Abstract
Mansonellosis is endemic in several regions of Africa, the Caribbean, and Latin America. Mansonella ozzardi and Mansonella perstans have been reported in Latin America, including the Amazon region. A morphological and molecular microfilariae study was performed in Pauini (Brazil). Blood samples were collected from 40 individuals, and were analyzed by Giemsa-stained blood film and by two different nested polymerase chain reactions which detect internal transcribed spacer-1 and the major sperm protein gene. By microscopy, 14 of 40 were positive: 11 as M. ozzardi and three as M. perstans–like infections. Both molecular methods detected 19 positive cases as M. ozzardi, including those 14 individuals detected by microscopy, without detectable genetic differences among any of the 19 positive samples. Molecular techniques showed an improvement of mansonellosis diagnosis and may become an effective tool to evaluate the present status of M. ozzardi and M. perstans in Latin America.
Introduction
Mansonellosis is a filarial disease caused by nematodes from the genus Mansonella that is transmitted by the bite of bloodsucking insects (Simulium in the Amazonia and Culicoides in the Caribbean regions, in Central and South America and in Africa).1 The mansonellosis disease is generally considered innocuous, with few symptoms, such as fever, headache, articular pain, and erythematous cutaneous plaques with pruritus.1 Microscopic examination of thick and thin peripheral blood smears stained with Giemsa or other appropriate stains is often regarded as the “gold standard” method for microfilariae diagnosis.1,2 Two species of Mansonella are described in Latin America, Mansonella ozzardi and Mansonella perstans, the latter being restricted to the Amazon regions of Venezuela, Colombia, and Guyana.3–7 In the last decades, a number of unidentified/atypical microfilariae have been described in the Amazonia areas of Brazil, Peru, and Venezuela based on their morphological characteristics, which suggest M. perstans-like.8–10 Morphologically, the most important features to identify microfilariae species are size, shape, and space of the tail, presence or absence of a sheath, and terminal arrangement of nuclei of the tail. However, all these morphological characteristics are not enough to identify these filaria species, and therefore, molecular methods are necessary.1,2,11
The aim of this study was to evaluate the possible presence of M. perstans in the Brazilian Amazonia and to characterize unidentified/atypical microfilariae using molecular characterization approaches.
Materials and Methods
For the molecular characterization of the atypical microfilaria in the Brazilian Amazonia, a study of the inhabitants of Pauini was performed. This area was chosen because in a previous study to validate the filaria nested polymerase chain reaction (FnPCR),12 it had the highest prevalence of mansonellosis in the studied communities (Ta-Tang T-H, Rubio JM, de Moura Abrahim CM, and others, unpublished data).
Forty individuals were included in the study. Samples were obtained as part of a study approved by the Research Ethical Committee of the Amazon Hematology and Hemotherapy Foundation, Brazil. Blood samples were analyzed by Giemsa-stained blood film and by different molecular methods. Morphological identification was performed by six expert microscopists, to whom the origin of the samples was unknown, in line with published guidelines.11 In the case of discrepancies, blood films were reexamined by the same microscopists. The molecular methods included a FnPCR that targets the internal transcribed spacer-1 (ITS-1) region of the ribosomal gene, which differentiates filarial species by the amplified fragment size and by gene sequence,12 and a nested PCR which detects the major sperm protein (MSP) gene. This nested PCR uses, as first amplification process, the MSP-PCR, developed by Hojas and Post13 and as the nested one, an in-house method, using primers MSP-1F (5′-ACCTGGTGACATCCACACACAAC-3′) and MSP-2R (5′-CCAGGACACCGCATGGTGGATC-3′) at 55°C for annealing.
PCR products from FnPCR were purified and sequenced in both directions by an automated sequencer ABI PRISM® 3700 DNA Analyzer (Foster City, CA). The sequences of the ITS-1 were aligned using CLUSTAL W (Cambridge, United Kingdom).14 After alignment, a phylogenetic tree was performed with Treecon software (Antwerpen, Belgium)15 by the neighbor-joining method.
Results
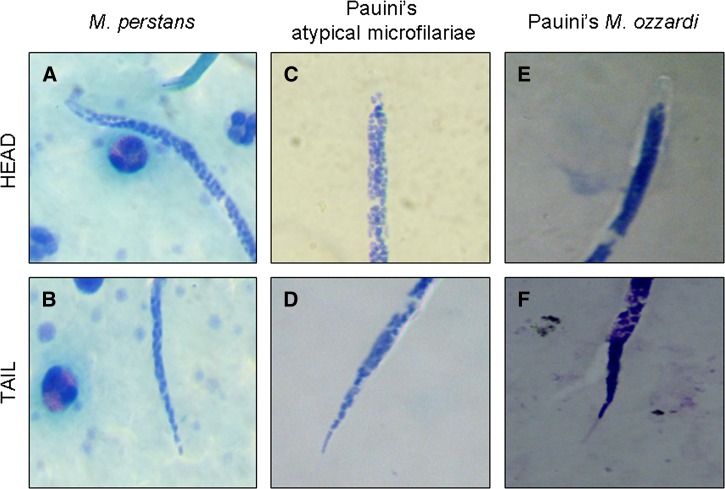
Fourteen of 40 blood films analyzed by microscopy showed microfilariae (35%). Eleven were characterized as M. ozzardi (78.6%) and the other three (21.4%) were characterized as M. perstans-like by five of the six microscopists and as M. ozzardi by the sixth. The morphological description of these atypical microfilariae included no sheath, short cephalic space, and tail shape with nuclei until the end which resemble the morphology of M. perstans (Table 1, Figure 1 ).
Table 1.
Comparison of morphological characteristics among Mansonella ozzardi, Mansonella perstans, and atypical microfilariae
| Characteristics species (type) | Length (μm) (average μm) (Ref.) | Width (μm) (average μm) (Ref.) | Sheath (Ref.) | Cephalic space (Ref.) | Tail and tail nuclei (Ref.) |
|---|---|---|---|---|---|
| M. perstans | 190–200 (195) (11) | 4–5 (11) | Absent (11) | Morphological feature not described in standard textbooks for microfilariae species diagnosis. | Tapered and bluntly. Nuclei to end of tail. (11) |
| M. ozzardi | 163–203 (183) (11) | 3–5 (11) | Absent (11) | After cephalic space, a single nucleus. (8–10) | Long and slender, with seven to nine nuclei that do not reach the tail end. (11) |
| Brazilian Amazon atypical microfilariae | Measures close to M. ozzardi (8) | Measures close to M. ozzardi (8) | Absent (8) | Two paired nuclei after the cephalic space, followed by a single one, and afterwards begin the proceeding characteristic column of nucleus (8). | The posterior extremity, where a nuclear column with 7–8 nuclei are present in a regular and aligned arrangement and a detachment from cuticle is very perceptible (8) |
| Peruvian Amazon atypical microfilariae | 120–130 (125) (9) | ND | Absent (9) | Two nuclei followed by a single nucleus just caudal to the cephalic space (9) | ND |
| Venezuelan Amazon atypical microfilariae: named as Microfilaria bolivarensis by the authors | 220–280 (250) (10) | 7–8 (7.5) (10) | Absent (10) | The cephalic space is short; typically, its length is only slightly greater than its width. (11) | Posterior one-fifth tapered rather sharply. Tail is shorter, less attenuated tail than M. ozzardi, is devoid of nuclei. (10) |
| Atypical microfilariae described in this work | 126–180 (153) | 3–3.6 (3.4) | Absent | Short cephalic space with two nuclei followed by single nucleus, just caudal to the cephalic space. | Nuclei reach the tip of the tail and short, blunt, and less attenuated tail. |
ND = no data.
Figure 1.
Giemsa-stained blood film. (A, B) Comparison of head and tail of Mansonella perstans microfilaria from Africa, (C, D) atypical microfilaria, and (E, F) Mansonella ozzardi microfilaria.
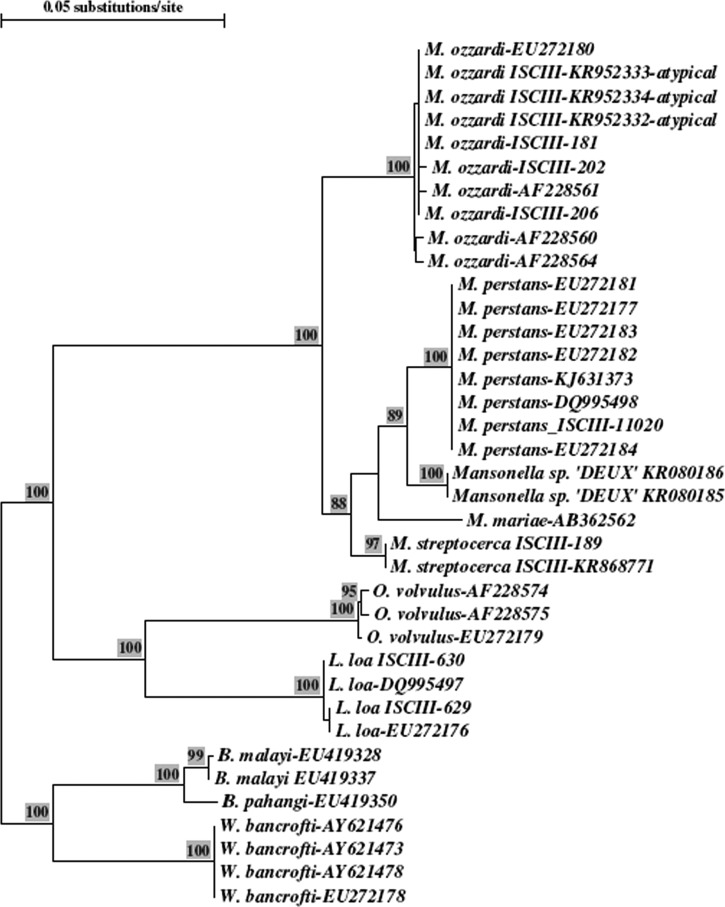
The FnPCR detected 19 filariae-positive cases (47.5%), five cases more than that detected by microscopy, showing higher sensitivity. The 19 cases yield a fragment of 305 base pairs (bp) corresponding to the expected size for M. ozzardi. The Basic Local Alignment Search Tool searches confirmed highest similarity with M. ozzardi sequences from GenBank. Furthermore, a phylogenetic tree performed by neighbor-joining method comparing the sequences from the atypical filariae with others from GenBank showed that atypical filarial sequences formed a monophyletic clade with M. ozzardi (Figure 2 ). The nested PCR detecting MSP gene showed that the same 19 samples were positive, showing the expected fragment of 406 bp which belonged to M. ozzardi; it was also used in proven M. perstans samples, giving a fragment size of 395 bp. Atypical microfilaria amplified fragments for ITS-1 and MSP were sequenced and M. ozzardi identification was confirmed (GenBank access no. for ITS-1: KR952332, KR952333, KR952334, M. ozzardi-MSP: KT224437, KT224438, KT224439 and M. perstans-MSP: KT224440).
Figure 2.
Phylogenetic tree of internal transcribed spacer-1 and flanking regions by neighbor-joining method with bootstrap analysis. The alignment file includes the Mansonella ozzardi atypical sequences, other filarial species identified in the laboratory named as ISCIII, plus the internal number and sequences from GenBank with the corresponding access number. The atypical microfilariae sequences cluster with the M. ozzardi group.
Discussion
More than 20% of microfilariae present in the Pauini inhabitants showed an atypical morphology, which several microscopists characterized as M. perstans-like. Using conventional parasitological techniques, differentiation between the microfilariae species is quite difficult, since there are no reliable morphological criteria to differentiate them.1 It becomes more complicated when two or more species are in sympatry, which could have medical significance, that is, in the monitoring for onchocerciasis recrudescence.16 In contrast with other microfilariae known to routinely infect humans in the Brazilian Amazon, the microfilariae of M. ozzardi are reported to be 163–203 × 3–5 μm, and to have no nuclei at the end of their tails; M. perstans are recorded as having dimensions of 190–200 × 4–5 μm and as having nuclei at the end of their tails (Table 1).11 The observed atypical Mansonella microfilariae in this report had uncommon sizes (ranging from 126–180 × 3–3.6 μm), smaller than both M. ozzardi and M. perstans, although the average was smaller, overlapped with the size of M. ozzardi. The method of specimen preparation is known to have a large effect on microfilariae body size measurements, but all samples were treated in the same way and length measurements are comparable with those published by previous authors for normal and atypical microfilariae.8–10 The cephalic space of these M. perstans-like had two nuclei followed by single nucleus, which is very similar to other atypical microfilariae reported from Brazilian Amazon,8 Peruvian Amazon,9 and Venezuelan Amazon,10 whereas in contrast, M. ozzardi has a single nucleus in this position. Furthermore, the posterior region has a blunt tail filled with nuclei similar to M. perstans (Table 1, Figure 1).
Molecular methods allow the differentiation of the species of filariae. In the present study, M. ozzardi was the unique species present, independent of the target used, ITS or MSP, with no detectable genetic differences among any of the 19 positive samples.
Records of distribution and prevalence of M. perstans in Latin America are restricted to Colombia, Guyana, and Venezuela, and all of them, except one,4 are from the late sixties to the early eighties of last century.3,5–7 Most recent reports describe atypical microfilariae besides M. ozzardi, but not M. perstans,8–10 and in the cases where some molecular identification has been performed, as in this report and one other,9 the unique species found was M. ozzardi.
The studies, in which M. perstans3–7 was described, were done in laboratories either without molecular diagnostic techniques or where these were not easily available. It is possible that these old studies might have misinterpreted atypical M. ozzardi as M. perstans because of the reliance on the morphological analysis. It would be interesting to carry out new field studies to analyze the present status of M. perstans in those communities. Molecular techniques show an improvement of mansonellosis diagnosis and may become an effective tool to evaluate the present geographical distribution and prevalence of M. ozzardi and M. perstans in Latin America.
Conclusions
There is a need to evaluate the real situation of M. perstans in Latin America, since the previous descriptions from the sixties to the eighties of the last century may be an atypical morphological presentation of M. ozzardi. Molecular techniques can have a special value to provide additional data and support evidences to confirm and to determine whether M. perstans is actually present in Latin America and to characterize those unidentified/atypical microfilariae.
ACKNOWLEDGMENTS
We would like to thank the people of Pauini, Brazilian Amazon, for participating in the study. Thuy-Huong Ta-Tang was financed by a project grant from the Sanitary Research Funds, Instituto de Salud Carlos III (no. CM07/00006) and by a contract associated with the Research Project MVP-1044/13 financed by EPISTEM LTD. We also thank James Lee Crainey for the careful reading and valuable correction of the manuscript in English.
Footnotes
Authors' addresses: Thuy-Huong Ta-Tang, Isabel de Fuentes, Marta Lanza, and José M. Rubio, Malaria and Emerging Parasitic Diseases Laboratory, Parasitology Department, Centro Nacional de Microbiologia, Instituto de Salud Carlos III, Madrid, Spain, E-mails: thuyhuong.tatang@gmail.com, ifuentes@isciii.es, mlanza@isciii.es, and jmrubio@isciii.es. Sergio L. B. Luz and Tatiana A. P. Almeida, Parasitology, Instituto Leônidas e Maria Deane, Fundação Oswaldo Cruz (FIOCRUZ), Manaus, Brazil, E-mails: sergioluz@amazonia.fiocruz.br and tatiana_almeida@amazonia.fiocruz.br. Francisco J. Merino, Microbiology and Parasitology, Severo Ochoa University Hospital, Madrid, Spain, E-mail: fmerino.hsvo@salud.madrid.org. Rogelio López-Vélez, Tropical Medicine and Clinical Parasitology Unit, Infectious Diseases Department, Hospital Ramón y Cajal, Madrid, Spain, E-mail: rogelio.lopezvelez@salud.madrid.org. Cláudia M. M. Abrahim, Fundação de Hematologia e Hemoterapia do Amazonas (HEMOAM), Manaus, Brazil, E-mail: cmmabrahim@hemoam.am.gov.br.
References
- 1.Walther M, Muller R. Diagnosis of human filariases (except onchocerciasis) Adv Parasitol. 2003;53:149–193. doi: 10.1016/s0065-308x(03)53004-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosenblatt JE. Laboratory diagnosis of infections due to blood and tissue parasites. Clin Infect Dis. 2009;49:1103–1108. doi: 10.1086/605574. [DOI] [PubMed] [Google Scholar]
- 3.Beaver PC, Neel JV, Orihel TC. Dipetalonema perstans and Mansonella ozzardi in Indians of southern Venezuela. Am J Trop Med Hyg. 1976;25:263–265. doi: 10.4269/ajtmh.1976.25.263. [DOI] [PubMed] [Google Scholar]
- 4.Gomez J, Guerrero R. Environmental factors and the distribution of mansonelliases in southern Venezuela. Parasite. 2000;7:71–76. doi: 10.1051/parasite/2000072071. [DOI] [PubMed] [Google Scholar]
- 5.Marinkelle CJ, German E. Mansonelliasis in the Comisaria del Vaupes of Colombia. Trop Geogr Med. 1970;22:101–111. [PubMed] [Google Scholar]
- 6.Kozek WJ, Palma G, Henao A, Garcia H, Hoyos M. Filariasis in Colombia: prevalence and distribution of Mansonella ozzardi and Mansonella (=Dipetalonema) perstans infections in the Comisaria del Guainia. Am J Trop Med Hyg. 1983;32:379–384. doi: 10.4269/ajtmh.1983.32.379. [DOI] [PubMed] [Google Scholar]
- 7.Orihel TC. Infections with Dipetalonema perstans and Mansonella ozzardi in the aboriginal Indians of Guyana. Am J Trop Med Hyg. 1967;16:628–635. doi: 10.4269/ajtmh.1967.16.628. [DOI] [PubMed] [Google Scholar]
- 8.Adami YL, Moraes MA, Lanfredi RM, Maia-Herzog M. An atypical microfilaria in blood samples from inhabitants of Brazilian Amazon. Parasitol Res. 2008;104:95–99. doi: 10.1007/s00436-008-1164-4. [DOI] [PubMed] [Google Scholar]
- 9.Marcos LA, Arrospide N, Recuenco S, Cabezas C, Weil GJ, Fischer PU. Genetic characterization of atypical Mansonella (Mansonella) ozzardi microfilariae in human blood samples from northeastern Peru. Am J Trop Med Hyg. 2012;87:491–494. doi: 10.4269/ajtmh.2012.11-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godoy GA, Orihel TC, Volcan GS. Microfilaria bolivarensis: a new species of filaria from man in Venezuela. Am J Trop Med Hyg. 1980;29:545–547. doi: 10.4269/ajtmh.1980.29.545. [DOI] [PubMed] [Google Scholar]
- 11.Eberhard ML, Lammie PJ. Laboratory diagnosis of filariasis. Clin Lab Med. 1991;11:977–1010. [PubMed] [Google Scholar]
- 12.Tang TH, Lopez-Velez R, Lanza M, Shelley AJ, Rubio JM, Luz SL. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon region. Mem Inst Oswaldo Cruz. 2010;105:823–828. doi: 10.1590/s0074-02762010000600016. [DOI] [PubMed] [Google Scholar]
- 13.Hojas RM, Post RJ. Regional genetic variation in the major sperm protein genes of Onchocerca volvulus and Mansonella ozzardi (Nematoda: Filarioidea) Int J Parasitol. 2000;30:1459–1465. doi: 10.1016/s0020-7519(00)00117-x. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 16.Luz SL, Crainey JL, Shelley AJ, Rubio M. Outstanding insecurities concerning the use of an Ov16-based ELISA in the Amazonia onchocerciasis focus. Mem Inst Oswaldo Cruz. 2014;109:506–508. doi: 10.1590/0074-0276140079. [DOI] [PMC free article] [PubMed] [Google Scholar]