Abstract
Naturally acquired immunity to Plasmodium falciparum presents a changing landscape as malaria control programs and vaccine initiatives are implemented. Determining which immunologic indicators remain surrogates of past infection, as opposed to mediators of protection, led us to compare stability of immune responses across regions with divergent malaria transmission intensities. A repeat cross-sectional study of Kenyan children from a malaria-holoendemic area and an epidemic-prone area was used to examine longitudinal antibody and interferon-gamma (IFN-γ) responses to the 3D7 and FVO variants of merozoite surface protein 1 (MSP1). Antibodies to MSP1 were common in both study populations and did not significantly wane over a 21-month time period. IFN-γ responses were less frequent and rapidly disappeared in children after a prolonged period of no malaria transmission. Antibody and IFN-γ responses rarely correlated with each other; however, MSP1-specific IFN-γ response correlated with lack of concurrent P. falciparum parasitemia of the same genotype, though only statistically significantly in the malaria-holoendemic region (odds ratio = 0.31, 95% confidence interval = 0.12–0.84). This study affirms that antimalarial antibodies are informative for evaluation of history of malaria exposure within individuals, whereas cell-mediated immunity, though short lived under natural exposure conditions, might provide an assessment of recent infection and protection from parasitemia.
Introduction
Plasmodium falciparum causes the most severe form of malaria, resulting in nearly 200 million cases and over 500,000 deaths in 2013.1 Burden of disease falls most heavily on children in sub-Saharan Africa, where the majority of both infections and deaths occur. Vector control, bed nets, and other interventions have decreased the incidence of malaria in many endemic areas, but an effective vaccine will be necessary to realize the goal of malaria elimination.2 Development of an effective vaccine has been hindered by our limited understanding of how immunologic memory to malaria is developed and sustained in humans and by difficulty in selecting malaria antigens that confer protective immunity as vaccine candidates.3
Immunity to clinical malaria develops gradually during childhood in endemic areas, but immunity is not sterilizing, as asymptomatic parasitemia is common in older children and adults living in areas of high transmission. In the absence of frequent exposure to P. falciparum, immunity to clinical disease wanes rapidly.4,5 The importance of humoral immunity was established in 1961, when infused gamma globulin was shown to be an effective treatment of experimental malaria infections.6 The critical role for antibodies in establishing clinical immunity appears to be their ability to reduce the density of blood-stage parasitemia, thereby preventing severe manifestations of malaria infection.7,8 Antibodies to most blood-stage vaccine candidate antigens prevent merozoites from attaching to and invading red blood cells, but which antibody or combination of antibodies confer protection from clinical malaria is unknown.8,9 Deconvoluting antimalarial immunity is further complicated by a vaccine antigen's ability to induce cross-reactive antibodies between different antigenic variants with shared epitopes, as has been seen for merozoite surface protein 1 (MSP1).10 The role of cell-mediated immunity is not as well characterized. Interferon-gamma (IFN-γ) responses appear to be protective against P. falciparum infection but are short lived or fall below the level of detection11–15; however, T cells likely play an important role in control of P. falciparum infections both directly and indirectly via interactions with B cells.16
MSP1, the most abundant surface protein on P. falciparum merozoites, is a malaria vaccine candidate. During schizogony, MSP1 undergoes several cleavage reactions. The 42-kDa region at the C-terminal (MSP142) is cleaved into 19- and 33-kDa fragments (MSP119 and MSP133, respectively) during merozoite invasion.17,18 MSP119 contains conserved B-cell epitopes, whereas MSP133 contains T-cell epitopes.19–21 Immune responses to these antigens as measured by enzyme-linked immunosorbent assay and enzyme-linked immunosorbent spot assays (ELISPOT) assays may serve as useful correlates of vaccine efficacy. A recent meta-analysis of population-based cohort studies found that individuals with IgG responses to the MPS119 antigen had lower risk of clinical malaria than those without IgG responses.9 However, at least one study has shown that as a vaccine candidate, MSP119 alone is not protective unless the MSP133 fragment is included to provoke cell-mediated responses.21 Thus, a better understanding of the relative contributions of humoral and cellular immunity to MSP1 are necessary for continued development and evaluation of this vaccine candidate.
Studies of naturally acquired malaria infections and clinical outcomes have been used to gain a fuller understanding of the development and maintenance of immunity to malaria. To recapitulate this goal, we analyzed responses to B- and T-cell epitopes from two MSP1 genotypes (3D7 and FVO) at two time points in a cohort of Kenyan children living in Kisumu, a region with holoendemic malaria, and Nandi, a highland region with hypoendemic malaria. Importantly, Nandi experienced an epidemic shortly before the first time point, exposing children with little prior exposure to a significant malaria burden, followed by regression to very low transmission intensity. This cohort allows us to compare individual immune responses of children who experienced persistent malaria exposure in Kisumu to those rarely exposed in Nandi.
Materials and Methods
Study sites and population.
We performed a secondary analysis of data collected for a study of the relationship between malaria and Epstein–Barr virus22 (see Supplemental Information). Clinical data and blood samples for microscopy, immunological testing, and parasite genotyping were collected from study participants approximately every 6 months. Infection with P. falciparum was determined by microscopy of thick and thin blood smears, and cases were defined as those with detectable blood-stage P. falciparum parasite. For this analysis, we focused on samples collected at two time points: February 2003 and November 2004. Although malaria is holoendemic in Kisumu, there are relative peaks in transmission intensity after the long rains (March–May) and short rains (October–December).23 Transmission intensity varied in Nandi, where in February 2003, there was a peak in malaria transmission, but between February 2003 and November 2004, there was little malaria reported.24,25
MSP1 antibody levels and IFN-γ ELISPOT.
Recombinant antigens for the 3D7 and FVO genotypes of the MSP142 antigen were expressed as described elsewhere.15,22 Testing for MSP1 antibodies used the same approach as previously described.26 IgG specific for the MSP142 3D7 and MSP142 FVO were detected using a bio-Plex (Hercules, CA) bead-based assay. One thousand beads of each malaria antigen were then placed in wells with plasma from participants and diluted to 1:5,000. Included on each plate were negative controls (U.S. residents with no history of malaria) and positive controls (pooled samples from Kisumu residents). Although antibody results were calculated as mean fluorescence intensity (MFI), slight plate-to-plate variation necessitated standardization of results by expressing them in arbitrary units (AU). For each plate, the participant's AU values were calculated by dividing each participant's MFI antibody response by the negative controls' mean MFI plus three standard deviations. AU values greater than 1.0 indicated a positive IgG response. Cellular responses were determined by ELISPOT for IFN-γ as described previously.27,28 In brief, peripheral blood mononuclear cells were incubated for 84 hours with 5 μL MSP142 3D7 or FVO antigen and the number of spot-forming units in the well was counted. A positive ELISPOT response was defined as a number of spot-forming units that was significantly greater than the number in the negative control well by Fisher's exact test with P < 0.05 (additional details are in the Supplemental Information).
MSP1 genotyping.
The block 16 section of the MSP1 gene, corresponding to T-cell epitopes in the MSP133 fragment, was then analyzed as previously described using allele-restricted polymerase chain reaction.29 Details are provided in the Supplemental Information.
Statistical analyses.
We analyzed cross-sectional data at each time point stratified by site because prevalence of parasitemia was very strongly correlated with residence in Kisumu, and site was an effect measure modifier for many of the analyses. Primary outcomes were presence and magnitude of antibody response, presence and magnitude of genotype-specific ELISPOT test, and parasitemia with specific genotypes, measured in February 2003 and November 2004. Exposures included age, sex, markers of immune function, and parasitemia. In bivariate analysis, dichotomous or polyomatous variables were compared using χ2 and Cochran–Armitage trend tests; continuous and ordinal variables were compared using Student's t test or linear regression for data that were normally distributed and Wilcoxon rank-sum or signed rank tests for data that were not. Spearman's rank correlation coefficient was used to assess correlation among the different P. falciparum antibodies measured as continuous AU values and ELISPOT responses. Children who had mixed infections with both 3D7 and FVO genotypes were included in analyses for each genotype when correlations between presence of antibody or IFN-γ response and a specific genotype were performed. Data analysis was performed in Stata 12 (StataCorp, College Station, TX) and SAS 9.2 (SAS, Cary, NC).
Ethical considerations.
Informed consent was obtained from each child's parent or guardian before study enrollment. The original study was approved by the Institutional Review Board at University Hospitals of Cleveland, Case Western Reserve University (AM's affiliation at the time) and the Ethical Review Committee of the Kenya Medical Research Institute. This analysis was exempted by the institutional review boards at the University of North Carolina at Chapel Hill and the University of Massachusetts Medical School.
Results
Study population characteristics.
There were 210 children sampled in February 2003 and 174 in November 2004. There were no statistical differences between children sampled at both time points and those who dropped out with respect to sex, study site, or prevalence of parasitemia in February 2003. Additional patient characteristics are described in Table 1 and the Supplemental Information.
Table 1.
Parasitemia and genotype data in Kisumu and Nandi
| Kisumu (lowland) | Nandi (highland) | |
|---|---|---|
| Demographic data | ||
| Mean age (SD) | 7.62 (0.38) | 7.91 (0.35) |
| Female sex | 62 (50%) | 53 (55%) |
| Plasmodium falciparum parasitemia | ||
| February 2003 | 73 (79%) | 14 (12%) |
| November 2004 | 65 (88%) | 1 (1%) |
| Genotype in February 2003* | ||
| 3D7 | 57 (79%) | 10 (71%) |
| FVO | 1 (1%) | 0 |
| Mixed | 9 (13%) | 1 (7%) |
| No amplification | 5 (7%) | 3 (21%) |
| Not parasitemic | 20 | 104 |
| Genotype in November 2004* | ||
| 3D7 | 29 (50%) | 0 |
| FVO | 1 (2%) | 0 |
| Mixed | 10 (17%) | 0 |
| No amplification | 18 (31%) | 1 (100%) |
| Not parasitemic | 16 | 99 |
SD = standard deviation. In February 2003, there were 92 observations in Kisumu and 118 in Nandi, and in November 2004, there were 74 observations in Kisumu and 100 in Nandi.
Percentages are of P. falciparum microscopy-positive samples.
Parasitemia rates and parasite genotyping.
As expected, P. falciparum parasitemia was much more common in children residing in holoendemic Kisumu (Table 1). Prevalence of parasitemia increased with time in Kisumu, whereas in Nandi, it declined precipitously between February 2003 and November 2004, when only one child (1%) in Nandi was parasitemic. In Nandi, parasitemic children were 3.8 years older than nonparasitemic children in February 2003 (P = 0.007). Age was not related to parasitemia in Kisumu. Sex was not related to parasitemia in Kisumu, but females had higher prevalence of parasitemia than males in Nandi in 2003 (odds ratio [OR] = 15.67, 95% confidence interval [CI] = 2.18, 681.37).
Genotyping was performed on samples from participants that were parasitemic by microscopy. Of these, approximately 90% were successfully amplified. Parasites containing the 3D7 MSP1 genotype were much more common than the FVO MSP1 genotype (Table 1).
MSP142 humoral immune responses.
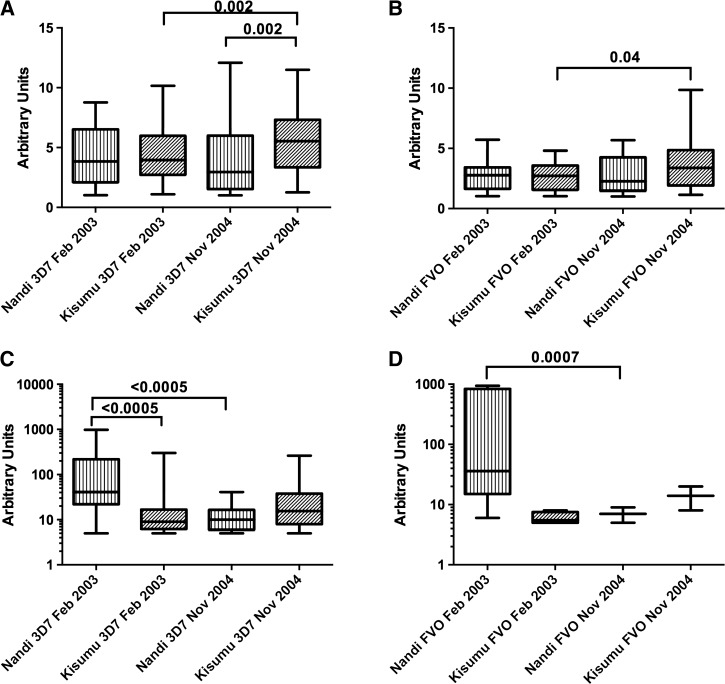
The median magnitude of antibody response to both antigens were higher in Kisumu than in Nandi except in 2003, when there was no difference for FVO (Figure 1A and B , Supplemental Table 1, Supplemental Information). In Kisumu, the prevalence of detectable IgG response increased between surveys for both antigens (P ≤ 0.04); however, there was a nonsignificant decrease in Nandi. The median antibody response significantly increased in magnitude between surveys in Kisumu. In Nandi, the opposite was seen, with decreasing magnitude in response to both antigens between surveys.
Figure 1.
Merozoite surface protein 142 humoral and cell-mediated immune responses. Panels (A) and (B) show humoral immune responses to recombinant 3D7 and FVO antigen, respectively. Data is presented as arbitrary units as described in the Materials and Methods section. The box represents the 25th to 75th percentile, with whiskers extending to the maximum and minimum values. Panels (C) and (D) show cell-mediated immune response for 3D7 and FVO recombinant antigen, respectively, and is plotted in a similar manner.
Most children with a positive antibody response in February 2003 retained it in November 2004. In Kisumu, 93% of responders to MSP142-3D7 and 88% of responders to MSP142-FVO remained seropositive to the same antigen; in Nandi, 80% and 74% retained responses to MSP142-3D7 and MSP142-FVO, respectively. Neither age nor sex were significantly associated with odds of retention of antibody response (Table 2).
Table 2.
Association of study site, age, and sex with retention of immune responses between February 2003 and November 2004
| Antibody response |
ELISPOT |
|||||
|---|---|---|---|---|---|---|
| 3D7 |
FVO |
3D7 |
||||
| OR | 95% CI | OR | 95% CI | OR | 95%CI | |
| Residence in Kisumu | 3.34 | 0.85–15.80 | 2.21 | 0.58–8.30 | 2.49 | 0.67–10.22 |
| Age (per year) | 1.01 | 0.86–1.20 | 1.04 | 0.90–1.11 | 1.04 | 0.89–1.23 |
| Female sex | 0.74 | 0.18–2.82 | 0.97 | 0.53–1.80 | 0.31 | 0.07–1.21 |
CI = confidence interval; ELISPOT = enzyme-linked immunosorbent spot assays; OR = odds ratio. ORs were not calculated for ELISPOT for FVO because no child with a positive interferon-gamma ELISPOT for FVO in 2003 retained it.
Age and sex had little effect on immunity. Age was modestly associated with antibody response only to FVO in 2004 in Kisumu (OR = 1.14, 95% CI = 1.01–1.28). Magnitude of antibody response increased modestly but significantly with age for both antigens at both time points in Nandi (OR = 1.11–1.21), but in Kisumu, this relationship was only statistically significant for MSP142-3D7 in 2004 (OR = 1.37, 95% CI = 1.13–1.68). Female children were more likely to have positive antibody responses in Nandi but less likely in Kisumu, though this finding was only significant for MSP142-FVO in 2004 in Kisumu (OR = 0.20, 95% CI = 0.06–0.67).
Presence of a positive MSP142 antibody response to a given allele was not associated with current parasitemia with the same genotype at either site in either year. Magnitude of antibody response was only associated with parasitemia during the malaria epidemic in 2003 in Nandi, where infection with the 3D7 genotype of P. falciparum was associated with greater magnitude of antibody response to both MSP142-3D7 (P < 0.005) and MSP142-FVO (P = 0.001).
MSP142 cellular immune responses.
Patterns of IFN-γ ELISPOT responses to 3D7 and FVO variants of MSP142 varied between sites with different levels of transmission as shown in Figure 1C and D. Both prevalence and magnitude of ELISPOT response to MSP142-3D7 increased slightly in Kisumu from 2003 to 2004; in Nandi, prevalence and magnitude of ELISPOT response to MSP142-3D7 decreased significantly (Supplemental Table 2, Supplemental Information). The magnitude of 3D7 ELISPOT (among those with a positive test) was significantly higher in Nandi than in Kisumu in February 2003 (P < 0.0005). In November 2004, however, the magnitude of 3D7 ELISPOT response was slightly higher in Kisumu, though this was not significant. In Kisumu, the magnitude of 3D7 ELISPOT response increased slightly (P < 0.0005) but decreased nonsignificantly in Nandi (P = 0.07). Very few children at either site responded to MSP142-FVO except in Nandi in February 2003, when 17% of the children responded. The number of positive ELISPOT responses to MSP142-FVO was too small to draw conclusions, but trends were similar to those seen for 3D7 genotype.
ELISPOT responses were not sustained between time points: of children with a positive MSP142-3D7 ELISPOT in February 2003, only 21% in Nandi and 36% in Kisumu retained a positive response, and no child with a positive IFN-γ ELISPOT for FVO in 2003 retained it. As shown in Table 2, children were more likely to retain responses to MSP142-3D7 in Kisumu than in Nandi, though the association did not reach statistical significance. Neither age nor sex was associated with retention of ELISPOT response.
Magnitude of IFN-γ ELISPOT responses to both antigens increased with age in Kisumu in 2004 (P = 0.003 for both antigens), as did prevalence of positive response (P = 0.02 for 3D7; P = 0.008 for FVO); in Nandi, this relationship was only significant for the prevalence of MSP142-3D7 ELISPOT response in 2004 (P = 0.009). Neither prevalence nor magnitude of ELISPOT responses differed by sex.
Positive IFN-γ ELISPOT response correlated with absence of infection with the same genotype at both sites in 2003, though only statistically significantly in Kisumu (Table 3). Because of scarcity of immune responses and parasitemia in Nandi, we were unable to calculate statistics for other time points and parasite-immune response combinations at that site. Magnitude of ELISPOT response was not associated with parasitemia except in Kisumu in 2003, when increased response to MSP142-3D7 modestly reduced odds of parasitemia with 3D7 (OR = 0.94, 95% CI = 0.89–0.99).
Table 3.
Relationship between cellular immune response to MSP1 antigens and genotype-specific parasitemia
| Kisumu | Nandi | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| 2003 | ||||
| 3D7 and MSP142-3D7 | 0.31 | 0.12–0.84 | 0.3 | 0.06–1.53 |
| FVO and MSP142-FVO | – | – | – | – |
| 3D7 and MSP142-FVO | 0.11 | 0.01–1.09 | – | – |
| FVO and MSP142-3D7 | 0.48 | 0.10–2.43 | – | – |
| 2004 | ||||
| 3D7 and MSP142-3D7 | 0.42 | 0.16–1.09 | – | – |
| FVO and MSP142-FVO | – | – | – | – |
| 3D7 and MSP142-FVO | – | – | – | – |
| FVO and MSP142-3D7 | 0.92 | 0.25–3.51 | – | – |
CI = confidence interval; MSP = merozoite surface protein; OR = odds ratio.
Relationships between humoral and cellular immune responses to MSP142.
In February 2003, presence of positive IFN-γ ELISPOT results for different MSP142 genotypes were significantly associated in Nandi (OR = 31.64, 95% CI = 4.44–1,340) but not Kisumu (OR = 2.15, 95% CI = 0.15–30.94). In November 2004, data were too sparse to calculate OR, but when stratified by site, this relationship was again only significant in Nandi (P = 0.002; P = 0.06 in Kisumu). As shown in Table 4, magnitude of humoral responses to 3D7 and FVO MSP142 antigens were correlated, but magnitude of cellular responses were rarely correlated to each other or to humoral responses. In Kisumu (Table 4), IFN-γ ELISPOT response to MSP142-3D7 antigen was correlated to the 3D7 antibody response in 2004 and to the FVO antibody response in 2003 but not 2004; and in 2004, humoral and cellular responses to the MSP1-FVO antigen were correlated. In Nandi (Table 5) in 2004, MSP142-FVO ELISPOT response was correlated to antibody response to MSP142-FVO and to ELISPOT response to MSP142-3D7. There was no evidence of synergy between humoral and cellular responses to protect against concurrent parasitemia with either genotype at either time point or site.
Table 4.
Spearman's rho values for the correlation between humoral (Aby) and cellular (Cell) immune responses showing the correlations in Kisumu
| 3D7 Aby 2003 | 3D7 Aby 2004 | FVO Aby 2003 | FVO Aby 2004 | 3D7 Cell 2003 | 3D7 Cell 2004 | FVO Cell 2003 | FVO Cell 2004 | |
|---|---|---|---|---|---|---|---|---|
| 3D7 Aby 2003 | 1 | |||||||
| 3D7 Aby 2004 | 0.53 | 1 | ||||||
| 0.0001 | ||||||||
| FVO Aby 2003 | 0.8 | 0.49 | 1 | |||||
| < 0.0005 | 0.0006 | |||||||
| FVO Aby 2004 | 0.38 | 0.87 | 0.52 | 1 | ||||
| 0.04 | < 0.0005 | 0.0002 | ||||||
| 3D7 Cell 2003 | 0.15 | 0.09 | 0.15 | 0.09 | 1 | |||
| NS | NS | NS | NS | |||||
| 3D7 Cell 2004 | 0.13 | 0.06 | 0.16 | 0.04 | 0.47 | 1 | ||
| NS | NS | NS | NS | 0.002 | ||||
| FVO Cell 2003 | −0.008 | −0.23 | 0.01 | −0.12 | 0.46 | 0.08 | 1 | |
| NS | NS | NS | NS | 0.0002 | NS | |||
| FVO Cell 2004 | 0.001 | 0.31 | 0.03 | 0.39 | 0.04 | 0.28 | −0.03 | 1 |
| NS | NS | NS | 0.02 | NS | NS | NS |
NS = nonsignificant. P values are Bonferroni-corrected for multiple comparisons.
Table 5.
Spearman's rho values for the correlation between humoral (Aby) and cellular (Cell) immune responses showing the correlations in Nandi
| 3D7 Aby 2003 | 3D7 Aby 2004 | FVO Aby 2003 | FVO Aby 2004 | 3D7 Cell 2003 | 3D7 Cell 2004 | FVO Cell 2003 | FVO Cell 2004 | |
|---|---|---|---|---|---|---|---|---|
| 3D7 Aby 2003 | 1 | |||||||
| 3D7 Aby 2004 | 0.52 | 1 | ||||||
| < 0.0005 | ||||||||
| FVO Aby 2003 | 0.95 | 0.53 | 1 | |||||
| < 0.0005 | 0.001 | |||||||
| FVO Aby 2004 | 0.5 | 0.97 | 0.54 | 1 | ||||
| < 0.0005 | < 0.0005 | < 0.0005 | ||||||
| 3D7 Cell 2003 | 0.06 | 0.005 | 0.03 | −0.008 | 1 | |||
| NS | NS | NS | NS | |||||
| 3D7 Cell 2004 | −0.04 | 0.04 | −0.04 | 0.04 | −0.09 | 1 | ||
| NS | NS | NS | NS | NS | ||||
| FVO Cell 2003 | −0.01 | −0.15 | −0.01 | −0.16 | 0.23 | −0.08 | 1 | |
| NS | NS | NS | NS | NS | NS | |||
| FVO Cell 2004 | 0.14 | 0.37 | 0.17 | 0.35 | −0.05 | 0.06 | −0.04 | 1 |
| NS | 0.005 | NS | 0.01 | NS | NS | NS |
NS = nonsignificant. P values are Bonferroni-corrected for multiple comparisons.
Discussion
This longitudinal study reiterates our understanding of immunological profiles observed in children who are constantly exposed to malaria in holoendemic regions compared with children who are infrequently infected with malaria during epidemics. Importantly, the dynamics and potential protection against subsequent malaria infections provided by humoral and cellular immune responses are based on intensity of exposure to malaria infections. This study confirmed prior findings that humoral immunity can persist for long periods of time within individuals, whereas cellular immunity, though protective against parasitemia in the short term, wanes quickly in the absence of constant exposure.15,30–32
As expected, we observed a higher prevalence of positive IgG responses to P. falciparum antigens in a holoendemic area than a hypoendemic area.30,31 In addition, we found that median antibody responses increased over time in Kisumu, as would be expected in a holoendemic region where children are constantly exposed to the parasite.30,32 In contrast, antibody responses in Nandi declined in magnitude between surveys as the epidemic resolved and transmission decreased (Figure 1). Interestingly, the prevalence of anti-FVO antibodies was modest (60%) in 2003 in Kisumu and increased to 72% by the second survey, yet FVO was the least common of the two variants found circulating in the population (Supplemental Table 2, Supplemental Information). This likely represents the known antibody cross-reactivity between FVO and 3D7 that share many B-cell epitopes.10 This finding suggests that polyclonal antibodies developed to MSP1 3D7 may cross-react with FVO and provide some degree of protection in the absence of exposure. Antibody titers to both isoforms remained high in Nandi children despite prolonged periods of no malaria transmission.33
Malaria transmission intensity has been shown to influence the development of protective immunity,34,35 yet studies examining the duration of malaria-specific antibody responses in areas of hypoendemic transmission are limited. Antibodies to MSP1 are reportedly short lived in children under 5 years of age,36 although a study from Thailand has shown that antibodies to some malaria antigens can persist for as long as 30 years without reexposure to the parasite.37 This was supported by recent data from another study in Nandi that suggests that the half-lives of MSP1 antibodies in older children and adults can be on the order of decades.38 Our work is consistent with these findings, supporting the notion that antimalarial antibody titers could be used as a surrogate of past exposure history in children and a tool for monitoring malaria control programs.39–41
Although we were underpowered to detect significant changes in cellular immune responses in most cases, some trends did emerge in our analysis. In contrast to humoral responses, when assessing cell-mediated immunity, we observed that children made robust IFN-γ responses to MSP1 3D7 and FVO, but they were short lived or fell below the threshold of detection in peripheral blood samplings over time in the absence of exposure to malaria.15 Furthermore, cell-mediated responses appeared more genotype specific than antibody responses (Tables 4 and 5). IFN-γ response was not protective against subsequent infection, similar to another recent report.42 Although we were underpowered to detect statistically significant relationships between parasitemia and antigen-specific IFN-γ responses, our results consistently suggested that IFN-γ MSP1-recall response was associated with lower risk of concurrent parasitemia (Tables 4 and 5). This short-term immunity has been demonstrated in other studies, including a recent treatment-reinfection study performed in the same holoendemic site of Kisumu that revealed that positive IFN-γ ELISPOT to 3D7-MSP142 at baseline conferred a 73% reduction in 3-month risk of reinfection with P. falciparum.14
Both magnitude and persistence of cellular immune responses were affected by malaria transmission intensity.15 In February 2003, at the end of an epidemic of P. falciparum in Nandi, both prevalence and magnitude of IFN-γ ELISPOT response to the 3D7 antigen were higher in Nandi than in Kisumu despite the fact that malaria transmission intensity and prevalence of parasitemia were much greater in Kisumu. There are several possible explanations for this phenomenon. Children in Nandi, who might not have been previously exposed to malaria, may have produced more vigorous responses characteristic of a primary immune response. Children in Kisumu, on the other hand, had smaller responses which may have been generated by a more moderate secondary immune response within the context of lifelong exposure to holoendemic malaria transmission. This finding would support the hypothesis that constant antigenic stimulation through repeated malaria infections can downregulate T-cell immunity to malaria, perhaps via Treg cells, allowing asymptomatic parasitemia to persist.43,44 Given the higher prevalence of parasitemia despite a high prevalence of positive IFN-γ ELISPOT responses, it suggests that cellular immunity generated to MSP142 under natural exposure conditions does not provoke immunologic memory. This would be consistent with the “Goldilocks principle” that posits a moderate, “just right” antigen dose induces a more robust and long-lasting T-cell response, whereas constant, high-level antigen stimulation leads to apoptosis of activated cells.45 It also has been suggested that intense, repeated malaria antigen exposure could lead to tolerance or even T-cell exhaustion resulting in an insufficient immune response during subsequent infections.46
Limitations within this cross-sectional study include our inability to address whether differences in IgG responses between the districts, or relative changes in antibody levels within an individual, were the result of malaria infections not detected and cleared before blood sampling. However, this caveat does not diminish our findings using region as an ecological variable for malaria exposure. We also did not measure functional immunity; however, other studies using functional antibody assays suggest duration of immune response engendered is an important variable to consider when assessing quality of protection against malaria.47 Finally, due to the limited power of our study, we were unable to examine more complex relationships between parasitemia and other covariates such as nutritional status and schistosomiasis coinfections.
In summary, this study supports past observations about the duration of immunity to malaria being dependent on the quality of exposure. However, it provides additional evidence for immunity developed by children in that a recent, primary infection may lead to higher MSP142 IFN-γ responses than in a child with repeated, secondary infections in a holoendemic region.15 In a previous study of adults, the half-life of IFN-γ secreting Th1 effector memory responses was reported to be about 3 years,15 whereas our study of children demonstrates a much shorter duration. Our study confirms previous work that antibodies to MSP142 can be long lived regardless of age.41 We found that IFN-γ responses to MSP142 correlate to absence of concurrent parasitemia, whereas antibody responses do not, suggesting that control of concurrent infection may be mediated via IFN-γ rather than antibodies. Lastly, our data provide evidence that IFN-γ responses to MSP142 are, by nature of T-cell epitope presentation, more genotype specific, whereas antibody responses that are polyclonal may be cross-reactive to alternate variants. This study supports the combined use of long-lived antibody titers for monitoring malaria control programs in conjunction with short-lived T-cell responses that would indicate recent infections; furthermore, these findings increase our understanding of naturally acquired immunity to MSP142, a potential vaccine antigen. Understanding the underlying dynamics of immunity under changing natural exposure conditions has implications for interpreting longevity of malaria vaccine efficacy and surrogate measures of protection testing in malaria-endemic settings.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Carol Long for her participation in the original study and her review of the manuscript.
Footnotes
Financial support: Natalie Bowman was supported by K23 AI113197-01, a Burroughs Wellcome Trust/American Society of Tropical Medicine and Hygiene, and Ruth Kirchner NRSA T32 grant AI715134-13. This work was partially supported by National Institutes of Health grants CTSA UL1RR025747, R01 AI089819, R01 AI43906, and K08 AI51565 (Ann M. Moormann), R01 CA134051 (Ann M. Moormann).
Authors' addresses: Natalie M. Bowman, Jonathan J. Juliano, and Oksana Kharabora, Division of Infectious Diseases, University of North Carolina, School of Medicine, Chapel Hill, NC, E-mails: natalie_bowman@med.unc.edu, jonathan_juliano@med.unc.edu, and oxsana.k@gmail.com. Cynthia J. Snider and Steven R. Meshnick, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC, E-mails: cjsnider@hotmail.com and meshnick@email.unc.edu. John Vulule, Biomedical Sciences, Center for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya, E-mail: jvulule@gmail.com. Chandy C. John, Ryan White Center for Pediatric Infectious Disease and Global Health, Indiana University, Indianapolis, IN, E-mail: chjohn@iu.edu. Ann M. Moormann, University of Massachusetts Medical School, Worcester, MA, E-mail: ann.moormann@umassmed.edu.
References
- 1.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013;19:168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- 4.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J, Network GeoSentinel Surveillance. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–1193. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 5.Pavli A, Maltezou HC. Malaria and travellers visiting friends and relatives. Travel Med Infect Dis. 2010;8:161–168. doi: 10.1016/j.tmaid.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 7.Bloland PB, Boriga DA, Ruebush TK, McCormick JB, Roberts JM, Oloo AJ, Hawley W, Lal A, Nahlen B, Campbell CC. Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg. 1999;60:641–648. doi: 10.4269/ajtmh.1999.60.641. [DOI] [PubMed] [Google Scholar]
- 8.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 9.Fowkes FJ, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsyula N, Angov E, Bergmann-Leitner E, Koech M, Khan F, Bennett J, Otieno L, Cummings J, Andagalu B, Tosh D, Waitumbi J, Richie N, Shi M, Miller L, Otieno W, Otieno GA, Ware L, House B, Godeaux O, Dubois MC, Ogutu B, Ballou WR, Soisson L, Diggs C, Cohen J, Polhemus M, Heppner DG, Jr, Ockenhouse CF, Spring MD. Results from tandem Phase 1 studies evaluating the safety, reactogenicity and immunogenicity of the vaccine candidate antigen Plasmodium falciparum FVO merozoite surface protein-1 (MSP142) administered intramuscularly with adjuvant system AS01. Malar J. 2013;12:29. doi: 10.1186/1475-2875-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent AE, Chelimo K, Sumba PO, Spring MD, Crabb BS, Moormann AM, Tisch DJ, Kazura JW. Temporal stability of naturally acquired immunity to merozoite surface protein-1 in Kenyan adults. Malar J. 2009;8:162. doi: 10.1186/1475-2875-8-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan KL, Mwangi T, Plebanski M, Odhiambo K, Ross A, Sheu E, Kortok M, Lowe B, Marsh K, Hill AV. Ex vivo interferon-gamma immune response to thrombospondin-related adhesive protein in coastal Kenyans: longevity and risk of Plasmodium falciparum infection. Am J Trop Med Hyg. 2003;68:421–430. [PubMed] [Google Scholar]
- 13.Moormann AM, John CC, Sumba PO, Tisch D, Embury P, Kazura JW. Stability of interferon-gamma and interleukin-10 responses to Plasmodium falciparum liver stage antigen-1 and thrombospondin-related adhesive protein in residents of a malaria holoendemic area. Am J Trop Med Hyg. 2006;74:585–590. [PubMed] [Google Scholar]
- 14.Moormann AM, Sumba PO, Chelimo K, Fang H, Tisch DJ, Dent AE, John CC, Long CA, Vulule J, Kazura JW. Humoral and cellular immunity to Plasmodium falciparum merozoite surface protein 1 and protection from infection with blood-stage parasites. J Infect Dis. 2013;208:149–158. doi: 10.1093/infdis/jit134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wipasa J, Okell L, Sakkhachornphop S, Suphavilai C, Chawansuntati K, Liewsaree W, Hafalla JC, Riley EM. Short-lived IFN-gamma effector responses, but long-lived IL-10 memory responses, to malaria in an area of low malaria endemicity. PLoS Pathog. 2011;7:e1001281. doi: 10.1371/journal.ppat.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udhayakumar V, Anyona D, Kariuki S, Shi YP, Bloland PB, Branch OH, Weiss W, Nahlen BL, Kaslow DC, Lal AA. Identification of T and B cell epitopes recognized by humans in the C-terminal 42-kDa domain of the Plasmodium falciparum merozoite surface protein (MSP)-1. J Immunol. 1995;154:6022–6030. [PubMed] [Google Scholar]
- 17.Blackman MJ, Ling IT, Nicholls SC, Holder AA. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–33. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 18.Holder AA, Lockyer MJ, Odink KG, Sandhu JS, Riveros-Moreno V, Nicholls SC, Hillman Y, Davey LS, Tizard ML, Schwarz RT, Robert RF. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature. 1985;317:270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- 19.Egan AF, Chappel JA, Burghaus PA, Morris JS, McBride JS, Holder AA, Kaslow DC, Riley EM. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP119, the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–466. doi: 10.1128/iai.63.2.456-466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holder AA. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–1456. doi: 10.1017/S0031182009990515. [DOI] [PubMed] [Google Scholar]
- 21.Pusic KM, Hashimoto CN, Lehrer A, Aniya C, Clements DE, Hui GS. T cell epitope regions of the P. falciparum MSP1-33 critically influence immune responses and in vitro efficacy of MSP1-42 vaccines. PLoS One. 2011;6:e24782. doi: 10.1371/journal.pone.0024782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, Kazura J, Rochford R. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191:1233–1238. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 23.ter Kuile FO, Terlouw DJ, Phillips-Howard PA, Hawley WA, Friedman JF, Kolczak MS, Kariuki SK, Shi YP, Kwena AM, Vulule JM, Nahlen BL. Impact of permethrin-treated bed nets on malaria and all-cause morbidity in young children in an area of intense perennial malaria transmission in western Kenya: cross-sectional survey. Am J Trop Med Hyg. 2003;68:100–107. [PubMed] [Google Scholar]
- 24.Ernst KC, Adoka SO, Kowuor DO, Wilson ML, John CC. Malaria hotspot areas in a highland Kenya site are consistent in epidemic and non-epidemic years and are associated with ecological factors. Malar J. 2006;5:78. doi: 10.1186/1475-2875-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snider CJ, Cole SR, Chelimo K, Sumba PO, Macdonald PD, John CC, Meshnick SR, Moormann AM. Recurrent Plasmodium falciparum malaria infections in Kenyan children diminish T-cell immunity to Epstein Barr virus lytic but not latent antigens. PLoS One. 2012;7:e31753. doi: 10.1371/journal.pone.0031753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piriou E, Kimmel R, Chelimo K, Middeldorp JM, Odada PS, Ploutz-Snyder R, Moormann AM, Rochford R. Serological evidence for long-term Epstein-Barr virus reactivation in children living in a holoendemic malaria region of Kenya. J Med Virol. 2009;81:1088–1093. doi: 10.1002/jmv.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, Martin LB, Saul AJ, Miller LH, Long CA. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun. 2006;74:4573–4580. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spring MD, Chelimo K, Tisch DJ, Sumba PO, Rochford R, Long CA, Kazura JW, Moormann AM. Allele specificity of gamma interferon responses to the carboxyl-terminal region of Plasmodium falciparum merozoite surface protein 1 by Kenyan adults with naturally acquired immunity to malaria. Infect Immun. 2010;78:4431–4441. doi: 10.1128/IAI.00415-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terrientes ZI, Vergara J, Kramer K, Herrera S, Chang SP. Restricted genetic diversity of Plasmodium falciparum major merozoite surface protein 1 in isolates from Colombia. Am J Trop Med Hyg. 2005;73:55–61. doi: 10.4269/ajtmh.2005.73.55. [DOI] [PubMed] [Google Scholar]
- 30.Badu K, Afrane YA, Larbi J, Stewart VA, Waitumbi J, Angov E, Ong'echa JM, Perkins DJ, Zhou G, Githeko A, Yan G. Marked variation in MSP-119 antibody responses to malaria in western Kenyan highlands. BMC Infect Dis. 2012;12:50. doi: 10.1186/1471-2334-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Supargiyono S, Bretscher MT, Wijayanti MA, Sutanto I, Nugraheni D, Rozqie R, Kosasih AA, Sulistyawati S, Hawley WA, Lobo NF, Cook J, Drakeley CJ. Seasonal changes in the antibody responses against Plasmodium falciparum merozoite surface antigens in areas of differing malaria endemicity in Indonesia. Malar J. 2013;12:444. doi: 10.1186/1475-2875-12-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC. Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis. 2014;210:1115–1122. doi: 10.1093/infdis/jiu219. [DOI] [PubMed] [Google Scholar]
- 33.Sutton PL, Clark EH, Silva C, Branch OH. The Plasmodium falciparum merozoite surface protein-1 19 KD antibody response in the Peruvian Amazon predominantly targets the non-allele specific, shared sites of this antigen. Malar J. 2010;9:3. doi: 10.1186/1475-2875-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 35.Lusingu JP, Vestergaard LS, Mmbando BP, Drakeley CJ, Jones C, Akida J, Savaeli ZX, Kitua AY, Lemnge MM, Theander TG. Malaria morbidity and immunity among residents of villages with different Plasmodium falciparum transmission intensity in north-eastern Tanzania. Malar J. 2004;3:26. doi: 10.1186/1475-2875-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, Liewsaree W, Riley EM, Hafalla JC. Long-lived antibody and B cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, Narum DL, Park GS, Ofulla AV, John CC. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis. 2014;210:1123–1132. doi: 10.1093/infdis/jiu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oduro AR, Conway DJ, Schellenberg D, Satoguina J, Greenwood BM, Bojang KA. Seroepidemiological and parasitological evaluation of the heterogeneity of malaria infection in the Gambia. Malar J. 2013;12:222. doi: 10.1186/1475-2875-12-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olotu A, Fegan G, Wambua J, Nyangweso G, Ogada E, Drakeley C, Marsh K, Bejon P. Estimating individual exposure to malaria using local prevalence of malaria infection in the field. PLoS One. 2012;7:e32929. doi: 10.1371/journal.pone.0032929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, Masika P, Mosha J, Bousema T, Shekalaghe S, Cook J, Corran P, Ghani A, Riley EM, Drakeley C. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS One. 2009;4:e6083. doi: 10.1371/journal.pone.0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jagannathan P. IFNγ responses to pre-erythrocytic and blood-stage malaria antigens exhibit differential associations with past exposure and subsequent protection. J Infect Dis. 2015;211:1987–1996. doi: 10.1093/infdis/jiu814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moormann AM. How might infant and paediatric immune responses influence malaria vaccine efficacy? Parasite Immunol. 2009;31:547–559. doi: 10.1111/j.1365-3024.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwenk RJ, Richie TL. Protective immunity to pre-erythrocytic stage malaria. Trends Parasitol. 2011;27:306–314. doi: 10.1016/j.pt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Burchill MA, Tamburini BA, Pennock ND, White JT, Kurche JS, Kedl RM. T cell vaccinology: exploring the known unknowns. Vaccine. 2013;31:297–305. doi: 10.1016/j.vaccine.2012.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illingworth J, Butler NS, Roetynck S, Mwacharo J, Pierce SK, Bejon P, Crompton PD, Marsh K, Ndungu FM. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J Immunol. 2013;190:1038–1047. doi: 10.4049/jimmunol.1202438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.John CC, O'Donnell RA, Sumba PO, Moormann AM, de Koning-Ward TF, King CL, Kazura JW, Crabb BS. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-119) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. J Immunol. 2004;173:666–672. doi: 10.4049/jimmunol.173.1.666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.