Abstract
Objectives.
We examined the degree to which online sentence processing and offline sentence memory differed among older adults who showed risk for amnestic and nonamnestic varieties of mild cognitive impairment (MCI), based on psychometric classification.
Method.
Participants (N = 439) read a series of sentences in a self-paced word-by-word reading paradigm for subsequent recall and completed a standardized cognitive test battery. Participants were classified into 3 groups: unimpaired controls (N = 281), amnestic MCI (N = 94), or nonamnestic MCI (N = 64).
Results.
Relative to controls, both MCI groups had poorer sentence memory and showed reduced sentence wrap-up effects, indicating reduced allocation to semantic integration processes. Wrap-up effects predicted subsequent recall in the control and nonamnestic groups. The amnestic MCI group showed poorer recall than the nonamnestic MCI group, and only the amnestic MCI group showed no relationship between sentence wrap-up and recall.
Discussion.
Our findings suggest that psychometrically defined sub-types of MCI are associated with unique deficits in sentence processing and can differentiate between the engagement of attentional resources during reading and the effectiveness of engaging attentional resources in producing improved memory.
Key Words: Aging, Mild cognitive impairment, Reading, Sentence memory, Sentence processing
Normative age-related changes in language comprehension are multifaceted in nature, with older adults showing patterns of both gains and losses. Although deficits in certain effortful aspects of language are found in normal aging (Federmeier, 2007; Payne, Grison, et al., 2014; Payne, Gross, et al., 2014; Payne & Stine-Morrow, 2012; Stine-Morrow et al., 2008; Wlotko, Lee, & Federmeier et al., 2010), many aspects of language comprehension and production are spared until very late in life among healthy older adults (see Burke & Shafto, 2008; Wingfield & Stine-Morrow, 2000 for reviews). In contrast, declines in language processing and comprehension are widespread and substantially accelerated among older adults with pathological cognitive impairments, including dementia of the Alzheimer’s type (DAT), frontotemporal dementia, and Parkinson’s disease (Grossman et al., 1996; Kempler, Almor, Tyler, Andersen, & MacDonald, 1998; MacDonald, Almor, Henderson, Kempler, & Andersen, 2001; Waters & Caplan, 2002; see Kempler & Goral, 2008 for a review). At the same time, little research has examined the extent to which language comprehension is impaired among older adults who show early signs of cognitive impairment, but who do not meet the clinical criteria for dementia. In the current study, we examined the degree to which online sentence processing and offline sentence memory differed among older adults who showed risk for amnestic and nonamnestic varieties of mild cognitive impairment (MCI) based on psychometric classification.
Mild Cognitive Impairment
MCI is a heterogeneous condition, characterized as a transient pre-demented state that occurs between normal and pathological cognitive aging (Petersen, 2004). Like DAT, MCI is a clinically diagnosed syndrome, marked by the following characteristics: concern regarding a change in cognition, impairment in one or more cognitive domains, preservation of independence of functional abilities, and no clinical diagnosis of dementia (Albert et al., 2011; Petersen, 2004). It is common to classify subgroups of MCI into amnestic (aMCI) types, which are characterized by a selective and severe deficit in episodic memory, and nonamnestic (nMCI) types, which are defined as a deficit in one or more nonmemory cognitive domains. Generally, individuals diagnosed with MCI show a substantially higher risk of transition to dementia (Geslani, Tierney, Herrmann, & Szalai, 2005; Morris et al., 2001).
A number of researchers have utilized data from prospective studies in order to develop psychometrically defined classifications for MCI risk based solely on performance on standardized neuropsychological tasks (Cook et al., 2013; Crowe et al., 2006; Ganguli et al., 2010; Jak et al., 2009; Ritchie, Artero, & Touchon, 2001; O’Connor, Edwards, Wadley, & Crowe, 2010; Wadley et al., 2007). The psychometric approach, which relies only on empirical data, has shown overlap in classification when compared with clinical consensus (Clark et al., 2013). In addition, psychometrically defined MCI predicts subsequent progression to Alzheimer’s disease and severe cognitive impairment (Cook et al., 2013; Damian et al., 2013; Ganguli et al., 2010), declines in functional abilities (Wadley et al., 2007), and memory complaints and later memory decline (Crowe et al., 2006; cf. Hutchens et al., 2013).
Aging, Sentence Processing, and Sentence Memory
We (Miller & Stine-Morrow, 1998; Payne, Gao, Noh, Anderson, & Stine-Morrow, 2012; Stine, 1990; Stine-Morrow, Milinder, Pullara, & Herman, 2001; Stine-Morrow, Miller, et al., 2008; Stine-Morrow, Shake, Miles, & Noh, 2006) have used the self-paced moving window paradigm along with item-level regression techniques (Aaronson & Scarborough, 1977; Just & Carpenter, 1980; Lorch & Myers, 1990; Millis, Simon, & Tenbroek, 1998; Schroeder, 2011) to examine age differences in online allocation during reading, and its relationship with subsequent text memory. Collectively, these studies have found that age differences in text memory can be explained to some extent by a reduced allocation of time to semantic integration processes during reading (see Stine-Morrow & Miller, 2009 for a review).
For example, the wrap-up effect is characterized by an increase in processing time at the ends of clause and sentence boundaries (Fallon, Peelle, & Wingfield, 2006; Payne & Stine-Morrow, 2012; Rayner et al., 2000; Stine-Morrow, Shake, Miles, Lee, & McConkie, 2010). Because the wrap-up effect increases as a function of variables and manipulations presumed to increase the difficulty of constructing a coherent semantic representation, it is often interpreted as a measure of attentional allocation to semantic integration. In fact, wrap-up (i.e., the extra time allocated to processing sentence-final words relative to baseline reading time) is correlated with subsequent recall such that deficits in text memory are magnified among older adults who fail to allocate attention at clause and sentence boundaries (Smiler, Gagne, & Stine-Morrow, 2003; Stine, 1990; see Stine-Morrow & Miller, 2009; Stine-Morrow, Miller, & Hertzog, 2006 for reviews). Such findings implicate wrap-up as a potential compensatory mechanism that older adults may be able to exploit during sentence processing to optimize comprehension in the face of cognitive declines. At the same time, there is evidence that wrap-up is cognitively demanding, especially for relatively larger language segments and especially for older readers (Payne & Stine-Morrow, 2012, 2014). This may be why older readers are sometimes found to allocate less time to sentence wrap-up (e.g., Miller & Stine-Morrow, 1998). Even when older readers do allocate time to wrap-up, it appears to require higher levels of wrap-up for them to reach equivalent levels of recall relative to their younger counterparts.
The Current Study
Our goal was to examine the extent to which sentence processing, and its effectiveness in contributing to memory performance, vary within an older group as a function of cognitive status. Although a small literature exists examining the effects of MCI on lexical processing and fluency (see Taler & Phillips, 2008 for a review), there are very few studies examining the extent to which sentence and text understanding is impaired among older adults who are at risk for MCI (but see Chapman et al., 2002). In the current study, we examined online sentence processing and sentence memory among a sample of older adults with psychometrically defined MCI (Cook et al., 2013). Because clinical consensus is the current “gold standard” for MCI classification (Petersen, 2004), we are conservative in our interpretation, referring to the psychometrically defined amnestic and nonamnestic groups as being at higher risk for these subtypes of MCI (cf. Clark et al., 2013).
Although we hypothesized that memory for text would be poorer in individuals at risk for MCI, our main interest was in characterizing the mechanisms underlying this effect, focusing in particular on semantic integration processes (Payne & Stine-Morrow, 2012; Stine-Morrow et al., 2008). We hypothesized that individuals at risk for MCI would show less allocation of attention to message-level semantic processing, which could, in part, explain deficits in subsequent sentence memory. However, given evidence for individual differences in processing effectiveness (Stine & Hindman, 1994; Stine-Morrow et al., 2008), we also explored the possibility that the allocation of time to these processes have less of an impact on recall among those at risk for MCI.
Method
Participants
Participants were 439 community-dwelling older adults. These data are reported from the Senior Odyssey project (Stine-Morrow et al., 2008; Stine-Morrow, Parisi, Morrow, Greene, & Park, 2007), a cognitive intervention study investigating the effects of intellectual engagement on cognition among a sample of relatively inactive (<10hr of planned activities) older adults. These data are based on baseline measures, before participants were randomly assigned to an experimental or control group. Demographics for the participants are included in Table 1. The participants had normal or corrected-to-normal vision and were screened for incident dementia risk such that all participants in the sample scored above 23 on the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975). However, even with the MMSE screening, there was a great deal of variability in performance in cognitive function and risk for MCI (cf. Cook et al., 2013).
Table 1.
Means and 95% Confidence Intervals for Demographics, Neuropsychological Test Performance, and Sentence Comprehension in UC, nMCI, and aMCI
| Controls (N = 281) | nMCI (N = 94) | aMCI (N = 64) | |
|---|---|---|---|
| Age | 70.99 [70.17, 71.81] | 73.39 [71.70, 75.08] | 77.55 [75.59, 79.51] |
| Proportion female | 0.77 [0.71, 0.82] | 0.78 [0.70, 0.86] | 0.53 [0.41, 0.65] |
| Education | 16.04 [15.74,16.33] | 14.27 [13.78, 14.76] | 14.85 [14.20, 15.50] |
| Neuropsychological performance | |||
| MoCA | 27.16 [26.90, 27.41] | 24.62 [24.13, 25.11] | 22.70 [21.86, 23.54] |
| MMSE | 28.88 [28.76, 28.99] | 27.9 [27.61, 28.19] | 27.05 [26.64, 27.46] |
| Psychomotor speed (z) | 0.30 [0.22, 0.38] | −0.53 [−0.67, −0.39] | −0.63 [−0.83, −0.43] |
| Verbal ability (z) | 0.37 [0.29, 0.45] | −0.85 [−1.03, −0.67] | −0.50 [−0.77, −0.22] |
| Visual–spatial (z) | 0.37 [0.29, 0.44] | −0.64 [−0.80, −0.48] | −0.68 [−0.88, −0.48] |
| Reasoning (z) | 0.36 [0.26, 0.46] | −0.65 [−0.77, −0.53] | −0.63 [−0.83, −0.43] |
| Episodic memory (z) | 0.34 [0.28, 0.39] | 0.03 [−0.07, 0.13] | −1.68 [−1.82, −1.54] |
| Sentence processing and sentence recall | |||
| Word length effect (in ms) | 44 [37, 52] | 55 [43, 66] | 51 [25, 77] |
| Word frequency effect (in ms) | 33 [29, 37] | 48 [39, 57] | 38 [27, 50] |
| New concept effect (in ms) | 91 [76, 105] | 91 [54, 128] | 69 [48, 89] |
| Sentence wrap-up effect (in ms) | 109 [94, 124] | 77 [51, 103] | 74 [44, 103] |
| Proportion of propositions recalled | 0.51 [0.49, 0.53] | 0.39 [0.35, 0.43] | 0.33 [0.29, 0.37] |
Note. Sentence processing effects are average unstandardized regression coefficients from subject-level multiple regression models (see text for more details).
Measures for Psychometric MCI Classification
The following measures were used from our broader neuropsychological battery, which were chosen to match closely with recent studies using psychometric MCI classification in the ACTIVE study (Cook et al., 2013; Crowe et al., 2006).
Episodic Memory.–
Episodic memory was measured using three variables derived from performance on the Hopkins Verbal Learning Test (Benedict, Schretlen, Groninger, & Brandt, 1998), each of which is based on memory accuracy; the total number of words remembered over three trials, delayed recall, and the recognition discrimination index.
Visuospatial Processing.–
Two instruments were used to identify a construct for visuospatial processing: the Card-Rotation and Hidden Patterns tasks (Ekstrom, French, Harman, & Dermen, 1976). These tasks require participants to identify visual patterns in an array in which spatial rotation and/or visual transformation is required. The final score for each task was the total number of correctly identified items within the allotted time limits.
Psychomotor Speed.–
Three tasks were used to identify a psychomotor speed construct: the letter and pattern comparison tasks (Salthouse & Babcock, 1991) and the identical pictures task (Ekstrom et al., 1976). Each of these tasks requires that the participants make speeded judgments in comparing simple stimuli. The final score for each task was the total number of correct items within the allotted time limits.
Verbal Ability.–
Verbal ability was measured with the extended range and advanced vocabulary tasks (Ekstrom et al., 1976) and the North American Adult Reading Test (Uttl, 2002). The final score for each task was the total number of accurate responses within the allotted time limits.
Executive Reasoning.—
Reasoning was measured with the Letter Sets, Number Sets, and Letter Series tasks (Ekstrom et al., 1976). These tasks require participants to identify patterns in a series of items and either generate the next item in the series or decide which item does not adhere to the pattern. The final score for each task was the total number of accurate responses within the allotted time limits.
The Montreal Cognitive Assessment.–
In addition to the earlier measures, which were used for the psychometric classification, we also assessed risk for MCI via the Montreal Cognitive Assessment (MoCA), allowing a validity check of our psychometric classification. The MoCA is a common clinical screening assessment to test for MCI and shows high sensitivity in both normal and clinical samples of older adults (Nasreddine et al., 2005; Smith, Gildeh, & Holmes, 2007). The test assesses several cognitive domains, including attention and executive functions, memory, language, and visuospatial abilities. The maximum possible score is 30, with cut-off scores ranging between 20 and 26 across different samples (Nasreddine et al., 2005; Waldron-Perrine & Axelrod, 2012).
Data Analyses and Psychometric Algorithm for MCI Assessment
In order to classify individuals in the current study into at-risk groups for amnestic and nonamnestic MCI, we adapted the psychometric classification algorithm used in the ACTIVE trials (Ball et al., 2002), described in detail in Cook et al. (2013) (see also Crowe et al., 2006; Wadley et al., 2007). One difference between the current classification scheme and that from Cook and colleagues is that we opted not to perform our classification separately across stratified demographic groups (e.g., age, education). Instead, these demographic covariates were statistically controlled in our models examining group differences. This allows us (a) to treat these covariates continuously, allowing for maximum power to detect effects (see, e.g., MacCallum, Zhang, Preacher, & Rucker, 2002), and (b) use a uniform single classification scheme for the whole sample, rather than classifying individuals within subgroups, which would result in unstable classification given the small subgroup sample sizes after demographic stratification. To estimate ability within the five domains of cognition (Episodic Memory, Psychomotor Speed, Verbal Ability, Visuospatial Processing, and Reasoning), scores for each measure were standardized and equal-weight composites were formed by averaging the standardized scores for each measure. Individuals whose composite scores were 1 SD below the mean (i.e., <~16th percentile) were considered impaired in that domain (see Jak et al., 2009). Because the distributions of the average composites were largely normal, there was very little deviation between classifications based on 1 SD cut-offs used in the current study and those based on average percentile scoring, as was done in Cook and colleagues. Using this impairment score, individuals were classified into one of three groups: Unimpaired Controls (UC; no impairment in any domain), Amnestic MCI (aMCI; an impairment in the episodic memory domain, regardless of whether impairments were evident in other domains), or Nonamnestic MCI (nMCI; an impairment in one or more domains, but no episodic memory impairment). Following classification of individuals into groups at risk for MCI, we then examined group differences in sentence processing and sentence recall.
Data analyses focused on effect size estimation and quantifying the precision of these effect sizes using 95% confidence intervals in order to assess degree of group differences across these outcome measures (Cumming, 2014; Kelley & Preacher, 2012). Cohen’s d is presented as a standardized effect size. Models are also presented correcting for demographic covariates using general linear regression. All data analyses were conducted with R (R Core Team, 2013).
Procedure
A word-by-word sentence-reading task was used in the current study. Participants read two-sentence passages that were presented on a 19-inch (48.3cm) Dell M782 monitor set to a resolution of 1024×768 pixels, controlled by a Dell 3.20 GHz computer. MATLAB software (Mathworks, Inc., Natick, MA) was used to control presentation and record millisecond reading times. Participants read each passage word by word, using the moving window method (Aaronson & Ferres, 1984). On a fixed but randomly selected third of the sentences, participants were instructed to recall the information from the sentence they had just read (immediate recall). Participants recalled sentences aloud, and this was recorded and transcribed for later scoring.
Sentence Processing Task
Each participant read a set of 24 sentences adapted from the stimuli in Stine-Morrow and colleagues (2001). Each sentence contained 18 words and was followed by a filler sentence to ensure that retrieval planning did not contaminate reading times on the sentence-final word. These filler sentences were not analyzed. Each of the words was coded for a set of four variables reflecting attentional allocation to text processing demands. Word-level variables included: (a) the number of syllables and (b) the natural logarithm of word-frequency, using norms from Francis and Kucera (1982). These two variables reflect the word-level automatic and obligatory processes of orthographic decoding and lexical access, respectively. Variables reflecting message-level semantic processing included: (c) whether a word was a new concept noun in the sentence (Haberlandt, Graesser, Schneider, & Kiely, 1986) and whether the word occurred at a sentence boundary (i.e., sentence wrap-up). These variables have consistently been shown to affect reading times (Chin et al., 2014; Payne et al., 2012; Schroeder, 2011; Stine-Morrow, Milinder, et al., 2001; Stine-Morrow, Miller, et al., 2008) among both younger and older readers.
Separate item-level regression models were fit to the data for each subject in order to decompose word-by-word reading times into components reflecting time allocation to each word-level predictor (e.g., Lorch & Myers, 1990; Stine-Morrow, Miller, et al., 2008). All words were analyzed. Reading times for each participant were regressed onto the number of syllables per word, word frequency, the presence of a new concept noun, and if the word marked a sentence boundary in a ordinary least squares multiple regression model. Thus, for each participant, each regression coefficient can be interpreted as the relative increase in reading time in response to a unit increase in the predictor, controlling for all other predictors. Subject-specific unstandardized regression coefficients were then analyzed as a function of MCI group.
Results
Psychometric Assessment of MCI
Our psychometric classification was used to create three groups. A total of 281 individuals performed better than 1 SD below the mean on all tasks and were designated as UC. There were 64 individuals who showed a deficit of 1 SD or more in the episodic memory domain (regardless of scores in nonmemory tasks) and were thus designated as at-risk for amnestic MCI (aMCI). Finally, 94 individuals showed a deficit of 1 SD or more on one or more nonmemory domains and were designated as at risk for nonamnestic MCI (nMCI).
The top rows of Table 1 present demographic differences between the groups. The nMCI group was older (Cohen’s d (d) = 0.31; 95% CI = [0.12, 0.51]) and had fewer years of education (d = 0.73; 95% CI = [0.51, 0.94]) than UC. The aMCI group was older (d = 0.88; 95% CI = [0.63, 1.15]), had fewer years of education (d = 0.46; 95% CI = [0.21, 0.71]), and had a larger proportion of men than the UC group (d = 0.53; 95% CI = [0.28, 0.78]). Education level did not differ between aMCI and nMCI groups, but the aMCI group was older (d = 0.53; 95% CI = [0.25, 0.83]) and had a larger proportion of men (d = 0.56; 95% CI = [0.27, 0.85]) than the nMCI group. These findings are in line with the prior literature showing higher risk for clinically assessed MCI among adults who are older, male, and have lower educational level (Roberts et al., 2012).
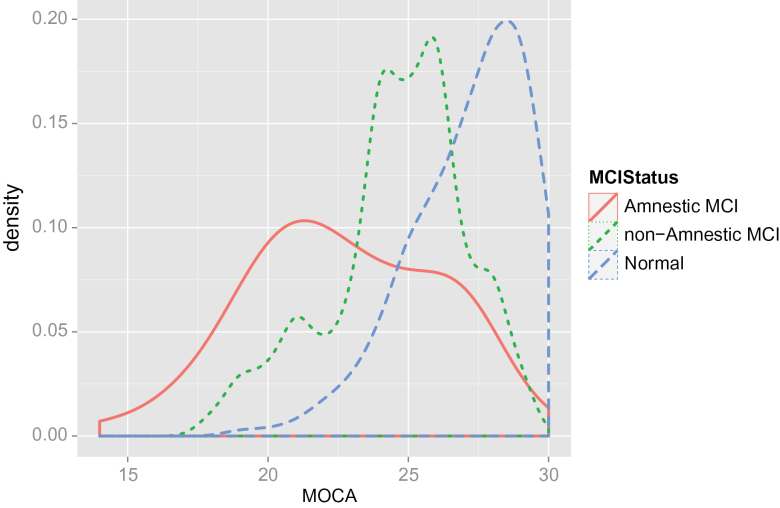
We also examined differences in MoCA scores across the three groups. We found that MoCA scores patterned with our psychometric MCI classification (see Table 1). However, the aMCI group had a larger variance in MoCA scores, suggesting that a small proportion of individuals with substantial memory impairments may still be able to score within the normal range on the MoCA (cf. Waldron-Perrine & Axelrod, 2012). This is presented graphically in Figure 1, which plots the smoothed density distribution for the three groups. The figure shows clear separation of distributions by group, with a larger variance in MoCA scores across the aMCI group.
Figure 1.
Smoothed probability density plot for distribution of MoCA scores of participants classified as normal, aMCI, and nMCI.
Online Sentence Processing Time
The lower rows of Table 1 present the mean reading time components for the effects of word length, frequency, new concept noun, and sentence boundary on reading times separately for UC, aMCI, and nMCI participants.
None of the groups differed significantly in effects of word length on reading times, suggesting that MCI risk did not affect orthographic processing. However, the nMCI group did show evidence for a larger effect of word frequency on reading times compared with the UC group (d = 0.40; 95% CI = [0.20, 0.61]), and the aMCI group (d = 0.22; 95% CI = [0.03, 0.44]). In other words, those in the nMCI group allocated differentially more time to process low-frequency words. To test for the unique effects of MCI status, a linear model was fit to the data with age, educational level, and gender entered as covariates. The adjusted model showed that nMCI individuals were still reliably slower at lexical processing compared with UC (b = 15ms; 95% CI = [7, 23]).
Older adults at risk for amnestic MCI showed weaker effects of new concept on reading times compared with the UC group (d = 0.24; 95% CI = [0.04, 0.43]). In a model adjusting for age, educational level, and gender, this effect failed to reach statistical significance (b = 23ms, 95% CI = [−18, 64]), suggesting that the group difference may be explained in part by demographic differences between the groups.
Sentence wrap-up effects were reliably smaller among groups at risk for nonamnestic MCI (M = 77ms) (d = 0.24; 95% CI = [0.06, 0.44]) and amnestic MCI (M = 74ms) (d = 0.29; 95%CI = [0.09, 0.48]), compared with controls (M = 109ms). In a model correcting for age, educational level, and gender, the difference in wrap-up between the nMCI and UC groups (b = 32ms; 95% CI = [3, 61] and the aMCI and UC groups (b = 35ms; 95% CI = [2, 68]) remained reliable.
Sentence Recall
The bottom row of Table 1 shows the proportion of propositions correctly recalled from test sentences for UC, aMCI, and nMCI groups. As expected, the proportion of propositions correctly recalled was lower for the nMCI group (M = 0.39) (d = 0.77; 95% CI = [0.57, 0.97]) and the aMCI group (M = 0.33) (d = 1.16; 95%CI = [0.94, 1.37]) compared with the UC older adults (M = 0.51). In addition, recall was reliably worse among the aMCI group compared with the nMCI group (d = 0.37; 95% CI = [0.19, 0.57]). All effects remained reliable in a model that adjusted for age, educational level, and gender.
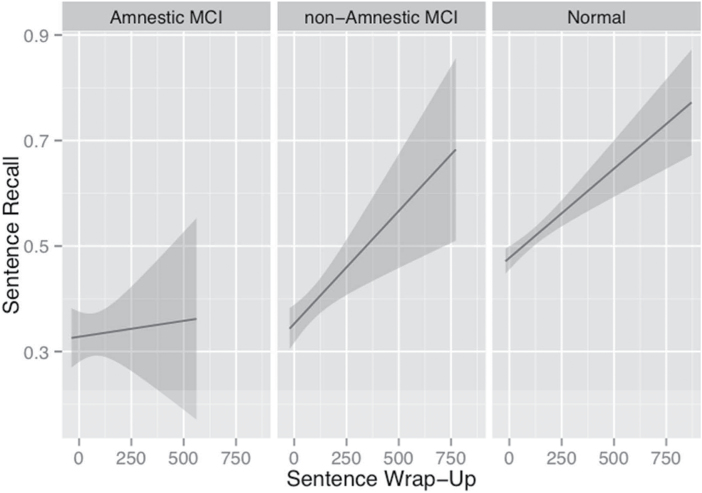
We have previously shown that, in healthy older adults, there is a robust relationship between the magnitude of sentence wrap-up effects and subsequent recall, suggesting that wrap-up reflects a cognitively demanding process in which message-level semantic information is integrated across clauses and sentences in order to enable a stable representation in memory (Payne et al., 2012; Payne & Stine-Morrow, 2012, 2014; Stine-Morrow et al., 2008). In order to test whether the relationship between attentional allocation at sentence boundaries and recall is disrupted among adults at risk for MCI, we fit a linear regression model to the recall data, including sentence wrap-up, MCI group (UC, nMCI, aMCI), and the interaction between sentence wrap-up and MCI group as predictors in the model. Because the MCI group has three levels, two contrasts were formed, with UC as the reference group (C1 = UC vs. nMCI, C2 = UC vs. aMCI). The results from this model are presented graphically in Figure 2. We found a reliable interaction between C2 and sentence wrap-up (b = 0.004, 95% CI = [0.003, 0.005]). As seen in Figure 2, this effect was found because there was a reliable relationship between sentence wrap-up and recall in the unimpaired control group (b = 0.0004, t = 4.12), and in the nonamnestic MCI group (b = 0.0004, t = 3.48), but no reliable relationship was found between wrap-up and recall among the amnestic MCI group (b = 6.04×10−5, t = 0.32). All effects remained reliable in a model that adjusted for age, educational level, and gender.
Figure 2.
Relationship between sentence wrap-up effect and recall for participants classified as normal, aMCI, and nMCI.
Discussion
The aim of the current study was to test whether risk for MCI was associated with deficits in online sentence processing and offline recall during reading. As expected, those with MCI showed poorer sentence memory relative to unimpaired controls. Our primary interest was whether we could localize the source of these memory deficits in comprehension processes during reading, in particular, semantic integration processes that support text memory.
Importantly, we found smaller wrap-up effects among both nMCI and aMCI groups relative to unimpaired controls. Prior work has shown that wrap-up effects are cognitively demanding among healthy older adults (Payne & Stine-Morrow, 2012, 2014; Smiler et al., 2003). For example, Payne and Stine-Morrow (2012) showed that the perceptual span during reading is reduced selectively at sentence boundary sites for older readers only, indicating an increase in cognitive workload that disrupts low-level parafoveal visual processing of upcoming words (cf. Henderson & Ferreira, 1990; White, Rayner, & Liversedge, 2005). Thus, older adults at risk for MCI may allocate less time overall at sentence boundaries in order to avoid the demands associated with sentence-level integration (cf. Miller & Stine-Morrow, 1998). However, there was still variability in wrap-up effects among both the nMCI and aMCI groups (see Table 1) such that that some individuals in these groups showed wrap-up effects similar to those in the unimpaired control group.
Even though nMCI and aMCI groups did not differ at wrap-up, they did differ in memory performance. This appeared to be a consequence of unequal benefits from allocation of time to semantic integration at sentence boundaries in nMCI and aMCI groups. Those individuals in the aMCI group who did allocate this effort showed no benefits in recall, whereas healthy adults and those at risk for nMCI did. Thus, the aMCI group, who showed substantial episodic memory impairments, appeared to reach a functional threshold whereby allocating disproportionate effort did not result in improved memory performance, as is seen in healthy older adults. This is analogous to the so-called “labor in vain” effect (cf. Nelson & Leonesio, 1988; Stine-Morrow, Shake, et al., 2006) in the memory literature whereby increased allocation of effort yields no benefits in terms of later performance.
Collectively, these findings suggest that sentence memory deficits in those at risk for MCI are attributable to two different factors: (a) the implementation of a semantic integration strategy for creating a robust memory representation of the text, and (b) the effectiveness of this strategy for creating and/or retaining the representation. Memory deficits in the nMCI group were attributable to reduced allocation to sentence integration; the effort they invested in this process was as effective as it was among cognitively intact older adults, suggesting that remediation centered on increasing wrap-up (e.g., Stine-Morrow, Noh, & Shake, 2010) may be a viable pathway to compensation in this group. Individuals at risk for aMCI, however, neither allocated as much time to conceptual integration as cognitively intact older adults, nor benefited from it, suggesting that any intervention with this group may need to focus on fundamental comprehension processes.
This raises the question as to why those in the aMCI group allocated effort to wrap-up at all. It is likely to some extent that micropauses associated with salient boundaries such as sentence endings are part of the proceduralized motor skills of reading (Hirotani, Frazier, & Rayner, 2006; Perfetti, 1989) that are resistant to declines. An open question is whether the representation is fleetingly created, but decays quickly (e.g., Radvansky, Zwaan, Curiel, & Copeland, 2001), or whether it is not constructed at all. More research is needed to examine these possibilities.
We also found MCI to be related to changes in lexical processing. The increased word-frequency effect found among nMCI adults suggests that this group had increased difficulty in processing lower frequency words during reading, compared with aMCI and control participants. This effect patterns with existing literature suggesting impairments in lexical semantic processing revealed by performance on standardized naming tasks (Dwolatzky et al., 2003; Grundman et al., 2004), and lexical decision tasks (Taler & Jarema, 2006). Duong, Whitehead, Hanratty, and Chertkow (2006) have argued that MCI impairs intentional and controlled lexical processing but spares automatic lexical processing. Because the lexical impairment found in the current study occurs during naturalistic reading, in a task without explicit response demands, this suggests that, at least during reading for comprehension, risk for nMCI can impair automatic aspects of lexical processing (cf. Taler & Jarema, 2006). Interestingly, the largest deficit in neuropsychological performance in the nMCI group was in verbal ability (see Table 1), indicating that a deficit in lexical knowledge was the largest factor contributing to the classification into this group, and is consistent with work in healthy older adults showing that the lexical processing is related to verbal ability (Stine-Morrow et al., 2008).
There currently exists very little research investigating changes in text understanding in aMCI adults. One exception to this is a study by Chapman and colleagues (2002), who examined differences in discourse comprehension and memory among healthy older adults, DAT patients, and older adults diagnosed with MCI. Levels of both gist and specific recall were lower among adults with MCI. In fact, MCI participants performed within the range of a sample of DAT patients. Similar to Chapman and colleagues (2002), we also found substantial deficits in text memory for adults at risk for nMCI and aMCI. However, our findings extend those of Chapman and colleagues by examining the processing mechanisms during comprehension that may, in part, be responsible for the observed age-related declines in memory for text.
Our findings have implications for clinical and applied domains of MCI research as well. First, language measures have not received a great deal of focus as part of diagnostic tools in MCI. Taler and Phillips (2008) argued that by focusing on lexical semantic processing, clinicians might be able to detect earlier and subtler declines in semantic abilities that are often seen in very early prodromal stages of Alzheimer’s disease. Our current findings are consistent with this, and extend this by suggesting that focusing on message-level semantic processing in sentence understanding provides a more complete picture of the neuropsychological profile of individuals at risk for MCI.
Additionally, our findings have implications for health and medical literacy, and the design of patient educational materials. Health-related literacy skills decline with normative and nonnormative aging, compromising the ability of older adults to comprehend health information from text (cf. Cahana-Amitay et al., 2013; Levinthal, Morrow, Tu, Wu, & Murray, 2008; Morrow, Hier, Menard, & Leirer, 1998; Morrow, Weiner, Steinley, Young, & Murray, 2007). Indeed, Chin and colleagues (2014) recently showed that both lexical processing and message-level semantic integration processes, such as clause- and sentence wrap-up, support understanding of health information in sentence processing in a sample of primarily hypertensive older adults. In the current study, we found that older adults at risk for amnestic MCI could not improve their recall, even among those who allocated disproportionately more time to semantic processing, at least as assessed by sentence wrap-up. Given the degree to which MCI affects both lexical and message-level semantic processing in reading, it may be possible to design health and medical educational material to mitigate such deficits, for example, by including more high-frequency words and simpler syntax with marked clause boundaries to signal more frequent clause wrap-up (Chin et al., 2014). Future work will benefit from examining whether adults with MCI can adopt other compensatory strategies such as re-reading, which may potentially aid in the recall of propositional information in this group. Moreover, because both the current study and Chin and colleagues (2014) have focused on sentence processing in isolation, it is important for future work to address the degree to which these findings generalize to larger multi-sentence discourses (cf. Stine-Morrow et al., 2008).
Some limitations in the current study should be addressed. First, this study did not employ a clinical consensus for MCI classification (Petersen, 2004). Instead, we relied on psychometric classification, based on participants’ performance on a wide variety of neuropsychological tasks (Cook et al., 2013; Ganguli et al., 2010; Jak et al., 2009; Ritchie et al., 2001). Thus, we can only draw the inference that the groups we identified reflect individuals with a higher risk factor for clinical MCI (see Clark et al., 2013). It is worth noting that our classification showed some external validity, patterning with the clinical literature in showing similar demographic characteristics (Roberts et al., 2012), and expected distributions of scores on the MoCA, a clinical screening tool for identifying MCI risk (Nasreddine et al., 2005).
Another limitation of the current study is that our sentence-processing task relied on a corpus-based approach, using naturalistic sentences (cf. Kliegl, Grabner, Rolfs, & Engbert, 2004; Kuperman & Van Dyke, 2011). In future research, the effects of MCI on sentence comprehension should be examined in well-designed experiments in which other potential linguistic influences have been completely controlled (cf. Payne & Stine-Morrow, 2012).
Despite these limitations, the current findings are valuable in showing that message-level semantic processing during reading is impaired among adults at risk for MCI, and that by examining both online processing and offline sentence memory, we can distinguish between the effects of MCI on the engagement of processing strategies during reading and their effectiveness in producing improved sentence memory.
Funding
This research was supported by the National Institute on Aging (R01 AG029475 and R01 AG013935). The first author was supported by a National Institutes of Health training grant (T32-HD055272) and a Beckman Institute predoctoral fellowship during the preparation of this manuscript.
References
- Aaronson D., Ferres S. (1984). The word-by-word reading paradigm: An experimental and theoretical approach. New Methods in Reading Comprehension Research, 31–68.
- Aaronson D., Scarborough H. S. (1977). Performance theories for sentence coding: Some quantitative models. Journal of Verbal Learning and Verbal Behavior, 16, 277–303. Retrieved from http://dx.doi.org/10.1016/S0022-5371(77)80052-2 [Google Scholar]
- Albert M. S., DeKosky S. T., Dickson D., Dubois B., Feldman H. H., Fox N. C., … Phelps C. H. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines from Alzheimer’s disease. Alzheimer’s & Dementia, 7, 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K., Berch D. B., Helmers K. F., Jobe J. B., Leveck M. D., Marsiske M. … & ACTIVE Study Group. (2002). Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA, 288, 2271–2281. Retrieved from http://dx.doi.org/10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R. H., Schretlen D., Groninger L., Brandt J. (1998). Hopkins Verbal Learning Test–Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist, 12, 43–55. Retrieved from http://dx.doi.org/10.1076/clin.12.1.43.1726 [Google Scholar]
- Burke D. M., Shafto M. A. (2008). Language and aging. In Craik F. I. M., Salthouse T. A. (Eds.), The handbook of aging and cognition (3rd ed., pp. 373–443). New York, NY: Psychology Press. [Google Scholar]
- Cahana-Amitay D., Albert M. L., Ojo E. A., Sayers J., Goral M., Obler L. K., Spiro A. (2013). Effects of hypertension and diabetes on sentence comprehension in aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68, 513–521. 10.1093/geronb/gbs085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S. B., Zientz J., Weiner M., Rosenberg R., Frawley W., Burns M. H. (2002). Discourse changes in early Alzheimer’s disease, mild cognitive impairment and normal aging. Alzheimer Disease and Associated Disorders, 16, 177–186. 10.1097/00002093-200207000-00008 [DOI] [PubMed] [Google Scholar]
- Chin J., Payne B. R., Gao X., Stine-Morrow E. A. L., Morrow D. G., Conner-Garcia T., … Murray M. D.(2014). Knowledge influences comprehension and memory for health information among older adults: Distinguishing the effects of domain-general and domain-specific knowledge. Memory. 10.1097/00002093-200207000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. R., Delano-Wood L., Libon D. J., McDonald C. R., Nation D. A., Bangen K. J., … Bondi M. W. (2013). Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society, 19, 1–11. 10.1017/S1355617713000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. E., Marsiske M., Thomas K. R., Unverzagt F. W., Wadley V. G., Langbaum J. B., Crowe M. (2013). Identification of mild cognitive impairment in ACTIVE: Algorithmic classification and stability. Journal of the International Neuropsychological Society, 19, 73. 10.1017/S1355617712000938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe M., Andel R., Wadley V., Cook S., Unverzagt F., Marsiske M., Ball K. (2006). Subjective cognitive function and decline among older adults with psychometrically defined amnestic MCI. International Journal of Geriatric Psychiatry, 21, 1187–1192. 10.1002/gps.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming G. (2014). The new statistics why and how. Psychological Science, 25, 7–29. 10.1177/0956797613504966 [DOI] [PubMed] [Google Scholar]
- Damian M., Hausner L., Jekel K., Richter M., Froelich L., Almkvist O., … Visser P. J. (2013). Single-domain amnestic mild cognitive impairment identified by cluster analysis predicts Alzheimer’s disease in the European prospective DESCRIPA study. Dementia and Geriatric Cognitive Disorders, 36, 1–19. 10.1159/000348354 [DOI] [PubMed] [Google Scholar]
- Duong A., Whitehead V., Hanratty K., Chertkow H. (2006). The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia, 44, 1928–1935. 10.1016/j.neuropsychologia.2006.01.034 [DOI] [PubMed] [Google Scholar]
- Dwolatzky T., Whitehead V., Doniger G. M., Simon E. S., Schweiger A., Jaffe D., Chertkow H. (2003). Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatrics, 3, 4. 10.1186/1471-2318-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom R.B., French J. W., Harman H. H., Dermen D. (1976). Manual for kit of factor referenced cognitive tests. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Fallon, M., Peelle, J. E., & Wingfield, A. (2006). Spoken sentence process-ing in young and older adults modulated by task demands Evidence from self-paced listening. The Journals of Gerontology Series B Psychological Sciences and Social Sciences, 61, 10–17. 10.1093/geronb/61.1.P10 [DOI] [PubMed]
- Francis W. N., Kucera H. (1982). Frequency Analysis of English Usage: Lexicon and Grammar. Boston, MA: Houghton Mifflin. [Google Scholar]
- Farias S. T., Chand V., Bonnici L., Baynes K., Harvey D., Mungas D., … Reed B. (2012). Idea density measured in late life predicts subsequent cognitive trajectories: Implications for the measurement of cognitive reserve. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 67, 677–686. 10.1016/j.neuropsychologia.2006.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier K. D. (2007). Thinking ahead: The role and roots of prediction in language comprehension. Psychophysiology, 44, 491–505. 10.1111/j.1469-8986.2007.00531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. Retrieved from http://dx.doi.org/10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Ganguli M., Chang C. C. H., Snitz B. E., Saxton J. A., Vanderbilt J., Lee C. W. (2010). Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. American Journal of Geriatric Psychiatry, 18, 674–683. 10.1097/JGP.0b013e3181cdee4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geslani D. M., Tierney M. C., Herrmann N., Szalai J. P. (2005). Mild cognitive impairment: An operational definition and its conversion rate to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 19, 383–389. Retrieved from http://dx.doi.org/10.1159/000084709 [DOI] [PubMed] [Google Scholar]
- Grundman M., Petersen R. C., Ferris S. H., Thomas R. G., Aisen P. S., Bennett D. A. … & Thal L. J. (2004) . Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology, 61, 59–66. 10.1001/archneur.61.1.59 [DOI] [PubMed] [Google Scholar]
- Haberlandt K. F., Graesser A. C., Schneider N. J., Kiely J. (1986). Effects of task and new arguments on word reading times. Journal of Memory and Language, 25, 314–322. Retrieved from http://dx.doi.org/10.1016/0749-596X(86)90004-5 [Google Scholar]
- Henderson J. M., Ferreira F. (1990). Effects of foveal processing difficulty on the perceptual span in reading: Implications for attention and eye movement control. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16, 417. 10.1037/0278-7393.16.3.417 [DOI] [PubMed] [Google Scholar]
- Hirotani M., Frazier L., Rayner K. (2006). Punctuation and intonation effects on clause and sentence wrap-up: Evidence from eye movements. Journal of Memory and Language, 54, 425–443. Retrieved from http://dx.doi.org/10.1016/j.jml.2005.12.001 [Google Scholar]
- Hutchens R. L., Kinsella G. J., Ong B., Pike K. E., Clare L., Ames D., … Parsons S. (2013). Relationship between control beliefs, strategy use, and memory performance in amnestic mild cognitive impairment and healthy aging. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68, 862–871. 10.1093/geronb/gbt016 [DOI] [PubMed] [Google Scholar]
- Jak A. J., Bondi M. W., Delano-Wood L., Wierenga C., Corey-Bloom J., Salmon D. P., Delis D. C. (2009). Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry, 17, 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just M. A., Carpenter P. A. (1980). A theory of reading: From eye fixations to comprehension. Psychological Review, 87, 329–354. Retrieved from http://dx.doi.org/10.1037/0033-295X.87.4.329 [PubMed] [Google Scholar]
- Kelley K., Preacher K. J. (2012). On effect size. Psychological Methods, 17, 137. 10.1037/a0028086 [DOI] [PubMed] [Google Scholar]
- Kempler D., Almor A., Tyler L. K., Andersen E. S., MacDonald M. C. (1998). Sentence comprehension deficits in Alzheimer’s disease: A comparison of off-line vs. online sentence processing. Brain and Language, 64, 297–316. Retrieved from http://dx.doi.org/10.1006/brln.1998.1980 [DOI] [PubMed] [Google Scholar]
- Kempler D., Goral M. (2008). Language and dementia: Neuropsychological aspects. Annual Review of Applied Linguistics, 28, 73–90. 10.1017/S0267190508080045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R., Grabner E., Rolfs M., Engbert R. (2004). Length, frequency, and predictability effects of words on eye movements in reading. European Journal of Cognitive Psychology, 16, 262–284. 10.1080/09541440340000213 [Google Scholar]
- Kuperman V., Van Dyke J. A. (2011). Effects of individual differences in verbal skills on eye-movement patterns during sentence reading. Journal of Memory and Language, 65, 42–73. 10.1016/j.jml.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal B. R., Morrow D. G., Tu W., Wu J., Murray M. D. (2008). Cognition and health literacy in patients with hypertension. Journal of General Internal Medicine, 23, 1172–1176. Retrieved from http://dx.doi.org/10.1007/s11606-008-0612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch R. F., Myers J. L. (1990). Regression analyses of repeated measures data in cognitive research. Journal of Experimental Psychology: Learning, Memory, and Cognition, 16, 149–157. Retrieved from http://dx.doi.org/10.1037/0278-7393.16.1.149 [DOI] [PubMed] [Google Scholar]
- MacCallum R. C., Zhang S., Preacher K. J., Rucker D. D. (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7, 19–40. Retrieved from http://dx.doi.org/10.1037/1082-989X.7.1.19 [DOI] [PubMed] [Google Scholar]
- MacDonald M. C., Almor A., Henderson V. W., Kempler D., Andersen E. S. (2001). Assessing working memory and language comprehension in Alzheimer’s disease. Brain and Language, 78, 17–42. 10.1006/brln.2000.2436 [DOI] [PubMed] [Google Scholar]
- Miller L. M. S., Stine-Morrow E. A. L. (1998). Aging and the effects of knowledge on on-line reading strategies. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 53, 223–233. 10.1093/geronb/53B.4.P223 [DOI] [PubMed] [Google Scholar]
- Millis K. K., Simon S., Tenbroek N. S. (1998). Resource allocation during the rereading of scientific texts. Memory & Cognition, 26, 232–246. Retrieved from http://dx.doi.org/10.3758/BF03201136 [DOI] [PubMed] [Google Scholar]
- Morris J. C., Storandt M., Miller J. P., McKeel D. W., Price J. L., Rubin E. H., Berg L. (2001). Mild cognitive impairment represents early-stage Alzheimer disease. Archives of Neurology, 58, 397–405. 10.1001/archneur.58.3.397 [DOI] [PubMed] [Google Scholar]
- Morrow D. G., Hier C. M., Menard W. E., Leirer V. O. (1998). Icons improve older and younger adults’ comprehension of medication information. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 53, 240–254. 10.1093/geronb/53B.4.P240 [DOI] [PubMed] [Google Scholar]
- Morrow D. G., Weiner M., Steinley D., Young J., Murray M. D. (2007). Patients’ health literacy and experience with instructions influence preferences for heart failure medication instructions. Journal of Aging and Health, 19, 575–593. Retrieved from http://dx.doi.org/10.1177/0898264307304448 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., … Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society, 53, 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nelson T. O., Leonesio R. J. (1988). Allocation of self-paced study time and the “labor-in-vain effect”. Journal of Experimental Psychology: Learning, Memory, and Cognition, 14, 676. 10.1037/0278-7393.14.4.676 [DOI] [PubMed] [Google Scholar]
- O’Connor M. L., Edwards J. D., Wadley V. G., Crowe M. (2010). Changes in mobility among older adults with psychometrically defined mild cognitive impairment. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65, 306–316. 10.1093/geronb/gbq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. R., Gao X., Noh S. R., Anderson C. J., Stine-Morrow E. A. L. (2012). The effects of print exposure on sentence processing and memory among older adults: Evidence for efficiency and reserve. Aging, Neuropsychology, and Cognition, 19, 122–149. 10.1080/13825585.2011.628376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. R., Grison S., Gao X., Christianson K., Morrow D. G., Stine-Morrow E. A. L. (2014). Aging and individual differences in binding during sentence understanding: Evidence from temporary and global syntactic attachment ambiguities. Cognition, 130, 157–173. 10.1016/j.cognition.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. R., Gross A., Parisi J., Marsiske M., Rebok G., Stine-Morrow E. A. L. (2014). Modeling longitudinal changes in older adults’ memory for spoken discourse: Findings from the ACTIVE cohort. Memory. 10.1080/09658211.2013.861916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. R., Stine-Morrow E. A. L. (2012). Aging, parafoveal preview, and semantic integration in sentence processing: Testing the cognitive workload of wrap-up. Psychology and Aging, 27, 638–649. 10.1037/a0026540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. R., Stine-Morrow E. A. L. (2014). Adult age differences in wrap-up during sentence comprehension: Evidence from ex-Gaussian distributional analyses of reading time. Psychology and Aging, 29, 213–228. 10.1037/a0036282 [DOI] [PubMed] [Google Scholar]
- Perfetti C. A. (1989). There are generalized abilities and one of them is reading. In Resnick L. B. (Ed.), Knowing, learning, and instruction (pp. 307–335). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Petersen R. C. (2004). Mild cognitive impairment. Journal of Internal Medicine, 256, 183–1–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Radvansky, G. A., Zwaan, R. A., Curiel, J. M., & Copeland, D. E. (2001). Situation models and aging. Psychology and Aging, 16, 145–160. Retrieved from http://dx.doi.org/10.1037/0882-7974.16.1.145 [DOI] [PubMed] [Google Scholar]
- Rayner, K., Kambe, G., & Duffy, S. A. (2000). The effect of clause wrap-up on eye movements during reading. The Quarterly Journal of Experimental Psychology Section A, 53, 1061–1080. Retrieved from http//dx..org/10.1080/713755934 [DOI] [PubMed]
- R Core Team. (2013). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013.
- Ritchie K, Artero S, & Touchon J. (2001). Classification criteria for mild cognitive impairment: A population-based validation study. Neurology, 56, 37–42. 10.1212/WNL.56.1.37 [DOI] [PubMed] [Google Scholar]
- Roberts R. O., Geda Y. E., Knopman D. S., Cha R. H., Pankratz V. S., Boeve B. F., … Petersen R. C. (2012). The incidence of MCI differs by subtype and is higher in men: The Mayo Clinic Study of Aging. Neurology, 78, 342–351. 10.1212/WNL.0b013e3182452862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A., Babcock R. L. (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27, 763–776. Retrieved from http://dx.doi.org/10.1037/0012-1649.27.5.763 [Google Scholar]
- Schroeder S. (2011). What readers have and do: Effects of students’ verbal ability and reading time components on comprehension with and without text availability. Journal of Educational Psychology, 103, 877. 10.1037/a0023731 [Google Scholar]
- Smiler A. P., Gagne D. D., Stine-Morrow E. A. L. (2003). Aging, memory load, and resource allocation during reading. Psychology and Aging, 18, 203–209. 10.1037/0882-7974.18.2.203 [DOI] [PubMed] [Google Scholar]
- Smith T., Gildeh N., Holmes C. (2007). The Montreal Cognitive Assessment: Validity and utility in a memory clinic setting. Canadian Journal of Psychiatry, 52, 329. [DOI] [PubMed] [Google Scholar]
- Stine E. A. L. (1990). On-line processing of written text by younger and older adults. Psychology and Aging, 5, 68–78. 10.1037/0882-7974.5.1.68 [DOI] [PubMed] [Google Scholar]
- Stine E. A. L., Hindman J. (1994). Age differences in reading time allocation for propositionally dense sentences. Aging and Cognition, 1, 2–16. [Google Scholar]
- Stine-Morrow E. A. L., Milinder L., Pullara P., Herman B. (2001). Patterns of resource allocation are reliable among younger and older readers. Psychology and Aging, 16, 69–84. 10.1037/0882-7974.16.1.69 [DOI] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Miller L. M. S. (2009). Aging, self-regulation, and learning from text. Psychology of Learning and Motivation, 51, 255–285. 10.1016/S0079-7421(09)51008-0 [Google Scholar]
- Stine-Morrow E. A. L., Miller L. M. S., Gagne D. D., Hertzog C. (2008). Self-regulated reading in adulthood. Psychology and Aging, 23, 131–153. 10.1037/0882-7974.23.1.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Miller L. M. S., Hertzog C. (2006). Aging and self regulated language processing. Psychological Bulletin, 132, 582–606. 10.1037/0033-2909.132.4.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Noh S. R., Shake M. C. (2010). Age differences in the effects of conceptual integration training on resource allocation in sentence processing. Quarterly Journal of Experimental Psychology, 63, 1430–1455. 10.1080/17470210903330983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Parisi J. M., Morrow D. G., Park D. C. (2008). The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychology and Aging, 23, 778–786. 10.1037/a0014341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Parisi J. M., Morrow D. G., Greene J. C., Park D. C. (2007). An engagement model of cognitive optimization through adulthood. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 62, 62–69. 10.1093/geronb/62.special_issue_1.62 [DOI] [PubMed] [Google Scholar]
- Stine-Morrow E. A. L., Shake M. C., Miles J. R., Lee K., McConkie G. W. (2010). Pay now or pay later: Aging and the role of boundary salience in self-regulation of conceptual integration in sentence processing. Psychology and Aging, 25, 168–176. 10.1037/a0018127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A., Shake M. C., Miles J. R., Noh S. R. (2006). Adult age differences in the effects of goals on self-regulated sentence processing. Psychology and Aging, 21, 790. 10.1037/0882-7974.21.4.790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler V., Jarema G. (2006). On-line lexical processing in AD and MCI: An early measure of cognitive impairment? Journal of Neurolinguistics, 19, 38–55. 10.1016/j.jneuroling.2005.07.002 [Google Scholar]
- Taler V., Phillips N. A. (2008). Language performance in Alzheimer’s disease and mild cognitive impairment: A comparative review. Journal of Clinical and Experimental Neuropsychology, 30, 501–556. 10.1080/13803390701550128 [DOI] [PubMed] [Google Scholar]
- Uttl B. (2002). North American Adult Reading Time: Age norms, reliability, and validity. Journal of Clinical and Experimental Neuropsychology, 24, 1123–1137. 10.1076/jcen.24.8.1123.8375 [DOI] [PubMed] [Google Scholar]
- Wadley V. G., Crowe M., Marsiske M., Cook S. E., Unverzagt F. W., Rosenberg A. L., Rexroth D. (2007). Changes in everyday function in individuals with psychometrically defined mild cognitive impairment in the Advanced Cognitive Training for Independent and Vital Elderly Study. Journal of the American Geriatrics Society, 55, 1192–1198. Retrieved from http://dx.doi.org/10.1111/j.1532-5415.2007.01245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron-Perrine B., Axelrod B. N. (2012). Determining an appropriate cutting score for indication of impairment on the Montreal Cognitive Assessment. International Journal of Geriatric Psychiatry, 27, 1189–1194. 10.1002/gps.3768 [DOI] [PubMed] [Google Scholar]
- Waters G., Caplan D. (2002). Working memory and online syntactic processing in Alzheimer’s disease: Studies with auditory moving window presentation. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 57, 298–311. 10.1093/geronb/57.4.P298 [DOI] [PubMed] [Google Scholar]
- White S. J., Rayner K., Liversedge S. P. (2005). Eye movements and the modulation of parafoveal processing by foveal processing difficulty: A reexamination. Psychonomic Bulletin & Review, 12, 891–896. 10.3758/BF03196782 [DOI] [PubMed] [Google Scholar]
- Wingfield A., Stine-Morrow E. A. L. (2000). Language and speech. In Craik F. I. M., Salthouse T. A. (Eds.), The handbook of aging and cognition (2nd ed., pp. 359–416). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Wlotko E., Lee C., Federmeier K. D. (2010). Language of the aging brain: Event-related potential studies of comprehension in older adults. Language and Linguistics Compass, 4, 623–638. 10.1111/j.1749-818X.2010.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]