Abstract
Accurate information regarding malaria prevalence at national level is required to design and assess malaria control/elimination efforts. Although many comparisons of microscopy and polymerase chain reaction (PCR)–based methods have been conducted, there is little published literature covering such comparisons in southeast Asia especially at the national level. Both microscopic examination and PCR detection were performed on blood films and dried blood spots samples collected from 8,067 individuals enrolled in a nationwide, stratified, multistage, cluster sampling malaria prevalence survey conducted in Cambodia in 2007. The overall malaria prevalence and prevalence rates of Plasmodium falciparum, Plasmodium vivax, and Plasmodium malariae infections estimated by microscopy (N = 8,067) were 2.74% (95% confidence interval [CI]: 2.39–3.12%), 1.81% (95% CI: 1.53–2.13%), 1.14% (95% CI: 0.92–1.40%), and 0.01% (95% CI: 0.003–0.07%), respectively. The overall malaria prevalence based on PCR detection (N = 7,718) was almost 2.5-fold higher (6.31%, 95% CI: 5.76–6.89%, P < 0.00001). This difference was significantly more pronounced for P. falciparum (4.40%, 95% CI: 3.95–4.90%, P < 0.00001) compared with P. vivax (1.89%, 95% CI: 1.60–2.22%, P < 0.001) and P. malariae infections (0.22%, 95% CI: 0.13–0.35%, P < 0.0001). The significant proportion of microscopy-negative but PCR-positive individuals (289/7,491, 3.85%) suggest microscopic examination frequently underestimated malaria infections and that active case detection based on microscopy may miss a significant reservoir of infection, especially in low-transmission settings.
Background
Over the past 15 years, Cambodia has made significant strides in controlling malaria with the introduction of artemisinin-based combinations, establishing rapid diagnosis tests (RDTs), and treatment through a system of village malaria health workers (set up in 2001 and scaled up in 2004) and the recent wide distribution of long-lasting insecticide-treated bed nets (LLITNs).1–4 In 2011, Cambodia committed itself to eliminating malaria by 2025. This goal may be challenged by the emergence of multidrug-resistant, including artemisinin-resistant Plasmodium falciparum (ART-R)5–8 and, now, the demise of dihydroartemisinin–piperaquine.9
Accordingly, conducting accurate, large-scale assessments of malaria prevalence, including pools of asymptomatic individuals detected by the polymerase chain reaction (PCR), is crucial for planning malaria control and elimination efforts and in measuring the impact of pertinent interventions. In Cambodia, efforts are being focused on containing the spread and ultimately the elimination of ART-R; thus, identifying and quantifying reservoirs of falciparum infection are seen as essential because these pools represent areas where there is ongoing transmission of ART-R.
Conventionally, quantitative malariometric surveys are mainly based on light microscopy (LM) diagnosis and, more recently, RDTs, which are tools with limits of detection that range from 50 to 200 malaria parasites/μL.10 In recent years, various molecular detection approaches like PCR, with lower theoretical limits of detection (0.5–5 parasites/μL), have been increasingly used for improving the detection of malaria infections. In malaria prevalence surveys, it has been shown that PCR may detect twice as many cases as microscopy,11 and that most of the positive cases harbor asexual and sexual parasites. Such asymptomatic cases are able to transmit infection to mosquitoes without coming to the attention of the health system, and so may complicate malaria control.12–14 Although submicroscopic parasitemias may be less infective to mosquitoes than microscopically detectable ones, they may nonetheless be an important source of transmission because they are severalfold more prevalent than microscopic parasitemias.15 These undetected reservoirs maintain parasite populations, especially multidrug-resistant parasites, and may contribute to the spread of ART-R within Cambodia through the movement of migrant and mobile populations.
Although studies have compared the performances of PCR and microscopy, few published studies have compared parasite prevalence rates estimated by these two methods at the national level.16,17 Nationwide malaria prevalence surveys have been conducted in Cambodia in 2004, 2007, 2010, and 2013; PCR detection was first used in 2007. Herein, we report the microscopy and PCR results of the 2007 national survey.
Materials and Methods
Survey design and study population.
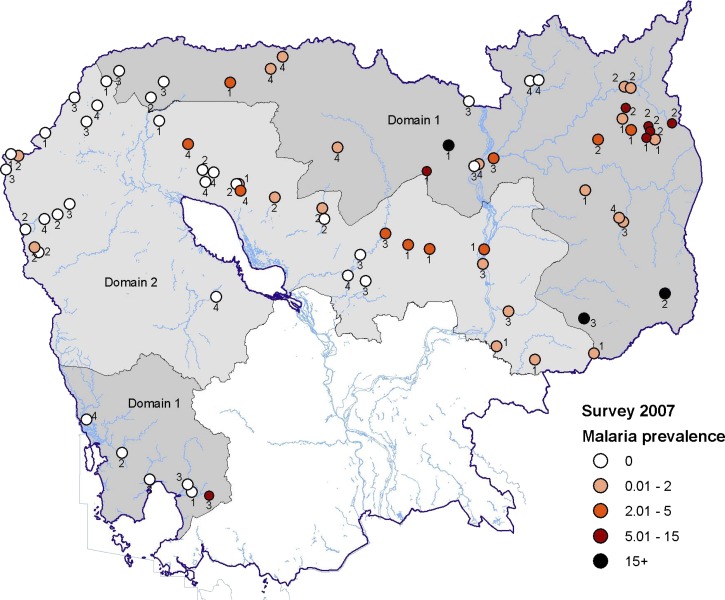
The 2007 Cambodian National Malaria Survey (CNMS) was a stratified, multistage cluster sampling design study. The country was stratified into three domains (Figure 1 ) based on the malaria prevalence results of the 2004 CNMS.18 Domain 1 consisted of the northeastern provinces of Mondulkiri, Oddor Meanchey, Preah Vihear, Rattanakiri, and Stung Treng and the southern province of Koh Kong (high-transmission areas); Domain 2 consisted of the central and western provinces of Banteay Meanchey, Battambang, Kampong Thom, Kratie, Pailin, Pursat, and Siem Reap (low-transmission areas). The Domain 3 includes the remaining provinces around Phnom Penh (not sampled here due to the very low malaria prevalence found in the 2004 survey).
Figure 1.
Malaria prevalence estimated by microscopy and distribution of the individual clusters, Cambodia, 2007. Domains 1 and 2 are shown in dark and light gray, respectively. The color assigned to each cluster corresponds to the malaria prevalence (%) estimated by microscopy (all species). The number beside each colored cluster indicates the risk zone: risk zone 1 (0–250 m from forest), risk zone 2 (250–1,000 m), risk zone 3 (1–2 km), and risk zone 4 (2–5 km).
Each domain was further stratified into risk zones based on the distance from the nearest forest margin. Risk zones 1, 2, 3, and 4 correspond to distances from the forest of 0–250 m, 250–1,000 m, 1–2 km, and 2–5 km, respectively. Within Domains 1 and 2, 38 clusters were randomly sampled: 14 from risk zone 1 and eight from each of the other three risk zones. Within each cluster, 40 households were randomly chosen from the village census list maintained by the village leaders. Within each household, a fingerprick blood sample was taken from four individuals, one from each of the following groups: one aged 0–4 years, one aged 5–14 years, one adult female, and one adult male. If there was more than one person in any of these groups, one was sampled randomly from all individuals falling in that group. In addition, any pregnant woman in the household who was not already sampled was included in the blood sampling.
Random selection of clusters was not based on probability proportional to size, and so was not self-weighting. Weights were determined by census data collected at the time of sampling, and took into account the probability of selection of individual subjects based on both cluster size and the distribution of age categories in individual households.
All study subjects gave informed consent; the study was approved by the National Ethics Committee for Health Research of the Cambodian Ministry of Health (097 NECHR dated October 26, 2007), and was conducted in compliance with the international standards for the protection of human research subjects, the participation of Naval Medical Research Unit 2 was approved by the NAMRU-2 Institutional Review Board.
Blood sampling.
Fingerprick blood samples from 8,067 individuals enrolled in a large-scale malaria prevalence survey in Cambodia conducted in October–December 2007 at the end of the rainy season, including 3,363 households in 13 provinces in Domains 1 and 2, were collected and used for microscopic examination of blood films and the detection of DNA using a PCR assay based on the Plasmodium mitochondrial cytochrome b gene from blood spots. The national malaria prevalence estimated both by microscopy and PCR was investigated and compared along with the main determinants associated with the malaria transmission in Cambodia.
Microscopic examination.
Using blood from a fingerstick, thick and thin films for malaria microscopy were prepared by field workers in the households during the survey, and were stained with Giemsa stain on the day of collection, according to the WHO protocol.19 Blood films were examined by a certified microscopist using ×1,000 oil immersion LM. At least, 200 ocular fields were reviewed before a slide was considered negative. Parasites were counted per 200 leukocytes and reported as parasites/μL, assuming a white blood cell count of 8,000/μL. A second, senior certified microscopist reviewed all positive smears and 10% of negative smears. In the event of discrepancies, the senior microscopist's reading was recorded.
DNA extraction and PCR assay.
From the same fingerprick, 2–3 drops of blood were blotted onto Whatman No. 3 filter paper (Sigma-Aldrich, Singapore), dried at ambient temperature, and placed in ziplock bags with a desiccant. DNA extraction, detection of Plasmodium DNA by nested PCR, and identification of the Plasmodium species (by sequencing the PCR products) were carried out as previously described.20 In brief, two sets of primers were used to amplify an 815–base pair sequence of Plasmodium mitochondrial cytochrome b. The species were determined by analysis of single nucleotide polymorphisms at 11 sites within the PCR product.
Statistical analysis.
All data were recorded on standardized case report forms, double entered into MS Access (Microsoft Inc., Redmond, WA), and exported for analysis to STATA (version 10.0; StataCorp LP, College Station, TX). The data were analyzed using survey analysis commands with eight strata (two domains and four risk zones in each domain); the primary sampling unit was the cluster, no finite population correction was used, and sample weights were calculated as the inverse of the probability of selecting each cluster. Linearized standard errors were calculated. Continuous data were compared by the unpaired t test (log transforming data, as needed), and proportional data were compared using the χ2 test. All statistical tests were two-tailed, and significance was defined as P < 0.05.
Results and Discussion
In total, 8,067 individuals were sampled from 3,363 households in 137 clusters in 13 provinces (Figure 1). Households numbered 2,110, 2,343, 1,820, and 1,794 from risk zones 1, 2, 3, and 4, respectively.
The male-to-female ratio was 0.89 and the median age was 23 years (95% confidence interval [CI]: 23–24 years). Of the sampled population, 63% was ≥ 15 years of age, 22% aged 5–14 years, and 15% were children aged from 0 to 4 years. Details of demographic data per domain and risk zone are provided in Table 1.
Table 1.
Demographic data of the surveyed population, Cambodia, 2007
| Demographic parameters | Sampling areas | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Domain 1 | Domain 2 | ||||||||||
| Risk zone 1 | Risk zone 2 | Risk zone 3 | Risk zone 4 | Total | Risk zone 1 | Risk zone 2 | Risk zone 3 | Risk zone 4 | Total | |||
| Population sampled | 8,067 | 992 | 1,299 | 1,039 | 934 | 4,264 | 1,118 | 1,044 | 781 | 860 | 3,803 | – |
| No. of clusters | 137 | 16 | 25 | 15 | 13 | 69 | 18 | 23 | 14 | 13 | 68 | – |
| Mean of individuals sampled per cluster | 58.9 | 62.0 | 52.0 | 69.3 | 718 | 61.8 | 62.1 | 45.4 | 55.8 | 66.2 | 55.9 | – |
| No. of households | 2,904 | 344 | 464 | 356 | 323 | 1,487 | 423 | 393 | 280 | 321 | 1,417 | – |
| Mean of individuals sampled per household | 2.8 | 2.9 | 2.8 | 2.9 | 29 | 2.9 | 2.6 | 2.7 | 2.8 | 2.7 | 2.7 | – |
| Median age in years (range) | 23 (0–95) | 22 (0–90) | 24 (0–84) | 24 (0–85) | 22 (0–90) | 23 (0–90) | 23 (0–95) | 24 (0–86) | 25 (0–87) | 25 (0–81) | 24 (0–95) | 0.06 |
| Population sampled by age group (%) | ||||||||||||
| 0–4 years | 14.9 | 15.5 | 14.8 | 15.6 | 16.2 | 15.5 | 15.8 | 10.0 | 10.0 | 13.3 | 14.4 | 0.75 |
| 5–14 years | 21.8 | 21.4 | 20.3 | 22.6 | 22.8 | 21.7 | 21.3 | 20.0 | 23.3 | 21.9 | 20.0 | |
| ≥ 15 years | 63.2 | 63.1 | 64.9 | 61.8 | 60.0 | 62.9 | 62.9 | 60.0 | 62.7 | 64.9 | 63.6 | |
| Gender (% male) | 47.1 | 47.7 | 49.2 | 47.5 | 45.9 | 47.7 | 45.6 | 46.8 | 46.5 | 46.6 | 46.4 | 0.75 |
| Pregnant women among females (%) | 11.6 | 14.7 | 13.1 | 9.2 | 12.1 | 12.3 | 10.8 | 11.4 | 11.6 | 9.2 | 10.8 | 0.42 |
| Resident in the sampling area (%) | 95.2 | 91.9 | 91.5 | 95.1 | 94.4 | 93.1 | 96.9 | 97.9 | 96.5 | 98.5 | 97.4 | P < 0.001 |
| Febrile individuals (Temperature ≥ 37.5°C, %) | 13.1 | 14.2 | 14.1 | 12.5 | 12.3 | 13.3 | 12.8 | 12.6 | 12.7 | 1,300, 0% | 12.8 | 0.84 |
| Forest worker (%) | 15.9 | 21.9 | 21.2 | 19.2 | 13.9 | 19.3 | 13.2 | 14.3 | 14.3 | 6.3 | 12.2 | P < 0.001 |
A total of 7,707 blood samples were tested both by microscopy and PCR among the 8,067 individuals enrolled in the national survey (95.4%). The distribution of the clusters sampled and the malaria prevalence in each cluster, determined by microscopy, are shown in Figure 1. The study did not sample enough sites in each province to produce reliable prevalence estimates at the provincial level. However, high prevalence clusters were mainly located in the northeastern provinces, known to be the highest malaria transmission settings in the country. As a consequence, the overall malaria prevalence rates estimated both by microscopy and PCR were significantly higher in high transmission areas: 4.5% and 9.8%, respectively, in Domain 1 compared with 0.7% and 2.2%, respectively, in Domain 2 (P < 0.0001).
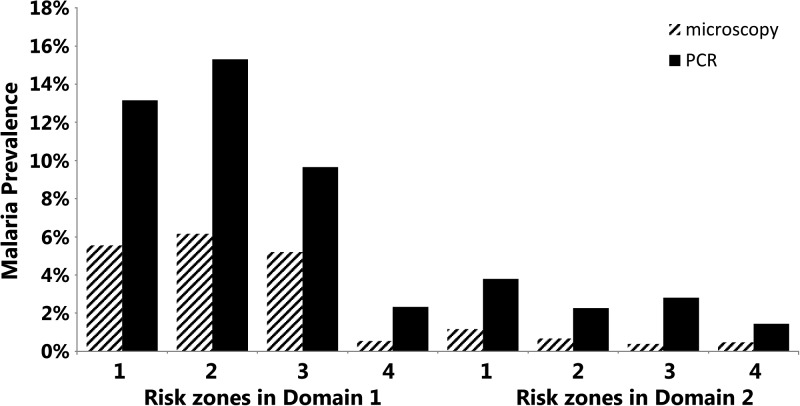
Comparing the malaria prevalence rates between the risk zones, the risk of being malaria positive decreased with increasing distance from the forest margin, regardless of the domain and the method of detection. In Domain 1, malaria prevalence by microscopic detection ranged from 6.2% in risk zone 1 to 0.5% in risk zone 4 (χ2 test for trend, P < 0.001), and from 15.3% in risk zone 1 to 2.3% in risk zone 4 by PCR (χ2 test for trend, P < 0.001). In Domain 2, malaria prevalence by microscopic detection ranged from 1.2% in risk zone 1 to 0.5% in risk zone 4 (χ2 test for trend, P = 0.04), and from 3.8% in risk zone 1 to 1.4% in risk zone 4 by PCR (χ2 test for trend, P < 0.01; Figure 2 ).
Figure 2.
Malaria prevalence by domain and risk zone, Cambodia, 2007. The malaria prevalences (any species) by domain and risk zone as measured by microscopy and polymerase chain reaction (PCR) are shown. Risk zones 1, 2, 3, and 4 correspond to distances from the forest of 0–250 m, 250–1,000 m, 1–2 km, and 2–5 km.
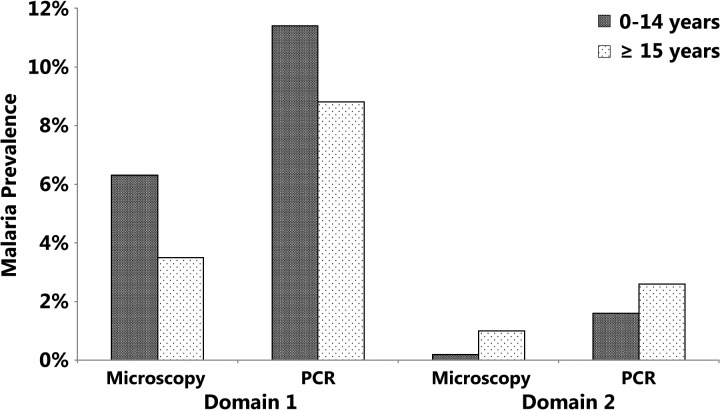
On the basis of microscopy or PCR, malaria prevalence rates declined with increasing age in the high malaria transmission areas of Domain 1 (Figure 3 ): 6.3% in 0–14 years age group versus 3.5% in ≥ 15 years age group (microscopy, P < 0.001) and 11.4% versus 8.8% (PCR, P < 0.01). These data are consistent with other findings in Africa, where age has been strongly associated with malaria infection (detected by RDT or PCR), especially for children aged 0–10 years.21 However, in low-transmission settings of Domain 2, the opposite trend was found for microscopy (0.2% in 0–14 years age group versus 1.0% in ≥ 15 years age group, P < 0.01). These data demonstrate how the malaria epidemiology can vary according to the ecological environment, the distribution of the vectors species, and human behavior, especially in unstable transmission areas.22–25 This trend may be explained partly by the intense efforts at malaria control in Domain 2, where prompt malaria treatment is achieved through the widespread use of malaria RDTs and artemisinin-combined therapies in health facilities and at community level coupled with the increased coverage of LLITNs and indoor residual spraying that have led to a substantial fall in malaria transmission. This may have led to a slowing of the development of malaria immunity in adults who are still at a high risk of contracting malaria because of their work in the forests.
Figure 3.
Malaria prevalence by diagnostic methods (microscopy and polymerase chain reaction [PCR]), Cambodia, 2007. The malaria prevalence rates (any species) found by microscopic examination and PCR detection are shown.
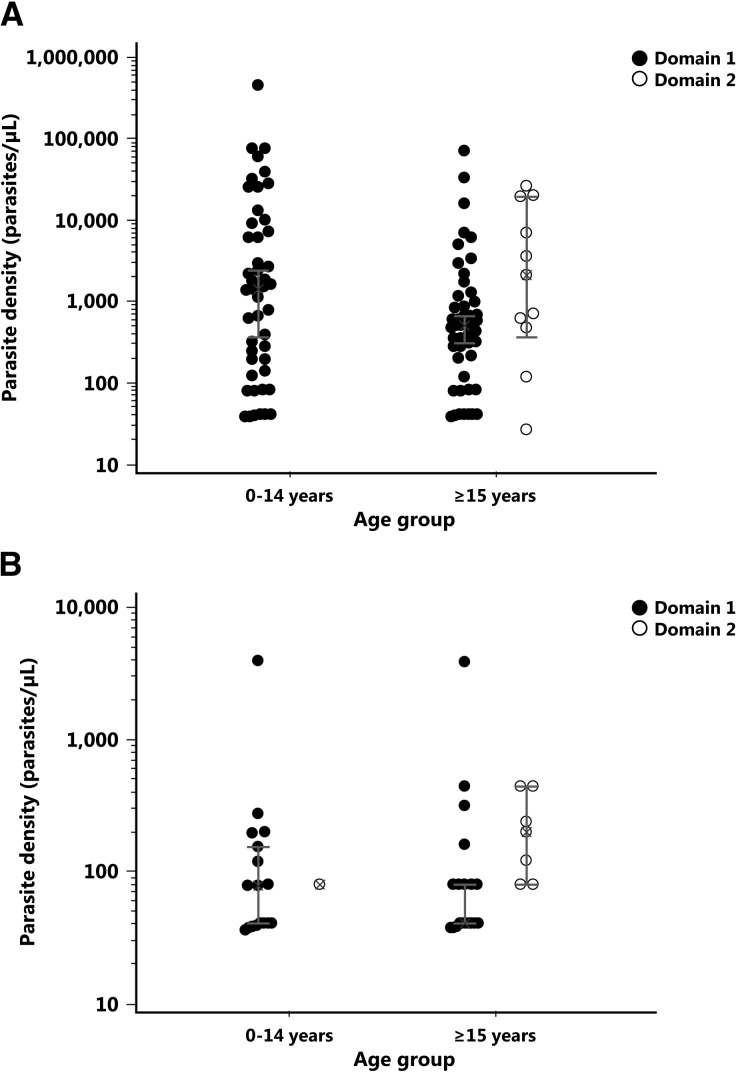
The distribution of parasite densities across the age groups is displayed in Figure 4 . For individuals infected by P. falciparum (Panel A), higher parasitemia was overrepresented in the 0–14 years age group compared with ≥ 15 years age group, with a nonsignificant difference between the geometric mean values (1, 050 parasites/μL in the 0–14 years age group versus 410 parasites/μL in the ≥ 15 years age, P = 0.1). Although not significant, the trend of lower parasitemia with increasing age in Domain 1 suggests the development of some clinical immunity to malaria.26,27
Figure 4.
Distribution of microscopic parasitemias by age group, Cambodia, 2007. The microscopic parasitemias by age group for each domain are presented in Panel A for Plasmodium falciparum infection and in Panel B for Plasmodium vivax infection.
By contrast, for individuals with vivax infections (Panel B), the geometric mean parasitemia was 50-fold lower and almost similar between age groups (84 parasites/μL in the 0–14 years age group versus 45 parasites/μL in the ≥ 15 years age, P = 0.9). Our parasitemia data are consistent with those of Baird and others, who demonstrated that the development of immunity to malaria was slower for P. vivax compared with P. falciparum.28,29
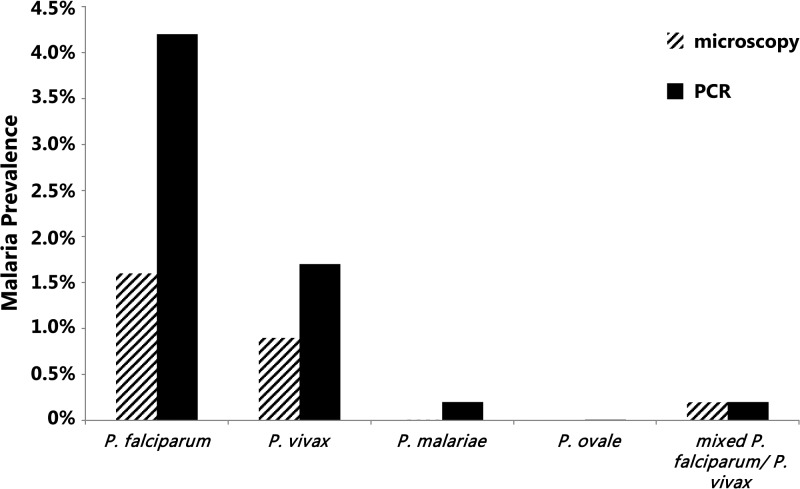
Overall malaria prevalence rates in both domains combined using microscopy or PCR are presented by species in Figure 5 . PCR-determined malaria prevalence rates were significantly higher for all malaria species compared with microscopy: 1) for P. falciparum (4.40%, 95% CI: 3.95–4.90% versus 1.6%, 95% CI: 1.4–2.0%, P < 0.00001), 2) P. vivax (1.89%, 95% CI: 1.60–2.22% versus 1.1%, 95% CI: 0.9–1.4%, P < 0.001), and 3) P. malariae infections (0.22%, 95% CI: 0.13–0.35% versus 0.01%, 95% CI: 0.0003–0.07%, P < 0.0001). Only one ovale infection was detected by PCR (0.01%) and missed by microscopy. The proportions of P. falciparum/P. vivax mixed infections detected by microscopy and PCR were not significantly different (0.20%, 95% CI: 0.12–0.32% versus 0.20%, 95% CI: 0.12–0.35%, P = 1). Among the samples tested as negative by microscopy, 3.85% (289/7,491) were found positive by PCR. This included 186 P. falciparum, 75 P. vivax, 16 P. malariae, 11 mixed P. falciparum/P. vivax and one Plasmodium ovale infections.
Figure 5.
Malaria prevalence by species. The estimated prevalences for the entire surveyed area for any Plasmodium species (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and mixed P. falciparum/P. vivax), as measured by microscopy and polymerase chain reaction (PCR) are shown.
Our findings demonstrate that large reservoirs of submicroscopic infections are common in Cambodia, and are approximately two to three times more common compared with microscopy and represent a significant source of transmission.30 It is worth noting that an elimination strategy based on microscopic mass screening and treatment would miss the majority of infected individuals, confirming our previous observations in Pailin Province.31 Similarly, at the village level, more than a third of villages which appear free of malaria based on microscopy alone actually harbor infection. Factors associated with submicroscopic P. falciparum and P. vivax infections were individuals living in high-transmission settings (Domain 1: 3.9% versus Domain 2: 0.9%, P < 0.0001 for P. falciparum and Domain 1: 1.6% versus Domain 2: 0.6%, P < 0.0001 for P. vivax), and living close to forested areas (risk zones 1 and 2: 3.0% and 3.8% versus risk zones 3 and 4: 2.2% and 0.8% for P. falciparum, P < 0.0001 and risk zones 1, 2, and 3: 1.1%, 1.7%, and 1.2% versus risk zone 4: 0.4% for P. vivax, P < 0.01). Strategies to protect high-risk individuals from contracting malaria need to be developed, taking into account that the two main forest-related vectors, Anopheles dirus and Anopheles minumus are outdoor biting mosquitoes.22,32
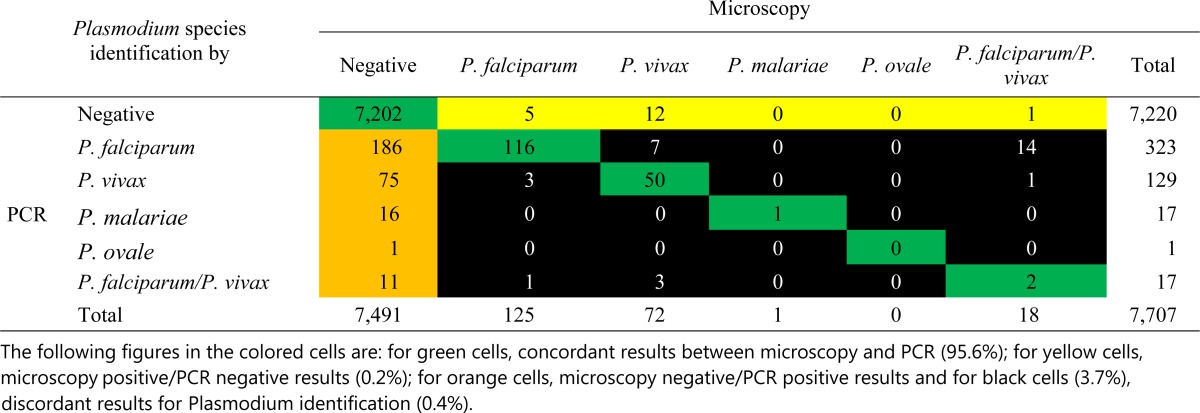
Surprisingly, the PCR failed to detect 18 infections (0.23%) found positive by microscopy (five P. falciparum, 12 P. vivax, and one mixed P. falciparum/P. vivax). All samples missed by PCR had a parasite density < 100 parasites/μL, except for two P. falciparum (20,000 and 3,560 parasites/μL) and one P. vivax infections (3,880 parasites/μL), representing clear PCR false-negative results probably due to technical errors (presence of PCR inhibitors for instance). Finally, there were 29/7,707 (0.38%) cases in which microscopy and PCR gave different species identifications: half cases (15 cases) involved cases that were reported as mixed P. falciparum/P. vivax infections by microscopy but were classified as monoinfections by PCR (14 P. falciparum and one P. vivax; Table 2).
Table 2.
Comparison of microscopy and PCR for the detection of asymptomatic malaria in 7,707 samples collected in endemic area in Cambodia in 2007
PCR = polymerase chain reaction; P. falciparum = Plasmodium falciparum; P. malariae = Plasmodium malariae; P. ovale = Plasmodium ovale; P. vivax = Plasmodium vivax.
In the table, green cells indicate concordant results between microscopy and PCR (95.6%); yellow cells indicate microscopy-positive/PCR-negative results (0.2%); orange cells indicate microscopy-negative/PCR-positive results; and black cells (3.7%) indicate discordant results for Plasmodium identification (0.4%).
The data presented in this study are reliable baseline estimates from which to assess the trends in malaria prevalence rates with successive national malaria surveys. Our findings reinforce previous observations11,33 in which the prevalence of infection measured by PCR was, on average, twice that measured by microscopy. The recent shift from control to elimination with elimination as the ultimate goal will require a rigorous assessment of reservoirs of infection, including submicroscopic infections. Cambodia has committed itself to the elimination of malaria by 2025. To reach this goal, asymptomatic reservoirs should be detected to assess the true prevalence of parasitemia to design an evidence-based elimination strategy and put in place a comprehensive surveillance system to assess the impact of the implemented strategies. Control methods based either on mass screening and treatment or targeted mass drug administration will only be effective if the screening method identifies submicroscopic reservoirs of infection. Cambodia may, therefore, need to shift its current emphasis on parasitological diagnosis based on RDTs and microscopy to high-throughput PCR with rapid communication to the field to find and eliminate hidden parasite reservoirs in transmission foci or to new molecular technologies implementable in the field, such as loop mediated isothermal amplification34 or capture and ligation probe-polymerase chain reaction.35 In addition, seroprevalence surveys of P. falciparum may complement current diagnostic tools and provide useful insight into malaria exposure levels and an estimation of the transmission intensity.36 Lastly, to improve the malaria surveillance and monitoring, more data are needed to assess the role of submicroscopic infections in maintaining the transmission cycle through the low-transmission dry season.
ACKNOWLEDGMENTS
We are grateful to the study participants and for the support of the National Institute of Health Research and Development of Indonesia, and of the Eijkman Institute.
Disclaimer: The views expressed herein are the private ones of the authors and do not purport to represent those of the U.S. Department of the Navy or of the Government of Cambodia.
Footnotes
Financial support: This study was supported by the Global Fund for Malaria, Tuberculosis and AIDS, the Institut Pasteur in Cambodia, and the U.S. Department of Defense Global Epidemic Information System. Jean Popovici is supported by a National Institutes of Health NIAID grant (no. R01 434 A103328).
Authors' addresses: Dysoley Lek, National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia and School of Public Health, National Institute of Public Health, Phnom Penh, Cambodia, E-mail: soleyl@cnm.gov.kh. Jean Popovici and Didier Menard, Institut Pasteur in Cambodia, Phnom Penh, Cambodia, E-mails: jpopovici@pasteur-kh.org and dmenard@pasteur-kh.org. Frederic Ariey, Institut Pasteur in Cambodia, Phnom Penh, Cambodia, Department of Parasites and Insect Vectors, Institut Pasteur, Paris, France, Institut Cochin, Institut National de la Santé et de la Recherche Médicale U1016 (INSERM U1016), Faculté de Médecine, Université Paris Descartes Sorbonne Paris Cité, Paris, France, and Laboratoire de Parasitologie-Mycologie, Hôpital Cochin, Assistance Publique–Hôpitaux de Paris (AP-HP), Paris, France, E-mail: frederic.ariey@yahoo.fr. Seshu Babu Vinjamuri, World Health Organization Lao People's Democratic Republic Office, Vientiane, Lao PDR, E-mail: vinjamuriv@wpro.who.int. Sylvia Meek and Jan Bruce, London School of Hygiene and Tropical Medicine, London, United Kingdom, E-mails: s.meek@malariaconsortium.org and jane.bruce@lshtm.ac.uk. Walter R. J. Taylor, Service de Médecine Tropicale et Humanitaire, Hôpitaux Universitaires de Genève, Geneva, Switzerland and Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand, E-mail: bob@tropmedres.ac. Duong Socheat, National Center for Parasitology, Entomology and Malaria Control, Phnom Penh, Cambodia, E-mail: socheatd@cnm.gov.kh. William O. Rogers, Naval Medical Research Unit 2 Detachment, Phnom Penh, Cambodia, E-mail: rogerswo@namrutwo.org.
References
- 1.Maude RJ, Nguon C, Ly P, Bunkea T, Ngor P, Canavati de la Torre SE, White NJ, Dondorp AM, Day NP, White LJ, Chuor CM. Spatial and temporal epidemiology of clinical malaria in Cambodia 2004–2013. Malar J. 2014;13:385. doi: 10.1186/1475-2875-13-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox J, Dy Soley L, Bunkea T, Sovannaroth S, Soy Ty K, Ngak S, Bjorge S, Ringwald P, Mellor S, Sintasath D, Meek S. Evaluation of community-based systems for the surveillance of day three-positive Plasmodium falciparum cases in western Cambodia. Malar J. 2014;13:282. doi: 10.1186/1475-2875-13-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasegawa A, Yasuoka J, Ly P, Nguon C, Jimba M. Integrating child health services into malaria control services of village malaria workers in remote Cambodia: service utilization and knowledge of malaria management of caregivers. Malar J. 2013;12:292. doi: 10.1186/1475-2875-12-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kheang ST, Duong S, Olkkonen A. Increasing access to early malaria diagnosis and prompted treatment in remote Cambodian villages. Am J Public Health. 2011;101:e6–e8. doi: 10.2105/AJPH.2011.300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Menard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Menard D. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. Tracking Resistance to Artemisinin Collaboration Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin Resistance in Cambodia 1 Study C Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 9.Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, Kim S, Witkowski B, Duru V, Domergue A, Khim N, Ringwald P, Menard D. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84:151–208. doi: 10.1016/B978-0-12-800099-1.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okell LC, Ghani AC, Lyons E, Drakeley CJ. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis. 2009;200:1509–1517. doi: 10.1086/644781. [DOI] [PubMed] [Google Scholar]
- 12.Karl S, Gurarie D, Zimmerman PA, King CH, St Pierre TG, Davis TM. A sub-microscopic gametocyte reservoir can sustain malaria transmission. PLoS One. 2011;6:e20805. doi: 10.1371/journal.pone.0020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, Sauerwein RW. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–474. [PubMed] [Google Scholar]
- 14.Shekalaghe SA, Bousema JT, Kunei KK, Lushino P, Masokoto A, Wolters LR, Mwakalinga S, Mosha FW, Sauerwein RW, Drakeley CJ. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop Med Int Health. 2007;12:547–553. doi: 10.1111/j.1365-3156.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 15.Babiker HA, Abdel-Muhsin AM, Ranford-Cartwright LC, Satti G, Walliker D. Characteristics of Plasmodium falciparum parasites that survive the lengthy dry season in eastern Sudan where malaria transmission is markedly seasonal. Am J Trop Med Hyg. 1998;59:582–590. doi: 10.4269/ajtmh.1998.59.582. [DOI] [PubMed] [Google Scholar]
- 16.Landman KZ, Jean SE, Existe A, Akom EE, Chang MA, Lemoine JF, Mace KE. Evaluation of case management of uncomplicated malaria in Haiti: a national health facility survey, 2012. Malar J. 2015;14:394. doi: 10.1186/s12936-015-0901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwesigwa J, Okebe J, Affara M, Di Tanna GL, Nwakanma D, Janha O, Opondo K, Grietens KP, Achan J, D'Alessandro U. On-going malaria transmission in the Gambia despite high coverage of control interventions: a nationwide cross-sectional survey. Malar J. 2015;14:314. doi: 10.1186/s12936-015-0829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Institute of Public Health CN, Malaria Consortium Report of the Cambodia National Malaria Baseline Survey 2004. 2005 http://www.malariasurveys.org/documents/CMBS2004report15jul.pdf Available at. Accessed November 3, 2015. [Google Scholar]
- 19.World Health Organization Malaria Microscopy Quality Assurance Manual: Version 2. 2009. http://apps.who.int/iris/bitstream/10665/204266/1/9789241549394_eng.pdf Available at. Accessed May 17, 2016.
- 20.Steenkeste N, Incardona S, Chy S, Duval L, Ekala MT, Lim P, Hewitt S, Sochantha T, Socheat D, Rogier C, Mercereau-Puijalon O, Fandeur T, Ariey F. Towards high-throughput molecular detection of Plasmodium: new approaches and molecular markers. Malar J. 2009;8:86. doi: 10.1186/1475-2875-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tusting LS, Willey B, Lucas H, Thompson J, Kafy HT, Smith R, Lindsay SW. Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet. 2013;382:963–972. doi: 10.1016/S0140-6736(13)60851-X. [DOI] [PubMed] [Google Scholar]
- 22.Gryseels C, Durnez L, Gerrets R, Uk S, Suon S, Set S, Phoeuk P, Sluydts V, Heng S, Sochantha T, Coosemans M, Peeters Grietens K. Re-imagining malaria: heterogeneity of human and mosquito behaviour in relation to residual malaria transmission in Cambodia. Malar J. 2015;14:165. doi: 10.1186/s12936-015-0689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in southeast Asia: a challenge for vector control. Trop Med Int Health. 2005;10:251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 24.Clark TD, Greenhouse B, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Staedke SG, Seto E, Kamya MR, Rosenthal PJ, Dorsey G. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. J Infect Dis. 2008;198:393–400. doi: 10.1086/589778. [DOI] [PubMed] [Google Scholar]
- 25.Alemu K, Worku A, Berhane Y. Malaria infection has spatial, temporal, and spatiotemporal heterogeneity in unstable malaria transmission areas in northwest Ethiopia. PLoS One. 2013;8:e79966. doi: 10.1371/journal.pone.0079966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodker R, Msangeni HA, Kisinza W, Lindsay SW. Relationship between the intensity of exposure to malaria parasites and infection in the Usambara Mountains, Tanzania. Am J Trop Med Hyg. 2006;74:716–723. [PubMed] [Google Scholar]
- 27.Schofield L, Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–221. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 28.Baird JK, Jones TR, Danudirgo EW, Annis BA, Bangs MJ, Basri H, Purnomo Masbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am J Trop Med Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 29.Baird JK, Purnomo Basri H, Bangs MJ, Andersen EM, Jones TR, Masbar S, Harjosuwarno S, Subianto B, Arbani PR. Age-specific prevalence of Plasmodium falciparum among six populations with limited histories of exposure to endemic malaria. Am J Trop Med Hyg. 1993;49:707–719. doi: 10.4269/ajtmh.1993.49.707. [DOI] [PubMed] [Google Scholar]
- 30.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- 31.Hoyer S, Nguon S, Kim S, Habib N, Khim N, Sum S, Christophel EM, Bjorge S, Thomson A, Kheng S, Chea N, Yok S, Top S, Ros S, Sophal U, Thompson MM, Mellor S, Ariey F, Witkowski B, Yeang C, Yeung S, Duong S, Newman RD, Menard D. Focused screening and treatment (FSAT): a PCR-based strategy to detect malaria parasite carriers and contain drug resistant P. falciparum, Pailin, Cambodia. PLoS One. 2012;7:e45797. doi: 10.1371/journal.pone.0045797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durnez L, Mao S, Denis L, Roelants P, Sochantha T, Coosemans M. Outdoor malaria transmission in forested villages of Cambodia. Malar J. 2013;12:329. doi: 10.1186/1475-2875-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson JC, Stresman GH, Baidjoe A, Okoth A, Oriango R, Owaga C, Marube E, Bousema T, Cox J, Drakeley C. Use of different transmission metrics to describe malaria epidemiology in the highlands of western Kenya. Malar J. 2015;14:418. doi: 10.1186/s12936-015-0944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cook J, Aydin-Schmidt B, Gonzalez IJ, Bell D, Edlund E, Nassor MH, Msellem M, Ali A, Abass AK, Martensson A, Bjorkman A. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015;14:43. doi: 10.1186/s12936-015-0573-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhibin C, Duoquan W, Xiaoyi T, Yu S, Xiaodong S, Ning X, Zhi Z. Capture and ligation probe-PCR (CLIP-PCR) for molecular screening, with application to active malaria surveillance for elimination. Clin Chem. 2015;61:821–828. doi: 10.1373/clinchem.2014.237115. [DOI] [PubMed] [Google Scholar]
- 36.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–582. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]