Abstract
In Thailand, the burden of liver abscess, a life-threatening infectious disease, has not been thoroughly evaluated. We developed a predictive scoring system to estimate survival of patients with liver abscess using information from the 2008–2013 Nationwide Hospital Admission Data to evaluate the burden of liver abscess in Thailand. All patients with primary diagnosis of pyogenic liver abscess (PLA) and amoebic liver abscess (ALA) were included. Epidemiological data, baseline characteristics, hospital course, and survival were analyzed. Overall, 11,296 admissions comprising 8,423 patients from 844 hospitals across Thailand were eligible for analysis. The mean age was 52 ± 17 years and 66.1% of patients were male. ALA was significantly prevalent in southern and western border regions of Thailand, and PLA occurred nationwide. The highest incidence of liver abscess occurred in the rainy season (June–November, P < 0.01). The median length of hospital stay was 8 days (interquartile range = 4–13 days), and mean direct cost of hospitalization was 846 ± 1,574 USD. The overall inhospital mortality rate was 2.8%. Incidence of ALA decreased over the 5-year study period, whereas PLA incidence increased (P < 0.01). Using multivariable Cox regression methods with stepwise variable selection, we developed a final model with five highly significant baseline parameters associated with increased 60-day mortality: older age, PLA, underlying chronic kidney disease, cirrhosis, and human immunodeficiency virus infection. Range of estimated probability of 60-day survival was 95–16% at cumulative risk score 0–13. This simplified score is practical, and may help clinicians prioritize patients requiring more intensive care.
Introduction
Liver abscess is a life-threatening infectious disease. The etiology of liver abscess can be majorly classified into pyogenic liver abscess (PLA) and amoebic liver abscess (ALA). Despite current advances in early diagnosis, effective antibiotic treatment, and therapeutic procedure, liver abscess is still one cause of fatal infectious diseases worldwide, especially in developing countries.
Mortality rates have decreased substantially over the past several decades, with recent studies reporting rates of 13–31%.1,2 The mean age of patients with PLA has increased, and the most common cause reported in recent series has shifted to biliary disease.3 Historically, Escherichia coli has been the predominant causative agent, but Klebsiella liver abscess has been reported to be an emerging disease in western countries, such as the United States.4 Klebsiella pneumoniae infection has also emerged as one of the most common causes of PLA in many Asian countries in the past three decades.5 The emerging serotype K1 K. pneumoniae with multilocus sequence type 23 has been strongly associated with the virulence of liver abscess and invasive syndrome.6,7
ALA has not been a topic of focus, but it is a potentially life-threatening complication by infection with the protozoan parasite Entamoeba histolytica.8 ALA is widely distributed throughout the tropics and subtropics, causing up to 40 million infections annually.9
The extent of liver abscess has remained unclear because of the lack of a population-based study. A recent previous report in Taiwan showed that PLA was endemic and that some factors including diabetes, malignancy, renal disease, and pneumonia were associated with a higher risk for the disease.10 To date, the outcomes in terms of disease burden and national economic impact of liver abscess have not been well studied. There is much knowledge regarding liver abscess in terms of diagnosis, microbiology, and risk factors. However, the burden of disease is unclear, especially in Thailand. This study evaluated the burden of liver abscess in Thailand and developed a simple predictive scoring method to estimate the survival of patients with liver abscess.
Materials and Methods
Study design.
A community-wide, retrospective cohort, observational study was conducted on the Thailand population. We analyzed liver abscess data from the 2008–2013 Nationwide Hospital Admission Data, National Health Security Office (NHSO), Thailand. All diagnoses were according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD10). All patients with a primary diagnosis of PLA (ICD10-K750) and ALA (ICD10-A064) by physicians were included. Epidemiological data, baseline characteristics, hospital course, and survival were analyzed.
Thailand adopted a universal public health-care system in 2002, which comprises three national health insurance categories—CivilServant Medical Benefit Scheme, Social Security Scheme, and Medical Welfare Scheme (MWFS). MWFS includes more than 80% of the population and more than 90% of all admissions included in the NHSO database. All data were retrieved from the national database from all government hospitals and 144 private hospitals registered with the NHSO.
Hospitals in Thailand are classified into three levels: primary hospitals are community hospitals for primary health care, secondary hospitals are hospitals for general health care, and tertiary hospitals are referral hospitals for complicated diseases and specialized health care.
Study population and cohort.
The study population comprised community-based data from 851 districts in all 77 provinces in Thailand. A mean of 5.6 million admissions occurred each year from all diseases and indications from 2008 to 2013. Overall, 11,296 admissions were patients with a primary diagnosis of PLA and ALA during the study period. Among all admissions, 8,424 individual patients from 844 hospitals across Thailand were included in this study analysis. Overall long-term survival was based on the national death registry at the date of data extraction.
Statistical analysis.
All statistical analyses were performed using SPSS version 13 software (SPSS Inc., Chicago, IL). The mean ± standard deviation was used to describe continuous variables where a percentage was used for categorical data. Temporal trends were assessed by the incidence rate ratio (IRR) derived from regression analysis. Continuous variables were compared between groups using an independent t test or one-way analysis of variance. Categorical variables were compared between groups using the χ2 or Fisher's exact test. Multiple logistic regression analysis was used to identify factors influencing mortality rate. The odds ratio (OR) and 95% confidence interval (CI) of each factor are presented. P values < 0.05 were considered statistically significant. For survival analysis, Kaplan–Meier survival curves between different groups were compared using the log-rank test. Cox regression models were used to identify risk factors associated with inhospital mortality caused by liver abscess. The strength of the association was represented by the hazard ratio (HR), 95% CI, Wald Statistics, and P value based on the χ2 distribution with one degree of freedom.
Survival risk score development.
HRs were converted into an easy-to-use integer risk score (0–13) to identify the risk of 60-day mortality. The probability of surviving for 60 days after admission was estimated as a weighted nonlinear function of scores from randomized cases from 90% of all liver abscess cases (training group). After the scoring system was developed, validation was performed with the other 10% of liver abscess cases (validating group). The χ2 test was used to compare the prediction of survival probability by using the proposed scoring with the observed data. Goodness of fit represented by R2 or the coefficient of determination was used to assess the scoring accuracy among subgroups from different hospital levels.
Ethical consideration.
This study was approved by the Gastroenterological Association of Thailand in collaboration with the NHSO, Thailand. All data used in this study were de-identified and released for research purposes. The research protocol was approved by the Institutional Review Board, Faculty of Tropical Medicine, Mahidol University (MUTM-EXMPT 2015-003).
Results
Study population characteristics.
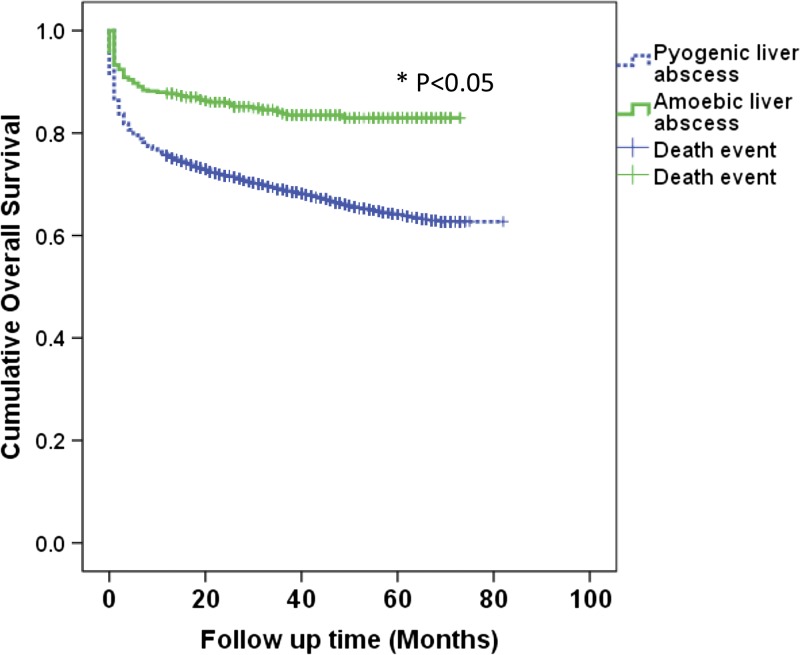
Overall, 7,975 patients had a primary diagnosis of PLA, whereas 448 patients had a primary diagnosis of ALA. The mean age was 52 ± 17 years and 66.1% of patients were male. The median length of hospital stay was 8 days, and mean cost of hospitalization was 846 ± 1,574 USD. The overall inhospital mortality rate was 2.8%. Long-term all-cause mortality after hospitalization with liver abscess was increased by time. The long-term overall mortality rate was very high, up to 15% in ALA and 30% in PLA (Table 1). Overall long-term survival was significantly higher among patients with ALA compared with PLA (HR = 0.54; 95% CI = 0.42–0.68; P < 0.001) (Figure 1 ).
Table 1.
Overall demographic data, duration of length of hospital stay, cost of hospitalization, inhospital mortality rate, and long-term all-cause mortality after hospitalization classified according to type of liver abscess
| Total (N = 8,423) | ALA (N = 448) | PLA (N = 7,975) | P value | |
|---|---|---|---|---|
| Age in years, mean ± SD | 52.1 ± 17.3 | 47.8 ± 16.4 | 52.3 ± 17.4 | < 0.01 |
| Male sex, N (%) | 5,570 (66.1) | 353 (78.8) | 5,217 (65.4) | < 0.01 |
| Length of stay in days, mean ± SD | 10.0 ± 10.1 | 9.3 ± 7.7 | 10.1 ± 10.3 | 0.02 |
| Hospital cost in USD, mean ± SD | 872 ± 1,623 | 704 ± 1,103 | 882 ± 1,646 | < 0.01 |
| Inhospital mortality, N (%) | 314 (2.8) | 7 (1.2) | 307 (2.9) | 0.02 |
| Long-term all-cause mortality after hospitalization with liver abscess, N (%) | ||||
| 30 days | 725 (8.6) | 20 (4.46) | 705 (8.54) | < 0.01 |
| 90 days | 1,335 (15.85) | 34 (7.59) | 1,301 (16.31) | < 0.01 |
| 1 year | 2,003 (23.78) | 55 (12.28) | 1,948 (24.42) | < 0.01 |
| 3 years | 2,451 (29.1) | 69 (15.4) | 2,382 (29.87) | < 0.01 |
| 5 years | 2,609 (30.97) | 71 (15.84) | 2,538 (31.82) | < 0.01 |
ALA = amoebic liver abscess; N = number; PLA = pyogenic liver abscess; SD = standard deviation; USD = U.S. dollar.
Figure 1.
Overall long-term survival of patients with amoebic liver abscess (ALA) compared with pyogenic liver abscess (PLA). Overall long-term survival was significantly higher among patients with ALA compared with those with PLA (hazard ratio [HR] = 0.54; 95% confidence interval = 0.42–0.68; P < 0.001). *Statistically significant by log-rank test.
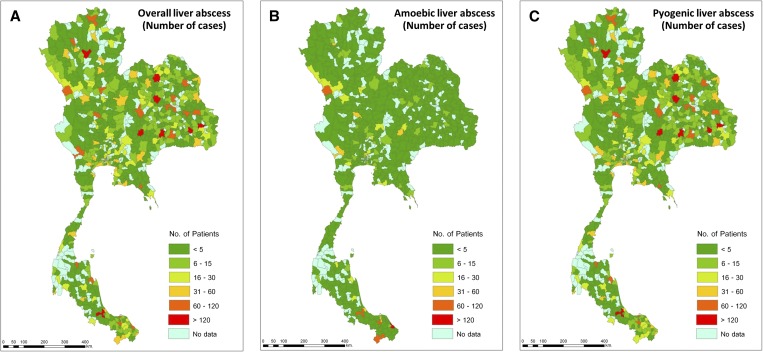
Overall cases of liver abscess were geographically distributed nationwide (Figure 2A ). However, after we classified the type of liver abscess into ALA (Figure 2B) and PLA (Figure 2C), ALA was shown to be predominant in some southern and western border regions of Thailand. Most patients with PLA were from the northeastern part of the country (40.1%), different from the patients with ALA who were mostly from the south (42.4%). ALA had a significantly high prevalence in Narathiwat Province, a southern border region (16.7% of total cases).
Figure 2.
Geographic distribution of patients hospitalized with (A) overall, (B) amoebic, and (C) pyogenic liver abscess from 2008 to 2013 in Thailand (classified by district).
Incidence of ALA decreased over the 5-year study period, whereas the incidence of PLA increased (P < 0.01). Inhospital mortality did not significantly change (P = 0.68), but the cost of treatment gradually increased over the 5-year period (P < 0.01) (Table 2).
Table 2.
Trend of inhospital mortality and cost of treatment of patients hospitalized with ALA and PLA over a 5-year study period
| Years | 2009 | 2010 | 2011 | 2012 | 2013 | P value |
|---|---|---|---|---|---|---|
| ALA cases | 133 | 110 | 114 | 109 | 109 | < 0.01 |
| PLA cases | 2,105 | 2,011 | 2,136 | 2,230 | 2,239 | < 0.01 |
| Hospitalization cost, mean ± SD (USD) | 799 ± 1,701 | 855 ± 1,546 | 853 ± 1,504 | 967 ± 1,837 | 883 ± 1,490 | < 0.01 |
| Inhospital mortality | 2.5% | 2.73% | 2.98% | 2.95% | 2.73% | 0.68 |
ALA = amoebic liver abscess; PLA = pyogenic liver abscess; SD = standard deviation; USD = U.S. dollar.
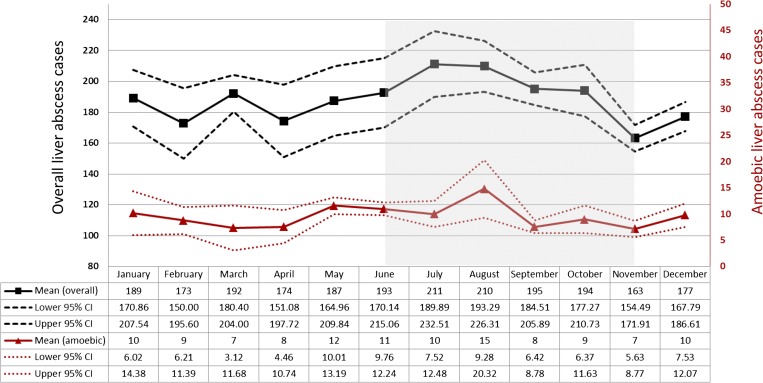
The 5-year mean and 95% CI of liver abscess incidence classified by month was analyzed. The highest incidence of liver abscess occurred in the rainy season (between June and November, IRR = 1.11; 95% CI = 1.07–1.16; P < 0.01) (Figure 3 ).
Figure 3.
Incidence of overall and amoebic liver abscess classified by month (5-year mean and 95% confidence interval).
The etiology of PLA was determined from documents in 1,029 cases (12.9%). The most common identified pathogen causing PLA was Burkholderia pseudomallei (56.5%), followed by K. pneumonia (22.2%), E. coli (10%), Staphylococcus spp. (4.1%), Pseudomonas aeruginosa (2.5%), and Salmonella spp. (2.3%). Of these, E. coli, K. pneumonia, and P. aeruginosa posed statistically significant increased risk of inhospital mortality (HR = 3.55; 95% CI = 1.82–6.94; P < 0.001, HR = 2.11; 95% CI = 1.20–3.69; P < 0.01, and HR = 4.48; 95% CI = 1.42–14.15; P = 0.01, respectively). Various inhospital complications were significantly associated with inhospital mortality including congestive heart failure, acute kidney injury, septic shock, requiring a ventilator, and hemodialysis (Table 3).
Table 3.
Significant risk factors (identified organisms and inhospital complications) associated with inhospital mortality in patients hospitalized with liver abscess
| Identified organisms | Risk factors |
|||
|---|---|---|---|---|
| Hazard ratio | 95% CI lower | 95% CI upper | P value | |
| Burkholderia pseudomallei | 0.472 | 0.233 | 0.958 | 0.038 |
| Klebsiella pneumonia | 2.512 | 1.470 | 4.293 | < 0.01 |
| Escherichia coli | 3.382 | 1.691 | 6.762 | < 0.01 |
| Pseudomonas aeruginosa | 4.404 | 1.319 | 14.704 | 0.016 |
| Entamoeba histolytica | 0.418 | 0.197 | 0.889 | 0.023 |
| Inhospital complications | ||||
| Congestive heart failure | 6.491 | 3.826 | 11.010 | < 0.01 |
| Acute kidney injury | 12.897 | 9.961 | 16.698 | < 0.01 |
| Septic shock | 15.558 | 10.635 | 22.759 | < 0.01 |
| Required ventilator | 24.091 | 17.961 | 32.313 | < 0.01 |
| Required hemodialysis | 29.815 | 16.374 | 54.288 | < 0.01 |
CI = confidence interval.
Survival risk scoring system.
Using multivariable Cox regression methods with stepwise baseline variable selection, we derived the final model with five highly significant baseline parameters associated with increased 60-day mortality: age, etiology of liver abscess, underlying chronic kidney diseases, cirrhosis, and human immunodeficiency virus (HIV) infection. HIV infection was found to be one of the most significant comorbidities associated with increased inhospital mortality (HR = 6.15; 95% CI = 4.58–8.27; P < 0.001). In contrast, ALA was found to be associated with a significantly reduced risk of inhospital mortality (HR = 0.52; 95% CI = 0.40–0.66; P < 0.001). HRs were converted into an easy-to-use integer risk score (0–13) to identify the risk of 60-day mortality (Table 4 ).
Table 4.
Predictive parameters, adjusted hazard ratio, simplified risk score, and estimated probability of 60-day survival from cumulative risk score*
| Parameters | Adjusted hazard ratio | 95% CI | P value | Score (total = 13) |
|---|---|---|---|---|
| Age | ||||
| < 40 years | 1.00 | 0 | ||
| 40–60 years | 2.07 | 1.79–2.38 | < 0.001 | 1 |
| > 60 years | 4.86 | 4.23–5.58 | < 0.001 | 4 |
| Chronic kidney diseases | 1.76 | 1.51–2.06 | < 0.001 | 0.75 |
| Cirrhosis | 2.20 | 1.86–2.60 | < 0.001 | 1.25 |
| Pyogenic liver abscess | 1.94 | 1.51–2.49 | < 0.001 | 1 |
| HIV infection | 6.15 | 4.58–8.27 | < 0.001 | 5 |
CI = confidence interval; HIV = human immunodeficiency virus.
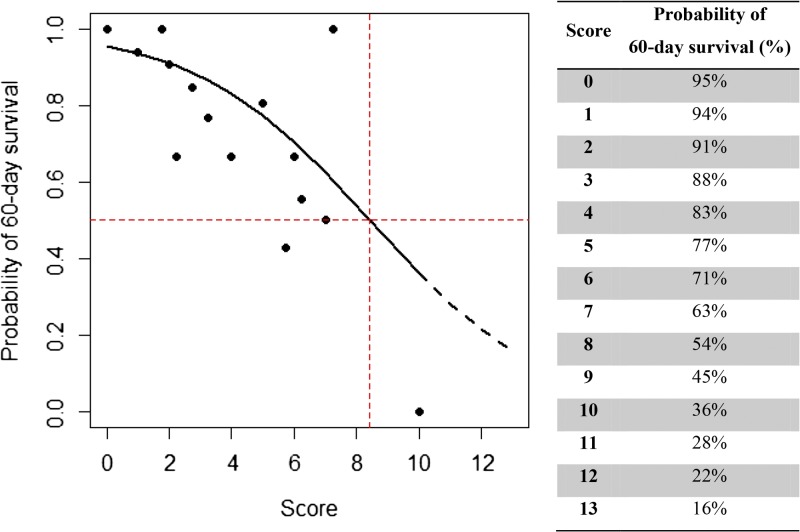
Estimated probability of 60-day survival from cumulative risk score (score: probability of 60-day survival [%]); score = 0–2: ∼91–95%, score = 3–5: ∼77–88%, score = 6–8: ∼54–71%, score = 9–11: ∼28–45%, and score = 12–13: ∼16–22%.
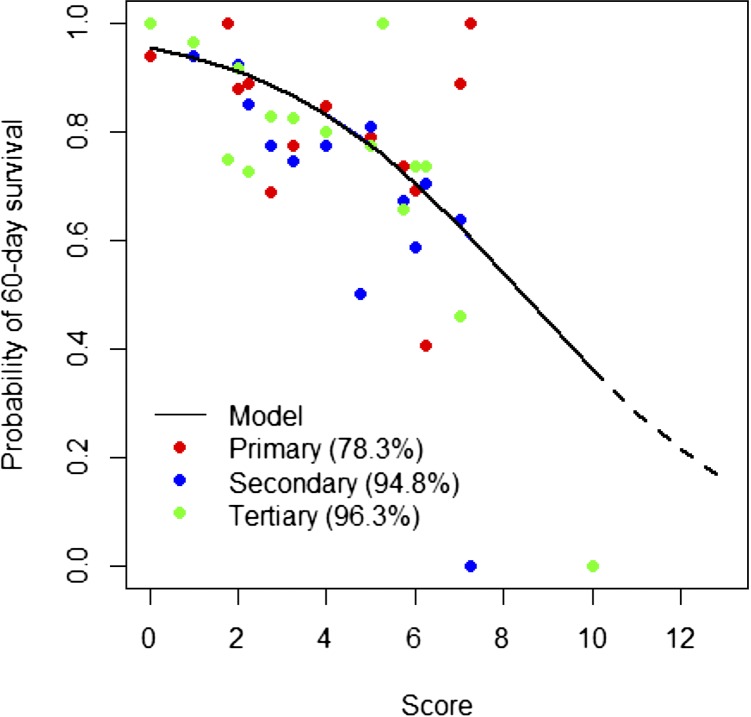
The probability of surviving 60 days after admission was estimated by weighted score (P [surviving 60 days]) = 1 − 1/(1 + 20.80 × exp[−0.36 × score]). The range of estimated probability of 60-day survival was 95–16% over the cumulative risk score of 0–13 (Figure 4 ). The scoring system was validated with different populations with significant accuracy of the expected number of survivals compared with observed data for any given score (χ2 = 3.4138; P = 0.9980) The goodness of fit of this scoring system (R2) was then assessed among subgroups from different hospital levels, that is, primary, secondary, and tertiary. The R2 for primary, secondary, and tertiary patient data was 78.3%, 94.8%, and 96.3%, respectively. This demonstrated that the scoring system accurately predicted the probability of survival in all levels of hospitals with the highest accuracy in tertiary level hospitals (Figure 5 ).
Figure 4.
Estimated probability of 60-day survival from cumulative risk score (score: probability of 60-day survival [%]).
Figure 5.
Validation of scoring system for estimated probability of 60-day survival in different levels of hospitals (R2).
Discussion
Between 2008 and 2013, 8,423 patients had an inpatient primary diagnosis of liver abscess across Thailand. Our data show that liver abscess is still a problem and a significant cause of morbidity and mortality. Inhospital mortality was 2.8%, but the long-term all-cause of mortality was much higher (1-year mortality of 23.78%). This might be explained by complications directly associated with liver abscess or comorbidity in patients with liver abscess. Interestingly, we discovered that the characteristics of patients with ALA and PLA were different. ALA patients were younger than those with PLA. In addition, almost 80% of amoebic cases were male. This might be explained by differences in occupation and environmental exposure. It has also been reported that testosterone influences susceptibility to ALA in a mouse model of the disease.11
Our data demonstrate different geographic distributions of endemic liver abscess. It is known that the northeastern part of Thailand has a very high incidence of B. pseudomallei.12 Presentation of hepatic abscess and/or splenic involvement is a common clinical manifestation of melioidosis.13 Therefore, this might be associated with the highest distribution of PLA in these areas. However, a causative organism was not defined for a significant proportion of PLA. This limitation might be explained by the use of diagnostic protocols from individual hospitals in Thailand and/or the reporting system in the national data collection process. This indicates the need for improvement in defining specific etiologies of PLA in the Thailand public health system. However, after we classified the type of liver abscess into ALA and PLA, ALA was shown to be predominant in some southern and western border regions of Thailand. This could be owing to personal hygiene or virulence factors of E. histolytica.14 Only 5–10% of those infected with E. histolytica developed symptomatic disease, and further analysis of the population structure of E. histolytica isolates in highly endemic areas is necessary.15 We do not have data regarding the environment of endemic and virulence factors of E. histolytica in Thailand. Investigation of the reasons why ALA occurs in that area might lead to disease control and prevention.
The seasonal incidence of liver abscess has been scantly reported. One previous study showed that ALA had the highest incidence during the peak rainy season (April–July).16 Consistent with this result, in the present study, the highest incidence of liver abscess occurred in the rainy season. The seasonal prevalence of human intestinal parasites including E. histolytica was reported.17 In Thailand, the agriculture season starts at the same time as the rainy season. Thus, the rainy season will increase the risk of occupational exposure to infections such as melioidosis.18
Among cases with determined organisms of liver abscess, B. pseudomallei was the most frequently reported. This might be due to endemic melioidosis, which is the major cause of community-acquired septicemia in northeast Thailand,19 and due to a high index of clinical suspicion among physicians in areas of endemic disease. The incidence of PLA from K. pneumonia has surpassed that of E. coli. This finding was consistent with emerging strains of Klebsiella spp. and reports that K. pneumonia is the dominant pathogen for liver abscesses in several Asian countries.20–22 However, there were some limitations of our retrospective data because less than 20% of cases had a defined causative organism. Thus, for the remaining ∼80% of cases, the causative organism remains unknown. The specific etiology of liver abscess also affected the inhospital mortality. We found that E. coli, Klebsiella spp., and Pseudomonas spp. increased the risk of inhospital death compared with melioidosis, and that amoebiasis showed a lower risk of inhospital death.
The burden associated with liver abscess remains a public health problem in Thailand, with an overall inhospital mortality rate of 2.8%. The direct cost of hospitalization was estimated to be 10 million USD. The overall length of stay, hospital cost, and inhospital mortality were higher in patients with PLA than ALA. Liver abscess had almost twice the burden in terms of cost and length of hospital stay than overall admissions. However, overall inhospital mortality from liver abscess did not differ from overall mortality of admitted cases (mortality rate of 2.7%).
After we analyzed the trend, we found that the cost of hospitalization had significantly increased yearly, but the inhospital mortality did not significantly change. However, pyogenic cases seemed to increase. In contrast, amoebic cases seemed to decrease yearly, which might be explained by increased urbanization and improved sanitation and personal hygiene in this region.
There are some previous reports of clinical or laboratory predictors for treatment outcome failure; however, most predictors used data from a single institution.23–25
This study developed a simple survival risk score using five baseline independent predictors associated with mortality from liver abscess in Thailand. Those parameters were age, chronic kidney disease, cirrhosis, HIV infection, and etiology of liver abscess. Such parameters can easily be observed and used as a bedside tool to predict survival of patients. This scoring system has been validated with a validating group and demonstrated accurate survival prediction in all levels of hospitals. This study was limited to the Thailand population. Although this is one of the strengths of the study, its use needs to be validated in other populations to further develop the risk scoring system.
In conclusion, liver abscess remains a public health problem in Thailand necessitating a lengthy hospital stay, high cost of admission, and high long-term overall mortality rate. The geography and season both have an impact on the incidence of liver abscess in Thailand. Various factors affecting patient survival include the etiology of liver abscess, baseline characteristics, comorbidities, and complications during admission. The scoring system that we developed is simple and practical, and may help clinicians with intensive care patient prioritization and case management in hospital settings.
ACKNOWLEDGMENTS
We would like to express our gratitude to the staff of the Department of Tropical Hygiene, the Office of Research Services, Faculty of Tropical Medicine, Mahidol University, the Faculty of Medicine, Chulalongkorn University, Vichaiyut Medical Center, and the National Health Security Office, Thailand.
Footnotes
Financial support: This work was supported by The Gastroenterological Association of Thailand and Mahidol University, an ICTM Grant of the Faculty of Tropical Medicine, and funding from the Department of Clinical Tropical Medicine of the Faculty of Tropical Medicine and Talent Management Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors' addresses: Kittiyod Poovorawan, Chatporn Kittitrakul, and Polrat Wilairatana, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: kittiyod.poo@mahidol.ac.th, chatporn.kit@mahidol.ac.th, and polrat.wil@mahidol.ac.th. Wirichada Pan-ngum, Ngamphol Soonthornworasiri, and Chotipa Kulrat, Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mails: wirichada.pan@mahidol.ac.th, ngamphol.soo@mahidol.ac.th, and chotipa.kul@mahidol.ac.th. Sombat Treeprasertsuk, Department of Medicine, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Chulalongkorn University, Bangkok, Thailand, E-mail: battan5410@gmail.com. Bubpha Kitsahawong and Kamthorn Phaosawasdi, Department of Medicine, Vichaiyut Hospital and Medical Center, Bangkok, Thailand, E-mails: kbubpha@gmail.com and kamthornphao@yahoo.com.
References
- 1.Alvarez Perez JA, Gonzalez JJ, Baldonedo RF, Sanz L, Carreno G, Junco A, Rodriguez JI, Martinez MD, Jorge JI. Clinical course, treatment, and multivariate analysis of risk factors for pyogenic liver abscess. Am J Surg. 2001;181:177–186. doi: 10.1016/s0002-9610(00)00564-x. [DOI] [PubMed] [Google Scholar]
- 2.Chen SC, Yen CH, Lai KC, Tsao SM, Cheng KS, Chen CC, Lee MC, Chou MC. Pyogenic liver abscesses with Escherichia coli: etiology, clinical course, outcome, and prognostic factors. Wien Klin Wochenschr. 2005;117:809–815. doi: 10.1007/s00508-005-0481-1. [DOI] [PubMed] [Google Scholar]
- 3.Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 4.Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu PF, Chang YY, Lin YT, Wang FD, Chan YJ, Fung CP. Clinical characteristics and economic consequence of Klebsiella pneumoniae liver abscess in Taiwan. J Microbiol Immunol Infect. 2015;48:190–197. doi: 10.1016/j.jmii.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Siu LK, Fung CP, Chang FY, Lee N, Yeh KM, Koh TH, Ip M. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol. 2011;49:3761–3765. doi: 10.1128/JCM.00977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore R, O'shea D, Geoghegan T, Mallon PW, Sheehan G. Community-acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41:681–686. doi: 10.1007/s15010-013-0408-0. [DOI] [PubMed] [Google Scholar]
- 8.Ximenez C, Moran P, Rojas L, Valadez A, Gomez A, Ramiro M, Cerritos R, Gonzalez E, Hernandez E, Oswaldo P. Novelties on amoebiasis: a neglected tropical disease. J Glob Infect Dis. 2011;3:166–174. doi: 10.4103/0974-777X.81695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wuerz T, Kane JB, Boggild AK, Krajden S, Keystone JS, Fuksa M, Kain KC, Warren R, Kempston J, Anderson J. A review of amoebic liver abscess for clinicians in a nonendemic setting. Can J Gastroenterol. 2012;26:729–733. doi: 10.1155/2012/852835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotter H, Helk E, Bernin H, Jacobs T, Prehn C, Adamski J, Gonzalez-Roldan N, Holst O, Tannich E. Testosterone increases susceptibility to amebic liver abscess in mice and mediates inhibition of IFNγ secretion in natural killer T cells. PLoS One. 2013;8:e55694. doi: 10.1371/journal.pone.0055694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apisarnthanarak P, Thairatananon A, Muangsomboon K, Lu DS, Mundy LM, Apisarnthanarak A. Computed tomography characteristics of hepatic and splenic abscesses associated with melioidosis: a 7-year study. J Med Imaging Radiat Oncol. 2011;55:176–182. doi: 10.1111/j.1754-9485.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 14.Ankri S, Padilla-Vaca F, Stolarsky T, Koole L, Katz U, Mirelman D. Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence. Mol Microbiol. 1999;33:327–337. doi: 10.1046/j.1365-2958.1999.01476.x. [DOI] [PubMed] [Google Scholar]
- 15.Haghighi A, Kobayashi S, Takeuchi T, Thammapalerd N, Nozaki T. Geographic diversity among genotypes of Entamoeba histolytica field isolates. J Clin Microbiol. 2003;41:3748–3756. doi: 10.1128/JCM.41.8.3748-3756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hai AA, Singh A, Mittal VK, Karan GC. Amoebic liver abscess. Review of 220 cases. Int Surg. 1991;76:81–83. [PubMed] [Google Scholar]
- 17.Amin OM. Seasonal prevalence of intestinal parasites in the United States during 2000. Am J Trop Med Hyg. 2002;66:799–803. doi: 10.4269/ajtmh.2002.66.799. [DOI] [PubMed] [Google Scholar]
- 18.Chen YL, Yen YC, Yang CY, Lee MS, Ho CK, Mena KD, Wang PY, Chen PS. The concentrations of ambient Burkholderia pseudomallei during typhoon season in endemic area of melioidosis in Taiwan. PLoS Negl Trop Dis. 2014;8:e2877. doi: 10.1371/journal.pntd.0002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, White NJ. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 20.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 21.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 22.Qu TT, Zhou JC, Jiang Y, Shi KR, Li B, Shen P, Wei ZQ, Yu YS. Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in east China. BMC Infect Dis. 2015;15:161. doi: 10.1186/s12879-015-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao WI, Sheu WH, Chang WC, Hsu CW, Chen YL, Tsai SH. An elevated gap between admission and A1C-derived average glucose levels is associated with adverse outcomes in diabetic patients with pyogenic liver abscess. PLoS One. 2013;8:e64476. doi: 10.1371/journal.pone.0064476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo JZ, Leow JJ, Ng PL, Lee HQ, Mohd Noor NA, Low JK, Junnarkar SP, Woon WW. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepatobiliary Pancreat Sci. 2015;22:156–165. doi: 10.1002/jhbp.174. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran S, Mishra K, Choudhury SR, Saxena R. Clinico-socio-demographic profile and predictors of poor outcome in children with liver abscess: a hospital-based study in northern India. Trop Doct. 2012;42:226–228. doi: 10.1258/td.2012.120266. [DOI] [PubMed] [Google Scholar]