Abstract
Schistosomiasis is the second most significant parasitic disease in children in several African countries. For this purpose, the “Programme National de Lutte contre les Bilharzioses” (PNLB) was developed in partnership with the World Health Organization (WHO) to control this disease in Senegal. However, geographic isolation of Bedik ethnic groups challenged implementation of the key elements of the schistosomiasis program in eastern Senegal, and therefore, a hospital was established in Ninefesha to improve access to health care as well as laboratory support for this population. The program we have implemented from 2008 in partnership with the PNLB/WHO involved campaigns to 1) evaluate schistosomiasis prevalence in children of 53 villages around Ninefesha hospital, 2) perform a mass drug administration following the protocol established by the PNLB in school-aged children, 3) monitor annual prevalence, 4) implement health education campaigns, and 5) oversee the building of latrines. This campaign led to a drop in schistosomiasis prevalence but highlighted that sustainable schistosomiasis control by praziquantel treatment, awareness of the use of latrines, and inhabitants' voluntary commitment to the program are crucial to improve Schistosoma elimination. Moreover, this study revealed that preschool-aged children, for whom praziquantel was not recommended until 2014 in Senegal, constituted a significant reservoir for the parasite.
Introduction
Schistosomiasis—also named bilharzia—is a major neglected tropical disease that affects more than 240 million people around the world, 90% of whom live in Africa.1 Approximately 800 million people are at risk, and the disease burden is thought to exceed 70 million disability-adjusted life years.2 Schistosomiasis is a water-borne disease caused by trematode parasites belonging to the genus Schistosoma, the two main species being Schistosoma mansoni (agent of intestinal bilharzia) and Schistosoma haematobium (agent of urogenital bilharzia). While urogenital schistosomiasis can easily be detected due to its classic hematuria, intestinal schistosomiasis is often ignored, as its onset is gradual and signs are more difficult to detect.3
Blocking the Schistosoma life cycle (through the prevention of freshwater contamination, elimination of host snails, and prevention of human contact with infected water) and treating infected humans with praziquantel constitute the key strategies of schistosomiasis control.4 For this purpose, many countries have launched large-scale control programs in partnership with the World Health Organization (WHO), facilitated by mass drug administration (MDA) of praziquantel (provided by the WHO) for school-aged children (from 6 to 14 years of age) to promote child health and development in Africa. Actually, studies have previously shown that children are particularly infected by Schistosoma (approximately 35% of infected people were school-aged children) and that they constitute the reservoir of the disease.1 In endemic regions, 60–80% of school-aged children can be infected, and teenagers constitute the age group that excretes the greatest quantity of eggs.4 Preschool-aged children (PSAC) had been excluded from mass treatment programs for control of schistosomiasis because of the limited documentation on the safety of praziquantel in this age group and lack of an appropriate formulation.5 However, during an informal meeting in Geneva, Switzerland, the WHO presented new evidence to consider these children as a high-risk group in endemic areas and to encourage changes in praziquantel formal licensing or off-label use in treatment of PSAC.6 However, these new recommendations have to be accepted at the national level.
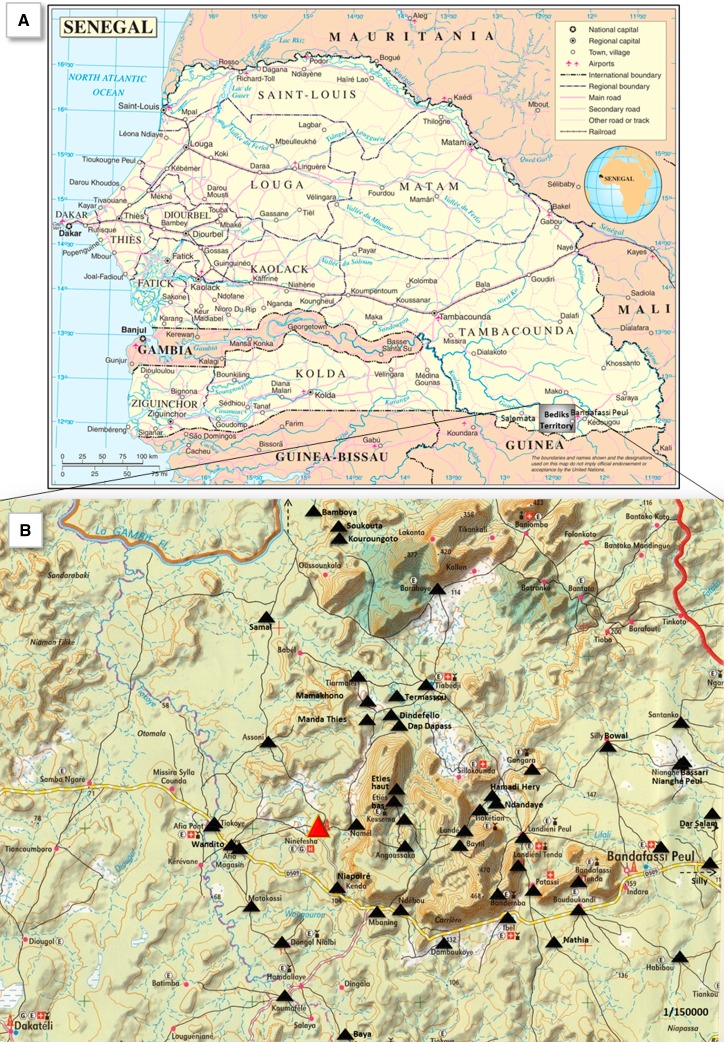
Concerning Senegal, schistosomiasis spread out in the northern part of this country since the construction of the Diama Dam in Richard-Toll in 1988, and today Senegal is considered as an endemic country for this disease.7,8 To fight against this pathology, Senegal, in agreement and in partnership with the WHO (who provided praziquantel), implemented a national control program against schistosomiasis called “Programme National de Lutte contre les Bilharzioses” (PNLB) in 1999. However, some parts of the country in eastern Senegal are really difficult to reach. Thus, to put an end to the isolation of several Bedik ethnic groups living on hills in steep relief, a hospital was built in Ninefesha (located in Kedougou District of eastern Senegal) (Figure 1A ) in 2002. The hospital parasitology laboratory establishment allowed the accidental discovery of S. mansoni eggs in stools of children coming from isolated villages around Ninefesha. A preliminary investigation conducted in 2006 showed that in 22 villages around Ninefesha, 44% of the school-aged children were positive for S. mansoni with prevalence reaching 100% in Assoni village. This finding confirmed the results of Larivière and others who had pointed out that there had been a focus of intestinal schistosomiasis near Salemata (eastern Senegal) since 1964.9 To realize a larger and in-depth study of the prevalence of this disease around Ninefesha, a campaign designed and funded by the French society “Kaïcedrat” and Normandy University was started in 2008 in agreement with the Senegalese health authorities and in partnership with the PNLB/WHO in 53 Bedik and Peul villages (Figure 1B). The main objectives were to 1) monitor the course of schistosomiasis prevalence in children between 6 and 14 years of age after treatment with praziquantel, 2) launch education campaigns for disease prevention (by educating populations about Schistosoma life cycle, by building latrines and raising awareness of use of latrines to interrupt the life cycle), and 3) study the role of reservoir played by Senegalese children under 6 years of age who could not benefit from praziquantel treatment until 2014 by assessing prevalence of schistosomiasis in this group of age.
Figure 1.
Location of the villages included in the program. (A) Bedik's territory was depicted on Senegal map (hatched square). (B) Location of all the 53 villages around Ninefesha area in Kedougou District.
Materials and Methods
Selection of villages.
The villages where our study was to take place were selected based on three criteria: the existence of at least one case of intestinal or urogenital schistosomiasis diagnosed at the Ninefesha hospital in 2006, the presence of a school, and close proximity (less than 6 km) of the village to a water supply system that remained in operation during the dry season and could constitute a long-lasting biotope for molluscs. In the vicinity of Ninefesha, 53 villages were selected, accounting for a population of about 9,000 people (Figure 1). It should be noted that it is extremely difficult—sometimes even impossible—to get access to these villages during the rainy season.
Assoni village (660 inhabitants) was found to have the strongest S. mansoni prevalence (100%) before praziquantel mass treatment implemented by PNLB/WHO in 2008. It was then decided to assess the prevalence of this disease not only in school-aged children but also in PSAC that had strong probability to be contaminated as well.
Organizational structure of the program.
The study was designed and funded by the French society “Kaïcedrat” and Normandy University. The head physician of the Ninefesha hospital was charged to implement this project in the 53 villages. Five health workers were trained by the head physician and provided with a motorcycle for the collection of stools and urine samples. They had to ensure education campaigns in partnership with the head physician and to supervise the building of latrines. A technician was trained by the head physician to perform an annual monitoring of schistosomiasis prevalence.
Schistosomiasis prevalence monitoring in children aged 6–14 years and treatment.
Prevalence monitoring.
Prevalence assessment was realized during the dry season (January–April) because tracks were more passable. From 2009 to 2014, schistosomiasis prevalence was determined in the 53 villages in one-third of all the children aged 6–14 years, whether they attended school or not and chosen at random. Stool and urine samples of children were collected from their household, and two direct microscopic examinations per stool were performed to reveal the presence of S. mansoni eggs. To detect the presence of S. haematobium eggs, microscopic examination of the sediment of 10 mL urine after detection of hematuria using a urine test strip was carried out.
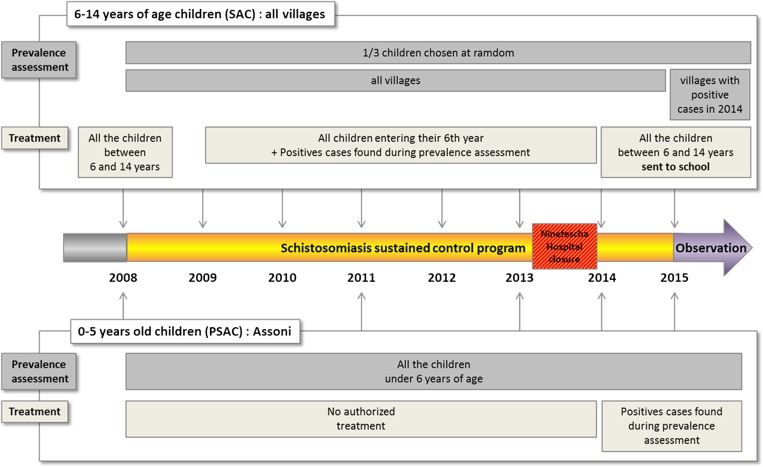
Since the beginning of 2015, we have been carrying out an observation program that consists of monitoring schistosomiasis prevalence in children sent to school or not, but only in the villages that were positive in 2014 (Figure 2 ).
Figure 2.
Scheme of the study. For each year, the monitoring of schistosomiasis prevalence and the treatment program were depicted for children 6–14 years of age and for children under 6 years of age in Assoni village.
Treatment.
In 2008, in total 3,324 children (aged 6–14 years, attended school or not) of the 53 villages were treated with praziquantel (40 mg/kg, provided by the WHO). The work of the mobile team led by the head physician was facilitated through the use of dose poles (as those developed by the WHO).
During 2009–2013, all of the children entering their sixth year (whether they attended school or not) were systematically treated, as well as the children 6–14 years of age (sent to school or not), who were found positive during the annual monitoring of the prevalence phase.
Since the Ninefesha hospital closed in July 2013, and as it was extremely difficult to reach some villages, only the children who can easily be supervised, that is, children aged 6–14 years attending school (98%), were treated by PNLB in 2014 and 2015 (Figure 2).
Schistosomiasis prevalence monitoring in PSAC and treatment.
Prevalence monitoring.
Prevalence in PSAC (from 0 to over 5 years of age) was only conducted in Assoni as mentioned in the “Organizational structure of the program” section. For this purpose, the stools of all the PSAC living in that village were examined, and the rate of intestinal schistosomiasis cases in different age groups (0–2, 2–4, and 4–5 years) was established in 2008, 2011, and from 2013 to 2015. Assessing prevalence in infants was a really difficult task. Moreover, before 2014, some mothers refused to include their children in the survey as they could not be treated thereafter, explaining why we could not monitor the prevalence in 2009, 2010, and 2012.
Treatment.
PSAC were not allowed to be treated by praziquantel in Senegal until 2014. However, thanks to the WHO authorities in Senegal, the medical regional authorities accepted to treat children under 6 years of age but only on an individual basis and under PNLB/WHO supervision. The treatment of PSAC was realized by a nurse who weighed the children to administer the right dose of praziquantel. The tablet was cut and crushed in drinkable water and the children were kept under observation for 30 minutes to check that the drug was correctly absorbed. Schedule of PSAC prevalence monitoring and treatment is depicted in Figure 2.
Health education campaigns.
Schistosomiasis life cycle illustrations.
During these meetings, our team used card games illustrating the life cycle of the parasite and short video reports about the way Schistosoma eggs hatch. Villagers were also shown the freshwater snails collected from the contaminated sites and were told about the release of cercariae. This health education campaign has to gather as many villagers as possible around the civil and religious chiefs to ascertain everybody especially mothers who bathed their children in the rivers was aware of the risks.
Latrine building.
Building pit latrines is the easiest way to interrupt the life cycle of the parasite.10 The model selected consisted of a pit measuring 2 × 2 × 2.5 m and covered with a reinforced concrete slab, which was poured by a bricklayer. The villagers were paid for digging the pit, but only after they had built and installed a fence around the latrine, thus making it possible to use it day and night.
Ethical guidelines.
The study protocol was approved by the WHO Neglected Tropical Diseases Inter-Country Support Team for West Africa, PNLB, Health and Social Action Ministry in Senegal. At the onset of the study, regional director of health sector, village authorities, and local community members were sensitized in detail about the objectives of the project.
Results
Prevalence of schistosomiasis in children aged 6–14 years.
Of the stool and urine samples, 1,108 were collected in 2009–2011, 1,225 in 2012, 1,047 in 2013, and 1,142 in 2014 (Figure 2).
Schistosoma mansoni prevalence.
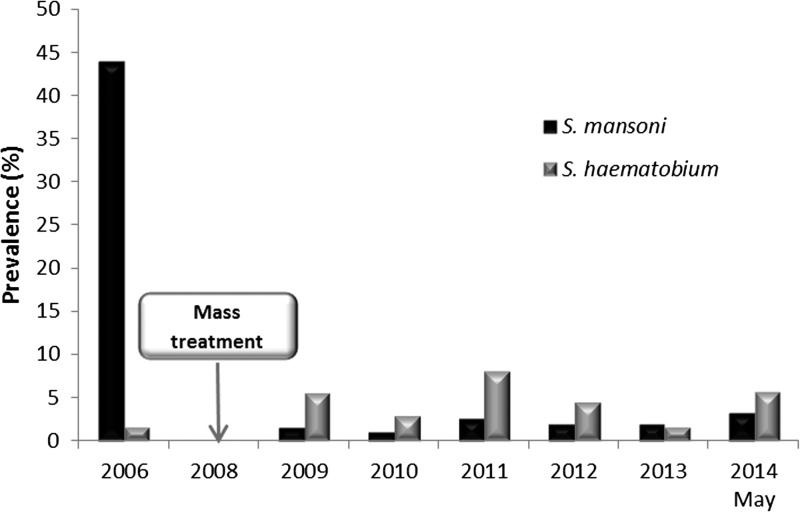
In 2009, namely a year after initiating mass treatment, we observed that the average prevalence for S. mansoni cases had gone down from 44% (in the pre-survey conducted in 2006) to 1.35%, to remain stable at a low rate over the following years. However, this prevalence slightly increased to 3.22% in 2014 (Figure 3 ).
Figure 3.
Average prevalence of Schistosoma mansoni and Schistosoma haematobium in children 6–14 years of age. For each year, the prevalence of S. mansoni (black bars) and S. haematobium (grey bars) was evaluated in school-aged children of the 53 villages.
If we focus on the prevalence for each village, 37/53 villages were totally exempted of S. mansoni and no positive case was detected in these villages across the study period.
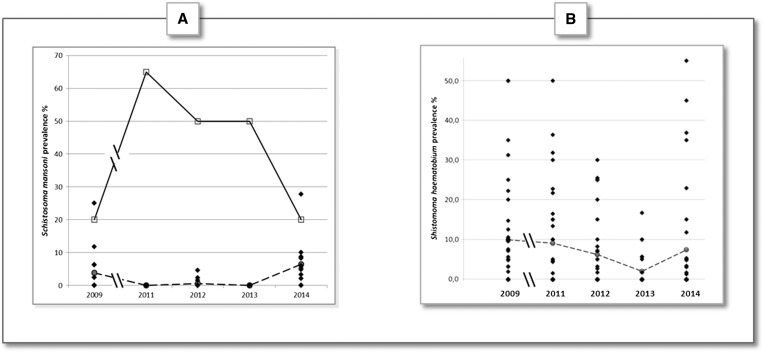
If we focus on the 16 villages that presented positive cases at least once between 2009 and 2014 (Figure 4A ), the results showed that the strongest prevalence was observed in Assoni. Actually, S. mansoni prevalence in this village fluctuated between 2009 and 2014 (with a peak in 2011: 65%) but never dropped below 20% (prevalence reported in 2009 and 2014) (Figure 4A, continuous line).
Figure 4.
Schistosoma mansoni and Schistosoma haematobium prevalence course of reported in villages that have at least one positive case between 2009 and 2014. (A) Schistosoma mansoni prevalence was assessed in each village from 2009 to 2014. Only the 16/53 villages which had positive at least once between 2009 and 2014 are represented. The continuous line linking empty squares represents S. mansoni prevalence in Assoni only. The dashed line linking the gray spots represents the average prevalence of S. mansoni in 15 other villages (i.e., excepted Assoni). (B) Schistosoma haematobium prevalence was assessed in each village from 2009 to 2014. Only 35/53 villages that had at least one positive case between 2009 and 2014 are represented. The dashed line linking the gray spots represents the average prevalence for all the 35 villages.
Among the 15 other villages, in 2009, six villages had positive cases with prevalence ranging from 2.38% in Bandafassi to 25% in Silly Bowal village (Supplemental Table 1). However, for all these 15 villages, S. mansoni prevalence dropped down the years after, and no positive case was observable in 2011 and 2013 in any of the villages tested. Only a low prevalence was observed in 2012 in Bandemba (1.45%), Hamadi Hery (2.33%), and Matakossi (4.55%), leading to an average prevalence of 0.6% for these 15 villages (Figure 4A, dashed line, and Supplemental Table 1). Nonetheless, in 2014, an increase in S. mansoni average prevalence was observed (6.5%) and 11/15 villages had positive cases with the highest prevalence for Nianghe Peul (27.8%). Moreover, among these 11 villages, eight (including Nianghe Peul) had never had cases of S. mansoni before 2014 (Supplemental Table 1).
Schistosoma haematobium prevalence.
Concerning S. haematobium, the average prevalence in the 53 villages remained low across the study period with a maximum of 7.9% in 2011 (Figure 3).
If we focus on the prevalence for each village, 18/53 villages were totally exempted of S. haematobium, and no positive cases were detected in these villages across the study period.
If we focus on the 35/53 villages that presented positive cases at least once between 2009 and 2014, the results showed that the prevalence varied a lot between these villages and for the years tested (Figure 4B). In general, S. haematobium average prevalence as well as the number of villages with positive cases decreased from 2009 (8.7% [20/35] villages with positive cases) to 2013 (1.9% [7/35] villages with positive cases). Moreover, the maximal prevalence reported across this period decreased from 50% to 16.7% (both obtained in the “Silly” village) (Figure 4B and Supplemental Table 2). However, in 2014, average prevalence as well as the number of villages with positive cases increased (7.4% [14/35] villages with positive cases). It is noteworthy that the highest prevalence ever reported during this study period was obtained in 2014 (55% in Nathia) (Figure 4B and Supplemental Table 2).
Prevalence in PSAC in Assoni.
The aim of our program was to assess the prevalence of S. mansoni in this age group, which reflects the impact of health education campaigns in the villages (described in Materials and Methods section) as this group of children was not allowed to be treated with praziquantel until 2014.
Only S. mansoni cases were reported in all of the PSAC in Assoni with a prevalence of 78% in children under 5 years of age (64/82 children) and 64% in children less than 2 years of age (9/14 children) in 2008 (Table 1). After health education campaigns led every year, average prevalence fell but remained significant (18% [16/88] children) in May 2014 (Table 1). It is noteworthy that the 4- to 5-year-old children group appeared to be the most infected across the study period.
Table 1.
Prevalence of Schistosoma mansoni in preschool-aged children 0–5 years of age in the Assoni village in 2008, 2009, 2011, 2013, 2014, and 2015
| Age (years) | n | Positive cases | Prevalence (%) |
|---|---|---|---|
| 2008 | |||
| 0–2 | 14 | 9 | 64.3 |
| 2–4 | 37 | 25 | 67.5 |
| 4–5 | 31 | 30 | 96.8 |
| Total | 82 | 64 | 78.0 |
| 2009 | |||
| 0–2 | 11 | 5 | 45.0 |
| 2–4 | 28 | 18 | 64.0 |
| 4–5 | 22 | 15 | 68.0 |
| Total | 61 | 42 | 59.0 |
| 2011 | |||
| 0–2 | 8 | 3 | 37.5 |
| 2–4 | 18 | 6 | 33.3 |
| 4–5 | 12 | 9 | 75.0 |
| Total | 38 | 18 | 47.4 |
| 0–2 | 36 | 3 | 8.3 |
| 2–4 | 36 | 7 | 19.4 |
| 4–5 | 16 | 6 | 37.5 |
| Total | 88 | 16 | 18.2 |
| November 2014 | |||
| 0–2 | 25 | 1 | 4.0 |
| 2–4 | 37 | 2 | 5.4 |
| 4–5 | 21 | 5 | 23.8 |
| Total | 83 | 8 | 9.6 |
| December 2015 | |||
| 0–2 | 53 | 6 | 11.3 |
| 2–4 | 31 | 1 | 3.2 |
| 4–5 | 24 | 7 | 29.0 |
| Total | 108 | 14 | 12.9 |
“N” represents the number of children in each group of age and “Positive cases” represent the number of carriers of S. mansoni in each group of age.
As praziquantel treatment was authorized for PSAC in 2014 in Senegal, all the 16 children found positive in May 2014 were then treated. New samples of stools and urines were collected in November 2014. Results showed that among the 83 children under 5 years of age living in Assoni, eight (10%) were still infected with S. mansoni (Table 1). It should be underlined that among these eight cases, two children between 4 and 5 years of age had been found positive and treated in May and got reinfected between May and November 2014. These children were treated again in December 2014, however the S. mansoni prevalence assessment in December 2015 showed that this disease was not eliminated and that 14/108 children (12.9%) were still infected (Table 1).
Building and use of latrines by inhabitants.
Before we started our program, there were no latrines in the villages, and the only latrines that could be found were in the schools of Ibel and Bandafassi. These latrines were made of concrete blocks with a corrugated iron roof and inadequate waste pipes (Figure 5A ). This standard eastern-Senegalese-school-latrine model was completely rejected and children preferred urinating and defecating in the backwaters nearby. Considering the high schistosomiasis prevalence in this area, we decided to implement latrines in the 53 villages. To be effective, the latrines model has to be accepted by the population, and the villagers that we worked with had strong opinions about the different types of latrines—more or less sophisticated—that we presented them during our first meetings. This made it clear that it was necessary for the concerned villagers to reach a consensus and voice their opinion. In the area where we operated, they all agreed quickly on the model described in section Materials and Methods: a pit measuring 2 × 2 × 2.5 m and covered with a reinforced concrete slab, which was poured by a bricklayer. A fence around the latrine was also added, thus making it possible to use it day and night (Figure 5B). The reason why villages are only progressively being equipped with latrines is that it is necessary to raise specific funds to finance such a project. By deciding to build one latrine per 10 inhabitants, it meant that 900 latrines had to be financed. So far, 757 latrines have been constructed, at a price of 100 euros per unit (Table 2).
Figure 5.
Latrine model and building. (A) Standard eastern-Senegalese-school-latrine model rejected by school-aged children was made with concrete blocks with a corrugated iron roof and inadequate waste pipes. (B) Latrine model accepted by inhabitants was made of a pit covered with a reinforced concrete slab, which was poured by a bricklayer. A fence around the latrine makes it possible to use it day and night.
Table 2.
Number of latrines built each year
| Year | Number of latrines built |
|---|---|
| 2010 | 86 |
| 2011 | 171 |
| 2012 | 192 |
| 2013 | 208 |
| 2014 | 78 |
| 2015 | 22 |
| Total | 757 |
Discussion
Intestinal schistosomiasis in an insidious disease and is a chronic public health problem in Africa. The WHO Regional Office for Africa has developed a strategic plan for schistosomiasis for the period 2011–2020, with the goal of eliminating schistosomiasis as a public health problem and to interrupt transmission, where possible.11 The results of our study showed that S. mansoni and S. haematobium are endemic in ethnic group villages around Ninefescha. Schistosoma haematobium distribution is more widespread than S. mansoni; however, its average prevalence among the 53 villages has always been low. Larivière had pointed out that there was a focus of intestinal schistosomiasis in Salemata (eastern Senegal), but no study had ever been performed in the isolated villages that we decided to include in our program.9 Prevalence before the start of the study was around 44%, a percentage also found in other studies, such as in Uganda in the “Schistosomiasis in Mothers and Infants” cohort.12 The strong overall decrease in schistosomiasis infestation rates in children aged 6–14 years highlights that the control program has a real, positive impact and that it should therefore be continued.
However, if we look at the results in each village, it can be seen that in Assoni, S. mansoni prevalence remained very high and did not drop below 20%. This could be explained because praziquantel does not kill immature Schistosoma and cannot prevent reinfection; its efficacy had therefore only temporary effect on transmission, and treatment has to be backed up by constant health education campaigns.13
To respond to this point, we organized and took part in health education campaigns to raise awareness of the ethnic groups about the risks of contaminated waters, and we began building latrines in the 53 villages.
As for avoiding contaminated waters, inhabitants tried to change their behaviors, however many circumstances led to behavioral drifts. For example, Assoni village lies over a great distance along the backwaters, which could explain the disappointing results obtained in that village only. At the entrance to the village, a large concrete footbridge was built in 2010 to get access to the fresh water well and thus bypass the infested backwaters. However, the school that was initially located near the footbridge was moved 1.5 km away. The fact that the only footbridge is located far from the school and the center of the village accounts for the many behavioral drifts of the children. In the other villages, it was also difficult to change behaviors: for example, Afia Pont and Nathia are both fishermen villages, which could explain the permanent contact of the population with infected water and the residual prevalence of schistosomiasis. Finally, in the Afia Magasin village, the well drilling was out of order, leading the inhabitants to return to the river. Moreover, another very important point is that the Senegalese population is very mobile and children who do not go to school travel a lot between the different villages to see their relatives. This leads them to be in contact with treated children, to indirectly infect them and thereby spread the disease.
As for use of pit latrines, when the conditions were met, they were frequently used and regularly kept clean, and the villagers seem to appreciate this improved comfort and recognize it as a tool to preserve the health of their children (fewer children with “big bellies”). They were willing to fully cooperate, and demand on their part was unanimously high.
A clear “praziquantel treatment gap” was observed when looking at the PSAC group, as this drug is not licensed for children under 6 years of age and infection can occur during the first year of life. The reasons are complex and include the absence of appropriate pediatric formulation, the uncertainties in levels of exposure of this age group to infected water sources, and unknown safety of praziquantel.14 To evaluate the prevalence of schistosomiasis in PSAC and the safety of praziquantel treatment in this age group, studies were led in Mali, Sudan, and Zimbabwe. During an informal meeting in Geneva, the WHO presented new evidence to consider these children as a high-risk group in endemic areas and to encourage changes in praziquantel formal licensing or off-label use in treatment of PSAC.6 For this purpose, infected PSAC should be provided with crushed tablets of praziquantel, a treatment that demonstrated to be safe for this age group.6,15 The investigation led in Assoni before 2014, that is, before praziquantel treatment was authorized for this age group in Senegal (A. F. Gabrielli, personal communication) highlighted the fact that PSAC constituted a reservoir of schistosomiasis and were contaminated at an early age, before 2 years of age. The prevalence rate we found was comparable with that found by Sousa-Figueiredo, who described that 62.3% of PSAC were infected with S. mansoni.12 Our results are also in line with several studies showing that children under 2 years of age have active infection with this helminth.16–19
Because of the efforts of the WHO authorities in Senegal, the medical regional authorities accepted to treat children under 6 years of age but only on an individual basis and under PNLB supervision in 2014. The study pointed out that these children were very difficult to treat and get reinfected very rapidly. Cases of reinfection were also observed in the study carried out by Stothard in Uganda, who found that even a 6-month period after two praziquantel administrations had little impact in decreasing prevalence.16 Moreover, a slight increase in S. mansoni prevalence was observed between 2014 and 2015. A possible explanation is that the backwaters are the main source of water used by mothers to bathe their children, which is how they get infested in the first place. These children, who are infected by the parasite, are a source of continuous reinfestation for the community and are therefore those who are likely to benefit the most from the program.18,20,21
It is difficult to determine the extent to which the treatment is effective in controlling the disease, and what the impact of the currently ongoing health education campaigns and construction of latrines program really is. It is therefore necessary to continue running health and hygiene programs for the village inhabitants because the role of sanitation and hygiene is crucial to interrupt Schistosoma life cycle.22 It seems particularly important to make mothers of infants understand why they have to use the safe fresh water from the local wells to bathe them, and not the contaminated water of the backwaters.
As mentioned above, the Ninefesha hospital closed in July 2013. As it was extremely difficult to reach some isolated villages, only the children who can easily be supervised, that is, those aged 6–14 years, attending school, were treated by PNLB since 2014.
However, as described previously, children who do not go to school were not offered this program, and therefore, their mobility could favor the spread of the disease. Moreover, as praziquantel in now administered by the teacher and not by a health worker aware of the serious risks of schistosomiasis, this could lead to a lack of compliance from the children, and praziquantel administration should be carefully monitored as also suggested in the study of Hodges and others conducted in Sierra Leone.23 It is therefore possible that for all these reasons, schistosomiasis was not eradicated in these villages and that this could explain why in our study schistosomiasis prevalence seemed to increase in 2014 especially in Nianghe Peul village. Reemergence of S. mansoni and S. haematobium after treatment in high-endemicity areas has previously been reported in Ivory Coast and Niger.24,25 An increase of S. mansoni prevalence was also observed in Senegal 10 months after praziquantel administration, in Mali after four rounds of praziquantel treatment, and in Ivory Coast 1 year after MDA.26–28
Since the beginning of 2015, we have been carrying out an observation program that monitors the schistosomiasis prevalence in one-third of children 6–14 years of age, whether they attend school or not, but only in the villages that had positive cases in 2014. As for PSAC, prevalence is still monitored in all the children less than 6 years of age in Assoni and only children positive for schistosomiasis are treated. We hope that the presence of a permanent and efficient health program to ensure the distribution of praziquantel to the local population along with the supervision of the activity of health workers, the construction of latrines to interrupt Schistosoma life cycle, the creation of footbridges, and the use of wells will make it possible to stop the spread of schistosomiasis in this part of the country.
Conclusion
The results of the current study showed that schistosomiasis is endemic in isolated villages of ethnic groups in eastern Senegal. Our intervention had an impact in reducing the prevalence of S. mansoni and S. haematobium. However, for sustainable schistosomiasis control in areas where transmission is high, it is crucial to treat children and PSAC and to engage the inhabitants in construction of latrines as well as hygiene behavioral changes so as to improve village-level sanitation and schistosomiasis elimination.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of “Le Kaïcedrat” organization.
Footnotes
Financial support: This campaign was supported by “Le Kaïcedrat” and Ministry of Higher Education and Research (French Government) for the microscopic material.
Authors' addresses: Monique N'Diaye, Charlotte Vernet, François Bessin, Dominique Barbier, and Pierre Georges, Parasitology Laboratory, College of Pharmacy, Normandy University, Caen, France, E-mails: monique.ndiaye@unicaen.fr, chachag007@hotmail.com, f.bessin@yahoo.fr, barbier.do@orange.fr, and georoc47@gmail.com. Elhadji M. Dioukhane, Centre de Santé, Kedougou, Senegal, E-mail: assdiokhane@gmail.com. Babacar Ndao, Ninefesha Hospital, Ninefesha, Senegal, E-mail: babacarndao73@hotmail.com. Kemo Diedhiou and Idrissa Talla, Neglected Tropical Diseases, World Health Organization, Dakar, Senegal, E-mails: kdiedhiou@yahoo.fr and idrissatalla@yahoo.fr. Lamine Diawara, Inter-Country Support Team for West Africa, World Health Organization, Ouagadougou, Burkina Faso, E-mail: elddiawara@gmail.com. Patrick Dewavrin, Le Kaïcedrat, Draveil, France, E-mail: patrickdewavrin@wanadoo.fr. Francis Klotz, Ecole du Val-de-Grâce, Paris, France, E-mail: fklotz2008@yahoo.fr.
References
- 1.Lai YS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, Mwinzi P, N'Goran EK, Raso G, Assaré RK, Sacko M, Schur N, Talla I, Tchuenté LA, Touré S, Winkler MS, Utzinger J, Vounatsou P. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15:927–940. doi: 10.1016/S1473-3099(15)00066-3. [DOI] [PubMed] [Google Scholar]
- 2.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 3.Duthe G, Faye SH, Guyavarch E, Arduin P, Kanté AM, Diallo A, Laurent R, Marra A, Pison G. Change of protocol in the verbal autopsy method and measure of malaria mortality in rural areas in Senegal [in French] Bull Soc Pathol Exot. 2010;103:327–332. doi: 10.1007/s13149-010-0078-4. [DOI] [PubMed] [Google Scholar]
- 4.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197–205. doi: 10.1016/j.pt.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Report of a Meeting to Review the Results of Studies on the Treatment of Schistosomiasis in Preschool-Aged Children. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 7.Talla I, Kongs A, Verle P. Preliminary study of the prevalence of human schistosomiasis in Richard-Toll (the Senegal River basin) Trans R Soc Trop Med Hyg. 1992;86:182. doi: 10.1016/0035-9203(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 8.Meurs L, Mbow M, Vereecken K, Menten J, Mboup S, Polman K. Epidemiology of mixed Schistosoma mansoni and Schistosoma haematobium infections in northern Senegal. Int J Parasitol. 2012;42:305–311. doi: 10.1016/j.ijpara.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Larivière M, Diallo S, Ranque P. Existence of foci of bilharziosis due to S. mansoni in the upper Casamance region and in eastern Senegal [in French] Bull Soc Med Afr Noire Lang Fr. 1964;9:288–289. [PubMed] [Google Scholar]
- 10.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Schistosomiasis: Progress Report 2001–2011 and Strategic Plan 2012–2020. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 12.Sousa-Figueiredo JC, Pleasant J, Day M, Betson M, Rollinson D, Montresor A, Kazibwe F, Kabatereine NB, Stothard JR. Treatment of intestinal schistosomiasis in Ugandan preschool children: best diagnosis, treatment efficacy and side-effects, and an extended praziquantel dosing pole. Int Health. 2010;2:103–113. doi: 10.1016/j.inhe.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, Coles G, Tchuem Tchuenté LA, Mbaye A, Engels D. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136:1825–1835. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 14.Mutapi F. Changing policy and practice in the control of pediatric schistosomiasis. Pediatrics. 2015;135:536–544. doi: 10.1542/peds.2014-3189. [DOI] [PubMed] [Google Scholar]
- 15.Mutapi F, Rujeni N, Bourke C, Mitchell K, Appleby L, Nausch N, Midzi N, Mduluza T. Schistosoma haematobium treatment in 1–5 year old children: safety and efficacy of the antihelminthic drug praziquantel. PLoS Negl Trop Dis. 2011;5:e1143. doi: 10.1371/journal.pntd.0001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stothard JR, Sousa-Figueiredo JC, Betson M, Green HK, Seto EY, Garba A, Sacko M, Mutapi F, Vaz Nery S, Amin MA, Mutumba-Nakalembe M, Navaratnam A, Fenwick A, Kabatereine NB, Gabrielli AF, Montresor A. Closing the praziquantel treatment gap: new steps in epidemiological monitoring and control of schistosomiasis in African infants and preschool-aged children. Parasitology. 2011;138:1593–1606. doi: 10.1017/S0031182011001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruganuza DM, Mazigo HD, Waihenya R, Morona D, Mkoji GM. Schistosoma mansoni among pre-school children in Musozi village, Ukerewe Island, north-western-Tanzania: prevalence and associated risk factors. Parasit Vectors. 2015;8:377. doi: 10.1186/s13071-015-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole H, Terlouw DJ, Naunje A, Mzembe K, Stanton M, Betson M, Lalloo DG, Stothard JR. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasit Vectors. 2014;7:153. doi: 10.1186/1756-3305-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulibaly JT, N'Gbesso YK, N'Guessan NA, Winkler MS, Utzinger J, N'Goran EK. Epidemiology of schistosomiasis in two high-risk communities of south Cote d'Ivoire with particular emphasis on pre-school-aged children. Am J Trop Med Hyg. 2013;89:32–41. doi: 10.4269/ajtmh.12-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139:835–841. doi: 10.1017/S0031182012000029. [DOI] [PubMed] [Google Scholar]
- 21.Garba A, Barkire N, Djibo A, Lamine MS, Sofo B, Gouvras AN, Bosqué-Oliva E, Webster JP, Stothard JR, Utzinger J, Fenwick A. Schistosomiasis in infants and preschool-aged children: infection in a single Schistosoma haematobium and a mixed S. haematobium-S. mansoni foci of Niger. Acta Trop. 2010;115:212–219. doi: 10.1016/j.actatropica.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors. 2015;8:156. doi: 10.1186/s13071-015-0766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodges MH, Paye J, Koroma MM, Nyorkor ED, Fofonah I, Zhang Y. High level of Schistosoma mansoni infection in pre-school children in Sierra Leone highlights the need in targeting this age group for praziquantel treatment. Acta Trop. 2012;124:120–125. doi: 10.1016/j.actatropica.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.N'Goran EK, Utzinger J, N'Guessan AN, Müller I, Zamblé K, Lohourignon KL, Traoré M, Sosthène BA, Lengeler C, Tanner M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Cote d'Ivoire. Trop Med Int Health. 2001;6:817–825. doi: 10.1046/j.1365-3156.2001.00785.x. [DOI] [PubMed] [Google Scholar]
- 25.Garba A, Lamine MS, Barkire N, Djibo A, Sofo B, Gouvras AN, Labbo R, Sebangou H, Webster JP, Fenwick A, Utzinger J. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop. 2013;128:334–344. doi: 10.1016/j.actatropica.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Ernould JC, Ba K, Sellin B. Increase of intestinal schistosomiasis after praziquantel treatment in a Schistosoma haematobium and Schistosoma mansoni mixed focus. Acta Trop. 1999;73:143–152. doi: 10.1016/s0001-706x(99)00013-3. [DOI] [PubMed] [Google Scholar]
- 27.Landoure A, Dembele R, Goita S, Kané M, Tuinsma M, Sacko M, Toubali E, French MD, Keita AD, Fenwick A, Traoré MS, Zhang Y. Significantly reduced intensity of infection but persistent prevalence of schistosomiasis in a highly endemic region in Mali after repeated treatment. PLoS Negl Trop Dis. 2012;6:e1774. doi: 10.1371/journal.pntd.0001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assare RK, Tian-Bi YN, Yao PK, N'Guessan NA, Ouattara M, Yapi A, Coulibaly JT, Meïté A, Hürlimann E, Knopp S, Utzinger J, N'Goran EK. Sustaining control of Schistosomiasis mansoni in western Cote d'Ivoire: results from a SCORE study, one year after initial praziquantel administration. PLoS Negl Trop Dis. 2016;10:e0004329. doi: 10.1371/journal.pntd.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.