Abstract
A gap in the leptospirosis field remains the lack of well-characterized sample collections that allow for comparison of new methods to standard ones. In the context of a flood-related outbreak of leptospirosis evaluated in Anuradhapura, Sri Lanka, a specimen bank was obtained with detailed metadata accompanied by gold standard diagnostic test results. Blood samples collected on admission and 14 days later from suspected cases of leptospirosis were tested using microscopic agglutination test (MAT) (17 serovars), an in-house enzyme-linked immunosorbent assay (ELISA) using a locally obtained strain of Leptospira kirschneri as sonicated antigen, a commercially available ELISA based on sonicated Leptospira biflexa, and a quantitative polymerase chain reaction (qPCR) assay targeting the pathogenic Leptospira-specific 16S rRNA gene. Of 62 patients presenting within the first 2 days of illness, 31 had confirmed leptospirosis based either on paired-sample MAT or qPCR. During the acute phase, qPCR was most sensitive, detecting 74% of definitively diagnosed cases; immunoglobulin G (IgG) ELISA (in-house), IgG ELISA (commercial), and MAT had sensitivities of 35.5%, 12.0%, and 22.6%, respectively, in detecting definitively diagnosed cases using acute phase serum. Of 40 patients with paired sera, 10 were qPCR positive. Of these, five samples were negative by paired-sample MAT. Of the 11 MAT-positive samples, only five were detected using qPCR confirming that both tests are needed for maximal sensitivity. Regional leptospiral serovar-specific IgG ELISA was superior to MAT. Knowing the regionally dominant serovars improves serological sensitivity in the analysis of acute specimens by ELISA, but qPCR was most sensitive in this patient population.
Introduction
Worldwide, around 1 million cases and 48,000 deaths are conservatively attributed annually to leptospirosis.1–4 In the tropics and subtropics, leptospirosis remains increasing as a major public health threat. The highest incidence of leptospirosis is in the Asia–Pacific region (Seychelles, India, Sri Lanka, and Thailand), South America, and Central America, and the Caribbean (Trinidad and Tobago, Barbados, Jamaica, El Salvador, Uruguay, Cuba, Nicaragua, and Costa Rica) with incidences over 10 per 100,000 people.5 Tropical countries without reported cases or small numbers of cases are most probably due to underdiagnosis or underreporting. An increasing number of leptospirosis outbreaks have been reported over the past few decades, mostly associated with environmental changes and travel.6 Leptospirosis is widely considered to be a reemerging tropical disease.7
Globally—in the industrialized and developing worlds alike—the point-of-care and clinically actionable diagnosis of leptospirosis remains a major challenge for clinicians and public health authorities. Large numbers of rapid tests are being developed, but the optimal test for leptospirosis diagnosis is yet to be developed.8 Traditionally, the microscopic agglutination test (MAT) has been used as the gold standard for leptospirosis diagnosis, but has been limited to use in reference laboratories.9 A large prospective study from Thailand convincingly showed that the MAT is not a perfect test.10 Other work has shown that the use of a quantitative polymerase chain reaction (qPCR) increases the diagnostic sensitivity even in comparison to the MAT using paired serum sample.11 This shows that a combination of MAT plus molecular testing ought to be considered as a gold standard.
Since 2008, studies from Sri Lanka have reported one of the highest global incidences of leptospirosis.12 Endemic disease and outbreaks associated with floods and recreational activities have been reported recently.13–17 In 2011, a major outbreak of leptospirosis was reported from Anuradhapura, a district located in a dry area in North Central part of the country. Control of this outbreak and treatment were a challenge to all clinicians due to two reasons; first the clinical presentation was unusual and the classical “Weil's disease” was not observed among these patients; second, diagnostic facilities were not available routinely for early diagnosis. We previously reported preliminary confirmation of the etiology of this outbreak using molecular methods,13 which showed that there were dengue and hantavirus infections mimicking leptospirosis during this outbreak as well.18 The purpose of the this study was to describe the clinical and biochemical features of this post-flood leptospirosis outbreak, with a particular focus on identifying the most useful laboratory methods to provide definitive, actionable diagnosis.
Methods
Details of the study methodology have been previously described.13 Briefly, this cross-sectional descriptive study was carried out in the Teaching Hospital Anuradhapura (THA), Sri Lanka. The study was carried out from February to May, 2011, during an outbreak of leptospirosis related to unusual heavy rains and floods.
All clinically suspected cases of leptospirosis were included in this study. Our previous work has shown that the surveillance case definition used by the epidemiological unit of Sri Lanka and proposed by the World Health Organization19 had a low sensitivity and usually included moderate to severe cases only. For this study, we included even the mild cases with fever, headache with or without myalgia, and at least one other symptom/sign (oliguria, polyuria, conjunctival hemorrhage, conjunctival suffusion, dyspnea, and chest pain), supported by epidemiological evidence. We used a previously published protocol for leptospirosis investigations for all the procedures with case definitions as described (Table 1).20
Table 1.
Case definitions
| Confirmed |
| Detection of pathogenic Leptospira spp. DNA in a suspected case of leptospirosis using qPCR |
| A positive MAT test |
| Seroconversion |
| Fourfold increase in MAT titer |
| A single high MAT titer ≥ 400 |
| Probable |
| Positive MAT titer < 1/400 |
| Positive ELISA test (IgM/IgG BfR, IgG Virion) |
| Possible |
| Clinically suspected case of leptospirosis (with negative or unconfirmed laboratory test) |
ELISA = enzyme-linked immunosorbent assay; IgG = immunoglobulin G; IgM = immunoglobulin M; MAT = microscopic agglutination test.
This study included patients at least 13 years of age because of the admission criteria of patients to “internal medicine” wards. A medically qualified graduate visited the wards every day to screen fever patients and select potential cases. A standardized clinical data checklist and an interviewer-administered questionnaire were used for data collection. A registered nurse obtained acute and convalescent blood samples. The first sample was taken at the time of recruitment and the second sample at least 14 days after the first sample (mostly through a follow-up clinic visit). All samples were stored in −20°C until analyzed.
Basic clinical laboratory diagnostic tests such as full blood counts were performed on blood samples at the THA microbiology department. Diagnostic tests were carried out in two laboratories. qPCR was performed at the University of California, San Diego, CA. Procedure of DNA extraction, qPCR primers, and conditions have been previously described.13
Two different ELISA tests and standard MAT tests were carried out in the Federal Institute for Risk Assessment, Berlin. For the in-house ELISA (ELISA-BfR), protein was extracted from 1–2 × 108 Leptospira kirschneri serovar Grippotyphosa/mL. Bacteria (5 mL) were washed thrice in phosphate buffered saline (PBS), freeze/thawed twice for 5 minutes in liquid nitrogen, and sonicated thrice for 30 seconds, with 0.5 seconds pulses with a Sonoplus HD 200 ultrasound homogenizer (Bandelin) at maximum amplitude. The protein extract was centrifuged at 20,000 × g for 30 minutes and the concentration determined by bicinchoninic acid method (Thermo Scientific Pierce). The optimal protein concentration (0.325 μg/mL) used for coating ELISA plates (50 μL/well) was determined by checkerboard analysis. Each human serum sample was diluted 1:100 in PBS Tween 20, added and incubated for 30 minutes at 37°C. The plates were washed four times with PBS. Subsequently, rabbit anti-human immunoglobulin M (IgM) (1:800) and/or IgG (1:6,000) peroxidase conjugate (Sigma-Aldrich) were added and incubated for 30 minutes at 37°C. The plates were washed four times with PBS, 100 μL TMB Microvell peroxidase substrate (KPL) added for 30 minutes at room temperature and the reaction stopped by addition of 100 μL TMB BlueStop (KPL). The optical density was measured at 630 nm using the MRX, Dynatech Plate Reader. The cut-off value was determined by receiver operating characteristic analysis.21 In addition to this in-house method, commercially available IgG ELISA kit (Institut Viron Serion GmgH, Warburg, Germany) was also used according to the manufacturer's instructions.
The MAT was performed according to the standard protocol.22 Seventeen reference strains comprising 14 serogroups and 17 serovars (Australis, Autumnalis, Bataviae, Bratislava, Canicola, Copenhageni, Grippotyphosa, Hardjo, Hebdomadis, Pomona, Saxkoebing, Sejroe, Tarassovi, Ballum, Icterohaemorrhagiae, Pyrogenes, and Javanica) were used.
Ethics statement.
The Ethics Review Committee of Faculty of Medicine and Allied Sciences, Rajarata University of Sri Lanka approved this study. All subjects more than 16 years old provided written informed consent to participate. Children of ages 14–16 years provided assent and, for them, written parental consent was obtained.
Results
A total of 96 clinically suspected cases of leptospirosis were studied. Paired serum samples were available from 40 patients.
Case confirmation.
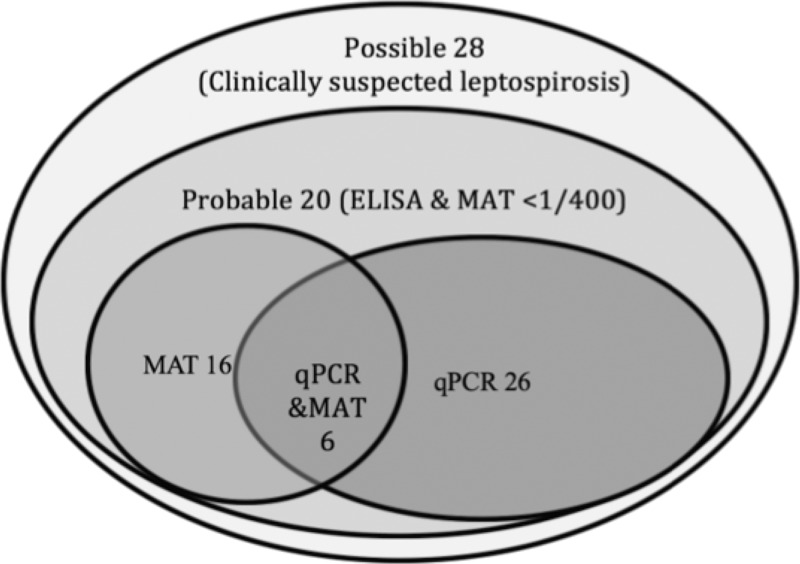
Of the 96 possible cases of leptospirosis, 48 (50%) were confirmed either by detection of Leptospira DNA in blood (N = 26), positive MAT test (N = 16), or both (N = 6) (Figure 1 ).
Figure 1.
Case confirmation of 96 suspected cases of leptospirosis.
Eight patients had a positive MAT result, but with a titer < 1/400. Another 12 patients showed a positive ELISA result in the IgM/, IgG BfR, or IgG Virion test (Table 2). All those 20 patients were classified as “probable” cases.
Table 2.
Laboratory confirmation of 96 clinically suspected cases of leptospirosis from Anuradhapura, Sri Lanka, 2011
| n (%) | |
|---|---|
| Confirmed (N = 48) | |
| qPCR only | 26 (27.1) |
| MAT only | |
| Seroconversion | 5 (5.2) |
| Fourfold rise | 2 (2.1) |
| Single MAT titer > 1/400 | 9 (9.4) |
| MAT and qPCR | |
| Four-fold rise | 1 (1) |
| Single MAT titer > 1/400 | 5 (5.2) |
| Probable (positive ELISA or positive MAT titer < 1/400) | 20 (20.8) |
| Possible (clinically suspected leptospirosis) | 28 (29.2) |
ELISA = enzyme-linked immunosorbent assay; MAT = microscopic agglutination test; qPCR = quantitative polymerase chain reaction.
Of the 48 confirmed cases, 33 (68.8%) were male. The mean age of the confirmed cases was 41 (standard deviation 12) years.
Clinical profile of the study sample.
Fever, chills, and headaches were the main presenting complaints on admission of all confirmed cases. Well-known clinical features of Weil's disease such as conjunctival suffusion (N = 20, 41.7%) and jaundice (N = 9, 18.7%) were observed in less than 50% of the patients (Table 3).
Table 3.
Clinical profile of 96 clinically suspected cases of leptospirosis from Anuradhapura, Sri Lanka, 2011
| Confirmed (N = 48) | Probable (N = 20) | Possible (N = 28) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Fever | 48 (100) | 20 (100) | 28 (100) |
| Chills | 48 (100) | 18 (90) | 27 (96.4) |
| Anorexia | 43 (89.6) | 15 (75) | 23 (82.1) |
| Arthalgia | 43 (89.6) | 17 (85) | 20 (71.4) |
| Muscle tenderness | 38 (79.2) | 17 (85) | 18 (64.3) |
| Myalgia | 37 (77.1) | 16 (80) | 23 (82.1) |
| Nausea and vomiting | 36 (75) | 17 (85) | 23 (82.1) |
| Headache | 35 (72.9) | 14 (70) | 16 (57.1) |
| Abdominal pain | 32 (66.7) | 8 (40) | 9 (32.1) |
| Conjuctival suffusion | 20 (41.7) | 5 (25) | 5 (17.9) |
| Prostration | 17 (35.4) | 5 (25) | 6 (21.4) |
| Oliguria | 15 (31.3) | 5 (25) | 8 (28.6) |
| Diarrhea | 11 (22.9) | 4 (20) | 5 (17.9) |
| Positive Kernig sign | 11 (22.9) | 6 (30) | 5 (17.9) |
| Cough | 9 (18.8) | 2 (10) | 4 (14.3) |
| Jaundice | 9 (18.8) | 6 (30) | 4 (14.3) |
| Shortness of breath | 9 (18.8) | 3 (15) | 3 (10.7) |
| Hypotension | 8 (16.7) | 3 (15) | 3 (10.7) |
| Neck stiffness | 8 (16.7) | 6 (30) | 6 (21.4) |
| Hematuria | 6 (12.5) | 2 (10) | 2 (7.1) |
| Hematemesis | 5 (10.4) | 1 (5) | 1 (3.6) |
| Skin rash | 5 (10.4) | 2 (10) | 3 (10.7) |
| Hepatomegaly | 4 (8.3) | 0 (0) | 3 (10.7) |
| Pharyngitis | 4 (8.3) | 3 (15) | 2 (7.1) |
| Photophobia | 4 (8.3) | 3 (15) | 3 (10.7) |
| Proteinuria | 3 (6.3) | 1 (5) | 1 (3.6) |
| Anuria | 2 (4.2) | 1 (5) | 0 (0) |
Hematological profiles in study population.
Leukopenia (< 4,000), leukocytosis (> 11,000), and thrombocytopenia (< 150,000) developed among 9 (18.8%), 16 (33.3%), and 36 (75%) patients (Table 4). Six (12.5%) patients had severe thrombocytopenia (< 20,000).
Table 4.
Biochemical and hematological test results of 96 clinically suspected cases of leptospirosis from Anuradhapura, Sri Lanka, 2011
| Confirmed (N = 48) | Probable (N = 20) | Possible (N = 28) | |
|---|---|---|---|
| median (IQR) | median (IQR) | median (IQR) | |
| Maximum WBC | 8,800 (7,450–12,250) | 13,200 (10,750–14,000) | 7,000 (6,000–12,050) |
| Minimum WBC | 6,200 (4,450–8,200) | 6,950 (5,150–8,000) | 5,100 (3,500–8,000) |
| Maximum % neutrophils | 83.15 (77–87) | 89 (82–90.45) | 80.9 (72–86) |
| Minimum % neutrophils | 70.5 (58–79) | 65.8 (57.9–74) | 70 (50.3–72) |
| Platelet count | 83,500 (30,000–154,000) | 106,500 (41,500–170,000) | 100,000 (70,000–190,000) |
| Serum creatinine (mg/dL) maximum | 1.15 (0.9–1.75) | 1.6 (1–2.3) | 1 (1–1.2) |
| Blood urea (mg/dL) maximum | 44 (35–78) | 38 (23–93) | 40 (30–47) |
| Serum bilirubin (mg/dL) maximum | 2.2 (2.1–2.6) | 2.6 (2.4–3.2) | 2.4 (0.9–5.8) |
| Maximum AST | 59.5 (41–79) | 68 (45–99) | 68 (42–99) |
| Maximum ALT | 40 (26–64) | 42 (32–80) | 43 (32–63) |
| Serum potassium | 3.75 (3.3–4.1) | 4 (3.4–4.8) | 4.2 (3.2–4.2) |
| Serum sodium maximum | 138 (133.5–141) | 140 (134–145) | 139 (135–144) |
| Erythrocyte sedimentation rate maximum | 55 (20–62) | 43 (19–50) | 12 (10–55) |
ALT = alanine transaminase; AST = aspartate aminotransferase; IQR = inter quartile range; WBC = white blood cell count.
Sequelae.
Acute renal failure (serum creatinine > 1.5 mmol/L) was confirmed in 10 confirmed cases. Of these, ultrasound showed renal parenchymal disease in three. Six confirmed cases developed acute myocarditis as defined by electrocardiogram changes; echocardiography demonstrated cardiac contractility impairment in two cases. Elevated blood urea (> 40 mg/dL), liver enzymes, and total bilirubin was observed in 37 (77.1%), 26 (54.2%), and 7 (14.6%) confirmed cases, respectively.
Median duration of hospital stay was 5 days for all possible, probable, and confirmed cases with an interquartile range of 4–6, 3–7, and 3–6 days, respectively.
Comparison of leptospirosis diagnostic methods during acute phase.
Of the 96 patients studied, only 12 (12.5%) patients presented to hospital on or before fever day 3. Another 27 (28.1%) presented between days 4 and 5 of fever, followed by 23 (24.0%) in between days 6 and 7. For the evaluation of clinically relevant diagnostic methods during the acute phase of the disease, we selected these 62 patients presented to the hospital within the first 7 days of illness (Table 5).
Table 5.
Results of testing acute (admission) samples in 62 suspected leptospirosis cases in Anuradhapura, Sri Lanka, 2011*
| Confirmed | Unconfirmed | ||
|---|---|---|---|
| n (%) | n (%) | ||
| MAT | Positive | 7 (22.6) | 0 (0) |
| Negative | 24 (77.4) | 31 (100) | |
| IgG/IgM BfR | Positive | 11 (35.5) | 8 (26.7) |
| Negative | 20 (64.50 | 22 (73.3) | |
| Virion | Positive | 4 (12) | 0 (0) |
| Negative | 27 (87.10) | 30 (100) | |
| qPCR | positive | 23 (74.2) | 0 (0) |
| negative | 8 (25.8) | 31 (100) | |
IgG = immunoglobulin G; IgM = immunoglobulin M; MAT = microscopic agglutination test; qPCR = quantitative polymerase chain reaction.
For some tests the total is not equal to 62 because the sample quantities were inadequate to carry out all tests.
Because qPCR directly identifies the presence of Leptospira in specimens at the time of active infection, while antibody tests are an indirect assessment of infection (with the confounding possibility of previous infection yielding positive results), we compared results of acute sample test results with MAT-confirmed cases. For BfR (both IgM and IgG combined), sensitivity was 66.7% (N = 8) compared with 58.3% (N = 7) for acute sample MAT. Although a titer of 1/400 was used here as the diagnostic cutoff,23 some authors have suggested that high endemic countries (such as Sri Lanka) should use an MAT titer cutoff of 1/800 to improve specificity for the diagnosis of acute leptospirosis. Of the acute sample MAT, only three had titers more than or equal to 1/800, which provided a sensitivity of 9.6%, which is not acceptable for diagnosis. Overall, nucleic acid amplification outperformed both MAT and ELISA antibody detection in the acute phase.
Case confirmation based on paired serum samples.
Because the MAT remains the reference diagnostic method for leptospirosis, we compared MAT and qPCR results in 40 patients, among whom, the paired samples were available (Table 6).
Table 6.
Microscopic agglutination test results in 40 paired sera samples by qPCR-based DNA detection
| MAT results | qPCR positive | qPCR negative |
|---|---|---|
| n (%) | n (%) | |
| Paired samples negative | 5 (50) | 19 (63.3) |
| Sero-conversion | 0 (0) | 5 (16.7) |
| Fourfold rise | 1 (10) | 2 (6.7) |
| Single high titer (≥ 1/400) | 2 (20) | 2 (6.7) |
| Possible past infection (MAT titer < 1/400) | 2 (20) | 2 (6.7) |
MAT = microscopic agglutination test; qPCR = quantitative polymerase chain reaction.
Of 10 qPCR-positive samples, five were serologically negative as determined by acute or convalescent MAT tests. All five qPCR-positive samples were confirmed to be true positives by retesting that used a single-tube nested PCR method with sequenced amplicons.
Discussion
Here, we demonstrate the use of a well-characterized clinical specimen collection, obtained in the context of a flood-associated leptospirosis outbreak in Sri Lanka, which confirms that nucleic acid amplification-based diagnosis outperforms antibody detection.
The laboratory confirmation of human leptospirosis is essential both for clinical management and for public health measures, because the clinical presentation is so variable. Leptospiral infection may be clinically inapparent,24 or may result in undifferentiated fever, fulminant disease, and mimics other tropical diseases such as malaria,25 dengue,26–29 hantaviral syndromes,18,30–32 scrub typhus,33,34 and others. During the acute phase of leptospirosis, timely confirmation is an important clinical priority to optimize both specific treatment and supportive management. Timely confirmation remains a major challenge. Early diagnostic tests currently available for such purposes include direct visualization of Leptospira in clinical samples (which suffers from issues of sensitivity, specificity, and operator error), nucleic acid amplification–based diagnostic tests, and antibody detection–based tests. In this study, we compared a qPCR assay, two ELISA-based methods, and the putatively gold standard MAT for diagnosis during the acute phase of disease, in the context of a flood-related outbreak. As expected, molecular methods were reliably confirmed acute leptospirosis, but the high cost of qPCR in resource-poor settings still limits availability. We specifically tested serology-based methods, because these tests are the only available methods in most high-burden countries, even though low sensitivity is expected during the acute phase due to delay in antibody response.
Compared with the previously reported positivity rate of 51.0% (95% confidence interval [CI] = 37.5–64.4%) for the same qPCR assay applied in a different context in Sri Lanka,11 this study showed a higher positivity rate (74.2%) without having a difference in duration of fever on admission. Because the sample collection protocol and procedures were similar in these two studies, the observed higher detection rate, while likely due to the nature of the outbreak, could partly be due to improved sample handling and storage compared with previous studies. In the previously reported 2008 outbreak investigation, tests were done 3 years after the initial sample collection and samples were thawed at least three times over this period of time. The data presented here were obtained within 3 months of sample collection and no thawing was done between final testing of samples and first freezing, which enhances the reliability and applicability of the results.
Previously published data indicate that the sensitivity of in-house and commercialized ELISA test can be more than 93% and 90%, respectively.8 However, in Sri Lankan settings, the reported sensitivity and specificity of commercially available ELISA test using the Leptospira biflexa Patoc antigen was 60.2% (95% CI = 49.8, 69.8) and 46.5% (95% CI = 35.4, 57.9), respectively.35 In the present study we observed important performance differences of in-house and commercially available methods. The antigen used in this in-house method was L. kirschneri serovar Grippotyphosa and the commercial ELISA test was based on L. biflexa. The difference between the sensitivity of two ELISA tests may be due to the differences in cross-reactivity of the antigen used. Whether the antigens from pathogenic leptospira having more cross-reactivity compared with saprophytic species needs further investigation.
Because ELISA is readily performed in clinical laboratories, this assay format is potentially more useful than MAT in providing actionable diagnostic data directly related to patient care. In our study, during the acute phase, in-house ELISA method provided the highest yield with 19 positives. However, the commercial ELISA test we used included only IgG, which is a major limitation of the interpretation of the validity of the particular test. Low sensitivity in the acute phase may be due to noninclusion of IgM in the test kits. There is a possibility of some false positives in the in-house test, which we are unable to comment on due to the lack of a gold standard for proper evaluation of specificity.10 Further, we did not have paired samples for some patients to exclude potential false positives in some of these ELISA-positive patients. Previous studies have consistently shown ELISA to be more sensitive than MAT in the acute phase.36 Sensitivity of MAT, even with properly obtained paired samples, is not 100% as specified in several other studies.10 We observed similar results in the 2008 Sri Lanka leptospirosis outbreak, with two additional patients detected only using qPCR. In this study, only five out of 10 qPCR positives showed any reaction in MAT, consistent with this previous observation. With the increasing body of evidence against the use of MAT as the gold standard test for leptospirosis diagnosis,10 new approaches are urgently needed for clinically actionable results that lead to management decisions.
In conclusion, we confirm the utility of qPCR as a valid early test for the diagnosis of leptospirosis. When ELISA is used, specific knowledge of circulating serovar(s) is important to yield useful results, and the use of IgG or IgG/IgM combination will yield better results in endemic settings.
ACKNOWLEDGMENTS
We are grateful for clinical contributions provided by the ward staff of the Teaching Hospital Anuradhapura, Sri Lanka, during this study.
Footnotes
Financial support: This work was supported by U.S. Public Health Service grants U19AI115658 (Suneth B. Agampodi and Joseph M. Vinetz), R01AI108276 (Joseph M. Vinetz), K24AI068903 (Joseph M. Vinetz) and Rajarata University grant RJT/R&P/2012/Med.&Alli/R03, RJT/RP&HDC/2013/Med.&Alli.Sci./R/06, RJT/RP&HDC/2014/FMAS/R/03 (Suneth B. Agampodi).
Authors' addresses: Suneth B. Agampodi, Department of Community Medicine, Faculty of Medicine and Allied Sciences, Saliyapura, E-mail: sunethagampodi@yahoo.com. Niroshana J Dahanayaka, Department of Medicine, Faculty of Medicine, University of Ruhuna, Karapitiya, Galle, Sri Lanka, E-mail: niroshanajd@yahoo.com. Karsten Nöckler and Mayer-Scholl Anne, Department Biological Safety, Federal Institute for Risk Assessment, Berlin Germany, E-mails: noeckler@bfr.bund.de and Anne.Mayer-Scholl@bfr.bund.de. Joseph M. Vinetz, University of California San Diego School of Medicine, Center for Tropical Diseases, La Jolla, CA, E-mail: jvinetz@ucsd.edu.
References
- 1.Abela-Ridder B, Bertherat E, Durski K. Global Burden of Human Leptospirosis and Cross-Sectoral Interventions for its Prevention and Control Prince Mahidol Award Conference, October 15--17, 2009. Bangkok, Thailand. Prince Mahidol Award Conference.2013. [Google Scholar]
- 2.Abela-Ridder B, Sikkema R, Hartskeerl RA. Estimating the burden of human leptospirosis. Int J Antimicrob Agents. 2010;36((Suppl 1)):S5–S7. doi: 10.1016/j.ijantimicag.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, Goris MG, Stein C, Ko AI, Abela-Ridder B. Global burden of leptospirosis: estimated in terms of disability adjusted life years. PLoS Negl Trop Dis. 2015;9:e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis. 2008;12:351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Bandara M, Ananda M, Wickramage K, Berger E, Agampodi S. Globalization of leptospirosis through travel and migration. Global Health. 2014;10:61. doi: 10.1186/s12992-014-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picardeau M, Bertherat E, Jancloes M, Skouloudis AN, Durski K, Hartskeerl RA. Rapid tests for diagnosis of leptospirosis: current tools and emerging technologies. Diagn Microbiol Infect Dis. 2014;78:1–8. doi: 10.1016/j.diagmicrobio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Hartskeerl RA, Smythe LD. The role of leptospirosis reference laboratories. Curr Top Microbiol Immunol. 2015;387:273–288. doi: 10.1007/978-3-662-45059-8_11. [DOI] [PubMed] [Google Scholar]
- 10.Limmathurotsakul D, Turner EL, Wuthiekanun V, Thaipadungpanit J, Suputtamongkol Y, Chierakul W, Smythe LD, Day NP, Cooper B, Peacock SJ. Fool's gold: why imperfect reference tests are undermining the evaluation of novel diagnostics: a reevaluation of 5 diagnostic tests for leptospirosis. Clin Infect Dis. 2012;55:322–331. doi: 10.1093/cid/cis403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agampodi SB, Matthias MA, Moreno AC, Vinetz JM. Utility of quantitative polymerase chain reaction in leptospirosis diagnosis: association of level of leptospiremia and clinical manifestations in Sri Lanka. Clin Infect Dis. 2012;54:1249–1255. doi: 10.1093/cid/cis035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agampodi S, Peacock SJ, Thevanesam V. The potential emergence of leptospirosis in Sri Lanka. Lancet Infect Dis. 2009;9:524–526. doi: 10.1016/S1473-3099(09)70211-7. [DOI] [PubMed] [Google Scholar]
- 13.Agampodi SB, Dahanayaka NJ, Bandaranayaka AK, Perera M, Priyankara S, Weerawansa P, Matthias MA, Vinetz JM. Regional differences of leptospirosis in Sri Lanka: observations from a flood-associated outbreak in 2011. PLoS Negl Trop Dis. 2014;8:e2626. doi: 10.1371/journal.pntd.0002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agampodi SB, Karunarathna D, Jayathilala N, Rathnayaka H, Agampodi TC, Karunanayaka L. Outbreak of leptospirosis after white-water rafting: sign of a shift from rural to recreational leptospirosis in Sri Lanka? Epidemiol Infect. 2014;142:843–846. doi: 10.1017/S0950268813001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agampodi SB, Peacock SJ, Thevanesam V, Nugegoda DB, Smythe L, Thaipadungpanit J, Craig SB, Burns MA, Dohnt M, Boonsilp S, Senaratne T, Kumara A, Palihawadana P, Perera S, Vinetz JM. Leptospirosis outbreak in Sri Lanka in 2008: lessons for assessing the global burden of disease. Am J Trop Med Hyg. 2011;85:471–478. doi: 10.4269/ajtmh.2011.11-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamage CD, Amarasekera J, Palihawadana P, Samaraweera S, Mendis D, Janakan N, Lee RB, Obayashi Y, Tamashiro H. Analysis of hospital-based sentinel surveillance data on leptospirosis in Sri Lanka, 2005–2008. Jpn J Infect Dis. 2012;65:157–161. [PubMed] [Google Scholar]
- 17.Koizumi N, Gamage CD, Muto M, Kularatne SA, Budagoda BD, Rajapakse RP, Tamashiro H, Watanabe H. Serological and genetic analysis of leptospirosis in patients with acute febrile illness in Kandy, Sri Lanka. Jpn J Infect Dis. 2009;62:474–475. [PubMed] [Google Scholar]
- 18.Dahanayaka NJ, Agampodi SB, Bandaranayaka AK, Priyankara S, Vinetz JM. Hantavirus infection mimicking leptospirosis: how long are we going to rely on clinical suspicion? J Infect Dev Ctries. 2014;8:1072–1075. doi: 10.3855/jidc.4115. [DOI] [PubMed] [Google Scholar]
- 19.Epidemiological Unit . Leptospirosis. Surveillance Case Definitions for Notifiable Diseases in Sri Lanka. Colombo: Epidemiology Unit, Ministry of Health; 2005. pp. 19–20. [Google Scholar]
- 20.Agampodi SB, Nugegoda DB, Thevanesam V. Determinants of leptospirosis in Sri Lanka: study protocol. BMC Infect Dis. 2010;10:332. doi: 10.1186/1471-2334-10-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev Vet Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 22.World Organization for Animal Health . Leptospirosis. Manual for Diagnostic Tests and Vaccines for Terrestrial Animal. Paris, France: World Organization for Animal Health; 2008. pp. 251–264. [Google Scholar]
- 23.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–327. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganoza CA, Matthias MA, Saito M, Cespedes M, Gotuzzo E, Vinetz JM. Asymptomatic renal colonization of humans in the Peruvian Amazon by Leptospira. PLoS Negl Trop Dis. 2010;4:e612. doi: 10.1371/journal.pntd.0000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swoboda P, Fuehrer HP, Ley B, Starzengruber P, Ley-Thriemer K, Jung M, Matt J, Fally MA, Mueller MK, Reismann JA, Haque R, Khan WA, Noedl H. Evidence of a major reservoir of non-malarial febrile diseases in malaria-endemic regions of Bangladesh. Am J Trop Med Hyg. 2014;90:377–382. doi: 10.4269/ajtmh.13-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valarezo-Sevilla D, Sarzosa-Teran V. Leptospirosis: case-series report in a prison of the coast in Ecuador [in Spanish] Rev Esp Sanid Penit. 2014;16:20–23. doi: 10.4321/S1575-06202014000100004. [DOI] [PubMed] [Google Scholar]
- 27.Perez Rodriguez NM, Galloway R, Blau DM, Traxler R, Bhatnagar J, Zaki SR, Rivera A, Torres JV, Noyd D, Santiago-Albizu XE, Rivera Garcia B, Tomashek KM, Bower WA, Sharp TM. Case series of fatal Leptospira spp./dengue virus co-infections-Puerto Rico, 2010–2012. Am J Trop Med Hyg. 2014;91:760–765. doi: 10.4269/ajtmh.14-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, Hossain MA, Brooks WA, Levett PN. Leptospirosis during dengue outbreak, Bangladesh. Emerg Infect Dis. 2005;11:766–769. doi: 10.3201/eid1105.041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J Trop Pediatr. 2005;51:174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- 30.Sunil-Chandra NP, Clement J, Maes P, De Silva HJ, Van Esbroeck M, Van Ranst M. Concomitant leptospirosis-hantavirus co-infection in acute patients hospitalized in Sri Lanka: implications for a potentially worldwide underestimated problem—Erratum. Epidemiol Infect. 2015;143:2029. doi: 10.1017/S0950268814003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clement J, Maes P, Muthusethupathi M, Nainan G, van Ranst M. First evidence of fatal hantavirus nephropathy in India, mimicking leptospirosis. Nephrol Dial Transplant. 2006;21:826–827. doi: 10.1093/ndt/gfi334. [DOI] [PubMed] [Google Scholar]
- 32.Santos VM, Rocha de Sa DA, Turra TZ, Ferreira Borges NM, Nascimento UM, Damasceno EA. Hantavirus pulmonary syndrome in Brasilia periphery: a diagnostic challenge. J Infect Dev Ctries. 2009;3:639–643. doi: 10.3855/jidc.558. [DOI] [PubMed] [Google Scholar]
- 33.Ramyasree A, Kalawat U, Rani ND, Chaudhury A. Seroprevalence of scrub typhus at a tertiary care hospital in Andhra Pradesh. Indian J Med Microbiol. 2015;33:68–72. doi: 10.4103/0255-0857.148381. [DOI] [PubMed] [Google Scholar]
- 34.Nachega JB, Bottieau E, Zech F, Van Gompel A. Travel-acquired scrub typhus: emphasis on the differential diagnosis, treatment, and prevention strategies. J Travel Med. 2007;14:352–355. doi: 10.1111/j.1708-8305.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 35.Agampodi SB, Thevanesam V, Senaratne T. Validity of a commercially available IgM ELISA test for diagnosing acute leptospirosis in high endemic districts of Sri Lanka. Sri Lankan J Infect Dis. 2014;4:83–89. [Google Scholar]
- 36.Levett PN, Branch SL. Evaluation of two enzyme-linked immunosorbent assay methods for detection of immunoglobulin M antibodies in acute leptospirosis. Am J Trop Med Hyg. 2002;66:745–748. doi: 10.4269/ajtmh.2002.66.745. [DOI] [PubMed] [Google Scholar]