Abstract
The world population, especially in developing countries, has experienced a rapid progression of urbanization over the last half century. Urbanization has been accompanied by a rise in cases of urban infectious diseases, such as malaria. The complexity and heterogeneity of the urban environment has made study of specific urban centers vital for urban malaria control programs, whereas more generalizable risk factor identification also remains essential. Ahmedabad city, India, is a large urban center located in the state of Gujarat, which has experienced a significant Plasmodium vivax and Plasmodium falciparum disease burden. Therefore, a targeted analysis of malaria in Ahmedabad city was undertaken to identify spatiotemporal patterns of malaria, risk factors, and methods of predicting future malaria cases. Malaria incidence in Ahmedabad city was found to be spatially heterogeneous, but temporally stable, with high spatial correlation between species. Because of this stability, a prediction method utilizing historic cases from prior years and seasons was used successfully to predict which areas of Ahmedabad city would experience the highest malaria burden and could be used to prospectively target interventions. Finally, spatial analysis showed that normalized difference vegetation index, proximity to water sources, and location within Ahmedabad city relative to the dense urban core were the best predictors of malaria incidence. Because of the heterogeneity of urban environments and urban malaria itself, the study of specific large urban centers is vital to assist in allocating resources and informing future urban planning.
Introduction
Over the past five decades, the world has seen an era of rapid urbanization, with 52.1% of the world population living in urban environments as of 2011.1 In 2002, India had the second largest urban population in the world, and this number is predicted to double in the next 30 years.2 As these urban centers rapidly, and often haphazardly, expand, their complexity and heterogeneous nature lead to new challenges in the study and control of urban infectious diseases, such as malaria.3–6 The diversity of urban environments makes population level studies and identification of environmental risk factors for malaria transmission difficult. Research has shown that urban environments can protect inhabitants from malaria transmission for various reasons including chemical pollution of water bodies, deforestation, and opportunity to increase the separation between population and vector by limiting the number of breeding sites.3,4,7 However, in practice, rapid development and increase in urban population may also lead to an acceleration in the degradation of urban environmental quality, creating increasingly supportive environments for malaria vectors to breed.3,4,6 The population density of urban centers also leads to a higher proximity of subjects to each other and increased ease of transmission of malarial parasites by its vector. Finally, limited water resources leading to unsafe storage practices, material pollution, difficulties in sewage management, and poorly constructed buildings lead to novel vector breeding sites by increasing the prevalence of standing water such as in water storage tanks, unfinished building foundations, or improperly disposed tires.8,9
At present, urban malaria accounts for approximately 15% of India's total malaria burden.10 To exacerbate the problem, Anopheles mosquitoes have undergone a speciation process where new taxonomic units have developed that are well adapted to live among humans in urban environments, in particular Anopheles stephensi.11,12 Although research in this area is increasing, a challenge in understanding urban malaria is that the complex urban environment leads to diverse and poorly generalizable risk factors. Commitment to local research in large urban centers will allow for the identification of area specific risk factors, which can be used for early and targeted interventions. Preemptive vector and parasite control based on the knowledge of local or generalizable risk factors will improve workforce and resource utilization, inform urban planning, and decrease risk for disease outbreaks.
Ahmedabad city (hereinafter “Ahmedabad”) is located in the western most state of Gujarat, India, where the population has risen by more than 50% over the past two decades. In recent years, Ahmedabad has seen a growing number of malaria cases despite efforts by the National Vector Borne Disease Control Program (NVBDCP) to slow transmission. The increased number of cases may be a result of improved detection; however, the largely malaria-naive population of Ahmedabad is at high risk for severe malaria and malaria epidemics, making efforts to curb transmission vital. A 2003 study, which included urban malaria scheme (UMS) surveillance data, indicated that the malaria burden in Ahmedabad was 37,431 cases, approximately nine times greater than the officially reported numbers.13
To guide targeting of the NVBDCP's efforts, we performed spatiotemporal analysis of malaria cases in Ahmedabad, first assessing for spatial clustering of cases and then evaluating the association between population, environmental, and geographic factors with malaria incidence.
Materials and Methods
Study area.
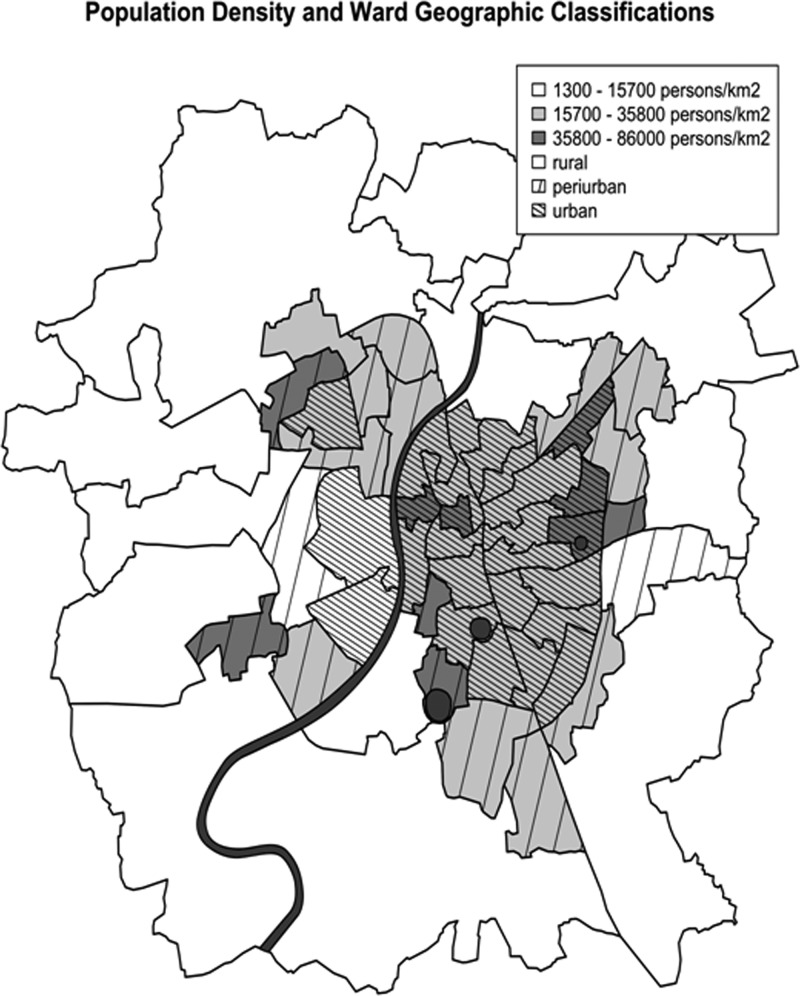
Ahmedabad, Gujarat, is the fifth largest city in India with a population of 5.6 million persons in an area of 581 km2.14 As of 2003, approximately 50% of this population lived in slums or peri-urban areas.13 It has a hot semiarid climate, lies at 53 m above sea level, receives approximately 740 mm of rain per year, has an average yearly low of 12°C and an average yearly high of 42°C, and has three seasons: winter from mid-November to mid-February, a hot and dry summer from mid-February to mid-June, and monsoon season from mid-June to mid-October.15 The Sabarmati River divides the city into new and old Ahmedabad in the west and east, respectively, and three urban lakes are located in the eastern half of the city (Figure 1 ). Finally, Ahmedabad is divided into 64 wards and has a large surrounding peri-urban area on its outskirts and a dense urban core centered just east of the Sabarmati River, which is home to a large portion of the city's urban poor and is one of the most densely populated areas of the city.
Figure 1.
Classification of population density (persons per km2) and ward geographic classifications based on location relative to the city's dense urban core and also considering the predominant land use type present in each ward (specifically urban vs. rural). Also included is the Sabarmati River that bisects the city and the three large urban lakes located in the eastern half of the city.
Epidemiological data.
The data used in this study were provided by the Ahmedabad Municipal Corporation (AMC), and includes monthly malaria incidence from 2010 to 2012 for 56 wards (during data collection, 16 wards were combined into eight wards due to recent changes in ward designation, 2010 data did not designate active versus passive collection, and therefore was not used in spatial analysis or model generation). These data were obtained through surveillance conducted under the UMS. Cases were confirmed by trained microscopists in public institutions within Ahmedabad. Malaria incidence was calculated per 1,000 persons in each ward assuming that the entire population of the ward was at risk. Cases recorded from migrant populations, which account for 1% of Plasmodium vivax and 1.7% of Plasmodium falciparum cases, were removed from the data set, as their location could not be specified to the ward level. Ward-wise population data from the 2011 census was used for calculation of malaria incidence and population density, and was obtained from the AMC.
Geographic and remote sensing data.
The initial mapping and observational analysis was performed in ArcGIS 10 (ESRI, Redlands, CA). AMC provided the original map, which was used for all spatial data analyses.16 Monthly Landsat 5 (National Aeronautics and Space Administration [NASA], Washington, DC) and Moderate-Resolution Imaging Spectroradiometer (NASA, Washington, DC) remote sensing data of Ahmedabad were obtained through Google Earth Engine (https://earthengine.google.org).17 The statistical software R (http://www.r-project.org/) was used to map P. vivax and P. falciparum case incidence by year. Finally, ArcGIS 10 was used to perform supervised maximum likelihood classification of land use each month for 2011, when images of adequate quality were available within the city of Ahmedabad (utilizing Landsat 5 imagery) to define the area per ward covered by “urban” environment and green vegetation, and to localize large water bodies (truthing by Google Earth imagery).18 To calculate the area of land covered by each land use type, we divided the pixels attributed to each land use type in a ward by the total number of pixels in that ward.
Data processing and analysis.
All statistical analyses were performed using the statistical software R. The Pearson's coefficient of correlation (r) was used to assess the inter- and intra-annual correlation within and between species of malaria. This statistic was also used to assess the association between cases and rainfall, and “low season” and “high season” malaria incidence.
Cluster analysis.
Spatial analysis for high-rate local clusters was performed using the Kulldorff's scan statistic in SaTScan (http://www.satscan.org/) on passive P. vivax and P. falciparum incidence for 2011 and 2012 separately. SaTScan uses a spatial window to evaluate clustering, based on Poisson-based likelihood ratios, around each possible geographic location in the study area (the center points of the wards).19 An elliptical window with a strong noncompactness penalty was chosen to allow for noncircular spatial clustering. A maximum cluster size of 10% of the population at risk was used to identify local clusters within Ahmedabad, while not limiting the cluster analysis to single high-incidence wards. Finally, secondary clusters were identified by removing the cases within primary clusters and then rerunning the statistic.
Historic incidence-based prediction of malaria incidence.
As environmental factors facilitating the transmission of malaria are relatively stable in urban centers and cases are expected to cluster in these areas, the utility of using “low season” (April/May) and prior year malaria incidence to predict future malaria incidence was assessed. To do this, we ranked the wards by incidence for P. vivax and P. falciparum for 2011, P. vivax for “low season” 2011, and P. vivax for “low season” 2012 resulting in four separate lists of wards ranked based on incidence (P. falciparum had essentially no low-season cases to use for prediction). These rank orders were applied to the ward-level data for P. vivax and P. falciparum in 2011 and 2012, and we assessed the ability of these rank lists to prospectively predict which wards would be the highest burdened wards. To demonstrate the efficiency of these ranks in targeting high-risk populations, we calculated the percent of total cases that would be accounted for by the approximately 10% and 20% of the population predicted to have the highest incidence. Because of our incidence data being limited to the ward level, exact calculations of the 10% and 20% most burdened portions of the population could not be made; therefore, we sequentially added wards according to their rank until at least 10% and 20% of the population was included.
Population, geographic, and environmental predictors of malaria incidence.
As 2010 data were not separated into active and passive case detection, only 2011–2012 passive P. vivax and P. falciparum cases were included in the regression analysis in an effort to remove bias from the analysis. Because of temporal correlation between incidences each month and the desire to focus on spatial prediction at the time when burden was highest, cases were aggregated into an average for “high season” from August to November for each year. A generalized estimating equation (GEE) model (R function geeglm, package geepack) was used to perform this analysis of 2011 and 2012 “high season” malaria case incidence.20–22 This model was used to assess the effects of multiple independent variables on P. vivax and P. falciparum incidence. The variables included population density, the designation of urban versus peri-urban versus rural, longitude, latitude, ward-wise literacy rate, difference between male and female ward-wise literacy rate, percentage of the ward classified as urban in the dry and wet season in 2011, the distance of the ward centroid from the Sabarmati River, the shortest distance of the ward centroid from the Sabarmati River or one of the urban lakes, the percentage of the ward classified as green vegetation in the dry and wet season in 2011, the percentage of the ward classified as water during the dry season in 2011 and the wet season in 2010, the land surface temperature during monsoon season in 2011, and the normalized difference vegetation index (NDVI) during monsoon season in 2011.14,16,17,23 Variables were first evaluated with univariate Poisson regression to identify variables for inclusion in the GEE model. Then a stepwise backward elimination based on t-statistics was used to identify the optimal GEE model. Finally, the most appropriate correlation structure was determined based on the quasi-likelihood under the independence model criterion of different models.
Results
Temporal statistical trends and stability of spatial case distribution.
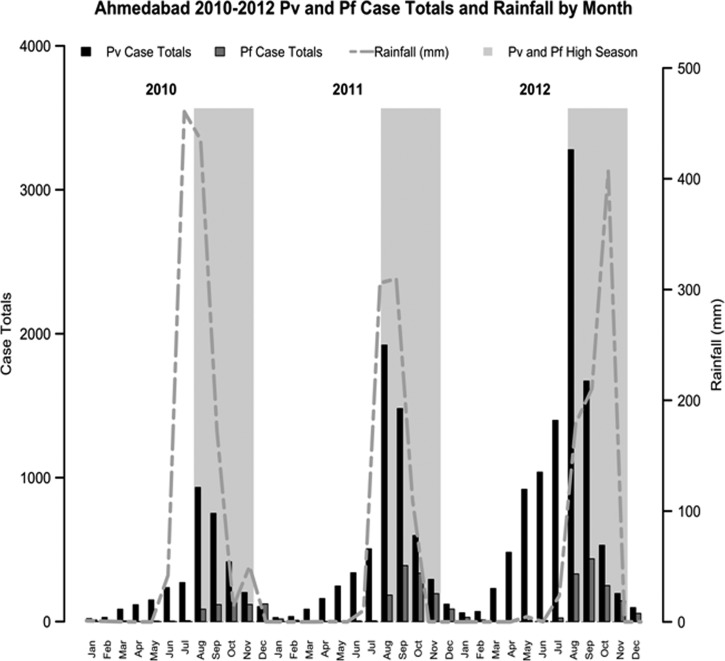
During the period of 2010–2012, the number of P. vivax cases was more than six times that of P. falciparum cases (P. vivax = 19,096 cases, P. falciparum = 3,162 cases). Plasmodium vivax annual incidence increased each year, whereas P. falciparum annual incidence leveled off after doubling from 2010 to 2011 (P. vivax annual incidence per 1,000 persons = 0.60, 1.04, 1.80, and P. falciparum annual incidence per 1,000 persons = 0.11, 0.22, 0.22 for 2010, 2011, 2012, respectively). The incidence rates for P. vivax and P. falciparum were both highly seasonal; P. vivax demonstrated a pattern of case escalation beginning annually in March, before the onset of monsoons, whereas P. falciparum abruptly rose in August after the beginning of monsoons (Figure 2 ). Plasmodium vivax and P. falciparum incidence strongly correlated with rainfall the prior month (r = 0.6 [P < 0.001], r = 0.78 [P < 0.001] for P. vivax and P. falciparum, respectively). In addition, the incidence of P. vivax and P. falciparum for each ward was relatively stable between 2011 and 2012 (r = 0.76 [P < 0.001] and r = 0.68 [P < 0.001] for P. vivax and P. falciparum, respectively). Finally, the distributions of annual incidence of P. vivax and P. falciparum between wards were highly related to each other (r = 0.69 [P < 0.001], r = 0.88 [P < 0.001] for 2011 and 2012, respectively).
Figure 2.
Total Plasmodium vivax and Plasmodium falciparum by month from 2010 to 2012 with coincident rainfall overlaid. Large grey boxes mark the high season of malaria transmission.
Observational spatiotemporal dynamics.
General observation was made of the geographic progression of ward-wise monthly P. vivax and P. falciparum incidence during the transmission season utilizing maps generated in ArcGIS. Specifically, we had hypothesized that we would find foci that could be identified as potential sources for cases in surrounding wards and that high incidence would progress from primarily peri-urban to urban at the start of high season, followed by a regression to peri-urban after monsoon. Observation for a temporal progression of P. vivax cases did not reveal obvious patterns in spatiotemporal progression. For P. falciparum, the abrupt escalation in cases in August made any observation of potential transmission patterns difficult.
Spatial clustering.
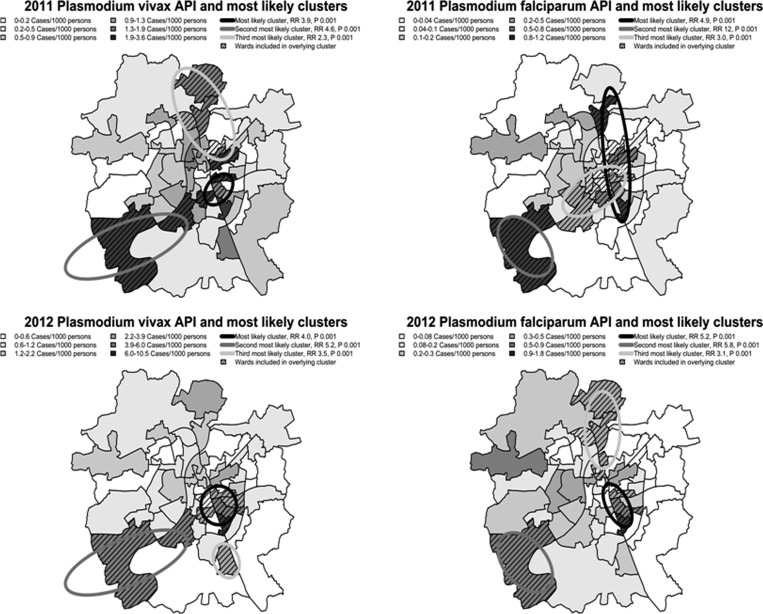
Kulldorff's scan statistic demonstrated several significant areas of clustering of P. vivax and P. falciparum incidence in 2011 and 2012 (Figure 3 ). The most likely clusters for P. vivax and P. falciparum in 2011 and 2012 were found in the central portion of Ahmedabad, generally referred to as old Ahmedabad and home to a large proportion of the city's slum and underserved populations. The primary clusters had relative risks of 3.86 with P = 0.001 (P. vivax 2011), 4.87 with P = 0.001 (P. falciparum 2011), 4.05 with P = 0.001 (P. vivax 2012), and 5.22 with P = 0.001 (P. falciparum 2012) indicating greatly increased risk for malaria infection in these central areas. Because of concerns that spatial clustering could be related to regional differences in case detection practices, we assessed for correlation between slide positivity rate and total cases. A high correlation with P < 0.005 was found suggesting that regional changes in incidence were unlikely due to case detection practices.
Figure 3.
Plasmodium vivax and Plasmodium falciparum incidence stratified by annual parasite index (API) into six quantiles. Also included are the top three most likely clusters of P. vivax and P. falciparum incidence for 2011 and 2012 with risk ratios and P values included.
Historic incidence-based prediction of malaria cases.
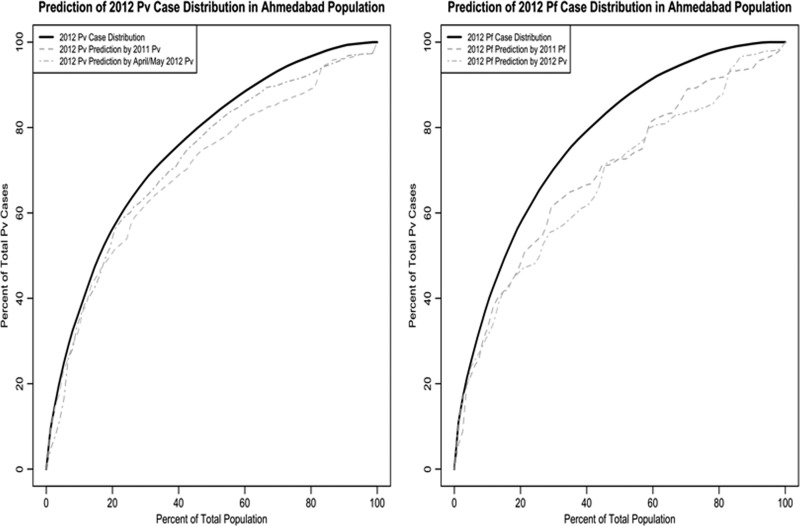
Taking advantage of the temporally stable spatial heterogeneity of malaria incidence in Ahmedabad, we evaluated the use of past incidence data to predict which wards would have highest burden of malaria cases in the future. The most burdened wards encompassing approximately 10% of the population accounted for between 37% and 40% of cases for both species in both years, whereas the most burdened wards encompassing approximately 20% of the population accounted for between 52% and 58% of cases (Table 1). The wards identified as the approximately 10% and 20% most burdened wards during the predictor periods, the same year's “low season” or the whole prior year, were assessed for what percentage of malaria cases they were able to predict in future seasons or years. For P. vivax, the “low season” prediction method using the approximately 20% highest burdened wards for 2011 and 2012 predicted on average 50% of the cases that occurred in Ahmedabad that year (Figure 4 ).However, an even better predictor was the prior year's approximately 20% highest burdened wards, which predicted 56% of the cases that occurred in Ahmedabad in 2012. For P. falciparum, similarly, the prior year prediction method using the approximately 20% highest burdened wards for 2011 predicted 49% of the cases that occurred in Ahmedabad in 2012. The “low season” data for P. falciparum was not used due to the scarcity of cases; however, the 2012 “low season” data for P. vivax predicted 47% of the P. falciparum cases that occurred in Ahmedabad in 2012.
Table 1.
Historic incidence-based prediction of malaria incidence utilizing 2011 low season, 2011 annual, and 2012 low season malaria incidence to predict Pv and Pf incidence in 2011 and 2012
| Predicted variables and predictors | % Population used in calculation | % Total cases in most burdened (approximately 10% population) | Cases/1,000 persons in most burdened approximately 10% of the population (cases/1,000 persons in study population) | % Population used in calculation | % Total cases in most burdened (approximately 20% population) | Cases/1,000 persons in most burdened approximately 20% of the population (cases/1,000 persons in study population) |
|---|---|---|---|---|---|---|
| Pv 2011 | 11.6 | 37 | 2.7 (0.86) | 20.7 | 52 | 2.2 (0.86) |
| Pv 2011 predicted by “low season” 2011 | 10.1 | 28 | 2.4 | 21.6 | 48 | 1.9 |
| Pv 2012 | 10.9 | 39 | 5.1 (1.4) | 20.9 | 57 | 3.9 (1.4) |
| Pv 2012 predicted by Pv 2011 | 11.6 | 39 | 4.7 | 20.7 | 56 | 3.8 |
| Pv 2012 predicted by Pv “low season” 2012 | 11.0 | 37 | 4.7 | 20.7 | 52 | 3.5 |
| Pf 2012 | 10.6 | 40 | 0.77 (0.2) | 20.5 | 58 | 0.58 (0.2) |
| Pf 2012 predicted by Pf 2011 | 10.1 | 33 | 0.67 | 20.3 | 49 | 0.49 |
| Pf 2012 predicted by Pv “low season” 2012 | 11.0 | 33 | 0.6 | 20.7 | 47 | 0.46 |
Pf = Plasmodium falciparum; Pv = Plasmodium vivax.
Figure 4.
Percent of total ward population vs. percent of total ward cases for both Plasmodium vivax and Plasmodium falciparum in 2012. The plots were generated by ranking incidence from greatest to least and then iteratively adding them to the plot. Included in the plots are predictions of P. vivax and P. falciparum for 2012 based on historic case data.
Geographic, population, and environmental risk factor analysis.
The final model for P. vivax included the following variables: location of the ward relative to urban city center, distance from rivers and lakes, percent ward area covered by water during monsoon, and NDVI during monsoon, which were all significantly associated with P. vivax incidence (Table 2). Surprisingly, the central/urban area was associated with higher P. vivax incidence than both the peri-urban and rural surrounding areas. As expected, increasing distance from the Sabarmati River and urban lakes was protective, and having more water area in a ward and a higher NDVI was associated with increased incidence. Literacy rates and difference between male and female literacy rates were not included in the model for P. vivax due to lack of significance in the multivariate model. The final model for P. falciparum included the following variables: location relative to urban city center, percent ward area covered by water during monsoon, and NDVI during monsoon, which all significantly contributed to P. falciparum incidence (Table 2). These factors demonstrated similar relationships with P. falciparum incidence as they did with P. vivax incidence. Distance from rivers and lakes was not included in the model for P. falciparum due to a lack of significance in the multivariate model.
Table 2.
Univariate and multivariate GEE Poisson generalized linear model of “high season” Pv and Pf API 2011–2012
| Ward attributes | Plasmodium vivax | Plasmodium falciparum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| RR | 95% CI | P value | RR | 95% CI | P value | RR | 95% CI | P value | RR | 95% CI | P value | |
| Geographic | ||||||||||||
| Population density (1,000 persons/km2) | 0.99 | 0.98–1.0 | 0.23 | 0.98 | 0.97–1.0 | 0.038 | ||||||
| Location relative to urban city center | ||||||||||||
| Peri-urban vs. central/urban | 0.42 | 0.26–0.67 | 0.00026 | 0.41 | 0.28–0.62 | 0.000017 | 0.41 | 0.22–0.76 | 0.0048 | 0.37 | 0.22–0.62 | 0.00018 |
| Rural vs. ventral/urban | 0.79 | 0.43–1.5 | 0.46 | 0.41 | 0.2–0.85 | 0.016 | 1.1 | 0.52–2.3 | 0.8 | 0.25 | 0.12–0.53 | 0.00025 |
| Longitude (km) | 1.0 | 0.96–1.0 | 0.97 | 0.96 | 0.90–1.0 | 0.18 | ||||||
| Latitude (km) | 0.95 | 0.90–1.0 | 0.088 | 0.97 | 0.89–1.1 | 0.41 | ||||||
| Socioeconomic | ||||||||||||
| Ward literacy rate | 0.94 | 0.90–0.99 | 0.023 | 0.97 | 0.91–1.03 | 0.3 | ||||||
| Difference between male and female ward literacy rate | 1.09 | 1.02–1.17 | 0.013 | 1.07 | 0.98–1.16 | 0.14 | ||||||
| Remote sensing | ||||||||||||
| % Area urban dry season 2011 | 1.0 | 0.99–1.0 | 0.72 | 0.99 | 0.98–1.0 | 0.39 | ||||||
| % Area urban monsoon season 2011 | 1.0 | 0.99–1 | 0.33 | 0.99 | 0.98–1.0 | 0.14 | ||||||
| % Urban classification dry season 2011 | ||||||||||||
| Medium density vs. high density | 1.2 | 0.59–2.4 | 0.61 | 1.1 | 0.45–2.6 | 0.87 | ||||||
| Low density vs. high density | 1.1 | 0.58–2.2 | 0.72 | 1.3 | 0.51–3.1 | 0.61 | ||||||
| % Urban classification monsoon season 2011 | ||||||||||||
| Medium density vs. high density | 1.5 | 0.70–3.0 | 0.31 | 1.4 | 0.53–3.5 | 0.52 | ||||||
| Low density vs. high density | 1.4 | 0.64–3.0 | 0.41 | 1.6 | 0.59–4.4 | 0.36 | ||||||
| Distance from rivers (km) | 0.94 | 0.86–1.0 | 0.12 | 0.92 | 0.80–1.1 | 0.27 | ||||||
| Distance from rivers and lakes (km) | 0.83 | 0.74–0.93 | 0.0015 | 0.81 | 0.79–0.93 | 0.00022 | 0.9 | 0.73–1.1 | 0.3 | |||
| % Area green vegetation dry season 2011 | 1.0 | 1.0 | 0.15 | 1.0 | 1.0–1.1 | 0.032 | ||||||
| % Area green vegetation monsoon season 2011 | 1.0 | 0.99–1.0 | 0.23 | 1.0 | 1.0 | 0.022 | ||||||
| % Area water monsoon season 2010 (excluding lakes and rivers) | 1.3 | 0.99–1.8 | 0.14 | 1.4 | 1.1–1.9 | 0.0095 | 1.3 | 0.86–1.9 | 0.22 | 1.6 | 1.15–2.1 | 0.005 |
| Land surface temperature monsoon season 2011 | 1.0 | 0.88–1.2 | 0.79 | 0.96 | 0.77–1.2 | 0.71 | ||||||
| % Area water dry season 2011 (excluding lakes and rivers) | 1.8 | 1.1–3.2 | 0.032 | 2.4 | 1.2–4.6 | 0.013 | ||||||
| Normalized difference vegetation index monsoon season 2011 | 4.1 | 0.36–47 | 0.25 | 84 | 4.0–1,800 | 0.0044 | 39 | 1.4–1,100 | 0.031 | 1,400 | 17–12,000 | 0.0013 |
API = annual parasite index; CI = confidence interval; GEE = generalized estimating equation; Pf = Plasmodium falciparum; Pv = Plasmodium vivax; RR = risk ratio.
Discussion
In this study, we showed that malaria burden in Ahmedabad was spatially heterogeneous, with high correlation between areas at risk for P. falciparum and P. vivax, and strong temporal stability of these high-risk areas. A simple strategy of ranking wards based on past incidence data was able to identify the population at highest risk of malaria with high accuracy. Finally, analysis of geographic and remote sensing data was able to identify potential risk factors driving this heterogeneity, such as location relative to urban city center, percent ward area covered by water during monsoon, and NDVI.
During the 2010–2012 period studied, the incidence of both P. vivax and P. falciparum malaria cases were noted to rise, consistent with the concerns over rising malaria cases that were the impetus for this study. In addition, there was strong inter- and intra-annual correlation between species and across species of malaria, as well as clear spatial clustering of cases demonstrated by Kulldorff's scan (Figure 3). This indicates that there are likely factors inherent to those wards consistently experiencing the highest burden of P. vivax and P. falciparum. Although the reservoir for P. falciparum during the dry season is unclear, the persistence of P. vivax cases during the dry season suggests an ongoing reservoir within Ahmedabad and may represent recrudescence among the previously affected population.
The primary clusters for both P. vivax and P. falciparum for 2011 and 2012 identified by SaTScan were located near the dense urban core of old Ahmedabad, just east of the Sabarmati River near the large urban lakes present in Ahmedabad. This area is home to a large portion of the city's urban poor and is among the most densely populated parts of Ahmedabad. On the western side of the Sabarmati, which is generally less urban, clusters of both P. vivax and P. falciparum were found in peri-urban wards at the northern and southern ends of the city (situated near the Sabarmati River, where the wards have higher densities of vegetation). The increased densities of vegetation and therefore more hospitable environments for vector breeding in these wards relative to the generally drier and more arid rest of peri-urban/rural Ahmedabad may explain the observed high-incidence wards in western Ahmedabad. This indicates a potential variety in ecologic habitats predisposing to malaria within the city of Ahmedabad: rural/peri-urban in the west, and urban in the east. Historically, the trend found in urban centers is that peri-urban cases lead to urban cases, and that a predominance of cases exists in the peri-urban areas.10 In Ahmedabad, these regions were found to have distinct malaria-transmission patterns, which do not appear to interact, and arise concurrently rather than in succession.
We have shown that malaria clusters in particular regions of Ahmedabad, and that these clusters are spatially stable from year to year. This stability allowed us to use 2011 P. vivax case incidence to accurately predict which 10% and 20% of the 2012 population were at highest risk for P. vivax simply by ranking wards based on the previous year's case data. Using such a strategy would have allowed NVBDCP interventions to, for example, target 56% of P. vivax cases in 2012 by focusing on the wards containing the highest risk 20% of the population in 2011. Even if spatial heterogeneity was not stable from year to year, we demonstrated that monitoring of cases based on the early months of P. vivax transmission during the escalation in “low season” was nearly as useful for predicting populations at highest risk of P. vivax as well as P. falciparum in the high season.
Finally, as indicated by regression analysis, local environment plays a key role in the local transmission of malaria, which has been well described in the malaria literature.7 Remotely sensed data such as land use characteristics, land surface temperature, and NDVI have been previously used widely in both rural and urban settings.7,10 In addition, environmental and population factors like distance from water bodies, local presence of water bodies, and population density have been demonstrated to influence malaria in both rural and urban settings.5 In this study, our analysis indicated that P. vivax incidence was associated with location relative to the urban city center, distance from rivers and lakes, percent ward area covered by water during monsoon, and NDVI during monsoon, all of which significantly affected P. vivax incidence. NDVI, which indicates the degree to which the local environment includes live green vegetation, is a complex factor including a diverse array of influences, many of which have been associated with increased malaria incidence. Urban farming and peri-urban vegetation both lead to an increase in NDVI and provide potential breeding habitats for urban Anopheles mosquitoes. Our findings are in keeping with previous studies, which have also demonstrated NDVI as a reliable predictor of malaria prevalence, although a 2011 study by Baeza and others24 implied that in the setting of high irrigation, effective malaria control measures are often sufficient to break down the relationship between NDVI and malaria epidemics in rural settings. Contrary to some studies,3,4,7 but consistent with others,6,7 highly urbanized areas were found to be a significant risk factor for urban malaria, whereas wards containing decreasing degrees of urbanization were protected. The fact that population density was not a significant predictor indicates that it may be the degradation of the environment, rather than the concentration of persons per se, that make highly urban areas a greater risk in Ahmedabad. Further, the socioeconomics and behavioral practices of such populations in terms of water storage, treatment nonadherence, and migrant worker immigration might contribute to high risk of malaria in highly urbanized areas. We assessed the impact of socioeconomic status, which has been demonstrated to be a predictor of urban malaria incidence, through the inclusion of literacy rates and male–female literacy rate disparity.25,26 Interestingly, in the univariate analysis, these covariates were significant predictors of P. vivax incidence, which may relate to treatment nonadherence mentioned above and recrudescence in these populations. Not surprisingly, the variance explained by this proxy for socioeconomic status was accounted for by the other covariates included in the final model. We do not consider this result owing to the lack of evidence of contribution of this factor to malaria incidence. In addition, targeted malaria interventions in socioeconomically disadvantaged areas could be contributing in part to this lack of significance. It is vital that well documented malaria interventions are incorporated into future studies to inform the impact of these interventions as well as considering their effects when assessing risk factors for transmission. Finally, as expected, wards that contain or are closer to more large water bodies are more at risk for a higher malaria incidence.25 Again, the similarities between P. vivax and P. falciparum models indicate that it is likely similar vector habitats are driving local malaria transmission, and continued targeting of vector habitats would be prudent.
As urbanization, and concurrently urban infectious disease, become increasingly prevalent, studies such as this evaluating incidence and transmission characteristics in large urban centers will be vital in targeting interventions and informing urban planning to prevent the spread and development of endemicity of urban infectious diseases such as malaria. Through in-depth investigation of a particular urban center, we were able to not only identify risk factors particular to Ahmedabad, but we also identified aspects of malaria-transmission patterns in Ahmedabad, such as the predominant peri-urban malaria in west Ahmedabad and urban malaria in east Ahmedabad, that will further inform intervention. Finally, we identified that in this particular center, there is stable inter- and intraspecies heterogeneity of incidence that will allow the prediction of populations at elevated risk utilizing data from the previous year or early in the transmission season.
ACKNOWLEDGMENTS
Justin Parizo is thankful to the United States International Education Fund, the Fulbright Fellowship, and the University of California, San Francisco, Resource Allocation Program for Trainees for funding this research and to National Institute of Malaria Research (ICMR), Delhi, India, for providing the facilities to Fellow. We thank the Ahmedabad Municipal Corporation, particularly, V. K. Kohli, Entomologist, for providing the malaria incidence data utilized in this study.
Footnotes
Financial support: This study was supported by the United States International Education Fund, the Fulbright Fellowship, and the University of California, San Francisco, Resource Allocation Program for Trainees.
Authors' addresses: Justin Parizo, Department of Medicine, Stanford University Medical Center, Palo Alto, CA, E-mail: jparizo@stanford.edu. Hugh J. W. Sturrock, Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA, E-mail: hugh.sturrock@ucsf.edu. Ramesh C. Dhiman, National Institute of Malaria Research (Indian Council of Medical Research), New Delhi, India, E-mail: r.c.dhiman@gmail.com. Bryan Greenhouse, Division of Infectious Diseases, Department of Medicine, University of California San Francisco, San Francisco, CA, E-mail: bryan.greenhouse@ucsf.edu.
References
- 1.United Nations United Nations Department of Economics and Social Affairs, Population Division. 2012. http://www.un.org/en/development/desa/population/ Available at. Accessed April 2012.
- 2.Fry S, Cousins B, Olivola K. Health of Children Living in Urban Slums in Asia and the Near East: Review of Existing Literature and Data. Arlington, VA: Environmental Health Project; 2002. [Google Scholar]
- 3.Fobil JN, Kraemer A, Meyer CG, May J. Neighborhood urban environmental quality conditions are likely to drive malaria and diarrhea mortality in Accra, Ghana. J Environ Public Health. 2011;2011:e484010. doi: 10.1155/2011/484010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keiser J, Utzinger J, Caldas de Castro M, Smith TA, Tanner M, Singer BH. Urbanization in sub-Saharan Africa and implication for malaria control. Am J Trop Med Hyg. 2004;71:118–127. [PubMed] [Google Scholar]
- 5.Peterson I, Borrell LN, El-Sadr W, Teklehaimanot A. A temporal-spatial analysis of malaria transmission in Adama, Ethiopia. Am J Trop Med Hyg. 2009;81:944–949. doi: 10.4269/ajtmh.2009.08-0662. [DOI] [PubMed] [Google Scholar]
- 6.Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. Am J Trop Med Hyg. 2003;68:169–176. [PubMed] [Google Scholar]
- 7.Machault V, Vignolles C, Pagés F, Gadiaga L, Gaye A, Sokhna C, Trape JF, Lacaux JP, Rogier C. Spatial heterogeneity and temporal evolution of malaria transmission risk in Dakar, Senegal, according to remotely sensed environmental data. Malar J. 2010;9:252. doi: 10.1186/1475-2875-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye C. Health and urban living. Science. 2008;319:766–769. doi: 10.1126/science.1150198. [DOI] [PubMed] [Google Scholar]
- 9.Liu JX, Bousema T, Zelman B, Gasase S, Hashim R, Maxwell C, Chandramohan D, Gosling R. Is housing quality associated with malaria incidence among young children and mosquito vector numbers? Evidence from Korogwe, Tanzania. PLoS One. 2014;9:e87358. doi: 10.1371/journal.pone.0087358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldas de Castro M, Yamagata Y, Mtasiwa D, Tanner M, Utzinger J, Keiser J, Signer BH. Integrated urban malaria control: a case study in Dar es Salaam, Tanzania. Am J Trop Med Hyg. 2004;71:103–117. [PubMed] [Google Scholar]
- 11.Chinery WA. Effects of ecological changes on the malaria vectors Anopheles funestus and the Anopheles gambiae complex of mosquitoes in Accra, Ghana. J Trop Med Hyg. 1984;87:75–81. [PubMed] [Google Scholar]
- 12.Favia G, Lanfrancotti A, Spanos L, Sidén-Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 13.Yadav RS, Bhatt RM, Kohli VK, Sharma VP. The burden of malaria in Ahmedabad city, India: a retrospective analysis of reported cases and deaths. Ann Trop Med Parasitol. 2003;97:793–802. doi: 10.1179/000349803225002642. [DOI] [PubMed] [Google Scholar]
- 14.Government of India, Ministry of Home Affairs Ahmedabad City Census 2011 Data. 2013. http://www.census2011.co.in/census/city/314-ahmedabad.html Available at. Accessed April 10, 2013.
- 15.Meteorologic Department . Ahmedabad. India: Extreme Weather Data. 2012. http://www.imd.gov.in/section/climate/extreme/ahmedabad2.htm Available at. Accessed April 2012. [Google Scholar]
- 16.Ahmedabad Municipal Corporation Ahmedabad City Map. 2011. http://ahmedabadcity.gov.in/portal/index.jsp Available at. Accessed February 15, 2013.
- 17.Google Inc Google Earth Engine. 2014. https://earthengine.google.org Available at. Accessed March 3, 2014.
- 18.Donnelly MJ, McCall PJ, Lengeler C, Bates I, D'Alessandro U, Barnish G, Konradsen F, Klinkenberg E, Townson H, Trape JF, Hastings IM, Mutero C. Malaria and urbanization in sub-Saharan Africa. Malar J. 2005;4:12. doi: 10.1186/1475-2875-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulldorf Martin. SaTScan User Guide. 2015. http://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download Available at. Accessed August 2015.
- 20.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11. [Google Scholar]
- 21.Yan J. geepack: yet another package for generalized estimating equations. R-News. 2002;2/3:12–14. [Google Scholar]
- 22.Yan J, Fine JP. Estimating equations for association structures. Stat Med. 2004;23:859–880. doi: 10.1002/sim.1650. [DOI] [PubMed] [Google Scholar]
- 23.Census 2011 Ahmedabad Urban Region: Ahmedabad Literacy Rate 2011. 2011 http://www.census2011.co.in/census/metropolitan/275-ahmedabad.html Available at. Accessed March 3, 2013. [Google Scholar]
- 24.Baeza A, Bouma MJ, Dobson A, Dhiman RC, Srivastava HC, Pascual M. Climate forcing and desert malaria: the effect of irrigation. Malar J. 2011;10:190. doi: 10.1186/1475-2875-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-Saharan Africa: a systematic review. J Trop Med. 2012;2012:819563. doi: 10.1155/2012/819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klinkenberg E, McCall PJ, Wilson MD, Akoto AO, Amerasinghe FP, Bates I, Verhoeff FH, Barnish G, Donnelly MJ. Urban malaria and anaemia in children: a cross-sectional survey in two cities of Ghana. Trop Med Int Health. 2006;11:578–588. doi: 10.1111/j.1365-3156.2006.01609.x. [DOI] [PubMed] [Google Scholar]