Abstract
Diarrheagenic Escherichia coli (DEC) are common pathogens of childhood gastrointestinal infections worldwide. To date, research tracking DEC has mainly been completed in urban areas. This study aims to determine the prevalence and pathotype distribution of DEC strains in children from rural Peruvian communities and to establish their association with malnutrition. In this prospective cohort, 93 children aged 6–13 months from rural communities of Urubamba (Andes) and Moyobamba (jungle) were followed for 6 months. Diarrheal and control stool samples were analyzed using multiplex real-time polymerase chain reaction to identify the presence of virulence genes of DEC strains. The overall isolation rate of DEC was 43.0% (352/820). Enteroaggregative E. coli (EAEC, 20.4%), enteropathogenic E. coli (EPEC, 14.2%), and diffusely aggregative E. coli (DAEC, 11.0%) were the most prevalent pathotypes. EAEC was more frequently found in Moyobamba samples (P < 0.01). EPEC was the only strain significantly more frequent in diarrheal than asymptomatic control samples (P < 0.01). DEC strains were more prevalent among younger children (aged 6–12 months, P < 0.05). A decline in height-for-age Z-score (HAZ) was observed in 75.7% of children overall. EAEC was more frequently isolated among children who had a greater HAZ decline (P < 0.05). In conclusion, DEC strains were frequently found in stool samples from children in rural communities of the highlands and jungle of Peru. In addition, children with a greater decline in their growth rate had higher EAEC isolation rates, highlighting the importance of this pathogen in child malnutrition.
Introduction
Diarrheagenic Escherichia coli (DEC) account for 30–40% of cases of childhood diarrhea,1 one of the main causes of morbidity and mortality among children in developing countries.2 DEC may also colonize the infant gastrointestinal tract and result in asymptomatic infection, which confounds diagnosis and treatment.3
DEC are classified into six groups or pathotypes based on their pathogenic mechanisms: enteroaggregative (EAEC), enteropathogenic (EPEC), enterotoxigenic (ETEC), enteroinvasive (EIEC), diffusely aggregative (DAEC), and shiga toxin–producing E. coli (STEC).3 Although several analytic methods distinguish pathotypes, polymerase chain reaction (PCR) has the highest sensitivity and specificity.4 Nonetheless, this methodology is usually available only in research facilities.
Although the World Health Organization (WHO) has named specific DEC strains as the highest priority for vaccine development after rotavirus,5 detection has not been routinely conducted in clinical laboratories, particularly in rural areas. Others have shown that prevalence and distribution of DEC pathotypes vary significantly between and within countries.6–8 However, studies have been mostly conducted in periurban and urban areas and less frequently in rural communities, especially those in the geographical areas of the highlands and jungle.9–12
Malnutrition and growth retardation have remained a significant population health challenge in low-income regions. Previous studies suggest that the presence of EAEC may contribute to growth impairment in children, even when diarrhea is not present.13 Further exploring this association in rural populations may help public health initiatives prioritize therapy.
The present study aims to determine the prevalence and pathotype distribution of DEC strains isolated from children in rural communities of Peruvian highlands and jungle, as well as to establish whether an association between DEC pathotypes and malnutrition exists.
Materials and Methods
Study design and population.
This research was conducted as a subgroup analysis of an environmental enteropathy project. A cohort of 93 children aged between 6 and 13 months were prospectively enrolled from the Peruvian rural communities of Urubamba (Yucay, Huayllabamba, and Ccotohuincho) and Moyobamba (Yantalo and Calzada), representing the highlands and jungle, respectively. Subjects were followed up for 6 months (December 2014 to June 2015). We excluded children with history of congenital disease, severe neonatal complications, prematurity, or previously diagnosed acute or chronic malnutrition. Length and weight were measured by trained field workers monthly and analyzed according to WHO standards (Anthro version 3.2.2 [Department of Nutrition, WHO, Geneva, Switzerland]).
Specimen collection and processing.
Stool samples were collected every 2 weeks and with each diarrheal episode (within 48 hours). Nondiarrheal (asymptomatic control) samples were collected and included when diarrhea was absent for 1 week before or after stool sample collection to evaluate colonization. Diarrhea was defined as having three or more liquid or semiliquid stools in a 24-hour period or at least one loose stool with visible blood.14 All specimens were placed in Cary–Blair transport medium and shipped in iced boxes at 4°C to the Instituto de Medicina Tropical Alexander von Humboldt in Lima (Moyobamba samples) and Cuzco (Urubamba samples) for processing. Average time to analysis postcollection was 96 hours for Moyobamba and 32 hours for Urubamba.
Samples were inoculated onto MacConkey agars and incubated for 24 hours at 37°C. Five lactose-fermenting colonies with typical E. coli morphology and two non–lactose fermenting colonies (when found) were selected and maintained in nutrient agar slants at room temperature (seven per stool sample). No biochemical tests for identification were carried out.
To determine specific DEC pathotypes, pooling of the seven isolated colonies was done after thermal shock DNA extraction for posterior multiplex real-time PCR. This method simultaneously detects the presence of the following genes: aggR (EAEC), st1, st2, eltA, eltB (ETEC), eae (EPEC), eae, stx1, stx2 (STEC), ipaH (EIEC), and daaD (DAEC).4 This methodology has been validated and used since 2005 in the Instituto de Medicina Tropical Alexander von Humboldt (Lima, Peru).
Statistical analysis.
The statistical analyses were performed using STATA, version 14 (StataCorp LP, College Station, TX). Categorical data were compared by χ2 test. Comparisons between means were made using two-sample t test or nonparametric test when applicable. A P value < 0.05 was considered statistically significant.
Ethical considerations.
The study was approved by the Ethical Review Board of the University of Texas Health Science Center at Houston and Universidad Peruana Cayetano Heredia. Written informed consent was obtained from the parent or guardian of each child.
Results
Of the 93 children enrolled, 41 were from Urubamba and 52 from Moyobamba. Urubamba children were younger, had a higher percentage of high-school graduate mothers, and lived in houses with better sanitation (i.e., sewage system present and fewer animals inside the house) (Table 1).
Table 1.
Sociodemographic characteristics of study population at enrollment
| Characteristic | Moyobamba (jungle) | Urubamba* (Andes) | Total (N = 93) |
|---|---|---|---|
| (N = 52) | (N = 41) | ||
| Male sex | 30 (57.7) | 25 (61.0) | 55 (53.4) |
| Age (months) | |||
| 6–9 | 28 (53.8) | 31 (75.6)† | 59 (57.3) |
| 10–13 | 24 (46.2) | 10 (24.4) | 34 (33.0) |
| Mean age (months) ± SD | 9.4 ± 2.4 | 8.7 ± 1.7 | 9.1 ± 2.1 |
| Breastfeeding of any duration | 52 (100.0) | 34 (100.0) | 86 (100.0) |
| Exclusive breastfeeding at 6 months | 30 (57.7) | 17 (63.0) | 47 (59.5) |
| Mean age at start of solid food (months) ± SD | 5.8 ± 1.2 | 5.8 ± 0.8 | 5.8 ± 1.0 |
| Maternal age, mean ± SD | 27.2 ± 6.7 | 26.8 ± 6.6 | 27.0 ± 6.6 |
| Maternal education | |||
| None | 8 (15.4) | 1 (2.9) | 9 (10.4) |
| Grammar school | 25 (48.0) | 11 (32.4) | 36 (41.9) |
| High school | 11 (21.2) | 16 (47.1)† | 27 (31.4) |
| Technical school or university | 8 (15.4) | 6 (17.6) | 14 (16.3) |
| Access to piped water inside house | 52 (100.0) | 34 (100.0) | 86 (100.0) |
| Domestic sewage | 26 (54.2) | 29 (85.3)† | 55 (67.1) |
| Boiling of water before consumption | 46 (93.9) | 27 (100.0) | 73 (96.1) |
| Having animals at house | 22 (45.8) | 2 (5.9)† | 24 (29.3) |
SD = standard deviation. Data are no. (%) of children, unless otherwise indicated.
Data not available for all children.
P < 0.05.
Nineteen children (20.4%) were lost during follow-up period; still, their specimens were processed and analyzed. A total of 820 stool samples were collected, of which 46 (5.6%) were diarrheal, whereas 774 (94.4%) were nondiarrheal or asymptomatic control samples. A median of 10 stool samples per child were collected.
Overall, diarreagenic E. coli were isolated in 43.0% (353/820) of stool samples. Of the 93 enrolled children, 75 (80.6%) had at least one stool positive for E. coli (median of 3, range 1–10 positive samples for 1–5 different pathotypes per child) during follow-up (Table 2). DEC were identified in 50% (N = 23) of diarrheal samples and 42.6% (N = 330) of control samples. EAEC (20.8%), EPEC (13.3%), and DAEC (11.1%) were the most frequent pathotypes—as single or mixed infections—in control samples, whereas EPEC (28.3%), EAEC (13.0%), and ETEC (10.9%) were the most common in diarrheal samples. EPEC was the only pathotype significantly more common in diarrheal than in control samples (P < 0.01).
Table 2.
Distribution of DEC strains found in stool samples
| DEC pathotype | Moyobamba (jungle) | Urubamba (Andes) | All children | |||
|---|---|---|---|---|---|---|
| Diarrhea (N = 23) | Control (N = 415) | Diarrhea (N = 23) | Control (N = 359) | Diarrhea (N = 46) | Control (N = 774) | |
| Single pathotype | ||||||
| EAEC (aggR) | 3 (13.0) | 81 (19.5)* | 0 (0.0) | 46 (12.8)* | 3 (6.5) | 127 (16.4) |
| EPEC (eaeA) | 4 (17.4) | 39 (9.4) | 5 (21.7) | 32 (8.9) | 9 (19.6)† | 71 (9.2)† |
| DAEC (daaD) | 0 (0.0) | 18 (4.3) | 2 (8.7) | 27 (7.5) | 2 (4.3) | 45 (5.8) |
| ETEC (st and/or lt) | 1 (4.3) | 12 (2.9) | 3 (13.0) | 18 (5.0) | 4 (8.7) | 30 (3.9) |
| STEC (stx1 and/or stx2, eae) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| EIEC (ipaH) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| DEC coinfections | ||||||
| EAEC + DAEC | 1 (4.3) | 10 (2.4) | 0 (0.0) | 6 (1.7) | 1 (2.2) | 16 (2.1) |
| EPEC + DAEC | 0 (0.0) | 6 (1.4) | 1 (4.3) | 9 (2.5) | 1 (2.2) | 15 (1.9) |
| EAEC + EPEC | 1 (4.3) | 8 (1.9) | 1 (4.3) | 5 (1.4) | 2 (4.3) | 13 (1.7) |
| DAEC + ETEC | 0 (0.0) | 3 (0.7) | 0 (0.0) | 2 (0.6) | 0 (0.0) | 5 (0.6) |
| EAEC + ETEC + DAEC | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 2 (0.3) |
| EAEC + DAEC + EPEC | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
| EPEC + ETEC | 1 (4.3) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (2.2) | 1 (0.1) |
| EAEC + EPEC + ETEC | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 1 (0.1) |
| All DEC | 11 (47.8) | 180 (43.4) | 12 (52.2) | 150 (41.8) | 23 (50.0) | 330 (42.6) |
DEC = diarrheagenic E. coli; DAEC = diffusely aggregative E. coli; EAEC = enteroaggregative E. coli; EIEC = enteroinvasive E. coli; EPEC = enteropathogenic E. coli; ETEC = enterotoxigenic E. coli; STEC = shiga toxin–producing E. coli. Data are no. (%) of children, unless otherwise indicated.
P = 0.005, for the comparison of EAEC in control samples between Moyobamba and Urubamba.
P = 0.004, for the comparison of EPEC between diarrhea and control samples.
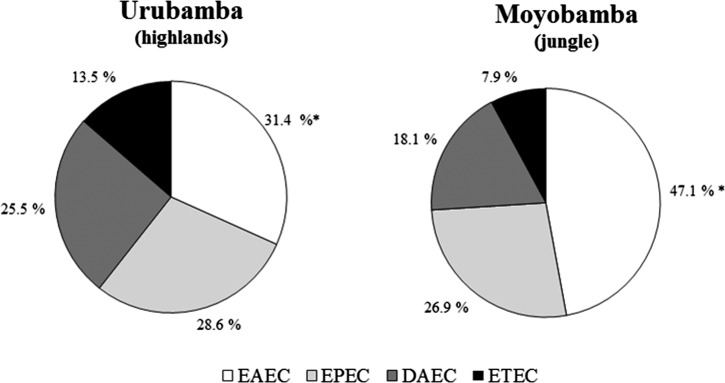
The prevalence of EAEC, as single or coinfection, was higher in control samples collected from the jungle site (Moyobamba) than in those from Urubamba, the highland site (102/415 [24.6%] versus 59/359 [16.4%], P < 0.01). Although pathotypes were similarly arranged by frequency, the distribution varied between both geographical regions (Figure 1 ).
Figure 1.
Distribution of diarrheagenic Escherichia coli (DEC) pathotypes in rural communities of the highlands and jungle. Urubamba N = 194 positive DEC samples; Moyobamba N = 227 positive DEC samples. * P < 0.05.
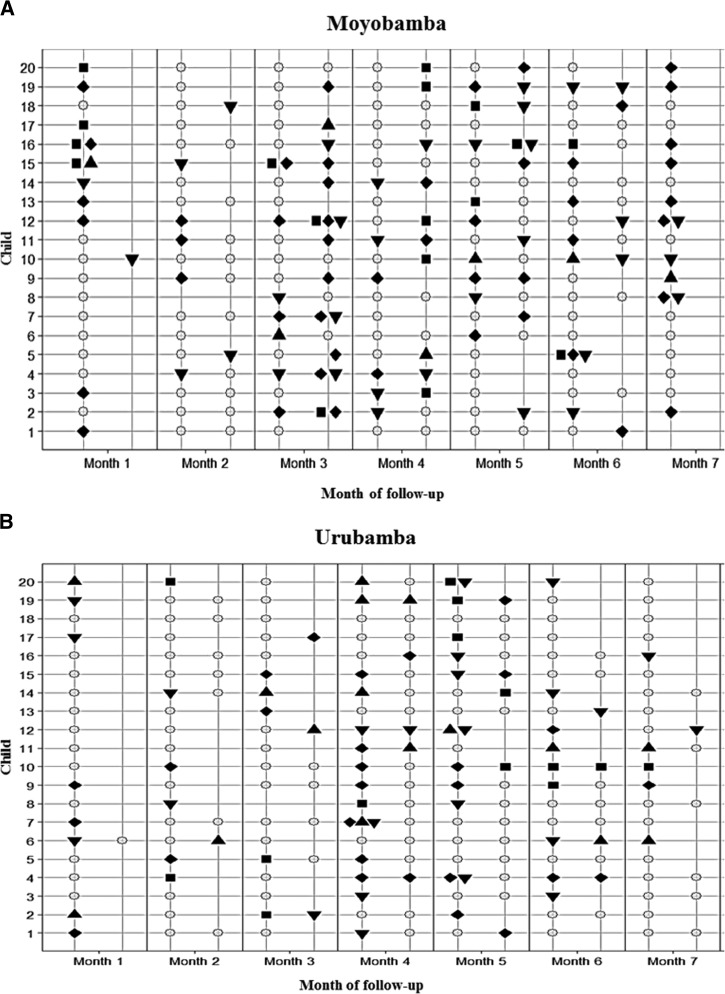
DEC colonization and behavior over time was frequent and heterogeneous in subjects from both communities (Figure 2 ). Children were found to be colonized with at least one DEC pathotype for about 50% of the time of follow-up (median number of months with positive stool was 3/6, interquartile range = 2–5). Reinfection with EAEC was common, being detected in 39 (54.9%) of 71 children with an initial infection (median of two infections per child, interquartile range = 2–3). This proportion was lower for EPEC (43.9% [29/66]), DAEC (30.8% [16/52]), and ETEC (21.9% [7/32]). On the other hand, shedding after a new infection, defined as the time elapsed from the first to the last positive consecutive sample, ranged from 2 to 16 weeks for EAEC, 2 to 5 weeks for EPEC, 2 to 7 weeks for DAEC, and 2 weeks for ETEC.
Figure 2.
Diarrheagenic Escherichia coli infections in children from both communities over time. Sample of 20 children randomly selected in each study site, among children with complete follow-up and at least one stool sample collected per month. ○ = negative sample, ♦ = positive for enteroaggregative E. coli, ▾ = positive for enteropathogenic E. coli, ▪ = positive for diffusely aggregative E. coli, ▴ = positive for enterotoxigenic E. coli.
To determine age-related susceptibility to infection with DEC, stool samples were divided into two groups according to the age at which they were obtained. DEC strains were more frequently isolated in samples from children aged 6–12 months than in the 13–19 months group (222/400 [55.5%] versus 197/420 [46.9%], P < 0.05), EAEC contributing most to this difference (P < 0.05).
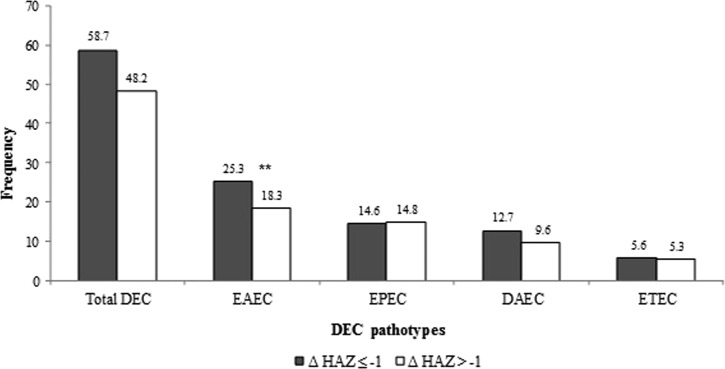
With regard to the evolution of nutritional status of those who completed follow-up (74/93), a decline in height-for-age Z-score (HAZ) was observed in 70.3% of children from Moyobamba (mean HAZ change of −0.49 ± 0.94) and 81.1% of children from Urubamba (mean HAZ change of −0.47 ± 0.73). Of these, six children from Moyobamba (6/37, 16.2%) and 11 from Urubamba (11/37, 29.7%) were clinically stunted (HAZ < −2) by the end of follow-up. EAEC was more frequently isolated among the children who had a decline greater than one unit in their HAZ score as opposed to those with a smaller change (P < 0.05) (Figure 3 ).
Figure 3.
Frequency of diarrheagenic Escherichia coli pathotypes according to variation of height-for-age Z-score (HAZ) during follow up. Sample size: N = 22 children in ΔHAZ ≤ −1 group, N = 52 children in ΔHAZ > −1. Significant difference was found among the enteroaggregative Escherichia coli (EAEC) groups, ** P < 0.05.
Discussion
This prospective cohort study was performed to determine the importance of DEC infections in children from rural communities of the highlands and jungle, which has not been thoroughly described previously. We found that the overall isolation rate of DEC strains in the present study (43.0%) was higher than previously reported in coastal periurban areas of Peru (31.5%)9 and some rural communities in other geographical areas of the world.15,16 Similar to other reports, no significant difference between the frequency of DEC infections in diarrheal (50%) and nondiarrheal samples (42.6%) was observed. This finding underscores the high prevalence of the carrier state in these areas.
Previous studies in Peru have predominantly been conducted in periurban Lima, located in the coast. In 2011, Ochoa and others studied the frequency and pathotypes of DEC in a cohort of 1,034 children aged 2–12 months with and without diarrhea followed for 1 year, and found DEC in 31.0% of 936 diarrheal samples and 31.8% of 424 asymptomatic control samples.9 In accordance to our findings, EAEC, EPEC, and DAEC were the most prevalent strains in asymptomatic infections. Similar results were obtained in another large study including 8,000 samples from children younger than 5 years, although ETEC was the third most common pathotype identified in that study.10 This data suggests that pathotype distribution in control samples is similar throughout the three major regions of Peru: coast, mountains, and jungle.
In congruence with our study, EAEC has been previously described as the DEC strain with the highest isolation rate overall.9,10,15,17 Nonetheless, its association with diarrhea requires further analysis: studies conducted in Brazil18,19 and Vietnam20 found EAEC to be significantly more prevalent in diarrheal than control samples, whereas others did not (including the present study).9,10,15,16 Notably, EAEC was more frequently identified in nondiarrheal samples from Moyobamba than Urubamba. This could be explained by the less favorable sociodemographic conditions (Table 1) or the distinct environmental and climatic characteristics in the former study site.
In diarrheal samples, EPEC was the most frequently isolated pathotype, followed by EAEC and ETEC. Furthermore, EPEC was significantly associated with diarrheal episodes, in concordance with previous reports by Estrada-Garcia and others21 in children < 2 years of age in Mexico and Moreno and others19 in infants from northeast Brazil. However, mostly atypical EPEC has been shown to contribute to the aforementioned association, information lacking in our study given that the bfpA gene was not sought for.
The role of DAEC in diarrhea is still undefined. This association has been described in several reports, which describe an age-related susceptibility in favor of older children.9,16,22 Although we also found DAEC to be an important cause of infection, these cases were mostly asymptomatic even after age stratification. Interestingly, DAEC was the most prevalent pathotype among DEC coinfections (Table 2).
The analysis of colonization over time demonstrates that children living in rural areas of Peru are frequently and repeatedly colonized with DEC in their first years of life; prolonged shedding, reinfections, and coinfections are common. The clinical implications of these findings need further investigation.
As mentioned before, we analyzed samples according to the age at which they were obtained to explore the age-related susceptibility to infection with DEC strains. We found that all pathotypes were more commonly identified in the younger group (6–12 months), especially EAEC, which was the only one with a significant difference between the two groups. This correlates with the results of Scaletsky and others in children < 2 years of age in Brazil for EAEC, EPEC, and DAEC.22
Several studies have suggested that EAEC plays a role in growth impairment mainly through intestinal inflammation and diminished nutrient absorption.11,23 To evaluate this effect, we compared the prevalence of EAEC infections in children with an HAZ decline greater than one standard deviation (SD) during follow-up (ΔHAZ ≤ −1) versus those with a decline smaller than one SD (ΔHAZ > −1). We observed that EAEC infection was more frequently detected in children with a greater decline in their HAZ (Figure 3). This association may reflect a microbiota preferred in the starvation state and is not necessarily causal, but an important observation nonetheless.
It is important to take the limitations of this study into account. The lack of complete demographic information may have altered our perspective of baseline characteristics in both study sites. The reduced number of diarrheal episodes may have contributed to the underestimation of the real importance of DEC strains as causal agents of symptomatic events. Delay in sample transportation from the Moyobamba study site to Lima, as well as the cold temperatures at which samples were kept (4°C), may have led to a decreased detection rate of DEC pathotypes. With regard to the diagnostic methods used, bfpA evaluation for further typification of isolated EPEC strains as typical or atypical would have allowed a more detailed insight on its association with diarrhea. Finally, the time interval between consecutive samples was very variable among participants, making it difficult to derive consistent information regarding shedding of pathogens after diarrheal events.
In summary, DEC strains were found to be an important cause of symptomatic and asymptomatic infection in rural communities of the Peruvian highlands and jungle. This information is valuable as it complements previous knowledge from coastal and periurban areas, provides further insight for countries with distinct geographical regions and completes the epidemiological panorama of DEC in Peru.
ACKNOWLEDGMENTS
We thank all the parents and children of Yantaló, Calzada, Yucay, Huayllabamba, and Ccotohuincho communities for their participation in this study. We would also like to thank the physicians (especially Renzo Gambetta), nurses, nurse aids, and community health workers that have collaborated in the study. Finally, a special mention to Renzo J. C. Calderón, who helped us design Figure 2 and John C. Densmore, for his valuable contribution to this paper.
Footnotes
Financial support: This work was supported by the Thrasher Research Funds (Thrasher Award No. 11955) to Mara Zambruni and Theresa J. Ochoa.
Authors' addresses: Gonzalo J. Acosta, Natalia I. Vigo, David Durand, Maribel Riveros, and Sara Arango, Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: gjacosta89@gmail.com, natalia.vigo@outlook.com, david.durand@upch.pe, maribel.riveros@upch.pe, and sara.arango29@gmail.com. Mara Zambruni, Department of Pediatric Infectious Diseases, University of Texas Medical Branch, Galveston, TX, E-mail: mazambru@utmb.edu. Theresa J. Ochoa, Instituto de Medicina Tropical Alexander von Humboldt, Universidad Peruana Cayetano Heredia, Lima, Peru, and University of Texas Health Science Center at Houston, School of Public Health, Houston, TX, E-mails: theresa.j.ochoa@uth.tmc.edu or theresa.ochoa@upch.pe.
References
- 1.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–136. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acácio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicentre Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 3.Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev. 2013;26:822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol. 2008;46:1752–1757. doi: 10.1128/JCM.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanata CF, Mendoza W. Improving Diarrhoea Estimates. 2002. http://www.who.int/child_adolescent_health/documents/pdfs/improving_diarrhoea_estimates.pdf Available at. Accessed February 24, 2016.
- 6.Al-Gallas N, Bahri O, Bouratbeen A, Haasen AB, Aissa RB. Etiology of acute diarrhea in children and adults in Tunis, Tunisia, with emphasis on diarrheagenic Escherichia coli: prevalence, phenotyping, and molecular epidemiology. Am J Trop Med Hyg. 2007;77:571–582. [PubMed] [Google Scholar]
- 7.Ifeanyi CI, Ikeneche NF, Bassey BE, Al-Gallas N, Ben Aissa R, Boudabous A. Diarrheagenic Escherichia coli pathotypes isolated from children with diarrhea in the Federal Capital Territory Abuja, Nigeria. J Infect Dev Ctries. 2015;9:165–174. doi: 10.3855/jidc.5528. [DOI] [PubMed] [Google Scholar]
- 8.Nataro JP, Mai V, Johnson J, Blackwelder WC, Heimer R, Tirrell S, Edberg SC, Braden CR, Glenn Morris J, Jr, Hirshon JM. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clin Infect Dis. 2006;43:402–407. doi: 10.1086/505867. [DOI] [PubMed] [Google Scholar]
- 9.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, Molina M, Amemiya I, Verastegui H, Hall ER, Cleary TG, Lanata CF. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from periurban areas in Lima, Peru. Clin Infect Dis. 2009;49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochoa TJ, Mercado EH, Durand D, Rivera FP, Mosquito S, Contreras C, Riveros M, Lluque A, Barletta F, Prada A, Ruiz J. Frequency and pathotypes of diarrheagenic Escherichia coli in Peruvian children with and without diarrhea. Rev Peru Med Exp Salud Publica. 2011;28:13–20. doi: 10.1590/s1726-46342011000100003. [DOI] [PubMed] [Google Scholar]
- 11.Levine MM, Ferreccio C, Prado V, Cayazzo M, Abrego P, Martinez J, Maggi L, Baldini MM, Martin W, Maneval D, Bradford K, Guers L, Lior H, Wasserman SS, Nataro JP. Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am J Epidemiol. 1993;138:849–869. doi: 10.1093/oxfordjournals.aje.a116788. [DOI] [PubMed] [Google Scholar]
- 12.Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun. 2007;75:3961–3968. doi: 10.1128/IAI.00459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steiner TS, Lima AA, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J Infect Dis. 1998;177:88–96. doi: 10.1086/513809. [DOI] [PubMed] [Google Scholar]
- 14.Baqui AH, Black RE, Yunus M, Hoque AR, Chowdhury HR, Sack RB. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol. 1991;20:1057–1063. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 15.Tobias J, Kassem E, Rubinstein U, Bialik A, Vutukuru SR, Navaro A, Rokney A, Valinsky L, Ephros M, Cohen D, Muhsen K. Involvement of main diarrheagenic Escherichia coli, with emphasis on enteroaggregative E. coli, in severe non-epidemic pediatric diarrhea in a high-income country. BMC Infect Dis. 2015;15:79. doi: 10.1186/s12879-015-0804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osawa K, Raharjo D, Wasito EB, Harijono S, Shigemura K, Osawa R. Frequency of diarrheagenic Escherichia coli among children in Surabaya, Indonesia. Jpn J Infect Dis. 2013;66:446–448. doi: 10.7883/yoken.66.446. [DOI] [PubMed] [Google Scholar]
- 17.Lozer DM, Souza TB, Monfardini MV, Vicentini F, Kitagawa SS, Scaletsky IC, Spano LC. Genotypic and phenotypic analysis of diarrheagenic Escherichia coli strains isolated from Brazilian children living in low socioeconomic level communities. BMC Infect Dis. 2013;13:418. doi: 10.1186/1471-2334-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Araujo JM, Tabarelli GF, Aranda KS, Fabbricotti SH, Mendes CMF, Fagundes-Neto U, Scaletsky IC. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea-associated pathotypes among Brazilian children. J Clin Microbiol. 2007;45:3396–3399. doi: 10.1128/JCM.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno AC, Fernandes Filho A, Gomes TAT, Ramos STS, Montemor KPG, Tavares VC, Santos Filho L, Irino K, Martinez MB. Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis. 2010;66:50–57. doi: 10.1016/j.diagmicrobio.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TV, Le Van P, Huy L, Gia KN, Weintraub A. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J Clin Microbiol. 2005;43:755–760. doi: 10.1128/JCM.43.2.755-760.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estrada-García T, Lopez-Saucedo C, Thompson-Bonilla R, Abonce M, Lopez-Hernandez D, Santos JI, Rosado JL, DuPont HL, Long KZ. Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among Mexican children and association of atypical enteropathogenic E. coli with acute diarrhea. J Clin Microbiol. 2009;47:93–98. doi: 10.1128/JCM.01166-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scaletsky IC, Fabbricotti SH, Carvalho RLB, Nunes CR, Maranhão HS, Morais MB, Fagundes-Neto U. Diffusely adherent Escherichia coli as a cause of acute diarrhea in young children in northeast Brazil: a case-control study. J Clin Microbiol. 2002;40:645–648. doi: 10.1128/JCM.40.2.645-648.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roche JK, Cabel A, Sevilleja J, Nataro J, Guerrant RL. Enteroaggregative E. coli (EAEC) impairs growth and malnutrition worsens EAEC infection: a novel murine model of the infection malnutrition cycle. J Infect Dis. 2010;202:506–514. doi: 10.1086/654894. [DOI] [PMC free article] [PubMed] [Google Scholar]