Abstract
Solitary cysticercus granuloma is a common neuroimaging abnormality in Indian patients with new-onset epilepsy. Calcific transformation of cysticercus granuloma is frequently associated with seizure recurrence. We evaluated predictors of lesion calcification in patients with solitary cysticercus granuloma and new-onset seizures. One hundred twenty-two patients, with new-onset seizures and a solitary cysticercus granuloma of the brain, were enrolled. All patients were clinically and radiologically evaluated and were treated with antiepileptic drug drugs. No patient received albendazole or corticosteroids. The follow-up period was of 1 year. Follow-up computed tomography was performed after 3 and 6 months. In 68 (54.8%) patients, solitary cysticercus granuloma had transformed into a calcified lesion. On logistic regression analysis, moderate-to-severe edema was a significant factor that predicted calcific transformation of the cysticercus granuloma (odds ratio: 3.325; 95% confidence interval: 1.502–7.362). During 1 year of follow-up, 19 (15.6%) patients experienced seizure recurrence. In 16 patients with seizure recurrence, cysticercus granuloma had transformed in to a calcified lesion. In conclusion, in solitary cysticercus granuloma, calcification of the lesion can be predicted if larger amount of perilesional edema is present. Calcification of the granuloma significantly predicts seizure recurrence.
Introduction
Neurocysticercosis is the most common parasitic infection of the central nervous system caused by the larvae of the human tapeworm Taenia solium. Neurocysticercosis is a leading cause of epilepsy in many resource-constrained countries including India.1,2 Solitary cysticercus granuloma is the most frequent form of neurocysticercosis seen in India and it is the commonest neuroimaging abnormality encountered in patients with new-onset seizures.3–6
Natural course of a neurocysticercus lesion in the brain parenchyma passes through four different stages. The “vesicular” stage represents a cystic structure filled with clear fluid having an eccentric scolex and evokes no demonstrable inflammatory reaction. In the next colloidal stage, the osmotic barrier of the cyst wall breaks down resulting in inflammatory changes in the cyst wall as well as in the surrounding brain parenchyma. Subsequently, progressive thickening of the cyst wall, hyaline degeneration, and mineralization of the cyst take place. Decrease in cyst size, mineralization of cystic fluid, and disintegration of the scolex lead to development of a “granular-nodular” stage. The last “calcified” stage represents a healed and completely mineralized cyst.7
Solitary cysticercus granuloma classically represents the colloidal or granular-nodular stage. On neuroimaging, solitary cysticercus granuloma is characterized with a ring or disc-shaped enhancement, a size of lesion less than 20 mm in diameter, and a variable amount of perilesional edema. The scolex is seen as an enhanced or a calcified eccentric dot within the granuloma. In its natural course, solitary cysticercus granuloma can either resolve spontaneously or can transform into a calcified nodule. Calcific transformation of cysticercus granuloma is often associated with seizure recurrence.8–11 In this study, we evaluated the predictors of lesion calcification in patients with solitary cysticercus granuloma and new-onset seizures. We also assessed the predictors of seizure recurrence in these patients.
Materials and Methods
This prospective study was carried out in the Department of Neurology, King George's Medical University, Lucknow, Uttar Pradesh, India, from August 2013 to August 2015. The study was approved by the Institutional Ethics Committee of our university. Written informed consent was obtained from all patients or their legal guardians, prior to the enrolment into the study.
Consecutive patients with new-onset seizures were subjected to contrast-enhanced computed tomography of the brain within 7 days of first seizure. Computed tomography was performed on PHILIPS Brilliance™ CT (64 slice scanner; Philips Medical Systems, The Netherlands) machine and a slice thickness of 0.3 mm was obtained.
Inclusion criteria.
Patients presenting within 7 days of first seizure and demonstrating a single ring/disc enhancing lesion of less than 20 mm in diameter, with variable amount of perilesional edema were included in the study. An eccentric nodule was supposed to represent the scolex.12
Exclusion criteria.
The patients with systemic malignancy, systemic tuberculosis, human immunodeficiency virus infection, pregnancy, progressive neurological deficit, raised intracranial tension, and hydrocephalus were excluded. Patients with previous history of seizures and those receiving corticosteroids or albendazole were also excluded.
Patient evaluation.
A detailed clinical history was obtained and neurological examination performed in all included subjects. The laboratory tests such as complete hemogram, erythrocyte sedimentation rate, renal function tests, liver function tests, and enzyme-linked immunosorbent assay for human immunodeficiency virus were performed. Electroencephalography (EEG) was also performed. EEG was done on a 21-channel machine, by using the International 10–20 system of electrode placement. EEG was considered abnormal if epileptiform activities were demonstrated.
All computed tomography records were independently evaluated by two neurologists and imaging findings were recorded with consensus. The site, size, presence or absence of scolex, and stage of the solitary cysticercus granuloma were noted. The perilesional edema, on computed tomography, appeared as a variable amount of hypodensity around the granuloma or the calcified lesion. The perilesional brain edema was categorized into three grades. Mild edema was defined as extension of edema to not more than 2 cm from the margin of the lesion. Moderate edema was defined as extension of edema beyond 2 cm from the margin of the lesion up to one half of the cerebral hemisphere, and severe perilesional brain edema was defined as extension of edema to more than half of the cerebral hemisphere.13 The scolex appeared as a hyperdense/enhancing tiny nodule, within the granuloma.
Ring lesions were characterized by ring-shaped contrast enhancement representing enhancement of cyst wall. Ring lesions characterized the colloidal stage. Disc lesions were characterized by a disc-like homogenous enhancement representing enhancement of cyst contents as well as the wall. Disc lesions described the granular-nodular stage.3,4
Treatment.
No patient was treated with albendazole or corticosteroids. All patients were treated only with antiepileptic drugs. Oxcarbazepine was given in dose of 10–15 mg/kg/day in two divided doses. Clobazam was added if seizure control was not achieved.
Follow-up.
Patients were followed up for 1 year. A follow-up computed tomography was done after 3 and 6 months. Seizure recurrence was defined as occurrence of another seizure at least 2 weeks after the institution of the antiepileptic therapy. Status epilepticus was defined as generalized convulsions exceeding more than 5 minutes in duration or convulsions with no recovery of consciousness between seizures. Serial seizures were defined as multiple seizures occurring within 24 hours. In case of seizure recurrence, patients were advised to report immediately to the investigator-in-charge.
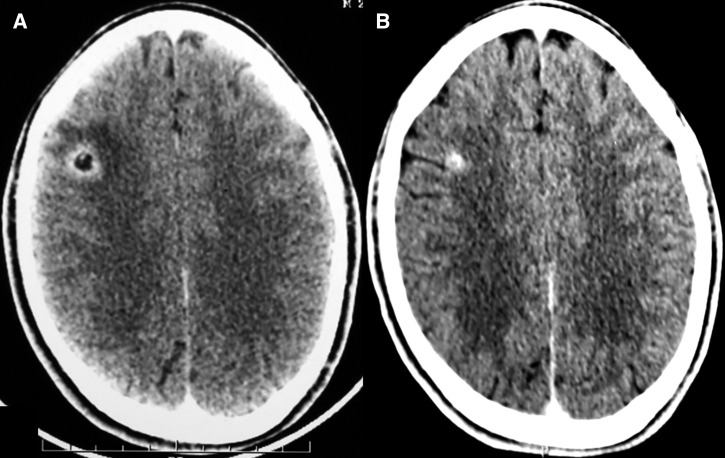
The lesion was considered as “calcified” if the follow-up non-contrast-enhanced computed tomography revealed a hyperdense lesion in place of the solitary cysticercus granuloma (Figure 1 ). The lesion was considered as “resolved” if the follow-up computed tomography was reported normal. Solitary cysticercus granuloma was considered “persistent,” if the contrast-enhancing ring/disc lesion was still visualized.
Figure 1.
Computed tomography of the brain depicts a solitary cysticercus granuloma (A) at baseline and (B) its evolution to a calcified lesion.
Statistical analysis.
The data were analyzed using the SPSS statistical software (version 16.0; SPSS Inc., Chicago, IL) and Microsoft Excel (Microsoft Corp., Redmond, WA). Factors associated with calcification of granuloma were identified by univariate and multivariate analyses. Multivariate analysis was performed by using the stepwise logistic regression model. To determine the predictors of seizure recurrence, seizure recurrence was taken as the dependent variable and all variables found statistically significant on univariate analysis (lesion size, stage of lesion, calcification, and abnormal EEG) were taken as independent variables. To look for predictors of calcification, calcification was kept as the dependent variable and variables found statistically significant on univariate analysis (moderate-to-severe perilesional edema and seizure recurrence) were kept as independent variables. The variables that were analyzed included age, sex, seizure semiology, seizure pattern, initial seizure frequency, seizure recurrence, family history of seizures, history of headache, Todd's palsy, lesion site, size, presence of scolex, and perilesional edema. Data available at 6 months of enrolment were used. A P value of < 0.05 was considered statistically significant and all analyses were two tailed.
Results
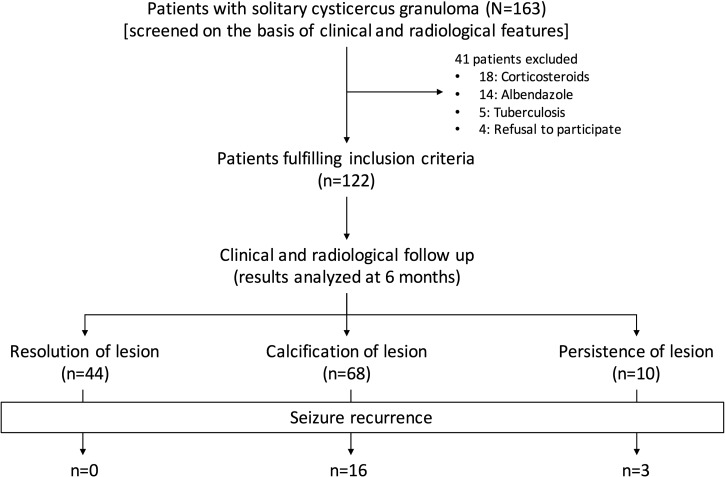
In our study, 163 consecutive patients, fulfilling clinical and neuroimaging criteria for solitary cysticercus granuloma, were screened. Forty-one patients were excluded from the study. Thirty-two patients had already received albendazole and/or corticosteroids and five patients had concomitant systemic tuberculosis. Four patients refused consent. Finally, a total of 122 patients were enrolled (Figure 2 ).
Figure 2.
Algorithm of the study.
Baseline characteristics.
In our study, the mean age (± standard deviation) of patients was 19.91 ± 10.34 (range 5–60) years. There were 76 (62.3%) males. Partial seizure with secondary generalization was the most common type of seizure. Sixty-eight (55.7%) patients presented with serial seizures. Moderate-to-severe perilesional edema was seen in 83 (68%) patients. EEG abnormalities were present in 21 (17.2%) patients. Other baseline characteristics are provided in Table 1.
Table 1.
Clinical and radiological characteristics of patients with solitary cysticercus granuloma
| Serial no. | Variables | No. of patients (N = 122) | Proportion (%) |
|---|---|---|---|
| 1 | Mean age ± SD in years | 19.91 ± 10.34 | – |
| Age ≤ 20 years | 79 | 64.75 | |
| 2 | Sex | ||
| Male | 76 | 62.3 | |
| Female | 46 | 37.7 | |
| 3 | Seizure types | ||
| Focal seizures | 41 | 33.1 | |
| Focal seizures with generalization | 51 | 41.8 | |
| Generalized tonic-clonic seizures | 30 | 24.6 | |
| 4 | Seizure pattern | ||
| Single seizure | 46 | 37.7 | |
| Serial seizures | 68 | 55.7 | |
| Status epilepticus | 8 | 6.6 | |
| 5 | Headache | 35 | 28.7 |
| 6 | Todd's palsy | 10 | 8.2 |
| 7 | EEG abnormality | 21 | 17.2 |
| 8 | Right lobar lesion | 67 | 54.9 |
| Left lobar lesion | 55 | 45.1 | |
| 9 | Lobar involvement | ||
| Frontal | 66 | 54.1 | |
| Parietal | 48 | 39.3 | |
| Temporal | 5 | 4.1 | |
| Occipital | 3 | 2.5 | |
| 10 | Lesion size | ||
| ≤ 1 cm | 70 | 57.4 | |
| > 1 cm | 52 | 42.6 | |
| 11 | Scolex | 49 | 40.2 |
| 12 | Morphology of granuloma | ||
| Ring | 100 | 82.0 | |
| Disc | 22 | 18.0 | |
| 13 | Perilesional edema | ||
| Mild | 39 | 32.0 | |
| Moderate | 81 | 66.4 | |
| Severe | 2 | 1.6 | |
| 14 | Computed tomography after 6 months | ||
| Resolution | 44 | 36.1 | |
| Calcification | 68 | 55.7 | |
| Persistent | 10 | 8.2 | |
EEG = electroencephalography; SD = standard deviation.
Follow-up.
In 68 (55.7%) patients, solitary cysticercus granuloma had transformed into a calcified nodule. The granuloma had resolved completely in 44 (36.1%) patients. The remaining 10 (8.2%) patients either had partial resolution or no resolution of the granuloma.
Predictors of calcification of granuloma.
Univariate analysis revealed that moderate-to-severe edema was significantly associated with calcification of the cysticercus granuloma (odds ratio: 3.325; 95% confidence interval: 1.502–7.362) (Table 2). On multivariate analysis, moderate-to-severe perilesional edema (P = 0.008) emerged as a significant predictor of calcific transformation.
Table 2.
Univariate analysis for evaluation of predictors of calcific transformation of solitary cysticercus granuloma
| Variable | Calcification | P value | Odds ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| No (N = 54) | Yes (N = 68) | Lower | Upper | |||
| Age | 20.04 ± 12.106 | 19.90 ± 9.955 | 0.944 | – | −3.783 | 4.060 |
| Sex | 0.609 | 0.824 | 0.393 | 1.726 | ||
| Male | 35 (64.8%) | 41 (60.3%) | ||||
| Female | 19 (35.2%) | 27 (39.7%) | ||||
| Generalized seizure | 0.271 | 1.527 | 0.717 | 3.252 | ||
| Present | 33 (61.1%) | 48 (70.6%) | ||||
| Absent | 21 (38.9%) | 20 (29.4%) | ||||
| Status epilepticus | 0.076 | 6.082 | 0.725 | 51.044 | ||
| Present | 1 (1.9%) | 7 (10.3%) | ||||
| Absent | 53 (98.1%) | 61 (89.7%) | ||||
| Seizure frequency before inclusion | 0.300 | 1.584 | 0.062 | 3.791 | ||
| < 3 | 44 (81.5%) | 50 (73.5%) | ||||
| > 3 | 10 (18.5%) | 18 (26.5%) | ||||
| Headache | 0.159 | 1.789 | 0.792 | 4.041 | ||
| Present | 12 (22.2%) | 23 (33.8%) | ||||
| Absent | 42 (77.8%) | 45 (66.2%) | ||||
| Todd's palsy | 0.093 | 3.467 | 0.705 | 17.057 | ||
| Present | 2 (3.7%) | 8 (11.8%) | ||||
| Absent | 52 (96.3%) | 60 (88.2%) | ||||
| Family history of seizures | 0.885 | 0.918 | 0.289 | 2.911 | ||
| Present | 6 (11.1%) | 7 (10.3%) | ||||
| Absent | 48 (88.9%) | 61 (89.7%) | ||||
| EEG abnormality | 0.112 | 2.264 | 0.813 | 6.305 | ||
| Present | 6 (11.1%) | 15 (22.1%) | ||||
| Absent | 48 (88.9%) | 53 (77.9%) | ||||
| Seizure recurrence | 0.010 | 5.231 | 1.437 | 19.044 | ||
| Present | 3 (5.6%) | 16 (23.5%) | ||||
| Absent | 51 (94.4%) | 52 (76.5%) | ||||
| Laterality | 0.390 | 1.371 | 0.666 | 2.822 | ||
| Right | 32 (59.3%) | 35 (51.5%) | ||||
| Left | 22 (40.7%) | 33 (48.5%) | ||||
| Lesion size | 0.457 | 1.317 | 0.637 | 2.721 | ||
| ≤ 1 cm | 33 (61.1%) | 37 (54.4%) | ||||
| > 1 cm | 21 (38.9%) | 31 (45.6%) | ||||
| Scolex | 0.626 | 0.834 | 0.403 | 1.728 | ||
| Present | 23 (42.6%) | 26 (38.2%) | ||||
| Absent | 31 (57.4%) | 42 (61.8%) | ||||
| Morphology of granuloma | 0.076 | 2.462 | 0.890 | 6.806 | ||
| Ring | 48 (88.9%) | 52 (76.5%) | ||||
| Disc | 6 (11.1%) | 16 (23.5%) | ||||
| Moderate-to-severe perilesional edema | 0.002 | 3.325 | 1.502 | 7.362 | ||
| Present | 29 (53.7%) | 54 (79.4%) | ||||
| Absent | 25 (46.3%) | 14 (20.6%) | ||||
CI = confidence interval; EEG = electroencephalography. Statistically significant P-values are depicted in bold.
Seizure recurrence.
During 1 year of follow-up, 19 (15.6%) patients experienced seizure recurrence. In all patients with seizure recurrence, follow-up computed tomography was abnormal. In 16 patients, cysticercus granuloma had transformed in to a calcified lesion and in remaining three patients, granuloma was still present. On univariate analysis, in addition to calcific transformation of the granuloma (P = 0.010), significant predictors of seizure recurrence were lesion size greater than 10 mm (P = 0.013), presence of a disc enhancement (P = 0.003), and an abnormal EEG (P = 0.014) (Table 3). On multivariate analysis, in addition to calcified granuloma (P = 0.040), a lesion size greater than 10 mm (P = 0.023), disc enhancement (P = 0.027), and an abnormal EEG (P = 0.026) were significant predictors of seizure recurrence.
Table 3.
Univariate analysis for evaluation of predictors of seizure recurrence in patients with solitary cysticercus granuloma
| Variable | Seizure recurrence | P value | Odds ratio | 95% CI | ||
|---|---|---|---|---|---|---|
| No (N = 103) | Yes (N = 19) | Lower | Upper | |||
| Age | 20.45 ± 11.598 | 17 ± 5.637 | 0.208 | – | –1.948 | 8.849 |
| Sex | 0.667 | 0.804 | 0.297 | 2.174 | ||
| Male | 65 (63.1%) | 11 (57.9%) | ||||
| Female | 38 (36.9%) | 8 (42.1%) | ||||
| Generalized seizures | 0.839 | 1.115 | 0.390 | 3.186 | ||
| Present | 68 (66.0%) | 13 (68.4%) | ||||
| Absent | 35 (34.0%) | 6 (31.6%) | ||||
| Status epilepticus | 0.355 | – | – | – | ||
| Present | 8 (7.8%) | 0 (0.0%) | ||||
| Absent | 95 (92.2%) | 19 (100%) | ||||
| Seizure frequency on presentation | 0.071 | 0.156 | 0.020 | 1.228 | ||
| < 3 | 76 (73.8%) | 18 (94.7%) | ||||
| > 3 | 27 (26.2%) | 1 (5.3%) | ||||
| Headache | 0.050 | 2.665 | 0.976 | 7.276 | ||
| Present | 26 (25.2%) | 9 (47.4%) | ||||
| Absent | 77 (74.8%) | 10 (52.6%) | ||||
| Todd's palsy | 0.612 | 0.580 | 0.069 | 4.866 | ||
| Present | 9 (8.7%) | 1 (5.3%) | ||||
| Absent | 94 (91.3%) | 18 (94.7%) | ||||
| Family history of seizures | 0.430 | 1.744 | 0.432 | 7.036 | ||
| Present | 10 (9.7%) | 3 (15.8%) | ||||
| Absent | 93 (90.3%) | 16 (84.2%) | ||||
| EEG abnormality | 0.014 | 3.708 | 1.248 | 11.021 | ||
| Present | 14 (13.6%) | 7 (36.8%) | ||||
| Absent | 89 (86.4%) | 12 (63.2%) | ||||
| Laterality | 0.198 | 0.509 | 0.179 | 1.442 | ||
| Right | 54 (52.4%) | 13 (68.4%) | ||||
| Left | 49 (47.6%) | 6 (31.6%) | ||||
| Lesion size | 0.013 | 3.556 | 1.249 | 10.121 | ||
| < 1 cm | 64 (62.1%) | 6 (31.6%) | ||||
| > 1 cm | 39 (37.9%) | 13 (68.4%) | ||||
| Scolex | 0.406 | 0.644 | 0.227 | 1.829 | ||
| Present | 43 (41.7%) | 6 (31.6%) | ||||
| Absent | 60 (58.3%) | 13 (68.4%) | ||||
| Moderate-to-severe perilesional edema | 0.116 | 2.866 | 0.783 | 10.493 | ||
| Present | 67 (65.0%) | 16 (84.2%) | ||||
| Absent | 36 (35.0%) | 3 (15.8%) | ||||
| Morphology of granuloma | 0.003 | 4.623 | 1.584 | 13.492 | ||
| Ring | 89 (86.4%) | 11 (57.9%) | ||||
| Disc | 14 (13.6%) | 8 (42.1%) | ||||
| Calcific transformation | 0.010 | 5.231 | 1.437 | 19.044 | ||
| Present | 52 (50.5%) | 16 (84.2%) | ||||
| Absent | 51 (49.5%) | 3 (15.8%) | ||||
CI = confidence interval; EEG = electroencephalography. Statistically significant P-values are depicted in bold.
Discussion
In this prospective study, we studied the natural course of 122 patients with solitary cysticercus granuloma presenting with new-onset seizure/s. In approximately a half of patients, cysticercus granuloma transformed in to a calcified nodule. Moderate-to-severe perilesional edema independently predicted calcification of the granuloma. Out of 19 patients with seizure recurrence, 16 patients had calcific transformation of the cysticercus granuloma.
The major strength of our study is that we included a large number of patients with solitary cysticercus granuloma and followed them for a year. We did not use albendazole and corticosteroids, thus we did not try to alter the natural course of the disease. In approximately 50% of patients, lesions healed with calcification. Our finding is important because a calcified lesion is a permanent source of seizure generation and may be a cause of refractory epilepsy.14 The factors responsible for calcific transformation of solitary cysticercus granuloma are not precisely known. We tried to find an answer to this issue. We observed that a large amount of perilesional edema around solitary cysticercus granuloma was associated with calcific transformation. We hypothesize that a large perilesional edema reflects the amount of inflammation in and around the solitary cysticercus granuloma, which may in turn predispose to calcific transformation of the cysticercus granuloma. The parasite is able to restrain the immune response when it is viable but once the scolex degenerates, brain parenchyma is no longer able to control the immune response leading to degeneration of the cyst and the development of perilesional edema around the degenerating cyst.15 Previously, Nash and coworkers had noted that in patients with seizures and a calcified neurocysticercosis lesion, neuroimaging often (50%; 12/24) demonstrated perilesional edema around the calcified lesion. They also noted that the perilesional edema was strongly associated with seizures. The exact mechanisms of perilesional edema in patients with calcified neurocysticercosis are not known. Possibly, intermittent release of cysticercal antigens from a calcified lesion produces an intense inflammatory reaction in the surrounding brain parenchyma resulting in perilesional edema.16 Perilesional inflammation and disruption of the blood brain barrier play a crucial role in the neurocysticercosis associated epileptogenesis.17,18 Cytokines and mediators of the innate and adaptive immune systems are important in the pathogenesis of seizures in patients with solitary cysticercus granuloma. Many cytokines and chemokines such as intercellular adhesion molecule, interleukin-1 beta, tumor necrosis factor-alpha, and matrix metalloproteinases participates in the process of inflammation in and around the neurocysticercal lesion. Matrix metalloproteinases (MMP-2 and MMP-9) have been shown to be responsible for maintaining blood–brain barrier permeability.19,20
The perilesional edema seen around a calcified lesion, possibly, has a different pathogenesis than perilesional edema present around a solitary cysticercus granuloma. Histopathologically, a degenerated cysticercus is characterized with intense intracystic and capsular inflammatory response (consisting of mononuclear cells, some plasma cells, and a few eosinophils). The central mass consists of a degenerated cyst with distinguishable scolex with suckers.15 A calcified dot in brain consists of a “dense mass of concentrated calcified calcareous corpuscles in the core” surrounded by a “dense collagenous wall making up the host capsule.” The surrounding brain parenchyma showed reactive gliosis.21 It has been argued that seizure recurrence in patients with calcified neurocysticercosis without perilesional edema may have a different mechanism of epileptogenesis. Perilesional cerebral parenchymal gliosis and concomitant mesial temporal sclerosis are possible reasons for seizure recurrence.3 Nash and coworkers suggested that small amount of inflammation that might remain undetected with routine neuroimaging techniques were responsible for seizure recurrences.17
Can immunosuppressive and anti-inflammatory agents be useful in preventing calcific transformation of cysticercus granuloma? There are some isolated reports indicating that anti-inflammatory treatments, such as immunosuppressive drugs and corticosteroids, may play a role in controlling or preventing seizures in these patients.22 However, the exact role of immunosuppressive drugs and corticosteroids can only be established after randomized controlled studies. Nash and coworkers noted that perilesional edema was slightly more frequent in patients who had received albendazole.16 Can treatment with albendazole, corticosteroids, or a combination of both reduce the chances of calcific transformation of a solitary cysticercus granuloma? A meta-analysis evaluated the effect of antiparasitic treatment and corticosteroids on the calcific transformation of cysticercus granuloma. None of regimens showed a better effect on reducing the risk of residual calcification.23
The use of computed tomography in our study might be contested. At one end, where it is an excellent modality to demonstrate calcification unequivocally, it may not be very sensitive in demonstrating perilesional edema. We could have used magnetic resonance imaging, which is superior in quantifying perilesional edema and in demonstration of scolex within the lesion.20 Association of calcified neurocysticercosis with hippocampal atrophy could have been studied in patients with refractory epilepsy, but which required the use of magnetic resonance imaging.
In conclusion, in patients with solitary cysticercus granuloma, calcification of the lesion can be predicted with larger amount of perilesional edema.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Lalit Mahajan, Hardeep Singh Malhotra, Ravindra Kumar Garg, Neeraj Kumar, Praveen Kumar Sharma, Rajesh Verma, and Imran Rizvi, Department of Neurology, King George Medical University, Lucknow, Uttar Pradesh, India, E-mails: lalitmahajan72@yahoo.in, drhsmalhotra@yahoo.com, garg50@yahoo.com, drneeraj2903@gmail.com, pspgimer@gmail.com, drrajeshverma32@yahoo.com, and imranrizvi09@gmail.com.
References
- 1.Burneo JG, Cavazos JE. Neurocysticercosis and epilepsy. Epilepsy Curr. 2014;14:23–28. doi: 10.5698/1535-7511-14.s2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg RK, Malhotra HS. Solitary cysticercus granuloma. Expert Rev Anti Infect Ther. 2012;10:597–612. doi: 10.1586/eri.12.35. [DOI] [PubMed] [Google Scholar]
- 4.Garg RK. Single enhancing computerized tomography-detected lesion in immunocompetent patients. Neurosurg Focus. 2002;12:e4. doi: 10.3171/foc.2002.12.6.5. [DOI] [PubMed] [Google Scholar]
- 5.Murthy JM, Yangala R, Srinivas M. The syndromic classification of the International League Against Epilepsy: a hospital-based study from south India. Epilepsia. 1998;39:48–54. doi: 10.1111/j.1528-1157.1998.tb01273.x. [DOI] [PubMed] [Google Scholar]
- 6.Singhi PD, Baranwal AK. Single small enhancing computed tomographic lesions in Indian children—II. Clinical features, pathology, radiology and management. J Trop Pediatr. 2001;47:266–270. doi: 10.1093/tropej/47.5.266. [DOI] [PubMed] [Google Scholar]
- 7.Escobar A. The pathology of neurocysticercosis. In: Palacios E, Rodriguez-Carbajal I, Taveras J, editors. Cysticercosis of the Central Nervous System. Chicago, IL: Charles C. Thomas; 1983. pp. 27–54. [Google Scholar]
- 8.Garg RK, Nag D. Single enhancing CT lesions in Indian patients with seizures: clinical and radiological evaluation and follow up. J Trop Pediatr. 1998;44:204–210. doi: 10.1093/tropej/44.4.204. [DOI] [PubMed] [Google Scholar]
- 9.Sethi PK, Kumar BR, Madan VS, Mohan V. Appearing and disappearing CT scan abnormalities and seizures. J Neurol Neurosurg Psychiatry. 1985;48:866–869. doi: 10.1136/jnnp.48.9.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi PP, Wadia RS, Kiyawat DP, Ichaporia NR, Kothari SS, Sangle SA, Wadhwa P. Ring or disc enhancing lesions in epilepsy in India. J Trop Med Hyg. 1994;97:347–353. [PubMed] [Google Scholar]
- 11.Rajshekhar V. Rate of spontaneous resolution of a solitary cysticercus granuloma in patients with seizures. Neurology. 2001;57:2315–2317. doi: 10.1212/wnl.57.12.2315. [DOI] [PubMed] [Google Scholar]
- 12.Rajshekhar V, Chandy MJ. Validation of diagnostic criteria for solitary cerebral cysticercus granuloma in patients presenting with seizures. Acta Neurol Scand. 1997;96:76–81. doi: 10.1111/j.1600-0404.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 13.Chernov MF, Kubo O, Hayashi M, Izawa M, Maruyama T, Usukura M, Ono Y, Hori T, Takakura K. Proton MRS of the peritumoral brain. J Neurol Sci. 2005;228:137–142. doi: 10.1016/j.jns.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: a surgically remediable syndrome? Epilepsia. 2013;54:1815–1822. doi: 10.1111/epi.12349. [DOI] [PubMed] [Google Scholar]
- 15.Nash TE, Bartelt LA, Korpe PS, Lopes B, Houpt ER. Calcified neurocysticercus, perilesional edema, and histologic inflammation. Am J Trop Med Hyg. 2014;90:318–321. doi: 10.4269/ajtmh.13-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH. Cysticercosis Working Group in Peru Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash TE, Mahanty S, Loeb JA, Theodore WH, Friedman A, Sander JW. Neurocysticercosis: a natural human model of epileptogenesis. Epilepsia. 2015;56:177–183. doi: 10.1111/epi.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RK, Awasthi R, Rathore RK, Verma A, Sahoo P, Paliwal VK, Prasad KN, Pandey CM, Narayana PA. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–625. doi: 10.1212/WNL.0b013e318248deae. [DOI] [PubMed] [Google Scholar]
- 19.Lalla RS, Garg RK, Malhotra HS, Jain A, Verma R, Pandey CM, Singh GP, Sharma PK. Cytokines, MMP-2, and MMP-9 levels in patients with a solitary cysticercus granuloma. Neurol India. 2015;63:190–196. doi: 10.4103/0028-3886.156279. [DOI] [PubMed] [Google Scholar]
- 20.Restrepo BI, Alvarez JI, Castaño JA, Arias LF, Restrepo M, Trujillo J, Colegial CH, Teale JM. Brain granulomas in neurocysticercosis patients are associated with a Th1 and Th2 profile. Infect Immun. 2001;69:4554–4560. doi: 10.1128/IAI.69.7.4554-4560.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. A calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg. 2011;85:460–463. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44:549–553. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]
- 23.Zhao BC, Jiang HY, Ma WY, Jin DD, Li HM, Lu H. Albendazole and corticosteroids for the treatment of solitary cysticercus granuloma: a network meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004418. doi: 10.1371/journal.pntd.0004418. [DOI] [PMC free article] [PubMed] [Google Scholar]