of the LFP. This clarification is important because direct evidence for contamination could greatly reduce the value of LFP as a tool for measuring the function of neural circuits. Previous studies with different techniques were unable to offer a definitive conclusion (Katzner et al., 2009; Eggermont et al., 2011; Kajikawa & Schroeder, 2011; Lindén et al., 2011; Gaucher et al., 2012; Einevoll et al., 2013).
The primary auditory cortex surface of the C57 mouse is about 2 × 1 mm2 and its spectral range spans up to 40 kHz (Zhang et al., 2005; Luo et al., 2009). This suggests that the frequency maps or representations in the mouse auditory cortex are denser than those in cats, guinea pigs and monkeys. If the broader frequency range of RFLFP is the result of volume conduction, the broadness of the frequency range (i.e. BWs) of RFLFP to RFSPK in mouse would need to be much greater than those found in larger animals. Our data showed the reverse. As described above, the BWs of RFLFP were only slightly wider than those of RFSPK and the broadness of RFLFP were even more significant at lower sound levels. Based on these findings, it is clear that the broader RFLFP cannot include the components of remote bioelectrical signals through volume conduction. Instead, it appears to represent the subthreshold alteration of membrane potentiation (Kaur et al., 2004; Berens et al., 2008; Eggermont et al., 2011). This conclusion is corroborated by our paired recordings from the multibarrel and tungsten electrodes. When SPKs and LFPs were largely or completely eliminated by focal application of muscimol (a GABAAR agonist) to the recorded neurons, the SPKs and LFPs of neighboring neurons (~100 μm apart) were reduced by < 50%. If this <50% deduction is considered to result from drug diffusion, the volume conduction can be excluded. However, if the diffusing impact of administered muscimol can be excluded (Fig. 5B2), these findings suggest that neural activities recorded by the tungsten electrode appear to contain the components of bioelectrical signals at remote sites where the multibarrel electrode was positioned. In other words, the neuronal activities of given neuron clusters may impact on the SPKs and LFPs within at least a 100‐μm range. Most importantly, this impact was unidirectional because the SPK and LFP could be largely and even completely eliminated at the sites where muscimol was applied (Fig. 5B2) but not at the site ~100 μm away (Fig. 5B2). As physical propagation (e.g. volume conduction) of bioelectrical signals has no directional preference, the unidirectional impact of neural activities of neighboring recording sites (Fig. 5) indicates that the remote signals were transmitted through local neural circuits. Our data therefore suggest that the remote signals of cortical LFPs are the integrative components of a local event but also represent the contribution of neurons from distant sites.
In conclusion, this study demonstrates that the differences in the BWs between RFLFP and RFSPK were much smaller than those observed in large animals. Does this mean that the inhibitory process is much weaker in the mouse auditory cortex particularly when volume conduction can be excluded? Our data further showed that the microiontophoresis of the GABAAR antagonist BMI increased SPK number and LFP amplitude by more than 160% while broadening BWs of RFSPK and RFLFP by about 10% only (Fig. 6). These findings suggest that the GABA‐mediated inhibitory processing in the mouse auditory cortex appears as strong as those in cats, guinea pigs and monkeys but its contribution to the sharpening of RFSPK is relatively small. Note that a study in rodents indicates that BMI has little impact on tuning broadening, probably due to BMI effects on calcium‐dependent potassium channels (a non‐GABAergic effect; Kurt et al., 2006). If viewed in the context of our muscimol data, the conclusion that lateral excitation contributes to a spectral range of cortical neurons is a viable possibility. A similar degree of broadening of RFSPK and RFLFP induced by the GABAAR antagonist appears to support the original assumption that the broader portion of cortical LFP depicts the subthreshold fEPSPs of thalamocortical inputs.
Supporting information
Fig. S1. The comparison of the receptive fields constructed on spikes that were isolated by using trigger levels 1.2 times and 2 times higher than the noise level.
Fig. S2. Example of the receptive fields constructed on action potentials and fEPSPs of single neurons recorded by the same glass electrode (loose patch recording).
Acknowledgements
We are grateful to Dr Jos J. Eggermont for his comments on this article. This work was supported by grants from the Canadian Institutes of Health Research (MOP164961, MOP274494), the Natural Sciences and Engineering Research Council of Canada (DG261338‐2009), Alberta Innovates – Health Solutions and the Campbell McLaurin Chair for Hearing Deficiencies, University of Calgary.
Abbreviations
- BF
best frequency
- BMI
bicuculline methiodiode
- BW
bandwidth
- FA
frequency–amplitude
- fEPSP
field excitatory postsynaptic potential
- GABAAR
γ‐aminobutyric acid receptor A
- LFP
local field potential
- MT
minimum threshold
- PST
peri‐stimulus time histogram
- PSTC
peri‐stimulus cumulative histogram
- RF
receptive field
- SPK
spike activity
References
- Barkat, T.R. , Polley, D.B. & Hensch, T.K. (2011) A critical period for auditory thalamocortical connectivity. Nat. Neurosci., 14, 1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens, P. , Keliris, G.A. , Ecker, A.S. , Logothetis, N.K. & Tolias, A.S. (2008) Feature selectivity of the gamma‐band of the local field potential in primate primary visual cortex. Front. Neurosci., 2, 199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg‐Graham, L.J. , Monier, C. & Frégnac, Y. (1998) Visual input evokes transient and strong shunting inhibition in visual cortex neurons. Nature, 393, 369–373. [DOI] [PubMed] [Google Scholar]
- Buracas, G.T. , Zador, A.M. , DeWeese, M.R. & Albright, T.D. (1998) Efficient discrimination of temporal patterns by motion‐sensitive neurons in primate visual cortex. Neuron, 20, 959–969. [DOI] [PubMed] [Google Scholar]
- Buzsáki, G. & Draguhn, A. (2004) Neuronal oscillations in cortical networks. Science, 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki, G. & Kandel, A. (1998) Somadendritic backpropagation of action potentials in cortical pyramidal cells of the awake rat. J. Neurophysiol., 79, 1587–1591. [DOI] [PubMed] [Google Scholar]
- Calford, M.B. , Webster, W.R. & Semple, M.M. (1983) Measurement of frequency selectivity of single neurons in the central auditory pathway. Hearing Res., 11, 395–401. [DOI] [PubMed] [Google Scholar]
- Canolty, R.T. , Edwards, E. , Dalal, S.S. , Soltani, M. , Nagarajan, S.S. , Kirsch, H.E. , Berger, M.S. , Barbaro, N.M. & Knight, R.T. (2006) High gamma power is phase‐locked to theta oscillations in human neocortex. Science, 313, 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cheveigné, A. , Edeline, J.M. , Gaucher, Q. & Gourévitch, B. (2013) Component analysis reveals sharp tuning of the local eld potential in the guinea pig auditory cortex. J. Neurophysiol., 109, 261–272. [DOI] [PubMed] [Google Scholar]
- Chrobak, J.J. & Buzsáki, G. (1998) Gamma oscillations in the entorhinal cortex of the freely behaving rat. J. Neurosci., 18, 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, K.S. (1968) Membranes, Ions and Impulses. University of California Press, Berkeley, CA. [Google Scholar]
- De Venecia, R.K. & McMullen, N.T. (1994) Single thalamocortical axons diverge to multiple patches in neonatal auditory cortex. Dev. Brain Res., 81, 135–142. [DOI] [PubMed] [Google Scholar]
- Denker, M. , Riehle, A. , Diesmann, M. & Grun, S. (2010) Estimating the contribution of assembly activity to cortical dynamics from spike and population measures. J. Comput. Neurosci., 29, 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker, M. , Roux, S. , Linden, H. , Diesmann, M. , Riehle, A. & Grun, S. (2011) The local field potential reflects surplus spike synchrony. Cereb. Cortex, 21, 2681–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe, A. , Contreras, D. & Steriade, M. (1999) Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J. Neurosci., 19, 4595–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, J.C. (1951) Interpretation of action potentials evoked in the cerebral cortex. Electroen. Clin. Neuro., 3, 449–464. [DOI] [PubMed] [Google Scholar]
- Eggermont, J.J. (1996) How homogeneous is cat primary auditory cortex? Evidence from simultaneous single‐unit recordings. Audit. Neurosci., 2, 79–96. [Google Scholar]
- Eggermont, J.J. (1998) Representation of spectral and temporal sound features in three cortical fields of the cat. Similarities outweigh differences. J. Neurophysiol., 80, 2743–2764. [DOI] [PubMed] [Google Scholar]
- Eggermont, J.J. , Munguia, R. , Pienkowski, M. & Shaw, G. (2011) Comparison of LFP‐based and SPK‐based spetro‐temporal receptive fields and cross‐correlation in cat primary auditory cortex. PLoS One, 5, e20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einevoll, G.T. , Pettersen, K.H. , Devor, A. , Ulbert, I. , Halgren, E. & Dale, A.M. (2007) Laminar population analysis: estimating firing rates and evoked synaptic activity from multielectrode recordings in rat barrel cortex. J. Neurophysiol., 97, 2174–2190. [DOI] [PubMed] [Google Scholar]
- Einevoll, G.T. , Kayser, C. , Logothetis, N.K. & Panzeri, S. (2013) Modelling and analysis of local field potentials for studying the function of cortical circuits. Nat. Rev. Neurosci., 14, 770–785. [DOI] [PubMed] [Google Scholar]
- Fiser, J. , Chiu, C. & Weliky, M. (2004) Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature, 431, 573–578. [DOI] [PubMed] [Google Scholar]
- Foeller, E. , Vater, M. & Kossl, M. (2001) Laminar analysis of inhibition in the gerbil primary auditory cortex. JARO, 2, 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, J.A. & Stone, J. (1969) A technique for current source density analysis of field potentials and its application to the frog cerebellum In Llinas R. (Ed.), Neurobiology of Cerebellar Evolution and Development. American Medical Association, Chicago, IL, pp. 421–430. [Google Scholar]
- Galván, V.V. , Chen, J. & Weinberger, N.M. (2001) Long‐term frequency tuning of local field potentials in the auditory cortex of the waking guinea pig. JARO, 2, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher, Q. , Edeline, J.M. & Gourévitch, B. (2012) How different are the local field potentials and spiking activities? Insights from multielectrodes arrays. J. Physiol., 106, 93–103. [DOI] [PubMed] [Google Scholar]
- Gieselmann, M.A. & Thiele, A. (2008) Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur. J. Neurosci., 28, 447–459. [DOI] [PubMed] [Google Scholar]
- Gustafsson, B. (1984) Afterpotentials and transduction properties in different types of central neurones. Arch. Ital. Biol., 122, 17–30. [PubMed] [Google Scholar]
- Hackett, T.A. , Barkat, T.R. , O'Brien, B.M. , Hensch, T.K. & Polley, D.B. (2011) Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J. Neurosci., 31, 2983–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel, M.F. , Jeschke, M. & Ohl, F.W. (2010) Spectral integration in primary auditory cortex attributable to temporally precise convergence of thalamocortical and intracortical input. J. Neurosci., 30, 11114–11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, J.A. (2003) Synaptic physiology and receptive field structure in the early visual pathway of the cat. Cereb. Cortex, 13, 63–69. [DOI] [PubMed] [Google Scholar]
- Hirsch, J.A. , Alonso, J.M. , Reid, R.C. & Martinez, L.M. (1998) Synaptic integration in simple striate cortical simple cells. J. Neurosci., 18, 9517–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa, Y. & Schroeder, C.E. (2011) How local is the local field potential? Neuron, 72, 847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner, S. , Nauhaus, I. , Benucci, A. , Bonin, V. , Ringach, D.L. & Carandini, M. (2009) Local origin of field potentials in visual cortex. Neuron, 61, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, S. , Lazar, R. & Metherate, R. (2004) Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J. Neurophysiol., 91, 2551–2567. [DOI] [PubMed] [Google Scholar]
- Kelly, R.C. , Smith, M.A. , Kass, R.E. & Lee, T.S. (2010) Local field potentials indicate network state and account for neuronal response variability. J. Comput. Neurosci., 29, 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet, T. , Bibitchkov, D. , Tsodyks, M. , Grinvald, A. & Arieli, A. (2003) Spontaneously emerging cortical representations of visual attributes. Nature, 425, 954–956. [DOI] [PubMed] [Google Scholar]
- Kreiman, G. , Hung, C.P. , Kraskov, A. , Quiroga, R.Q. , Poggio, T. & DiCarlo, J.J. (2006) Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron, 49, 433–445. [DOI] [PubMed] [Google Scholar]
- Kurt, S. , Crook, J.M. , Ohl, F.W. , Scheich, H. & Schulze, H. (2006) Differential effects of iontophoretic in vivo application of the GABAA‐antagonists bicuculline and gabazine in sensory cortex. Hearing Res., 212, 224–235. [DOI] [PubMed] [Google Scholar]
- Kurt, S. , Moeller, C.K. , Jeschke, M. & Schulze, H. (2007) Differential effects of iontophoretic application of the GABAA‐antagonists bicuculline and gabazine on tone‐evoked local field potentials in primary auditory cortex: interaction with ketamine anesthesia. Brain Res., 1220, 58–69. [DOI] [PubMed] [Google Scholar]
- Kyriazi, H.T. & Simons, D.J. (1993) Thalamocortical response transformations in simulated whisker barrels. J. Neurosci., 13, 1601–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos, P. , Shah, A.S. , Knuth, K.H. , Ulbert, I. , Karmos, G. & Schroeder, C.E. (2005) An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol., 94, 1904–1911. [DOI] [PubMed] [Google Scholar]
- Lindén, H. , Tetzlaff, T. , Potjans, T.C. , Pettersen, K.H. , Grün, S. , Diesmann, M. & Einevoll, G.T. (2011) Modeling the spatial reach of the LFP. Neuron, 72, 859–872. [DOI] [PubMed] [Google Scholar]
- Liu, R.C. & Galindo‐Leon, E.E. (2010) Predicting stimulus‐locked single unit spiking from cortical local field potentials. J. Comput. Neurosci., 29, 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. & Newsome, W.T. (2006) Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci., 26, 7779–7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, N.K. (2003) The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci., 23, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No, R. (1947) Analysis of the distribution of the action currents of nerve in volume conductors. Stud. Rockefeller Inst. Med. Res. Repr., 132, 384–477. [PubMed] [Google Scholar]
- Luo, F. , Wang, Q. , Kashani, A. & Yan, J. (2008) Corticofugal modulation of initial sound processing in the brain. J. Neurosci., 28, 11615–11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, F. , Wang, Q. , Farid, N. , Liu, X. & Yan, J. (2009) Three‐dimensional tonotopic organization of the C57 mouse cochlear nucleus. Hearing Res., 257, 75–82. [DOI] [PubMed] [Google Scholar]
- Machens, C.K. , Wehr, M.S. & Zador, A.M. (2004) Linearity of cortical receptive fields measured with natural sounds. J. Neurosci., 24, 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, A.R. (1976) The effect of membrane capacitance on non‐linear summation of synaptic potentials. J. Theor. Biol., 59, 179–187. [DOI] [PubMed] [Google Scholar]
- Metherate, R. , Lazar, R. & Simranjit, K. (2004) Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J. Neurophysiol., 91, 2551–2567. [DOI] [PubMed] [Google Scholar]
- Metherate, R. , Kaur, S. , Kawai, H. , Lazar, R. , Liang, K. & Rose, H.J. (2005) Spectral integration in auditory cortex: mechanisms and modulation. Hearing Res., 206, 146–158. [DOI] [PubMed] [Google Scholar]
- Miller, L.M. , Escabi, M.A. , Read, H.L. & Schreiner, C.E. (2001) Functional convergence of response properties in the auditory thalamocortical system. Neuron, 32, 151–160. [DOI] [PubMed] [Google Scholar]
- Miller, L.M. , Escabi, M.A. , Read, H.L. & Schreiner, C.E. (2002) Spectrotemporal receptive fields in the lemniscal auditory thalamus and cortex. J. Neurophysiol., 87, 516–527. [DOI] [PubMed] [Google Scholar]
- Mitzdorf, U. (1985) Current source‐density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev., 65, 37–100. [DOI] [PubMed] [Google Scholar]
- Moeller, C.K. , Kurt, S. , Happel, M.F. & Schulze, H. (2010) Long‐range effects of GABAergic inhibition in gerbil primary auditory cortex. Eur. J. Neurosci., 31, 49–59. [DOI] [PubMed] [Google Scholar]
- Mormann, F. , Fell, J. , Axmacher, N. , Weber, B. , Lehnertz, K. , Elger, C.E. & Fernández, G. (2005) Phase/amplitude reset and theta‐gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus, 15, 890–900. [DOI] [PubMed] [Google Scholar]
- Muller, C.M. & Scheich, H. (1988) Contribution of GABAergic inhibition to the response characteristics of auditory units in the avian forebrain. J. Neurophysiol., 59, 1673–1689. [DOI] [PubMed] [Google Scholar]
- Noreña, A.J. & Eggermont, J.J. (2002) Comparison between local field potentials and unit cluster activity in primary auditory cortex and anterior auditory field in the cat. Hearing. Res., 166, 202–213. [DOI] [PubMed] [Google Scholar]
- Noreña, A.J. , Gourévitch, B. , Pienkowski, M. , Shaw, G. & Eggermont, J.J. (2008) Increasing spectrotemporal sound density reveals an octave‐based organization in cat primary auditory cortex. J. Neurosci., 28, 8885–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez, P.L. (2006) Electric Fields of the Brain: The Neurophysics of EEG. Oxford University Press, Oxford. [Google Scholar]
- Rasch, M. , Logothetis, N.K. & Kreiman, G. (2009) From neurons to circuits: linear estimation of local field potentials. J. Neurosci., 29, 13785–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, C.E. , Mehta, A.D. & Givre, S.J. (1998) A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb. Cortex, 8, 75–592. [DOI] [PubMed] [Google Scholar]
- Suga, N. , Zhang, Y. & Yan, J. (1997) Sharpening of frequency tuning by inhibition in the thalamic auditory nucleus of the mustached bat. J. Neurophysiol., 77, 2808–2814. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Caspary, D. & Salvi, R.J. (2000) GABA‐A antagonist causes dramatic expansion of tuning in primary auditory cortex. NeuroReport, 11, 1137–1140. [DOI] [PubMed] [Google Scholar]
- Wang, J. , McFadden, S.L. , Caspary, D. & Salvi, R. (2002) Gamma‐aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res., 944, 219–231. [DOI] [PubMed] [Google Scholar]
- Wehr, M. & Zador, A.M. (2005) Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron, 47, 437–445. [DOI] [PubMed] [Google Scholar]
- Xing, D. , Yeh, C.I. & Shapley, R.M. (2009) Spatial spread of the local field potential and its laminar variation in visual cortex. J. Neurosci., 29, 11540–11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, J. & Ehret, G. (2002) Corticofugal modulation of midbrain sound processing in the house mouse. Eur. J. Neurosci., 16, 119–128. [DOI] [PubMed] [Google Scholar]
- Yan, J. & Zhang, Y. (2005) Sound‐guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. Eur. J. Neurosci., 21, 563–576. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Dyck, R.H. , Hamilton, S.E. , Nathanson, N.M. & Yan, J. (2005) Disrupted tonotopy of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptor. Hearing Res., 201, 145–155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The comparison of the receptive fields constructed on spikes that were isolated by using trigger levels 1.2 times and 2 times higher than the noise level.
Fig. S2. Example of the receptive fields constructed on action potentials and fEPSPs of single neurons recorded by the same glass electrode (loose patch recording).


