Abstract
In order to elucidate the chemical structure of black to brown pigments, neuromelanins (NMs), in the substantia nigra (SN) and the locus coeruleus (LC) in the central nervous system of humans and other mammalian species during aging, chemical degradative methods are powerful tools. HPLC analysis after hydroiodic acid hydrolysis detected aminohydroxyphenylethylamines, aminohydroxyphenylacetic acids, and aminohydroxyethylbenzenes, which confirmed that SN‐NM and LC‐NM contain melanin derived not only from dopamine and norepinephrine (NE) but also from several other catecholic metabolites, such as 3,4‐dihydroxyphenylalanine, 3,4‐dihydroxyphenylacetic acid, 3,4‐dihydroxymandelic acid, 3,4‐dihydroxyphenylethanol, and 3,4‐dihydroxyphenylethylene glycol, in addition to the corresponding Cys‐derivatives in varying degrees. However, hydroiodic acid hydrolysis showed that LC‐NM produced the same degradation products as were detected in SN‐NM. Thus, we needed to develop a new chemical detection method to validate the existence of NE in LC‐NM. In the present study, we report that HCl hydrolysis of LC‐NM in the presence of thioglycolic acid yields new products arising from substitution of the hydroxyl group by thioglycolic acid at the benzyl position of NE and cysteinyl‐NE. This is the first chemical evidence showing that NE and cysteinyl‐NE are incorporated into LC‐NM.
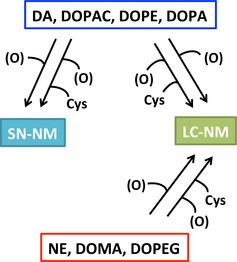
Using the chemical degradation methods for the determination of catechols in neuromelanin (NM), we have shown that dopamine (DA), 3,4‐dihydroxyphenylacetic acid (DOPAC), 3,4‐dihydroxyphenylethanol (DOPE), and 3,4‐dihydroxyphenylalanine (DOPA) are mainly responsible for the structure of NM from substantia nigra (SN), while norepinephrine (NE), 3,4‐dihydroxymandelic acid (DOMA), and 3,4‐dihydroxyphenylethylene glycol (DOPEG) are additionally responsible for the structure of NM from locus coeruleus (LC).
Keywords: HCl hydrolysis, locus coeruleus, neuromelanin, Parkinson's disease, substantia nigra
Abbreviations used
- 5‐S‐Cys‐CMT‐DA
5‐S‐cysteinyl β‐(carboxymethylthio)dopamine
- 5‐S‐Cys‐DA
5‐S‐cysteinyl‐dopamine
- 5‐S‐Cys‐NE
5‐S‐cysteinyl‐norepinephrine
- AHEB
aminohydroxyethylbenzene
- AHPAA
aminohydroxyphenylacetic acid
- AHP
aminohydroxyphenylalanine
- AHPEA
aminohydroxyphenylethylamine
- CMT‐DA
β‐(carboxymethylthio)dopamine
- Cys
cysteine
- DA
dopamine
- DOMA
3,4‐dihydroxymandelic acid
- DOPA
3,4‐dihydroxyphenylalanine
- DOPAC
3,4‐dihydroxyphenylacetic acid
- DOPE
3,4‐dihydroxyphenylethanol
- DOPEG
3,4‐dihydroxyphenylethylene glycol
- HI
hydroiodic acid
- LC
locus coeruleus
- LC‐NM
NM from LC tissue
- NE
norepinephrine
- NM
neuromelanin
- PDCA
pyrrole‐2,3‐dicarboxylic acid
- PD
Parkinson's disease
- PTCA
pyrrole‐2,3,5‐tricarboxylic acid
- SN‐NM
NM from SN tissue
- SN
substantia nigra
- TDCA
thiazole‐4,5‐dicarboxylic acid
- TTCA
thiazole‐2,4,5‐tricarboxylic acid
Neuromelanins (NMs) are complex polymeric compounds present in the human central nervous system that are localized in cytoplasmic organelles surrounded by a double membrane, together with lipid bodies and proteins (Sulzer et al. 2008; Zecca et al. 2008). NM pigment itself is composed of different compounds: melanin, proteins, lipids, and metal ions (Zecca et al. 2008; Engelen et al. 2012; Wakamatsu et al. 2012). NM is synthesized mainly in dopaminergic neurons of the substantia nigra (SN) and in noradrenergic neurons of the locus coeruleus (LC), however, it has been demonstrated that NM is also synthesized and accumulates in neurons of other brain areas (Zecca et al. 2008). The synthesis and accumulation of NM inside neurons arise during brain aging and there is evidence that NM is involved in the pathogenesis of neurodegenerative diseases such as Parkinson's disease (PD) (Mann and Yates 1983; Marsden 1983; Zecca et al. 2006). Notably, the SN and LC are the brain areas with the highest concentration of NM pigment in the brain (Zecca et al. 2004).
In contrast to cutaneous melanin, the biosynthesis and structure of NM remain poorly understood, especially NM derived from the LC (LC‐NM) due to the paucity of material and the difficulty of its isolation. In fact, the majority of structural studies on NMs have employed NM isolated from the SN (SN‐NM) and other large areas of the human brain (Wakamatsu et al. 2003, 2012; Zecca et al. 2008).
We have previously performed chemical analyses in order to elucidate the structure of NM in the SN (Wakamatsu et al. 2003, 2012; Zecca et al. 2008). In addition, it was recently suggested that various catecholic metabolites are incorporated into NM, including 3,4‐dihydroxyphenylalanine (DOPA), 3,4‐dihydroxyphenylacetic acid (DOPAC), 3,4‐dihydroxymandelic acid (DOMA), 3,4‐dihydroxyphenylethanol (DOPE) and 3,4‐dihydroxyphenylethylene glycol (DOPEG), which are metabolites of DA and norepinephrine (NE) formed by the oxidative deamination by monoamine oxidase followed by oxidation/reduction (Fig. 1) (Wakamatsu et al. 2014).
Figure 1.
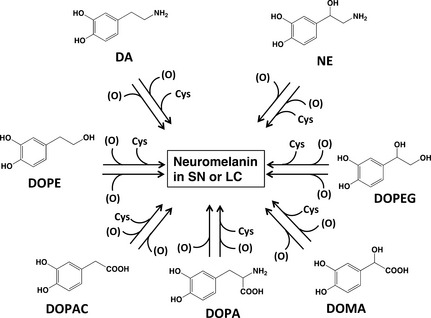
Possible participation of various catecholic metabolites known to be present in various regions of the brain that may be incorporated into neuromelanin (NM). In addition to dopamine (DA) and norepinephrine (NE) and the corresponding Cys‐derivatives, these other metabolites are also thought to be incorporated into NM. (O) represents the oxidants.
To estimate the incorporation of various melanin precursors into NM, we used the chemical degradation approach of SN‐NM originally adapted for the analysis of cutaneous melanin (Ito and Fujita 1985; Ito and Wakamatsu 2003). The reductive hydroiodic acid (HI) hydrolysis of SN‐NM and LC‐NM from the human brainstem followed by HPLC analysis using newly synthesized o‐aminophenol compounds as standards (Fig. 2), i.e., aminohydroxyphenylethylamine isomers (AHPEAs), aminohydroxyphenylacetic acid isomers (AHPAAs) and aminohydroxyethylbenzene isomers (AHEBs), made it possible to confirm that SN‐NM and LC‐NM contain melanin derived not only from DA and NE but also from several other catecholic metabolites, such as DOPA, DOPAC, DOMA, DOPE, and DOPEG, in addition to the corresponding Cys‐derivatives in varying degrees (Wakamatsu et al. 2014). Those results indicated that most of the catechol derivatives noted above were incorporated into SN‐NM and LC‐NM during their biosynthesis. However, upon HI hydrolysis, LC‐NM produced the same degradation products, AHPEAs, AHPAAs, and AHEBs, as SN‐NM did (Wakamatsu et al. 2014). This is because the hydroxyl group at the benzyl position of NE (and its metabolites) is easily eliminated to produce DA (and its metabolites) under the conditions of reductive HI hydrolysis. Thus, we could not distinguish LC‐NM from SN‐NM by HI reductive hydrolysis.
Figure 2.
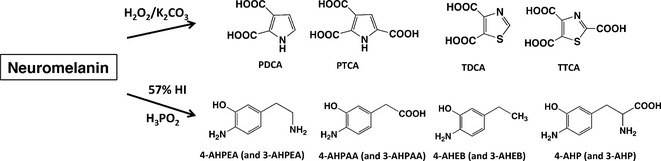
Various melanin markers of neuromelanin (NM) produced by chemical degradative methods. On alkaline H2O2‐mediated oxidation, melanins derived from 3,4‐dihydroxyphenylalanine (DOPA), dopamine (DA), norepinephrine (NE), and the corresponding Cys‐derivatives gave pyrrole‐2,3‐dicarboxylic acid (PDCA), pyrrole‐2,3,5‐tricarboxylic acid (PTCA), thiazole‐4,5‐dicarboxylic acid (TDCA) and thiazole‐2,4,5‐tricarboxylic acid (TTCA), while melanins from Cys‐derivatives of 3,4‐dihydroxyphenylacetic acid (DOPAC), 3,4‐dihydroxymandelic acid (DOMA), 3,4‐dihydroxyphenylethanol (DOPE) and 3,4‐dihydroxyphenylethylene glycol (DOPEG) gave only TDCA and TTCA. On hydroiodic acid (HI) hydrolysis, melanins derived from the Cys‐derivatives of DA (and NE), DOPAC (and DOMA), DOPE (and DOPEG) and DOPA gave aminohydroxyphenylethylamine (AHPEA) isomers, aminohydroxyphenylacetic acid (AHPAA) isomers, aminohydroxyethylbenzene (AHEB) isomers, and aminohydroxyphenylalanine (AHP) isomers, respectively.
Thus, as far as we know, there have been no reports regarding whether NE is incorporated into NM, and to what extent NE and NE metabolites are present, especially in LC‐NM, which accumulates in LC neurons that contain high levels of NE. Thus, we developed a new chemical detection method to validate the existence of NE in LC‐NM, and the contents of NE metabolites in LC‐NM. We report here the first chemical evidence regarding the existence of NE and NE metabolites in LC‐NM.
Materials and methods
DA, NE, Cys, DOMA, DOPEG, mushroom tyrosinase (EC.1.14.18.1, 1,715 units/mg), and 57% HI were purchased from Sigma‐Aldrich (St Louis, MO, USA). DOPE was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). 50% H3PO2, 30% H2O2 and sodium 1‐octanesulfonate were purchased from Nacalai Tesque, Inc (Kyoto, Japan). DOPAC and Dowex 50W‐X2 (200–400 mesh) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Preparations of pyrrole‐2,3,5‐tricarboxylic acid (PTCA), pyrrole‐2,3‐dicarboxylic acid (PDCA), thiazole‐2,4,5‐tricarboxylic acid (TTCA), thiazole‐4,5‐dicarboxylic acid (TDCA), 4‐amino‐3‐hydroxyphenylalanine (4‐AHP), 3‐amino‐4‐hydroxyphenylalanine (3‐AHP), 4‐amino‐3‐hydroxyphenylethylamine (4‐AHPEA) and 3‐amino‐4‐hydroxyphenylethylamine (3‐AHPEA) were described previously (d'Ischia et al. 2013). 5‐S‐cysteinyl‐dopamine (5‐S‐Cys‐DA) and 5‐S‐cysteinyl‐norepinephrine (5‐S‐Cys‐NE) were prepared as described by Ito et al. (1986). 4‐amino‐3‐hydroxyphenylacetic acid (4‐AHPAA), 3‐amino‐4‐hydroxyphenylacetic acid (3‐AHPAA), 4‐amino‐3‐hydroxyethylbenzene (4‐AHEB) and 3‐amino‐4‐hydroxyethylbenzene (3‐AHEB) were prepared according to Wakamatsu et al. (2014). Pheomelanins derived from the Cys‐derivatives of DA, NE, DOPAC, DOMA, DOPEG, DOPE, and DOPA, were prepared according to methods previously reported by our laboratory (Ito et al. 1986; Ito 1989). Alkaline H2O2‐mediated oxidation and reductive HI hydrolysis were performed as described in Ito et al. (2011), and Wakamatsu et al. (2002), respectively. All other chemicals were of the highest purity commercially available. UV‐visible spectra were recorded with a JASCO V‐630 UV/VIS spectrophotometer (JASCO Co., Tokyo, Japan). The HPLC systems consisted of a JASCO 880‐PU pump, a JASCO C18 column (JASCO Catecholpak; 4.6 × 150 mm; 7 μm particle size) with a Shiseido 3005 SI‐2 (Shiseido, Tokyo, Japan) electrochemical detector or a Shiseido C18 column (Shiseido Capcell Pak MG; 4.6 × 250 mm; 5 μm particle size) with a JASCO UV detector (JASCO Co.) and a flow rate of 0.7 mL/min. 1H‐NMR (400 MHz) and 13C‐NMR (100 MHz) spectra were recorded on a Bruker AVANCE400 spectrometer (Bruker, Billerica, MA, USA) and are reported as the chemical shift δ (ppm) downfield from sodium 2,2‐dimethyl‐2‐silapentane‐5‐sulfonate used as an internal reference. Coupling constants (J) are expressed in Hz and signals are expressed as s (singlet), d (doublet), or t (triplet). Elemental analyses were carried out by the Organic Elemental Analysis Research Center (Kyoto University, Japan). Mass spectra were obtained with an Applied Biosystems, a Biospectrometry Workstation Mariner (mode: electrospray ionization − time of flight, positive; ESI (+)‐TOF) (Applied Biosystems, Foster City, CA, USA), and ion trap liquid chromatography/mass spectrometry was carried out using an Esquire HCT Plus Ion Trap Mass Spectrometer (Bruker) connected to a HPLC system 1260 Infinity (Agilent Technologies, Santa Clara, CA, USA). Optical rotation was measured using a Polarimeter (JASCO DIP‐370; Tokyo, Japan).
Human NM pigments were isolated from SN and LC tissues as described previously (Wakamatsu et al. 2003, 2012; Zecca et al. 2008; Engelen et al. 2012). SN‐NM was isolated from a pool of five subjects (average age 82 years old; 3 males and 2 females). LC‐NM was isolated from a pool of 35 subjects (average age 77 years old; 22 males and 13 females). Brain samples were obtained during autopsies of male and female subjects who died without evidence of neuropsychiatric and neurodegenerative disorders. All tissues samples were analyzed anonymously. Samples of SN and LC were carefully dissected and then stored at −80°C until used for the isolation of NM pigment. In order to obtain comparable amounts of LC‐NM and SN‐NM, we had to pool LC tissues from many subjects since the LC brain area is much smaller than the SN. This study was approved by the Ethical Committee of the National Research Council of Italy—Institute of Biomedical Technologies (Segrate, Milan, Italy) and was carried out in agreement with the Policy of National Research Council of Italy.
HPLC conditions
For analysis of β‐(carboxymethylthio)dopamine (CMT‐DA) and other catecholamine metabolites formed upon HCl hydrolysis, the mobile phase was 0.1 M sodium citrate buffer, pH 3.0, containing 1 mM sodium octanesulfonate and 0.1 mM EDTA.2Na (buffer A): methanol, 95 : 5 (v/v) (Wakamatsu et al. 2003). Analyses were performed at 35°C with an electrochemical detector set at +700 mV versus Ag/AgCl electrode. For the analysis of 5‐S‐Cys‐NE and 5‐S‐cysteinyl β‐(carboxymethylthio)dopamine (5‐S‐Cys‐CMT‐DA), we used a Capcell pak C18 column with 0.1 M potassium phosphate buffer, pH 2.1:methanol, 99 : 1 (v/v), at 25°C, with a UV‐VIS detector set at 290 nm. For the preparative separation of 5‐S‐Cys‐NE, we used a Capcell pak C18 column (type MG, 20 × 250 mm, 5 μm particle size; from Shiseido) attached with a Capcell pak C18 column (20 × 35 mm, 5 μm particle size; from Shiseido), with 0.1 M potassium phosphate buffer, pH 2.1:methanol, 99 : 1 (v/v), at 55°C, respectively, with a UV‐VIS detector set at 290 nm, at a flow rate of 7.0 mL/min. Determination of melanin markers was performed using HPLC as previously reported (Wakamatsu et al. 2002, 2003; Ito et al. 2011).
HCl hydrolysis of human NM
One hundred μL of a suspension (1 mg/mL) of NM were transferred to a sealed‐capped tube with 300 μL 6 M HCl containing 5% thioglycolic acid and 1% phenol. The tube was purged with a stream of argon, then sealed and heated at 110°C for 16 h. After cooling, the hydrolysate was mixed with 10 μL of an internal standard solution (methyl‐5‐S‐Cys‐DOPA, 15 μmol/L), extracted twice with 1 mL diethyl ether to remove phenol, and treated with alumina to extract catecholic compounds as follows. A 120 μL aliquot of the hydrolysate was transferred into an Eppendorf tube containing 50 mg acid‐washed alumina and 200 μL 1% Na2S2O5‐1% EDTA.2Na. To the mixture, 500 μL 2.7 M Tris.HCl‐2% EDTA.2Na (pH 9.0) was added and immediately mixed vigorously for 5 min on a microtube mixer. After centrifugation, the aqueous layer was removed by aspiration, and the remaining alumina was washed with 1 mL water three times. The catecholic compounds were then eluted with 100 μL 0.4 M HClO4 by shaking for 2 min. A 30 μL aliquot of the HClO4 extract was injected into the HPLC system.
Synthesis of CMT‐DA
A solution of 144 mg (0.7 mmol) NE·HCl in 20 mL 6 M HCl containing 5% thioglycolic acid and 1% phenol was refluxed for 30 min at 110°C. Thioglycolic acid and phenol were essential to avoid polymerization of the starting material NE. The reaction mixture was washed twice with 20 mL ether to remove phenol. The water layer was evaporated to dryness and then subjected to Dowex 50W‐X2 chromatography (1.6 × 10 cm in 2 M HCl). The column was eluted with 2 M HCl and fractions of 10 mL were collected. Fractions 9–16 were evaporated to give 159 mg (80% yield) of CMT‐DA·HCl·H2O salt as a colorless solid (HPLC purity: > 99%). UV λmax 282 nm (ε 3,260) in 0.1 M HCl. 1H‐NMR (DCl): δ = 3.32 (dd, 2H, J = 16 Hz, 16 Hz), 3.47 (m, 2H), 4.19 (t, 1H, J = 8.0 Hz), 6.85 (dd, 1H, J = 2.3 Hz, J = 8.3 Hz), 6.93 (d, 1H, J = 8.3 Hz), 6.94 (d, 1H, J = 2.3 Hz). 13C‐NMR (DCl): δ = 35.24, 45.53, 49.08, 118.26, 119.18, 123.47, 131.55, 147.17, 147.20, 176.79. Mass spectrum: m/z 244.0567 (M+H)+ for C10H14N1O4S1 (−2.1 mDa). Elemental analysis: calculated for C10H13N1O4S1·HCl·H2O. C, 40.34%; H, 5.42%; N, 4.70%; S, 10.77%; Cl, 11.91%; found C, 40.72%; H, 5.27%; N, 4.72%; S, 10.79%; Cl, 13.05%.
Synthesis of 5‐S‐Cys‐CMT‐DA
A solution of 47 mg (0.16 mmol) 5‐S‐Cys‐NE in 9.4 mL 6 M HCl containing 5% thioglycolic acid and 1% phenol was refluxed for 30 min at 110°C. The reaction mixture was washed twice with 20 mL ether to remove phenol. The water layer was evaporated to dryness and then subjected to Dowex 50W‐X2 chromatography (1.6 × 10 cm in 2 M HCl). The column was eluted with 2 M HCl and fractions of 10 mL were collected. Fractions 16–26 were evaporated to give 26 mg (14% yield) of 5‐S‐Cys‐CMT‐DA·HCl salt as a colorless solid (HPLC purity: 96%). UV λmax 292 nm (ε 1,340) in 0.1 M HCl. 1H‐NMR (DCl): δ = 3.48 (m, 2H), 3.55 (m, 4H), 4.21 (m, 2H), 6.99 (d, 1H, J = 2.0 Hz), 7.10 (d, 1H, J = 2.4 Hz). 13C‐NMR (DCl): δ = 35.2, 36.4, 45.4, 49.0, 54.7, 119.3, 121.0, 128.5, 131.7, 147.7, 148.6, 172.7, 176.7. 13C‐NMR shows that 5‐S‐Cys‐CMT‐DA consists of a mixture of diastereomers evidenced by a double series of signals. Mass spectrum: m/z 363.08 (M+H)+ for C13H18N2O6S2.
Ion trap liquid chromatography/mass spectrometry of CMT‐DA
Ion Trap Liquid Chromatography/Mass Spectrometry was performed using a liquid chromatography/mass spectrometry system by Esquire HCT Plus Mass Spectrometer (Bruker) connected to an HPLC system 1260 Infinity (Agilent Technologies) with a Cadenza CD‐C18 column (2.0 ×75 mm) (Imtakt Corp., Kyoto, Japan) with 10% CH3OH – 0.1% HCOOH, at flow rate of 0.1 mL/min.
Statistical analyses
We did not perform any statistical analyses, but values obtained by H2O2‐mediated oxidation and HI hydrolysis of synthetic pheomelanins prepared from the Cys‐derivatives of DA, NE, DOPAC, DOMA, DOPEG, DOPE, and DOPA, were obtained as a mean of two determinations.
Results
Alkaline H2O2‐mediated oxidation and reductive HI hydrolysis
PDCA, PTCA, TDCA, and TTCA (Fig. 2) were measured using H2O2‐mediated oxidation of synthetic pheomelanins prepared from Cys‐DA, Cys‐NE, Cys‐DOPAC, Cys‐DOPE, Cys‐DOPA, Cys‐DOMA, and Cys‐DOPEG (Table 1). Pheomelanins prepared from Cys‐DOPAC, Cys‐DOPE Cys‐DOMA, and Cys‐DOPEG did not give detectable levels of PTCA or PDCA, but produced only TDCA and TTCA, two specific markers of pheomelanin (Table 1). This is consistent with the fact that those Cys‐catechols do not possess an amino group in their side chains.
Table 1.
Chemical analysis of synthetic melanins prepared from Cys‐DA, Cys‐NE, Cys‐DOPAC, Cys‐DOPE, Cys‐DOPA, Cys‐DOMA and Cys‐DOPEG, and human NMs isolated from SN and LC tissues
| Melanin | PDCA | PTCA | TDCA | TTCA | 4‐AHPEA | 4‐AHPAA | AHEBsa | 4‐AHP | DA |
|---|---|---|---|---|---|---|---|---|---|
| nmol/mg | |||||||||
| Cys‐DAb | 13.6 | 0.60 | 25.8 | 62.5 | 644 | NDc | 23.7 | ND | 20.5 |
| Cys‐NEb | 4.39 | 0.40 | 16.2 | 77.9 | 326 | ND | 11.7 | ND | ND |
| Cys‐DOPACb | ND | ND | 18.6 | 40.8 | ND | 1256 | ND | ND | ND |
| Cys‐DOPEb | ND | ND | 32.8 | 37.8 | ND | ND | 866 | ND | ND |
| Cys‐DOPAb | 5.67 | 15.3 | 12.0 | 34.1 | ND | ND | ND | 539 | ND |
| Cys‐DOMAb | ND | ND | 24.5 | 67.4 | ND | 537 | ND | ND | ND |
| Cys‐DOPEGb | ND | ND | 21.4 | 68.9 | ND | ND | 151 | ND | ND |
| SN‐NMd | 1.3 | 0.27 | 3.5 | 4.1 | 2.8e | 4.4e | 0.24e | 0.32e | 8.0e |
| LC‐NMd | 2.6 | 0.34 | 3.8 | 6.4 | 5.3e | 3.6e | 0.55e | 0.26e | 6.2e |
AHEBs were estimated as a mixture of 4‐AHEB and 3‐AHEB.
Value obtained as a mean of two determinations.
Not detected.
Value obtained in a single determination.
Data from Wakamatsu et al. 2014.
On HI hydrolysis, DA‐related pheomelanins, i.e., Cys‐DA‐melanin, Cys‐DOPAC‐melanin and Cys‐DOPE‐melanin, gave high levels of pheomelanin markers, i.e., 4‐AHPEA, 4‐AHPAA, and AHEB (as a mixture of 4‐AHEB and 3‐AHEB), respectively (Table 1 and Fig. 2). On the other hand, NE‐related pheomelanins, i.e., Cys‐NE‐melanin, Cys‐DOMA‐melanin, and Cys‐DOPEG‐melanin, gave lower (a half to one‐fifth of the corresponding DA‐related pheomelanins) levels of those markers. Cys‐DOPA‐melanin gave a high level of 4‐AHP.
Alkaline H2O2‐mediated oxidation of SN‐NM and LC‐NM produced TDCA, TTCA, PDCA, and PTCA at levels similar between SN‐NM and LC‐NM. Levels of TDCA and TTCA were greater than those of PDCA and PTCA both in SN‐NM and LC‐NM, which is consistent with the incorporation of Cys as a benzothiazole moiety (Wakamatsu et al. 2012).
HI hydrolysis of SN‐NM and LC‐NM gave a similar pattern of catechols, i.e., DOPA, DA, and DOPAC and their aminohydroxyphenyl counterparts, i.e., 4‐AHP, 4‐AHPEA, and 4‐AHPAA. AHEBs isomers were also detected (Table 1). DA was detected at the highest levels in both types of NMs, while NE was not detected (Wakamatsu et al. 2014). In this study, we did not detect 6‐(2‐amino‐2‐carboxyethyl)‐2‐carboxy‐4‐hydroxybenzothiazole or 7‐(2‐amino‐2‐carboxyethyl)‐2‐carboxy‐4‐hydroxybenzothiazole in alkaline H2O2‐mediated oxidation mixtures known as any benzothiazole markers (Greco et al. 2009). These results suggest two alternative possibilities: (i) that SN‐NM and LC‐NM have common structural features characterized by the incorporation of DOPA, DA, and DOPAC, or (ii) that LC‐NM is produced, at least in part, from NE (and DOPEG and DOMA), but the resulting structural features cannot be confirmed because of the reduction in the benzylic hydroxyl group to the hydrogen atom in NE (and DOPEG and DOMA).
Syntheses of CMT‐DA and 5‐S‐Cys‐CMT‐DA
HCl hydrolysis of NM pigments is one approach to characterize the structure of NM. A high level of DA was previously detected in an HCl hydrolysate of SN‐NM (Zecca et al. 2008). In order to establish a method to detect NE or NE‐derived products, we performed HCl hydrolysis of NE itself. Hydrolysis with 6 M HCl containing 5% thioglycolic acid and 1% phenol was carried out using the method of protein hydrolysis at 110°C for 16 h (Murakami et al. 2007; Zecca et al. 2008). Phenol is usually added when proteins are hydrolyzed with HCl. Phenol plays a role in protecting phenolic and catecholic compounds from oxidation (Gieseg et al. 1993). As expected, NE (with a retention time of 4.7 min) disappeared from the HPLC chromatogram, and instead an unknown compound newly appeared at the retention time of 16.1 min. In the absence of thioglycolic acid, the reaction resulted in the production of a black polymeric material. The new compound was prepared in a large scale and purified by ion‐exchange chromatography in a 81% yield. We performed instrumental analyses, including 1H‐NMR, 13C‐NMR, High Resolution Mass Spectrometry (HRMS) spectra, Ion Trap Liquid Chromatography/Mass Spectrometry, and elemental analysis, to determine the structure of this compound. 1H‐NMR and 13C‐NMR spectra indicated the existence of one benzyl proton (in addition to other protons), three aliphatic carbons, six aromatic carbons, and one carbonyl carbon. The elemental analysis of the compound confirmed the existence of sulfur and chlorine atoms in a ratio of 1 : 1, and HRMS monitored by mass spectrometry in positive mode showed a 244 m/z ion peak for a 243‐dalton compound (see Supporting Information). Extracted‐ion chromatogram (EIC) in Ion Trap Liquid Chromatography/Mass Spectrometry of CMT‐DA appeared as a broad peak at 4.5 min, which may contain a mixture of enantiomers of CMT‐DA because this compound is racemic, as proved by its optical rotation ([α]D = 0°). Thus, we identified the new unknown compound as CMT‐DA. On the other hand, HCl hydrolysis of DA under the same conditions did not afford CMT‐DA.
We also synthesized 5‐S‐Cys‐CMT‐DA in a 14% yield by HCl hydrolysis of 5‐S‐Cys‐NE in the presence of thioglycolic acid and phenol. The structure of 5‐S‐Cys‐CMT‐DA was identified as the 5‐S‐Cys derivative of CMT‐DA by several instrumental analyses, including 1H‐NMR, 13C‐NMR and HRMS (see Supporting Information). HRMS monitored by mass spectrometry in positive mode showed a 363 m/z ion peak for a 362‐dalton compound, 5‐S‐Cys‐CMT‐DA (see Supporting Information).
HCl hydrolysis of SN‐NM and LC‐NM
HCl hydrolysis of SN‐NM in the presence of thioglycolic acid and phenol gave DA (31.4 nmol/mg) and DOPA (19.7 nmol/mg) as major products, in addition to DOPAC (5.4 nmol/mg), Cys‐DA (1.7 nmol/mg), Cys‐DOPA (0.9 nmol/mg), and Cys‐DOPAC (1.4 nmol/mg) (Figs 3a and 4a). CMT‐DA and 5‐S‐Cys‐CMT‐DA were not detected on the HPLC chromatogram (Fig. 3a).
Figure 3.
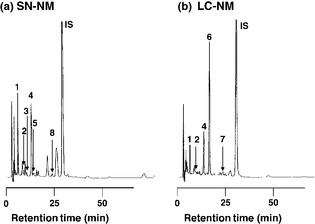
HPLC chromatograms of HCl hydrolysates of NM from SN tissue (SN‐NM) and NM from LC tissue (LC‐NM). Hydrolysis of SN‐NM (a) and LC‐NM (b) by 6 M HCl was performed in the presence of 5% thioglycolic acid and 1% phenol for 16 h at 110°C. The hydrolysates were treated with alumina to extract catecholic compounds in the presence of the IS (internal standard) α‐methyl‐Cys‐DOPA (Wakamatsu and Ito 1994). #1: 3,4‐dihydroxyphenylalanine (DOPA), #2: 3,4‐dihydroxyphenylacetic acid (DOPAC), #3: 5‐S‐Cys‐DOPA, #4: dopamine (DA), #5: 5‐S‐Cys‐DOPAC, #6: β‐(carboxymethylthio)dopamine (CMT‐DA), #7: 5‐S‐cysteinyl β‐(carboxymethylthio)dopamine (5‐S‐Cys‐CMT‐DA), #8: 5‐S‐Cys‐DA. IS is methyl Cys‐DOPA as an internal standard.
Figure 4.
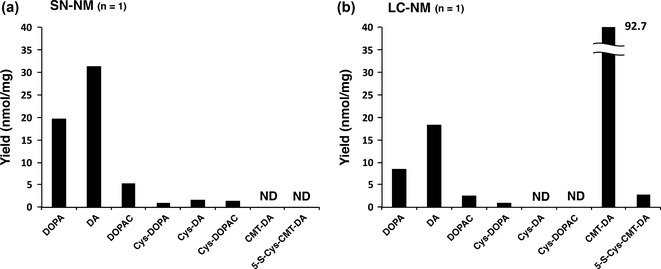
Hydrolysis of NM from SN tissue (SN‐NM) (a) and NM from LC tissue (LC‐NM) (b) by 6 M HCl in the presence of thioglycolic acid. ND, not detected.
HCl hydrolysis of human LC‐NM in the presence of thioglycolic acid and phenol produced CMT‐DA (92.7 nmol/mg) as an exceedingly major product, in addition to DA (18.4 nmol/mg), DOPA (8.5 nmol/mg) and DOPAC (2.7 nmol/mg). A low level of 5‐S‐Cys‐CMT‐DA (2.7 nmol/mg) was also detected. Cys‐DOPA was detected in a low amount (1.1 nmol/mg), while Cys‐DA and Cys‐DOPAC were below the detection limits (Figs 3b and 4b). CMT‐DA and 5‐S‐Cys‐CMT‐DA were identified by co‐injection with authentic samples. The Ion Trap Liquid Chromatography/Mass Spectrometry of the HCl hydrolysate of LC‐NM showed two EICs which were 5‐S‐Cys‐CMT‐DA at 3.8–4.0 min and CMT‐DA at 4.4 min. 5‐S‐Cys‐CMT‐DA at 3.8–4.0 min and CMT‐DA at 4.4 min in EIC appeared at the separated and broad peaks, respectively. The reason why EIC of 5‐S‐Cys‐CMT‐DA showed two peaks may be explained by 13C‐NMR that 5‐S‐Cys‐CMT‐DA consists of a mixture of diastereomers evidenced by a double series of signals. (see Supporting Information). The above results indicate that in addition to smaller amounts of DA and DOPA as biosynthetic precursors of NE, a greater amount of NE (and 5‐S‐Cys‐NE) is incorporated into LC‐NM.
Discussion
NM mainly accumulates in catecholaminergic neurons of the SN and LC (Zecca et al. 2004) and its structure differs from peripheral melanins produced in the hair, skin, and eyes (Bogerts 1981; Saper and Petito 1982; Zecca et al. 2003).
In contrast to the rather extensive studies on the biosynthetic origin and structure of SN‐NM (Wakamatsu et al. 2003, 2012; Zecca et al. 2008), the chemical constituents of LC‐NM have not been studied in detail as far as we know. In particular, whether NE is actually incorporated in LC‐NM is not known. In this study, the H2O2‐mediated oxidation and HI hydrolysis of SN‐NM and LC‐NM failed to clearly differentiate LC‐NM from SN‐NM but confirmed their similarities. In continuous efforts to find a new clue for the incorporation of NE into LC‐NM, we performed HCl hydrolysis of SN‐NM and LC‐NM. Our previous study (Zecca et al. 2008) showed that approximately 90% of catechols, DOPA, DA, Cys‐DOPA, and Cys‐DA, are present in the protein‐bound form. Therefore, in this study, we did not attempt to separate catechols into the free and protein‐bound forms. The HCl hydrolysis of SN‐NM yielded DOPA, DA, and DOPAC, together with Cys‐DOPA, Cys‐DA, and Cys‐DOPAC (Figs 3a and 4a). On the other hand, the HCl hydrolysis of LC‐NM gave a high level of an unknown catechol, in addition to DOPA, DA, and DOPAC (Figs 3b and 4b). This compound was then identified as CMT‐DA, produced via the substitution of the hydroxyl group of NE with the carboxymethylthio group of thioglycolic acid. Thus, we propose a mechanism (see Supporting Information) for the production of CMT‐DA showing that an addition reaction of thioglycolic acid may occur at the benzyl position of NE via the quinonemethide produced from the elimination of a hydroxyl group at the benzyl position. HCl hydrolysis of DA under the same conditions did not afford CMT‐DA. This showed that the production of CMT‐DA does not proceed through oxidation of DA to the quinonemethide, but the elimination of a hydroxyl group at the benzyl position of NE. Another possible mechanism may be via a carbonium ion at the benzyl position after elimination of the hydroxyl group. These mechanisms are supported by the experimental result that this compound is racemic, as proved by its optical rotation ([α]D = 0°). 5‐S‐Cys‐DMT‐DA, arising from 5‐S‐Cys‐NE, was also detected in a small amount. Although the exact nature of NE binding to LC‐NM proteins (and/or pigment) was not clarified in this study, one possibility may be that NE is trapped by proteins/pigment via Schiff base formation (nitrogen‐carbon double bond).
This study also suggests that DA and its metabolites, DOPAC and DOPE, are responsible for the production of SN‐NM, while NE and its metabolites, DOMA and DOPEG, are responsible for the production of LC‐NM. In reductive HI hydrolysis of LC‐NM, comparable amounts of DA and 4‐AHPEA were obtained, as previously reported (Wakamatsu et al. 2014). This suggests that they may be produced by the elimination of hydroxyl groups from NE and the corresponding aminohydroxy derivative having a hydroxyl group at the benzyl position of 4‐AHPEA (derived from Cys‐NE‐melanin). Moreover, DOPA and DA, present in LC neurons as precursors of NE synthesis, are incorporated in LC‐NM.
NM pigment is formed by the oxidative polymerization of catecholamines in SN neurons (Mann and Yates 1974; Graham 1979; Bogerts 1981; Sulzer et al. 2000; Napolitano et al. 2011). It has been reported that NM is a compound containing a melanic component (eumelanic dihydroxyindole and pheomelanic benzothiazine/benzothiazole units) bound to polyisoprenic and proteic components (Zecca et al. 2000, 2008; Engelen et al. 2012; Wakamatsu et al. 2012). It has been demonstrated in brain tissue that DA‐quinone synthesized by the non‐enzymatic autoxidation of DA (Zhang and Dryhurst 1993) reacts with the thiol group of l‐Cys/glutathione forming 5‐S‐Cys‐DA/5‐S‐glutathionyl‐DA, the latter being subsequently hydrolyzed by peptidases to produce 5‐S‐Cys‐DA (Napolitano et al. 2011; Segura‐Aguilar et al. 2014). The presence of 5‐S‐Cys‐DA in the human SN and in other brain regions rich in dopamine (DA) has been described, suggesting the possible incorporation of 5‐S‐Cys‐DA into NM pigment (Rosengren et al. 1985).
DA‐quinone was reported to cyclize several‐fold more slowly to produce the corresponding aminochrome than does DOPA‐quinone. This makes DA‐quinone highly reactive and toxic (Land and Riley 2000; Segura‐Aguilar et al. 2001; Ito and Wakamatsu 2008). Thus, DA may exert its toxicity through oxidation to DA‐quinone followed by binding to SH enzymes that are essential for cell proliferation and/or survival. Interestingly, levels of Cys‐DA are known to be elevated in the brains of patients who died from PD (Vauzour et al. 2008). The toxicity results from the facile autoxidation of these intermediates, thus producing reactive oxygen species. PD selectively targets catecholaminergic neurons, with a variable degree of involvement of the LC compared to the SN (Paulus and Jellinger 1991; Gesi et al. 2000; Zarow et al. 2003; Cebrián et al. 2014). It was clearly shown that patients with PD show a severe decrease of NM levels in their SN (around 60% compared to age‐matched controls) due to the loss of neurons containing NM (Zecca et al. 2002).
On the other hand, the noradrenergic neurotransmitter NE is also very easily oxidized at physiological pH to an o‐quinone that normally cyclizes and subsequently oxidatively polymerizes to black melanin. However, the oxidative behavior of NE is not much different from that of DA because the chemical reactivity is primarily dictated by the catechol moiety (Napolitano et al. 2011). As a matter of fact, besides the enzymatic oxidative deamination to DOMA and H2O2 and the subsequent degradation to various metabolites, NE also may take part in the formation of NM via a process that involves oxidation to NE‐o‐quinone followed by intramolecular cyclization. These reactive NE‐o‐quinones can also react with Cys or glutathione to produce neurotoxic compounds (Xin et al. 2000; Shen and Dryhurst 2001). For example, it is likely that NE‐o‐quinone could be scavenged by Cys leading to 5‐S‐Cys‐NE by the same mechanism as known for DA, thus influencing the vulnerability of noradrenergic neurons. An alternative (protective) pathway could be the following oxidation which may contribute to NM formation via a complex polymerization process (Manini et al. 2007). As previously described for NM biosynthesis in dopaminergic SN neurons (Sulzer et al. 2000), the synthesis of NM inside LC neurons could also be a protective process which removes these NE‐derived toxic compounds.
In conclusion, we demonstrated in this study that NE and 5‐S‐Cys‐NE are incorporated into LC‐NM by identifying two new compounds, CMT‐DA and 5‐S‐Cys‐CMT‐DA. We found that SN‐NM and LC‐NM contain melanin derived not only from DA and NE but also from several other catecholic metabolites that occur in catecholaminergic neurons such as DOPAC, DOPE, DOPA, DOMA, and DOPEG, in addition to the corresponding Cys‐derivatives in varying degrees. It is reasonable to conclude that DA, DOPAC, DOPE, and DOPA are mainly responsible for the structure of SN‐NM, while NE, DOMA, and DOPEG are responsible for the structure of LC‐NM in addition to DA, DOPAC, DOPE, and DOPA since abundant amounts of DA occur in LC neurons as a precursor of NE synthesis.
Supporting information
Figure S1. 1H‐NMR spectra of CMT‐DA.
Figure S2. 13C‐NMR spectra of CMT‐DA.
Figure S3. HRMS spectra of CMT‐DA.
Figure S4. 1H‐NMR spectra of 5‐S‐Cys‐CMT‐DA.
Figure S5. 13C‐NMR spectra of 5‐S‐Cys‐CMT‐DA.
Figure S6. HRMS spectra of 5‐S‐Cys‐CMT‐DA.
Figure S7. Extracted‐ion chromatogram (EIC) in Ion Trap LC‐MS of CMT‐DA.
Figure S8. Extracted‐ion chromatogram (EIC) in Ion Trap LC‐MS of LC‐NM.
Figure S9. Proposed mechanism for the production of CMT‐DA by hydrolysis of NE with 6 M HCl in the presence of thioglycolic acid and phenol.
Acknowledgments and conflict of interest disclosure
This work was supported by grants from the Japan Society for the Promotion of Science (JSPS) (No. 21500358, No. 24500450) given to KW and SI. FAZ and LZ were supported by a Lombardy Region—CNR 2013‐2015 Framework Agreement, an MbMM Project (18089/RCC) and by Italian Ministry of Education, University, and Research (MIUR)—Research Projects of National Interest (PRIN) 2010‐2011 prot. 2010M2JARJ. The authors also thank the Section of Legal Medicine and Insurances, Department of Biomedical Sciences for Health at the University of Milano for providing brain tissue samples. The authors declare no conflict of interest.
All experiments were conducted in compliance with the ARRIVE guidelines.
References
- Bogerts B. (1981) A brainstem atlas of catecholaminergic neurons in man, using melanin as natural marker. J. Comp. Neurol. 197, 63–80. [DOI] [PubMed] [Google Scholar]
- Cebrián C., Zucca F. A., Mauri P. et al (2014) MHC‐I expression renders catecholaminergic neurons susceptible to T‐cell‐mediated degeneration. Nat. Commun. 5, 3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M., Vanna R., Bellei C., Zucca F. A., Wakamatsu K., Monzani E., Ito S., Casella L. and Zecca L. (2012) Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS ONE 7, e48490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M., Soldani P., Giorgi F. S., Santinami A., Bonaccorsi I. and Fornai F. (2000) The role of the locus coeruleus in the development of Parkinson's disease. Neurosci. Biobehav. Rev. 24, 655–668. [DOI] [PubMed] [Google Scholar]
- Gieseg S. P., Simpson J. A., Charlton T. S., Duncan M. W. and Dean R. T. (1993) Protein‐bound 3,4‐dihydroxyphenylalanine is a major reductant formed during hydroxyl radical damage to proteins. Biochemistry 32, 4780–4786. [DOI] [PubMed] [Google Scholar]
- Graham D. G. (1979) On the origin and significance of neuromelanin. Arch. Pathol. Lab. Med. 103, 359–362. [PubMed] [Google Scholar]
- Greco G., Wakamatsu K., Panzella L., Ito S., Napolitano A. and d'Ischia M. (2009) Isomeric cysteinyldopas provide a (photo) degradable bulk component and a robust structural element in red human hair pheomelanin. Pigment Cell Melanoma Res. 22, 319–327. [DOI] [PubMed] [Google Scholar]
- d'Ischia M., Wakamatsu K., Napolitano A. et al (2013) Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 26, 616–633. [DOI] [PubMed] [Google Scholar]
- Ito S. (1989) Optimization of conditions for preparing synthetic pheomelanin. Pigment Cell Res. 2, 53–56. [DOI] [PubMed] [Google Scholar]
- Ito S. and Fujita K. (1985) Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 144, 527–536. [DOI] [PubMed] [Google Scholar]
- Ito S. and Wakamatsu K. (2003) Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 16, 523–531. [DOI] [PubMed] [Google Scholar]
- Ito S. and Wakamatsu K. (2008) Chemistry of mixed melanogenesis: pivotal roles of dopaquinone. Photochem. Photobiol. 84, 582–592. [DOI] [PubMed] [Google Scholar]
- Ito S., Fujita K., Yoshioka M., Sienko D. and Nagatsu T. (1986) Identification of 5‐S‐ and 2‐S‐cysteinyldopamine and 5‐S‐glutathionyldopamine formed from dopamine by high‐performance liquid chromatography with electrochemical detection. J. Chromatogr. 375, 134–140. [Google Scholar]
- Ito S., Nakanishi Y., Valenzuela R. K., Brilliant M. H., Kolbe L. and Wakamatsu K. (2011) Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 24, 605–613. [DOI] [PubMed] [Google Scholar]
- Land E. J. and Riley P. A. (2000) Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Res. 13, 273–277. [DOI] [PubMed] [Google Scholar]
- Manini P., Panzella L., Napolitano A. and d'Ischia M. (2007) Oxidation chemistry of norepinephrine: partitioning of the O‐quinone between competing cyclization and chain breakdown pathways and their roles in melanin formation. Chem. Res. Toxicol. 20, 1549–1555. [DOI] [PubMed] [Google Scholar]
- Mann D. M. A. and Yates P. O. (1974) Lipoprotein pigments‐ their relationship to ageing in the human nervous system. II. The melanin content of pigmented nerve cells. Brain 97, 489–498. [DOI] [PubMed] [Google Scholar]
- Mann D. M. A. and Yates P. O. (1983) Possible role of neuromelanin in the pathogenesis of Parkinson's disease. Mech. Ageing Dev. 21, 193–203. [DOI] [PubMed] [Google Scholar]
- Marsden C. D. (1983) Neuromelanin and Parkinson's disease. J. Neural Transm. Suppl. 19, 121–141. [PubMed] [Google Scholar]
- Murakami K., Wakamatsu K., Nakanishi Y., Takahashi H., Sugiyama S. and Ito S. (2007) Serum levels of pigmentation markers are elevated in patients undergoing hemodialysis. Blood Purif. 25, 483–489. [DOI] [PubMed] [Google Scholar]
- Napolitano A., Manini P. and d'Ischia M. (2011) Oxidation of chemistry of catecholamines and neuronal degeneration: an update. Curr. Med. Chem. 18, 1832–1845. [DOI] [PubMed] [Google Scholar]
- Paulus W. and Jellinger K. (1991) The neuropathologic basis of different clinical subgroups of Parkinson's disease. J. Neuropathol. Exp. Neurol. 50, 743–755. [DOI] [PubMed] [Google Scholar]
- Rosengren E., Linder‐Eliasson E. and Carlsson A. (1985) Detection of 5‐S‐cysteinyldopamine in human brain. J. Neural. Transm. 63, 247–253. [DOI] [PubMed] [Google Scholar]
- Saper C. B. and Petito C. K. (1982) Correspondence of melanin‐pigmented neurons in human brain with A1‐A14 catecholamine cell groups. Brain 105, 87–101. [DOI] [PubMed] [Google Scholar]
- Segura‐Aguilar J., Metodiewa D. and Baez S. (2001) The possible role of one‐electron reduction of aminochrome in the neurodegenerative process of the dopaminergic system. Neurotox. Res. 3, 157–165. [DOI] [PubMed] [Google Scholar]
- Segura‐Aguilar J., Paris I., Muñoz P., Ferrari E., Zecca L. and Zucca F. A. (2014) Protective and toxic roles of dopamine in Parkinson's disease. J. Neurochem. 129, 898–915. [DOI] [PubMed] [Google Scholar]
- Shen X. M. and Dryhurst G. (2001) Influence of glutathione on the oxidation chemistry of 5‐S‐cysteinyldopamine: potentially neuroprotective reactions of relevance to Parkinson's disease. Tetrahedron 57, 393–405. [Google Scholar]
- Sulzer D., Bogulavsky J., Larsen K. E. et al (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl Acad. Sci. USA 97, 11869–11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Mosharov E., Talloczy Z., Zucca F. A., Simon J. D. and Zecca L. (2008) Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. J. Neurochem. 106, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauzour D., Ravaioli G., Vafeiadou K., Rodriguez‐Mateos A., Angeloni C. and Spencer J. P. E. (2008) Peroxynitrite induced formation of the neurotoxins 5‐S‐cysteinyl‐dopamine and DHBT‐1: implications for Parkinson's disease and protection by polyphenols. Arch. Biochem. Biophys. 476, 145–151. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K. and Ito S. (1994) Improved HPLC determination of 5‐S‐cysteinyldopa in serum. Clin. Chem. 40, 495–496. [PubMed] [Google Scholar]
- Wakamatsu K., Ito S. and Rees J. L. (2002) Usefulness of 4‐amino‐3‐hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Res. 15, 225–232. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Fujikawa K., Zucca F. A., Zecca L. and Ito S. (2003) The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 86, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Murase Y., Zucca F. A., Zecca L. and Ito S. (2012) Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 25, 793–802. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Tanaka H., Tabuchi K., Ojika M., Zucca F. A., Zecca L. and Ito S. (2014) Reduction of the nitro group to amine by hydroiodic acid to synthesize o‐aminophenol derivatives as putative degradative markers of neuromelanin. Molecules 19, 8039–8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin W., Shen X. M., Li H. and Dryhurst G. (2000) Oxidative metabolites of 5‐S‐cysteinylnorepinephrine are irreversible inhibitors of mitochondrial complex I and the alpha‐ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes: possible implications for neurodegenerative brain disorders. Chem. Res. Toxicol. 13, 749–760. [DOI] [PubMed] [Google Scholar]
- Zarow C., Lyness S. A., Mortimer J. A. and Chui H. C. (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 60, 337–341. [DOI] [PubMed] [Google Scholar]
- Zecca L., Costi P., Mecacci C., Ito S., Terreni M. and Sonnino S. (2000) Interaction of human substantia nigra neuromelanin with lipids and peptides. J. Neurochem. 74, 1758–1765. [DOI] [PubMed] [Google Scholar]
- Zecca L., Fariello R., Riederer P., Sulzer D., Gatti A. and Tampellini D. (2002) The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Lett. 510, 216–220. [DOI] [PubMed] [Google Scholar]
- Zecca L., Zucca F. A., Wilms H. and Sulzer D. (2003) Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 26, 578–580. [DOI] [PubMed] [Google Scholar]
- Zecca L., Stroppolo A., Gatti A. et al (2004) The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl Acad. Sci. USA 101, 9843–9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L., Zucca F. A., Albertini A., Rizzio E. and Fariello R. G. (2006) A proposed dual role of neuromelanin in the pathogenesis of Parkinson's disease. Neurology 67(7 Suppl 2), S8–S11. [DOI] [PubMed] [Google Scholar]
- Zecca L., Bellei C., Costi P. et al (2008) New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl Acad. Sci. USA 105, 17567–17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. and Dryhurst G. (1993) Oxidation chemistry of dopamine: possible insights into the age‐dependent loss of dopaminergic nigrostriatal neurons. Bioorg. Chem. 21, 392–410. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 1H‐NMR spectra of CMT‐DA.
Figure S2. 13C‐NMR spectra of CMT‐DA.
Figure S3. HRMS spectra of CMT‐DA.
Figure S4. 1H‐NMR spectra of 5‐S‐Cys‐CMT‐DA.
Figure S5. 13C‐NMR spectra of 5‐S‐Cys‐CMT‐DA.
Figure S6. HRMS spectra of 5‐S‐Cys‐CMT‐DA.
Figure S7. Extracted‐ion chromatogram (EIC) in Ion Trap LC‐MS of CMT‐DA.
Figure S8. Extracted‐ion chromatogram (EIC) in Ion Trap LC‐MS of LC‐NM.
Figure S9. Proposed mechanism for the production of CMT‐DA by hydrolysis of NE with 6 M HCl in the presence of thioglycolic acid and phenol.


