Abstract
In Senegal, tick-borne relapsing fever (TBRF) is a major cause of morbidity and a neglected public health problem. Borreliosis cases commonly detected in two villages led us to implement a borreliosis preventive control including cementing of floors in bedrooms and outbuildings attended by inhabitants to avoid human contacts with tick vectors. Epidemiological and medical monitoring of the TBRF incidence was carried out at Dielmo and Ndiop by testing the blood of febrile patients since 1990 and 1993, respectively. Intra-domiciliary habitat conditions were improved by cementing, coupled with accompanying measures, from March 2013 to September 2015. Application of this strategy was associated with a significant reduction of borreliosis incidence. This was more evident in Dielmo, dropping from 10.55 to 2.63 cases per 100 person-years (P < 0.001), than in Ndiop where it changed from 3.79 to 1.39 cases per 100 person-years (P < 0.001). Thirty-six cases of TBRF were estimated to be prevented at a cost of €526 per infection. The preventive control strategy was successful in Dielmo and Ndiop, being associated with decreased incidence by 89.8% and 81.5%, respectively, suggesting that TBRF may be widely decreased when the population is involved. Public health authorities or any development stakeholders should adopt this effective tool for promoting rural health through national prevention programs.
Introduction
In Africa, tick-borne relapsing fever (TBRF) due to Borrelia infections is contracted through bites of argasid ticks of the genus Ornithodoros. TBRF is a major cause of disease in several African regions. Untreated TBRF patients experience relapsing illness characteristic of the disease, and severe meningoencephalitic complications,1–3 as well as spontaneous abortion in pregnant women,4 can ensue.
TBRF persists in endemic foci around the world, where each Borrelia species that cause relapsing fever appear to be specific to its tick vector.4 TBRF is endemic to sub-Saharan Africa. In eastern and southern Africa, TBRF occurs mainly due to Borrelia duttonii, which is transmitted by the tick vectors Ornithodoros moubata and Ornithodoros porcinus belonging to the O. moubata group.5 In west Africa and the most arid parts of north Africa, TBRF is caused mainly by Borrelia crocidurae, which is transmitted by Ornithodoros sonrai ticks.6 In coastal areas of north Africa, TBRF is caused by Borrelia hispanica, and is transmitted by ticks of the Ornithodoros marocanus group, whereas in Western Sahara and in Morocco potentially pathogenic “Borrelia merionesi” is hosted by Ornithodoros merionesi and Ornithodoros costalis ticks.6 TBRF is rarely detected in northern Africa and Mediterranean countries.1,7,8 In several African nations, TBRF remains a significant public health problem. For example, in Tanzania, TBRF due to B. duttonii is one of the main causes of mortality in children under 5 years of age, with an alarming perinatal mortality rate of 463/1,000 in endemic regions.9,10
The epidemiology and geographic distribution of TBRF borreliosis in west and north Africa with regard to the main foci of transmission were recently reviewed.6 Small mammals act as reservoir hosts for Borrelia infections,2,11 but the Ornithodoros ticks are also potential reservoirs of most Borrelia spp. because of their longevity,12 and the vertical transmission of Borrelia in tick vectors.12
In Senegal, the spirochete B. crocidurae was first described after isolation from the blood of a shrew from Dakar,13 and was later identified as the cause of relapsing fever borreliosis in Senegal14 and other countries of west Africa.3,15 Borrelia crocidurae infection is a common cause of fever in rural Senegal.3,16 The only known vectors of B. crocidurae are O. sonrai ticks.6 These ticks live in burrows and crevices, and human contact with O. sonrai occurs when burrows colonized by the tick vector open inside bedrooms.16
In natural habitats and crops areas, it is difficult to combat TBRF, but a strategy for reducing the frequency of human contact with O. sonrai ticks combined with rodent captures may be performed inside houses, as using insecticides to kill ticks inside burrows would expose villagers to risks of toxic contamination, especially young children.17
Epidemiological data reported from Senegal showed that since 1934,14 only 12 cases of borreliosis were detected in 1962,18 and 23 more cases were diagnosed in Dakar between 1979 and 1982.19 A prevalence of 4.2% was observed in children 10–14 years of age living in the Thies region, located 70-km east of Dakar.3 In the village of Dielmo (Sine-Saloum), the average incidence of TBRF was 5% in the early 1990s.15 A longitudinal study conducted in the same village reported that an average incidence of 11 per 100 person-years from the population develop TBRF each year16; however, this incidence has fluctuated between 3% and 27%.16 In 2010, using specific quantitative polymerase chain reaction (qPCR), a prevalence of 13% was observed in samples obtained from febrile patients who were followed for 7 months.20 The proportion of burrows harboring O. sonrai inside homes and fields of Dielmo was observed to be 8–14% in 2005 (G. Diatta, unpublished data), and 7.1–8.5% in 1992.2 Since 1998, imported or sporadic human cases of TBRF have been diagnosed in Ndiop, which is located south of Dielmo; however in 2011, a large number of TBRF cases were detected in Ndiop.21
These recent epidemiologic data indicate that TBRF occurs commonly in both populations, but no measures have been taken to avoid the occurrence of borreliosis cases within the community. This situation, along with our long-term relations with the population of two Senegalese villages, Dielmo and Ndiop,22 led us to implement a borreliosis control trial plan including 1) cementing (i.e., covering the floor with a 5-cm layer of clay mixed in equal proportions with cement) of all bedrooms and attics attended by inhabitants, then tracking the emergence of new burrows and crevices coupled with the catching of small mammals, and measures of sanitary hygiene including sweeping of bedrooms and attics to prevent the risk and reduce the frequency of human contacts with tick vectors; 2) evaluation of O. sonrai tick infestations inside homes at Ndiop and Dielmo, followed by detection of Borrelia infection in O. sonrai ticks; 3) to compare borreliosis morbidity before and during the period of intervention based on the data from point-of-care (POC) laboratory serving all inhabitants of Dielmo and Ndiop villages. The rodent capture approach will reduce massively the presence of rodents and their role in dispersing the tick vector O. sonrai inside homes, and the frequency of rodent burrows. This measure of the intervention strategy may highly contribute to the reduction of TBRF incidence in both villages. All these measures related to the intervention strategy and prevention of borreliosis aim to address the question of whether by combating O. sonrai tick vectors and small mammal reservoirs inside human dwellings, coupled with intra-domiciliary cleanliness, would widely reduce the frequency and incidence of TBRF disease?
Materials and Methods
Study sites.
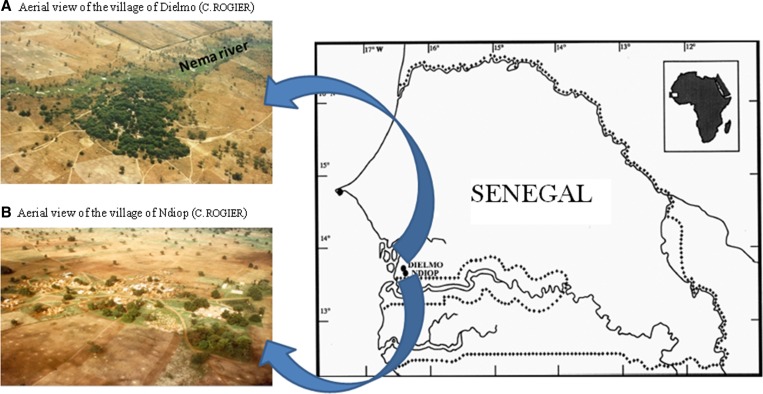
Recent data from Pasteur institute of Dakar surveys (J. F. Faye, unpublished data) indicated that Ndiop (13°41′N, 16°23′W) and Dielmo (13°43′N, 16°24′W) are two villages with 510 and 481 inhabitants, respectively, located in the Sine-Saloum area of Senegal (Figure 1 ). Ndiop is south of Dielmo, which is located 280-km southeast of Dakar. The vegetation and climate associated with both villages correspond to the Sudanese savanna area. Rainfall occurs over a 4-month period, from late June to mid-October. The village of Ndiop is populated by the Wolof people, whereas Dielmo is populated by the Serers. The houses are usually built in bricks consolidated with or without cement rendering, and the soil is noncemented dirt in most homes. In both villages, farming is a family activity. Agriculture is the main activity during the rainy season and extends through the dry season at which point gardening becomes more common.
Figure 1.
Locations of collection sites for tick samples studied in January 2012, Sine-Saloum, Senegal. (A) Aerial view of the village of Dielmo, Sine-Saloum, Senegal (Christophe Rogier); (B) Aerial view of the village of Ndiop, Sine-Saloum, Senegal (C. Rogier).
Population monitoring.
Before the beginning of the study, all the population, including each participant of each household, provided written individual informed consent for enrolling their residences. The National Ethics Committee of Senegal approved the project as part of program of identification and prevention of emergent pathogens, no. 00.87 MSP/DS/CNERS.
Medical and epidemiologic monitoring of the population began in 1990 in Dielmo and 1993 in Ndiop, when a dispensary was provided in each village. Two nurses and two technicians were replaced every 15 days, and four continuous field investigators were present every day (7 days/week). All febrile (defined as axillary temperature > 37.5°C) patients were examined by a nurse of the village dispensaries of Dielmo or Ndiop. This staffing provision at the dispensaries is really necessary to ensure all medical and epidemiological monitoring within Dielmo and Ndiop populations because of presence of malaria, borreliosis, rickettsioses, influenza, and so forth. Each agent had a specific function. All bedrooms of households from each village were visited by the field investigators. From 1990 to 2010, the thick blood smear technique was used to detect mainly malaria and Borrelia in all cases of fever monitored in the two villages. Since 2011, however, this technique has been reinforced with a functional POC diagnostic laboratory implemented in Dielmo (and serving both villages) to search for infections with Borrelia, Plasmodium falciparum, rickettsiae, and other fever-causing pathogenic agents using molecular diagnostics, thus enabling the rapid treatment of patients.23
Preventive control strategy of TBRF implemented and accompanying measures.
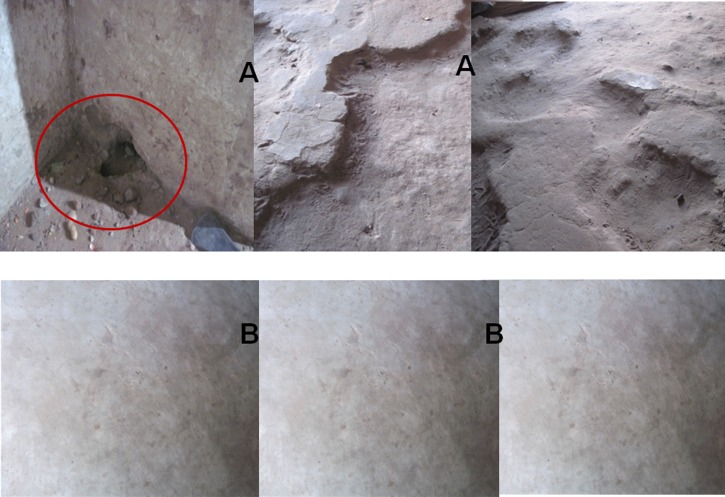
We initiated the present borreliosis control project in March 2013, when we began to cement the floors (or rodent burrows if cement floors already existed) of all bedrooms and outbuildings with a high human use, to prevent the risk of human contacts with tick vectors. Before cementing, risk factors were identified and counted in priority bedrooms of each household to understand the risk of human exposure to the O. sonrai tick. Risk factors included the presence of burrows and crevices that can harbor O. sonrai ticks (Figure 2A ). The cement was purchased from a local dealer, along with granulated rat poison. Cementing and action monitoring were performed by workers recruited in each village, who also tracked down the burrows and crevices reappearing to prevent any emergence. Meetings were held in each village to ask each head of household to be involved during the work by providing enough sand, gravel, and water, while the cement was provided by the project. Accompanying measures consisted of 1) captures of small rodents and insectivores inside homes with a nontoxic liquid glue (Trapcoll; Kenda Farben, Ferrera Erbognone, Italy); 2) placement of granulated rat poison inside open burrows (Figure 3 ); 3) treatment of any termite tunnels destroyed during the work with insecticide powder; 4) systematic tracking of the state of cemented floors for the reappearance of rodent burrows and refilling them with cement; and 5) lectures and training of women from both villages on hygienic measures aimed to reduce borreliosis cases, encouraging them to embrace the intervention strategy.
Figure 2.
Presence of intra-domiciliary rodent burrow and crevices in Dielmo and Ndiop homes (A) before and (B) after the intervention of cementing.
Figure 3.
Liquid glue (nontoxic) used to capture small mammals; granulated rat poison used for backfilling rodent burrows.
Evaluation of the cost of the implemented strategy.
We measured costs for the complete period of intervention implementation, that is, from March 2013 to September 2015. The costing perspective taken was that of the government. We considered the following costs: cement costs, bricklayers' salary, liquid glue and granulated rat poison costs, as well as costs for monitoring and field supervision including manager's salary, transportation, and phone costs. Thus, we calculated the total cost as the sum of all individual health-care resources used by participants, multiplied by the unit cost of these resources as well as the cost per avoided infection.
Sampling of O. sonrai ticks in burrows from Ndiop and Dielmo.
In January 2012, Ornithodoros ticks were collected exclusively from burrows inside homes in Ndiop and then Dielmo (Figure 4 )6 to evaluate the level of tick infestation. In Ndiop, sampling was conducted first of all in the homes of patients diagnosed with borreliosis in 2011 and then in other housings.24,25 Ticks were collected using a portable petrol-powered aspirator as previously described.25 All ticks specimens were kept in 125-mL pots with perforated caps at room temperature during collection (Elvetec, Meyzieu, France). After collection, live ticks were stored in a desiccator containing a potassium hydroxide solution at a relative humidity of 68–70%. Collected Ornithodoros ticks were identified according to standard morphological criteria.26 After security guarantees were provided to the carrier by the World Health Organization (WHO) Collaborative Center of Marseille, all tick samples were sent to Unité de Recherche sur les Maladies Infectieuses et Tropicales Émergentes in Marseille, France, for molecular analysis. Beyond the security guarantees provided by the WHO Collaborative Center of Marseille, all specimens of ticks sampled were stored into a guarantee box established for precautionary measures of vector-borne infection transportation. The ticks sampled from four burrows in one household in Ndiop village and two other burrows from two households in Dielmo were used for blood feeding to demonstrate Borrelia transmission in Ndiop. It is epidemiologically important to know a like situation for a future intervention project through rural endemic foci from Senegal or west Africa.
Figure 4.
Sampling of Ornithodoros ticks from rodent burrows inside homes in Ndiop village.
DNA extraction and detection of Borrelia infections from ticks.
Individual dead and live ticks from Ndiop and Dielmo villages were used for DNA extraction. All tick samples were washed twice with sterile water, then preserved in sterile microcentrifuge tubes. DNA was extracted from half or a quarter of each tick sample using the EZ1 DNA Tissue kit (QIAGEN, Courtaboeuf, France) according to the manufacturer's instructions. Genomic DNA was stored at 4°C until used as a template in PCR assays. The remaining portion of the tick was kept at −80°C for future analyses. Borrelia DNA was detected by real-time PCR using the primers Bor16S3F, 5′-AGCCTTTAAAGCTTCGCTTGTAG-3′ and Bor16S3R, 5′-GCCTCCCGTAGGAGTCTGG-3′, along with a probe (Bor16S3P, 5-6FAM-CCGGCCTGAGAGGGTGAACGG-TAMRA) designed to amplify a 148-bp fragment of the 16S RNA–encoding gene.21 Each real-time PCR reaction was performed with Light Cycler 2.0 equipment and software (Roche Diagnostics GmbH, Mannheim, Germany). Negative controls (sterile water and DNA from a sterile biopsy specimen) were included in each assay. All positive samples were tested with B. crocidurae-specific PCR using primers specific for the glpQ gene.27
Case definition.
We considered borreliosis cases as those with 1) a fever (axillary temperature > 37.5°C), whether accompanied or not by other clinical signs, and 2) carrying Borrelia infection identified in the blood either microscopically (before 2010) or by molecular methods. Molecular identification criteria included two positive qPCR (Borrelia genus specific and B. crocidurae specific).
Statistical analysis.
Daily monitoring of Dielmo inhabitants for the occurrence of febrile episodes from 1990 showed that borreliosis cases were detected at the beginning, while in Ndiop, indigenous cases seemed to appear in October 2010. The statistical unit used for the survey was the person-year. The incidence (expressed in person-time) was calculated each year using the number of diagnosed borreliosis cases multiplied by the number days-year (365.25) divided by the number of person-days of surveillance. Borreliosis cases diagnosed were identified before cementing, and the corresponding average incidence was calculated for each village. The average number of borreliosis cases before and during intervention was also calculated. For comparison between the average incidence observed before and during the intervention period, χ2 test (on contingency tables) adapted to data expressed in person-time was used. The relative risk (RR) of developing TBRF was estimated in each village according to calculated person-time data, and the corresponding 95% confidence interval (CI) was determined. The preventive risk fraction in each village was so evaluated from the estimated RR value.
Results
TBRF preventive control strategy implemented and accompanying measures.
In both villages, 90 households were taken into account during this intervention, including 48 in Dielmo and 42 in Ndiop. The risk factors identified before cementing consisted of 733 burrows and crevices, of which 239 and 198 burrows, totaling 437, were found inside houses of Dielmo and Ndiop, respectively, while 296 crevices were listed inside targeted dwellings of Dielmo and Ndiop, including 157 in Dielmo and 139 in Ndiop. Overall, 150 bedrooms and attics were cemented, 90 small rooms in Dielmo and 60 larger ones in Ndiop, using 9.55 tons of cement (Figure 2B). In some bedrooms and attics, burrows were opened and filled, and 81 pouches of granulated rat poison were inserted into the nests of all deep burrows. Small rodents and insectivores inside houses were captured using 1,877 tubes of 135 g of nontoxic liquid glue representing 253,395 g of glue distributed to inhabitants. Hygiene training courses for women carried out in the two villages were accepted timidly.
Measure of effectiveness of implemented strategy and cost assessment.
In Dielmo, during 2,399,045 person-days of surveillance, 693 Borrelia infections were detected from 1990 to 2012, whereas in Ndiop there were 366,158 person-days of surveillance with 38 cases of Borrelia infection diagnosed from 2009 to 2012. During the intervention period, we noted that in Dielmo, 360,511 person-days of surveillance comprise 118,040 person-days in 2013, 140,179 in 2014, and 102,292 in 2015, whereas in Ndiop 366,140 person-days of surveillance included 112,514 person-days in 2013, 140,399 in 2014, and 113,227 in 2015.
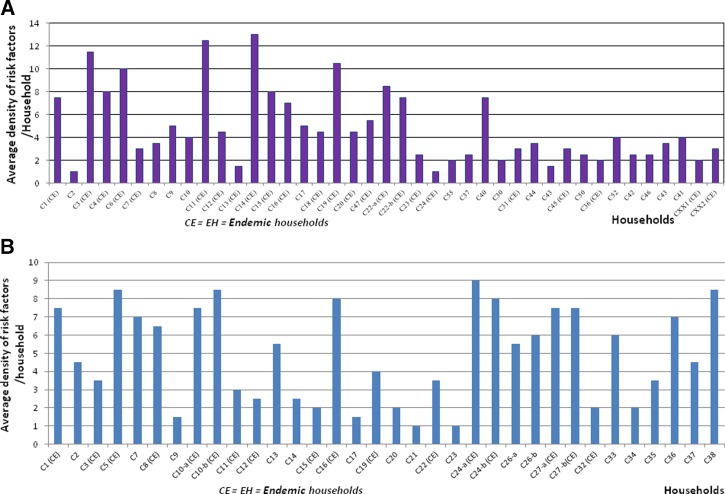
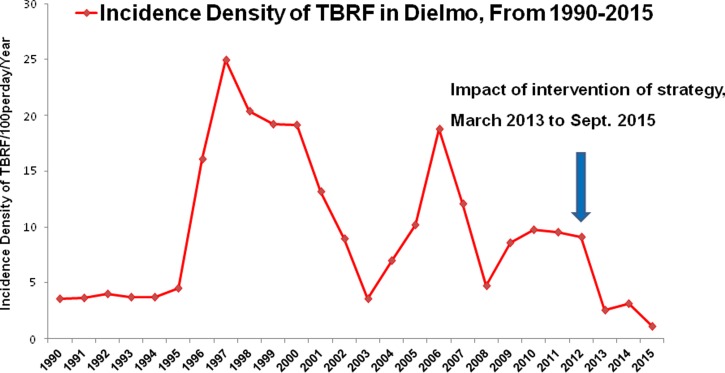
On average, 31 cases of TBRF were recorded annually in Dielmo between June 1990 and 2012, and the average incidence of infection was 10.55 cases per 100 person-years and up to nine separate episodes of borreliosis in the same person were observed during the study period. In Ndiop, however, where the first autochthonous cases were reported in October 2010,19 10 cases were recorded (2010–2012) with an average incidence of 3.79 cases per 100 person-years (Table 1). During the period of intervention—from March 2013 to September 2015—26 cases of TBRF were detected in Dielmo and 14 in Ndiop, with respective average incidences of 2.63 cases per 100 person-years in Dielmo and 1.39 cases per 100 person-years in Ndiop (Table 1). Average risk factor density was high in TBRF-endemic households from both villages (Figure 5A and B ). A respective incidence of 3.64 cases per 100 person-years and 3.04 cases per 100 person-years was recorded in the villages of Dielmo and Ndiop in 2013, respectively, whereas in 2014 and 2015, this incidence decreased from 3.13 to 1.07 cases per 100 person-years in Dielmo and from 0.78 to 0.72 cases per 100 person-years in Ndiop (Table 1, Figure 6 ). The progression of tick-borne borreliosis in Dielmo was marked by six distinct periods, of which the first three have been previously described16: 1) from 1990 to 1995, TBRF incidence was relatively low and stable; 2) from 1996 to 2002, there was a rise in TBRF, with a fast increase in incidence in 1997 and decreasing from 1998 to 2002; 3) in 2003, the incidence was at the low level of 1990; 4) from 2004 to 2007, there was a new increase of the disease, with a high incidence in 2006 that was however lower than in 1997, decreasing slowly from 2007 to 2008 at a level higher than the standard of 1990; then, 5) from 2009 to 2012, the incidence rate rose to reach a high stationary level; and 6) finally dropping significantly due to the intervention of the preventive strategy from 2013 to 2015.
Table 1.
Positive cases of tick-borne relapsing fever in Dielmo and Ndiop occurred before and during the preventive control strategy of borreliosis
| Annual monitoring | Diagnostic method | No. of borreliosis cases/village | Incidence/100 person-year | ||
|---|---|---|---|---|---|
| TBS and/or PCR | Dielmo | Ndiop | Dielmo | Ndiop | |
| 1990 (June in Dielmo) | TBS | 5 | 3.57 | ||
| 1991 | TBS | 8 | 3.63 | ||
| 1992 | TBS | 9 | 4.03 | ||
| 1993 (July in Ndiop) | TBS | 9 | 0 | 3.69 | 0 |
| 1994 | TBS | 9 | 0 | 3.7 | 0 |
| 1995 | TBS | 11 | 0 | 4.53 | 0 |
| 1996 | TBS | 44 | 0 | 16.06 | 0 |
| 1997 | TBS | 65 | 0 | 24.92 | 0 |
| 1998 | TBS | 56 | 0 | 20.35 | 0 |
| 1999 | TBS | 52 | 0 | 19.24 | 0 |
| 2000 | TBS | 55 | 0 | 19.15 | 0 |
| 2001 | TBS | 37 | 0 | 13.14 | 0 |
| 2002 | TBS | 25 | 0 | 8.93 | 0 |
| 2003 | TBS | 10 | 0 | 3.56 | 0 |
| 2004 | TBS | 21 | 0 | 6.96 | 0 |
| 2005 | TBS | 28 | 0 | 10.22 | 0 |
| 2006 | TBS | 53 | 0 | 18.73 | 0 |
| 2007 | TBS | 39 | 0 | 12.06 | 0 |
| 2008 | TBS/PCR QIAGEN (December)* | 17 | 0 | 4.77 | 0 |
| 2009 | TBS/PCR QIAGEN | 35 | 0 | 8.58 | 0 |
| 2010 | TBS/PCR POC (November)† | 36 | 5 | 9.78 | 6.04 |
| 2011 | PCR | 35 | 19 | 9.57 | 5.95 |
| 2012 | PCR | 34 | 14 | 9.08 | 4.08 |
| Total cases of any method | 693 | 38 | 10.55 | 3.79 | |
| Average annual | 31 (30.8) | 10 (9.5) | 10.55 | 3.79 | |
| January and February 2013 (before intervention) | 4 | 3 | |||
| Intervention period March 2013 (10 months) | 10 | 8 | 2.58 | 2.16 | |
| 2013 (for all cases detected) | 14 | 11 | 3.64 | 3.04 | |
| 2014 (intervention, monitoring, and tracking of risk factors) | 12 | 3 | 3.13 | 0.78 | |
| January to September 2015 (9 months) | 4 | 3 | 1.07 | 0.72 | |
| Cases during the intervention period (March 2013 to September 2015) | 26 | 14 | 2.63 | 1.39 | |
| Average cases in course of intervention‡ | 10 (10.07) | 5 (5.42) | 2.63 | 1.39 | |
PCR = polymerase chain reaction, POC = point-of-care, TBS = thick blood smear.
DNA extraction from patients with fever using QIAGEN columns.
PCR in Dielmo POC laboratory.
Cases occurred in the intervention period of 2 years 7 months.
Figure 5.
(A) Average density of risk factors (rodent burrows and crevices) in Dielmo; (B) average density of risk factors (rodent burrows and crevices) in Ndiop.
Figure 6.
Evolution of incidence density of borreliosis in Dielmo from 1990 to 2012, and impact of preventive control of borreliosis from March 2013 to September 2015.
The RR of developing borreliosis in Dielmo, despite the implementation of the strategy, was RRDL = 0.1014 = 10.14% with a 95% CI of 0.17–0.37. The proportion of cases that would have occurred if the preventive control strategy had not been implemented, or the preventive fraction of risk, was 1 − RRDL = 1− 0.1014 = 0.8986 = 89.8%, and the number of cases that were avoided due to the implementation of the strategy was 31 × 0.8986 = 28 cases. There is a significant difference between the average incidence of borreliosis before and during the cementing work (χ2 = 56.51; P < 0.001). By contrast, in Ndiop, RR was RRNDP = 0.1847 = 18.47% with 95% CI of 0.12–0.68. The preventive fraction of risk was 1 − RRNDP = 1 − 0.1847 = 0.8153 = 81.5%, and the number of cases avoided due to the implementation of the strategy was 10 × 0.8153 = 8 cases. A significant difference was observed between average incidence of borreliosis before and after the intervention (χ2 = 11.07; P < 0.001). The total cost of the intervention leading to a successful preventive control strategy of tick-borne borreliosis was estimated at €19,050, including €9,472 in Dielmo and €9,577 in Ndiop. The cost per avoided infection was estimated at €526.
Sampling of Ornithodoros ticks in burrows from Ndiop and Dielmo.
Overall, in January 2012, 10 and seven households were visited in Ndiop and Dielmo, respectively. A total of 60 burrows were investigated in homes from Ndiop and Dielmo (Table 2). Of the 60 burrows sampled (Table 2), 9/30 (30%) were infested with Ornithodoros ticks in Ndiop and 10/30 (33.3%) in Dielmo. All Ornithodoros ticks collected from both villages were identified as O. sonrai tick vector. In total, 180 Ornithodoros ticks were collected, 118 were used for molecular identification of Borrelia infection: 24 ticks collected from Ndiop and 94 from Dielmo, whereas the remaining 62 ticks were used for Borrelia isolation in a mouse model (data not shown). In Ndiop, the infested burrows were located in one household in the village (13°41′ 09″N and 16°23′ 04.02″W) and one other household southwest of the village (13°41′ 06.06″N and 16°23′ 09.03″W). However, among the 51 neighboring villages investigated, 33 were positive for the presence of O. sonrai ticks. All positive villages comprised between 13°46′N and 13°37′N have infested burrows with Ornithodoros ticks inside houses or around human dwellings with mostly of soil indoors without cementing and/or cement deteriorated where populations may be frequently exposed to tick vectors bites (G. Diatta, unpublished data).
Table 2.
Collection site of Ornithodoros sonrai ticks and infected specimens with Borrelia in Ndiop and Dielmo villages, Sine-Saloum, Senegal
| Collection site | No. of burrows investigated | No. of ticks tested | Tick species collected | No. of burrows infested | Molecular detection qPCR 16S RNA Borrelia spp. | No. of ticks kept live in livestock | Total ticks collected | |
|---|---|---|---|---|---|---|---|---|
| Study village | Household ID | No. of infected ticks | ||||||
| Ndiop | No. 3 | 1 | 2 | O. sonrai | 1/1 | 0/2 | 0 | 2 |
| No. 5 | 8 | 22 | O. sonrai | 8/8 | 8/22 | 32 | 54 | |
| Nos. 1, 8, 10, 11, 12, 27, 32, 37 | 21 | – | 0 | 0/21 | – | 0 | 0 | |
| Total | 10 | 30 | 24 | 1 | 9/30 (30%) | 8/24 (33.3%) | 32 | 56 |
| Dielmo | No. 13 | 3 | 9 | O. sonrai | 2/3 | 1/9 | 0 | 9 |
| No. 16 | 6 | 8 | O. sonrai | 1/6 | 0/8 | 10 | 18 | |
| No. 19 | 7 | 73 | O. sonrai | 5/7 | 6/73 | 20 | 93 | |
| No. 20 | 4 | 4 | O. sonrai | 2/4 | 0/4 | 0 | 4 | |
| Nos. 6, 11, 18 | 10 | – | 0 | 0/10 | – | 0 | 0 | |
| Total | 7 | 30 | 94 | 1 | 10/30 (33.3%) | 7/94 (7.4%) | 30 | 124 |
| Grand total | 17 | 60 | 118 | 19/60 (31.6%) | 15/118 (13%) | 62 | 180 | |
qPCR = quantitative polymerase chain reaction.
Detection of Borrelia infection by qPCR in Ndiop and Dielmo.
Of the 118 Ornithodoros ticks tested (57 dead and 61 alive) by qPCR, 15/118 (13%) were infected with Borrelia, including 8/24 (33.3%) ticks from Ndiop and 7/94 (7.4%) from Dielmo (Table 2). All Borrelia spp. infections identified by genus-specific 16S rDNA-based qPCR from tick samples tested positive by B. crocidurae-specific qPCR for the glpQ gene. Amplification and sequencing of the intergenic speacer gene 16S-23S from the Borrelia-positive specimens was performed on 13 B. crocidurae-positive samples 13/118 (11%), including 7/24 (29.1%) ticks from Ndiop and 6/94 (6.3%) from Dielmo (Table 2).
Discussion and Conclusion
The present study shows that improving intra-domiciliary habitat conditions in Dielmo and Ndiop by cementing, coupled with accompanying measures including the capture of small mammals and sanitary hygiene, was associated with a significant reduction of the incidence of tick-borne borreliosis, dropping from 10.55 cases per 100 person-years to 2.63 cases per 100 person-years (P < 0.001) in Dielmo and from 3.79 cases per 100 person-years to 1.39 cases per 100 person-years (P < 0.001) in Ndiop. The observed decline in incidence of borreliosis resulted after the implementation of the intervention strategy and the accompanying measures, but we did not notice a decrease in the incidence without intervention. We speculate that this is coincidental. Some antimalarials were used to protect the populations of Dielmo and Ndiop, and sometimes only antibiotics such as doxycycline/erythromycin were given when borreliosis or rickettsioses cases and/or other pathogenic bacteria were detected. Before or during the period of the intervention strategy, there is no unrelated project that acted and used within both populations a massive distribution of antimicrobials. The rodent capture approach reduced massively the presence of rodents and their role in dispersing the tick vector O. sonrai inside homes, and the frequency of rodent burrows. This measure of the intervention strategy has highly contributed to the significant reduction of TBRF incidence in both villages. We think that this strategy, tested here for the first time, contributed to avoid the occurrence of 28 cases of borreliosis in Dielmo and eight cases in Ndiop. The RR of developing TBRF in Dielmo (RRDL = 0.1014 = 10.14%) and in Ndiop (RRNDP = 0.1847 = 18.47%) was < 1, which indicates that the preventive method of tick-borne borreliosis control is a protective factor that has considerably reduced TBRF in Dielmo, and has played an important role in preventing the spread of this disease in Ndiop, where it appeared only in October 2010.21 The incidence rate of TBRF in Dielmo of 11 per 100 person-years and 9.7 cases per 100 person-years,16,21 along with 2.4 cases per 100 person-years in Ndiop,21 was considered as the highest in Africa. As part of the rise and fall of malaria in the Dielmo rural community of Sine-Saloum,28 TBRF seems presently to be the first frequent infectious disease in Dielmo after malaria and rickettsioses.22 The proposed strategy seems to be effective and appropriate for the control of borreliosis in endemic areas.3,15,20 In addition, our results suggest that with an evaluated cost of €526 per avoided infection over the study period, the intervention is economically affordable for low-income countries. We also estimated that the cost per avoided infection would drop to €262 in 2016 considering recent data on TBRF cases in Dielmo and Ndiop. The intervention strategy and the accompanying measures have been transferred to the community level since January 2016. This is why we believe that the best way to make sustainable our intervention strategy is to ensure that populations use the techniques provided, that is, each inhabitant must track down burrows and attics reappearing in his house while we continue to provide cement and nontoxic liquid glue to capture rodents and insectivores. By transferring the strategy to the community level in 2016, we decided to continue providing the nontoxic liquid glue every 3 months for anticipating on the duration this protection in each village, as both rural populations living in poverty would not be able to take the charge.
All human cases detected in Dielmo were initially diagnosed by thick blood smear technique from June 1990 to November 2008, thick blood smear and qPCR from December 2008 to November 2010, and then, before and during the intervention period, by qPCR alone. On average, only nine cases of borreliosis were observed from 1990 to 1994. These data were probably lower than the real number of cases that may be explained by the lack of experienced microscopists at the beginning of the project and by the lower sensitivity of microscopy for the detection of Borrelia compared to PCR.20 The average number of cases diagnosed annually during the intervention period was 10 in Dielmo and five in Ndiop, whereas before the intervention, there were 31 cases in Dielmo and 10 in Ndiop.
The high proportion of Ornithodoros tick-infested burrows in the village of Dielmo (33%) may indicate that the presence of burrows harboring O. sonrai inside bedrooms is the main factor in disease transmission.16 In Ndiop, only 20% of households investigated were infested with O. sonrai, compared with 57% in Dielmo, confirming the clinical data21 that the distribution of these tick vectors seems more recent in Ndiop village than in Dielmo, and it should also be noted that Ndiop is at the southern distribution limit and most infested burrows came from the same household. The high infestation of burrows inside bedrooms, of which many contained Borrelia-infected ticks allowed us to propose the following routes of transmission depending to the previous data24,25: 1) most likely, the villagers are infected inside their homes, especially when the burrows open into the bedroom and/or inhabitants sleep on mats or mattresses on the floor; 2) transmission may also occur during household work or in surrounding fields during various outdoor activities; and 3) infection may occur during a travel through high-risk areas outside the village.
The prevalence of infection with O. sonrai was higher in Ndiop (33.3%) than in Dielmo (7.4%), and there is no obvious explanation for this difference, though the fact that O. sonrai ticks were collected in Ndiop from the same endemic household may be suspected. This prevalence of infected ticks in Ndiop (33.3%) seems close to that recently reported in Senegal (26%) by Trape and others.6 In Mali, 12.5% of O. sonrai ticks,6 and 18% of other specimens,29 were recently reported to carry B. crocidurae, while in Tunisia, 15% of O. erraticus “small variety,”30 and 6.5% of O. sonrai6 were reported to carry B. crocidurae. In a recent study performed in Morocco, Diatta and others31 found a 10% infection rate in O. erraticus s.l., including B. hispanica in 33% of Borrelia-positive ticks, B. crocidurae in 41% of Borrelia-positive ticks, and B. merionesi in 26% of Borrelia-positive ticks.
The results of this study indicate that the strategy to improve intra-domiciliary habitat conditions with accompanying measures was associated with the decrease in cases of borreliosis and significant reduction of the incidence of the disease in Dielmo and Ndiop. This preventive control of borreliosis, aimed at combating tick vectors by avoiding contact with humans, deserves to be considered as an effective tool in preventing disease transmission within main foci of western African countries where traditional housing is predominant. This strategy and the accompanying measures have been transferred to the community level in 2016 to durably sustain the satisfactory results obtained. A rapid tick-borne borreliosis screening technique must be implemented to strengthen the management of febrile patients in sanitary structures. Our findings support the hypothesis that, taking action inside houses against the factors that give rise to TBRF, such as tick vectors, small rodents, and insectivore reservoirs, connected with promoting hygienic conditions, can really help to reduce the frequency and incidence of borreliosis in humans.
ACKNOWLEDGMENTS
We would like to thank the populations of Dielmo and Ndiop villages for allowing us to conduct our investigations in their homes, along with the staff of the Dielmo Point-of-Care laboratory, especially Khadim Mbacke Leye and Sawdiattou Djiba, the nurses at the Dielmo and Ndiop dispensaries, notably Domonique Manga, Abdoulaye Badiane, and Assane Camara. We are grateful to C. Nappez for his assistance during the laboratory experiments, J. M Berenger for the photos taken during the tick blood feedings.
Footnotes
Financial support: This work was supported by grants from La Fondation Méditerranée Infection, Marseille, and l'Unité de Recherche sur les Maladies Infectieuses et Tropicales Émergentes (URMITE), UMR 198 of Institut de Recherche pour le Développement (IRD).
Authors' addresses: Georges Diatta, Cheikh Sokhna, Hubert Bassène, and Gilles Chauvancy, Institut de Recherche pour le Développement, Campus International de Recherche IRD/UCAD de Hann, Dakar, Senegal, E-mails: georges.diatta@ird.fr, cheikh.sokhna@ird.fr, hubert.bassene@ird.fr, and gilles.chauvancy@ird.fr. Oleg Mediannikov, Florence Fenollar, Philippe Parola, and Didier Raoult, Institut de Recherche pour le Développement, Campus International de Recherche IRD/UCAD de Hann, Dakar, Senegal, and Unité de Recherche sur les Maladies Infectieuses et Tropicales Émergentes, UM63, CNRRS7278, IRD 198, INSERM 1095, Aix Marseille Université, Marseille, France, E-mails: oleg.mediannikov@ird.fr, florence.fenollar@univ-amu.fr, philippe.parola@univ-amu.fr, and didier.raoult@gmail.com. Sylvie Boyer, Sciences Economiques and Sociales de la Santé and Traitement de l'Information Médicale, Institut National de la Santé et de la Recherche Médicale, Aix Marseille Université, Marseille, France, E-mail: boyersyl@gmail.com. Abdoul Aziz Ndiaye, Département Santé Communautaire, Université Alioune Diop de Bambey, Bambey, Senegal, and Service de Santé des Armées, Camp Dial Diop, Dakar, Senegal, E-mail: ndiaziz2000@yahoo.fr. Fatoumata Diene, Institut Pasteur de Dakar, Dakar, Senegal, E-mail: fdsarr@pasteur.sn.
References
- 1.Cutler SJ, Abdissa A, Trape JF. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect. 2009;15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 2.Trape JF, Godeluck B, Diatta G, Rogier C, Legros F, Albergel J, Pepin Y, Duplantier JM. The spread of tick-borne borreliosis in west Africa and its relationship to sub-Saharan drought. Am J Trop Med Hyg. 1996;54:289–293. doi: 10.4269/ajtmh.1996.54.289. [DOI] [PubMed] [Google Scholar]
- 3.Trape JF, Duplantier JM, Bouganali H, Godeluck B, Legros F, Cornet JP, Camicas JL. Tick-borne borreliosis in west Africa. Lancet. 1991;337:473–475. doi: 10.1016/0140-6736(91)93404-w. [DOI] [PubMed] [Google Scholar]
- 4.Golvan YJ. Élément de Parasitologie Médicale. Paris, France: Flammarion Médecine-Sciences; 1983. pp. 360–372. [Google Scholar]
- 5.Walton GA. London, United Kingdom: Royal Zoological Society; 1962. The Ornithodoros moubata superspecies problem in relation to human relapsing fever epidemiology. Aspects of Disease Transmission by Ticks (Royal Zoological Society of London Symposium Series) pp. 83–153. [Google Scholar]
- 6.Trape JF, Diatta G, Arnathau C, Bitam I, Sarih M, Belghyti D, Bouattour A, Elguero E, Vial L, Mané Y, Baldé C, Pugnolle F, Chauvancy G, Mahé G, Granjon L, Duplantier JM, Durand P, Renaud F. The epidemiology and geographic distribution of relapsing fever borreliosis in west and north Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida) PloS One. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assous MV, Wilamowski A, Bercovier H, Marva E. Molecular characterization of tickborne relapsing fever Borrelia, Israel. Emerg Infect Dis. 2006;12:1740–1743. doi: 10.3201/eid1211.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyplosz B, Mihaila-Amrouche L, Baixench MT, Bigel ML, Berardi-Grassias L, Fontaine C, Hornstein M, Izri A, Baranton G, Postic D. Imported tick-borne relapsing fever, France. Emerg Infect Dis. 2005;11:1801–1803. doi: 10.3201/eid1111.050616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell J. Tick-borne relapsing fever under-reported. Lancet Infect Dis. 2003;3:604. doi: 10.1016/s1473-3099(03)00787-4. [DOI] [PubMed] [Google Scholar]
- 10.Barclay AJ, Coulter JB. Tick-borne relapsing fever in central Tanzania. Trans R Soc Trop Med Hyg. 1990;84:852–856. doi: 10.1016/0035-9203(90)90106-o. [DOI] [PubMed] [Google Scholar]
- 11.Boiron H. Considérations sur la fièvre récurrente à tiques au Sénégal. L'importance du rat comme réservoir de virus. Bull Soc Pathol Exot. 1949;42:62–70. [PubMed] [Google Scholar]
- 12.Lecompte Y, Trape JF. La fièvre récurrente à tiques d'Afrique de, l'Ouest. Ann Biol Clin (Paris) 2003;61:541–548. [PubMed] [Google Scholar]
- 13.Léger A. Spirochète de la musaraigne (Crocidura stampflii Jentink) Bull Soc Pathol Exot. 1917;10:280–281. [Google Scholar]
- 14.Bergeret C, Raoult A. Notes sur les formes nerveuses de la fièvre récurrente. Fièvre récurrente à tiques en Afrique occidentale française. Bul Med Afrique Occidentale Française. 1934;27:593–598. [Google Scholar]
- 15.Trape JF, Godeluck B, Diatta G, Rogier C, Legros F, Albergel J, Pepin Y, Duplantier JM. Tick-borne borreliosis in west Africa: recent epidemiological studies. Ann Acad Med Bialostociensis. 1996;41:136–141. [PubMed] [Google Scholar]
- 16.Vial L, Diatta G, Tall A, Ba EH, Bouganali H, Durand P, Sokhna C, Rogier C, Renaud F, Trape JF. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet. 2006;368:37–43. doi: 10.1016/S0140-6736(06)68968-X. [DOI] [PubMed] [Google Scholar]
- 17.Kishi M. Acutely Toxic Pesticides. Geneva, Switzerland: World Health Organization; 2002. [Google Scholar]
- 18.Reynaud R, Masssat R, Picca M, Revil M. A propos de douze cas de fièvres récurrentes à tiques. Bull Soc Med Afr Noire Lang Fr. 1965;10:1–4. [PubMed] [Google Scholar]
- 19.Aubry P, Renambot J, Teyssier J, Buisson Y, Granic G, Brunetti G, Dano P, Bauer PH. Les borrélioses à tiques au Sénégal: à propos de 23 observations. Dakar Méd. 1983;28:413–420. [PubMed] [Google Scholar]
- 20.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassène H, Trape JF, Raoult D. Tick-borne relapsing fever borreliosis, rural Senegal. Emerg Infect Dis. 2011;17:883–885. doi: 10.3201/eid1705.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mediannikov O, Socolovschi C, Bassène H, Diatta G, Ratmanov P, Fenollar F, Sokhna C, Raoult D. High incidence of Borrelia crocidurae in acute febrile patients in Senegal. Emerg Infect Dis. 2014;20:1335–1338. doi: 10.3201/eid2008.130550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P, Brahimi K, Faye O, Druilhe P, Da Sylva LP. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 23.Sokhna C, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape JF, Drancourt M, Raoult D. Point-of-care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis. 2013;7:e1999. doi: 10.1371/journal.pntd.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathis C, Durieux C, Advier M. Transmission naturelle et expérimentale à l'homme du spirochète infectant dans la nature, à Dakar, la tique: Ornithodorus erraticus var. marocanus. Ann Inst Pasteur (Paris) 1934;52:166–178. [Google Scholar]
- 25.Diatta G, Duplantier JM, Granjon L, Ba K, Chauvancy G, Ndiaye M, Trape JF. Borrelia infection in small mammals in west Africa and its relationship with tick occurrence inside burrows. Acta Tropica. 2015;152:131–140. doi: 10.1016/j.actatropica.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Sautet J, Witkowski M. A propos d'un ornithodore trouvé à Gao. Bull Soc Pathol Exot. 1944;37:182–188. [Google Scholar]
- 27.Elbir H, Raoult D, Drancourt M. Relapsing fever Borreliae in Africa. Am J Trop Med Hyg. 2013;89:288–292. doi: 10.4269/ajtmh.12-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, Mazenot C, Richard V, Badiane A, Dieye-Ba F, Faye J, Ndiaye G, Diene Sarr F, Roucher C, Bouganali C, Bassene H, Toure-Balde A, Roussilhon C, Perraut R, Spiegel A, Sarthou JL, Da Silva LP, Mercereau-Puijalon O, Druilhe P, Rogier C. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–488. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 29.Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, McCoy BN, Safronetz D, Sogoba N, Maïga O, Traoré SF. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, west Africa and the potential for human infection. PLoS Negl Trop Dis. 2012;6:e1924. doi: 10.1371/journal.pntd.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouattour A, Garnier M, M'Ghirbi Y, Sarih M, Gern L, Ferquel E, Postic D, Cornet M. Borrelia crocidurae infection of Ornithodoros erraticus (Lucas, 1849) ticks in Tunisia. Vector Borne Zoonotic Dis. 2010;10:825–830. doi: 10.1089/vbz.2009.0151. [DOI] [PubMed] [Google Scholar]
- 31.Diatta G, Souidi Y, Granjon L, Arnathau C, Durand P, Chauvancy G, Mané Y, Sarih M, Belghyti D, Renaud F, Trape JF. Epidemiology of tick-borne borreliosis in Morocco. PLoS Negl Trop Dis. 2012;6:e1810. doi: 10.1371/journal.pntd.0001810. [DOI] [PMC free article] [PubMed] [Google Scholar]