Abstract
Environmental enteropathy (EE), a subclinical intestinal disorder characterized by mucosal inflammation, reduced barrier integrity, and malabsorption, appears to be associated with increased risk of stunting in children in low- and middle-income countries. Fecal biomarkers indicative of EE (neopterin [NEO], myeloperoxidase [MPO], and alpha-1-antitrypsin [AAT]) have been negatively associated with 6-month linear growth. Associations between fecal markers (NEO, MPO, and AAT) and short-term linear growth were examined in a birth cohort of 246 children in Bangladesh. Marker concentrations were categorized in stool samples based on their distribution (< first quartile, interquartile range, > third quartile), and a 10-point composite EE score was calculated. Piecewise linear mixed-effects models were used to examine the association between markers measured quarterly (in months 3–21, 3–9, and 12–21) and 3-month change in length-for-age z-score (ΔLAZ). Children with high MPO levels at quarterly time points lost significantly more LAZ per 3-month period during the second year of life than those with low MPO (ΔLAZ = −0.100; 95% confidence interval = −0.167 to −0.032). AAT and NEO were not associated with growth; however, composite EE score was negatively associated with subsequent 3-month growth. In this cohort of children from an urban setting in Bangladesh, elevated MPO levels, but not NEO or AAT levels, were associated with decreases in short-term linear growth during the second year of life, supporting previous data suggesting the relevance of MPO as a marker of EE.
Introduction
Chronic malnutrition contributes substantially to global child morbidity and mortality. Over a third of children under of 5 years of age in south Asia and sub-Saharan Africa are stunted (length-for-age z-score [LAZ] < −2).1 Stunting is associated with a 5-fold increased risk of mortality among children under 5 years of age2,3; largely as a result of increased mortality due to diarrhea, pneumonia, and other respiratory illnesses.4–6 Stunting is also associated with decreased cognitive development, school readiness and performance, and reduced economic productivity later in life.5–10 In addition, women who are stunted in childhood have increased risk of maternal mortality and morbidity and increased risk of delivering small-for-gestational-age infants.4–6,11 Children born to women who are stunted are more likely to be stunted themselves and have a higher risk of death in the first 5 years of life.11
Environmental enteropathy (EE), also known as environmental enteric dysfunction, a subclinical intestinal disorder observed among children living in settings of poor hygiene and sanitation, is highly prevalent among children in resource-limited settings and is associated with reduced linear growth.12 EE is marked by mucosal and systemic inflammation, reduced intestinal barrier integrity, bacterial translocation, and reduced intestinal absorptive capacity.13–15 The intestinal histology of children with EE often includes villus atrophy, villus crypt proliferation, and lymphocyte infiltration of the lamina propria.12
Obtaining specimens to examine histology requires invasive endoscopy and biopsy procedures. As a result, less invasive biomarkers of EE are of interest for defining children at risk.16–19 Candidate EE fecal markers include those measuring intestinal inflammation (neopterin [NEO] and myeloperoxidase [MPO]) and those evaluating intestinal permeability (alpha-1-antitrypsin [AAT]). NEO is a molecule produced and released by macrophages and dendritic cells upon stimulation by activated T lymphocytes, and NEO concentration in stool is used as a marker of intestinal Th1 immune activation.20 MPO is a lysosomal protein contained within primary granules that are released into the gut lumen by activated neutrophils and other phagocytes in acute inflammation.21,22 Higher concentrations of MPO in the stool suggest lymphocytic infiltration of the lamina propria, one of the histological findings associated with EE.12 AAT is a protease inhibitor abundant in serum which appears to protect cells from inflammatory proteases secreted by neutrophils and macrophages. AAT is a large, polar, molecule that does not cross the luminal barrier unless there is significantly aberrant permeability. As a result, clearance of AAT is a useful marker of intestinal permeability and it has been used as a marker of protein-losing enteropathy.23–25 All three fecal markers (NEO, MPO, and AAT) have been shown to be negatively associated with subsequent 6-month linear growth in children under 12 months of age in multiple settings in the multisite Malnutrition and Enteric Diseases (MAL-ED) birth cohort.17,18 In addition, a disease activity score (composite EE score) which combined these markers was more predictive of long-term growth faltering than any individual marker.18
The first 2 years of life represent a critical period of growth, and is also one in which children are frequently exposed to multiple enteric pathogens, particularly as they wean from breast milk.26 As the normal rate of linear growth varies considerably in the first 2 years of life, examination of the relationship between fecal markers and growth over short intervals is important for identifying period(s) during which these markers may be most predictive. Although previous studies, including the large multisite MAL-ED study have reported an association between fecal markers and 6-month growth, the relationship of these markers with growth over shorter periods has not been described.18 In addition, there may be specific differences in the performance of these markers based on the population or geography in which they are tested. The present study describes the longitudinal patterns of AAT, MPO, and NEO among a cohort of children in Bangladesh with high rates of stunting to determine the contribution of EE to linear growth failure.
Materials and Methods
Study protocols were reviewed and approved by several institutional review boards and regional health authorities as part of the multisite MAL-ED birth cohort, including the Ethical Review Committee of the International Center for Diarrheal Disease Research, Bangladesh (icddr,b).27
Study population.
The study used data from the Bangladesh MAL-ED site, a 2-year prospective observational study. Details of the parent study have been published previously.27,28 In brief, healthy newborns living in the Bauniabadh area of section 11 of Mirpur, an urban slum in Dhaka, Bangladesh, were recruited by field workers within the first 17 days of life between February 2010 and February 2012.27 Enrolled children were visited every other day for 2 years by field research assistants who interviewed their parents about morbidity and breastfeeding behavior. Exclusion criteria for the cohort study were “maternal age of < 16 years, not a singleton pregnancy, another child already enrolled in the MAL-ED study, severe disease requiring hospitalization before recruitment, and severe acute or chronic conditions diagnosed by a physician (e.g. neonatal disease, renal disease, chronic heart failure, liver disease, cystic fibrosis, congenital conditions).”27
Data collection.
Assessment of household/maternal information was done at enrollment, and caregivers reported morbidity and breastfeeding behavior every other day. Child anthropometry was assessed at monthly intervals, children were weighed using metric pediatric balances with a certified accuracy of 100 g, and length was measured using a marked platform with a sliding footboard.29 Stool samples were collected without fixative by field health-care workers during home visits and frozen at −70°C pending processing.17 Stool was collected at monthly intervals in the first year and at quarterly intervals in the second year. Caregiver-reported diarrhea was defined as ≥ 3 loose stools/day. NEO, MPO, and AAT concentrations were measured in stool samples from months 3, 6, 9, 12, 15, 18, and 21 (quarterly) to ensure consistent time periods over the study period and to avoid inclusion of overlapping periods of growth. Stool samples were excluded from children who had acute diarrhea or diarrhea symptoms within 7 days before collection and from those who had dual sugar permeability testing within one day before collection. These factors alter stool water content to a variable extent, making the interpretation of fecal biomarker concentrations unreliable in the absence of obtaining dry weights. Obtaining dry weights was considered unfeasible given the number of specimens processed.17
Laboratory methods.
All laboratory procedures were conducted in laboratories at icddr,b in Dhaka, Bangladesh. AAT, NEO, and MPO were measured in stool samples using commercially available enzyme-linked immunosorbent assays (ELISAs).17,18 ELISAs were run per instructions on the package insert, except that for MPO (Alpco, Salem, NH) the initial dilutions run were 1:500 and NEO (GenWay Biotech, San Diego, CA) was diluted 1:1,000 in 0.9% saline. AAT (Biovendor, Candler, NC) was run according to the package insert at a dilution of 1:500. Samples out of range of the standard curve for any of the assays were run at higher or lower concentration (as appropriate).18
Statistical methods.
All analyses were performed using STATA version 12.1 IC (College Station, Texas). World Health Organization Anthro software was used to calculate z-scores from raw anthropometric data. Length-for-age data from regular intervals (3, 6, 9, 12, 15, 18, 21, and 24 months of age) were included in the analyses. All fecal marker results from ±15 days of the child's indicated age were included. Fecal marker concentrations were categorized based on the distribution of all measurements: low (in first quartile), medium (in the interquartile range [IQR]), or high (in fourth quartile). At each time point, the composite EE score (0–10) was calculated from the three fecal markers, as described in a previous study.18 Categories were assigned values as 0 (low), 1 (medium), or 2 (high). The formula for the composite EE score is as follows:
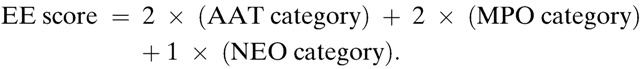 |
To test the association between fecal markers and short-term linear growth, the individual fecal marker or the composite EE score was the primary exposure and the subsequent 3-month change in LAZ was the outcome. Growth periods of 3 months were chosen to account for differences in age-dependent growth rates and to examine potential determinants of growth velocity unrelated to age. This allowed for the inclusion of seven nonoverlapping 3-month growth periods which followed stool sample collection. The relationship between EE markers and growth was also examined separately in the first and second year, given the contribution of low birthweight to stunting and the fact that most stunting occurs in the first year of life. Diarrhea was included as a covariate in all models to view associations between fecal markers and growth beyond that explained by diarrhea. Diarrhea was parameterized as the proportion of days that a child experienced diarrhea in the 3-month period after stool collection. Exclusive breastfeeding was not included as a covariate due to collinearity with age.
Piecewise linear mixed effects models with first-order autoregressive residual structures were used to test the association between markers and short-term linear growth. The equations below depict the parameterization of the models for short-term growth and association with fecal markers for the full 21-month period (stool from months 3–21), early period (months 3–9), and second year (months 12–21), respectively. In the equations, the bj term is a random intercept (unique for each study child), test = med indicates the marker was in the IQR, and test = high indicates that the marker was in the fourth quartile. LAZ at time of stool collection was included as a covariate in all models as it is highly correlated with subsequent growth. In models assessing the association between short-term growth and composite EE score, β1 and β2 below were replaced by a single grouped linear term for composite EE score.
For comparison with the multisite study findings, the relationships between fecal markers and subsequent 6-month growth were also examined with the same modeling approach and residuals structure as in a previous paper of the multisite MAL-ED study.18
Results
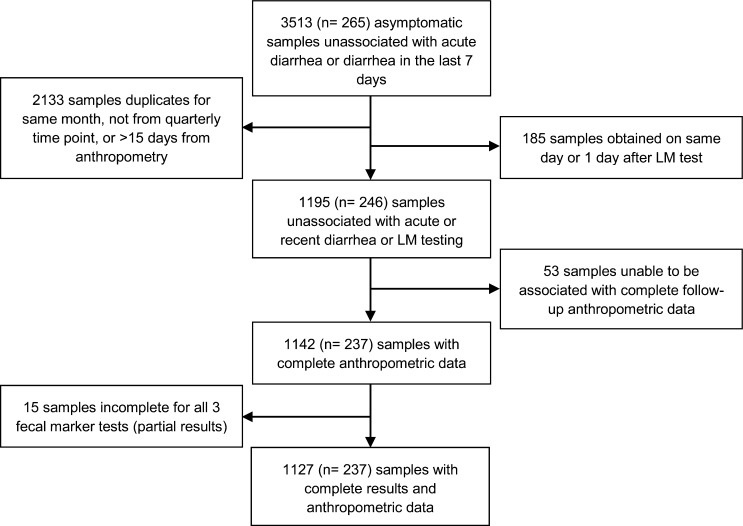
Overall, 265 children were enrolled. A total of 246 children provided stool samples which were unassociated with diarrhea or previous intestinal permeability testing. Summary statistics are provided for these 246 children and their mothers (Table 1). Gender was equally represented in this cohort. Of the 1,195 stool samples provided, 1,142 (N = 237) were accompanied by subsequent 3-month growth data, and 1,127 of these were tested for all three fecal markers (Figure 1 ). Of the 246 children whose samples were included in this study, 36 (14.6%) were lost to follow-up before 24 months of age, with 14 (5.7%) classified as lost to follow-up before 12 months of age.
Table 1.
Characteristics of participants (N = 246, unless otherwise noted)
| n (%) or median (IQR) | |||
|---|---|---|---|
| Infant | Female | 124 (50.4) | |
| Birth weight (kg) | 2.75 (2.47 to 3.06) | ||
| Birth length (cm) | 48.1 (47.0 to 49.5) | ||
| Anthropometry at birth | LAZ | −0.99 (−1.68 to −0.40) | |
| Stunting | 41 (16.7) | ||
| WAZ | −1.30 (−1.88 to −0.61) | ||
| Low birth weight (< 2,500 g) | 68 (27.6) | ||
| WHZ | −0.93 (−1.65 to −0.31) | ||
| Anthropometry (3 months) | LAZ | −1.17 (−1.80 to −0.47) | |
| Stunting | 38 (15.6) | ||
| WAZ | −0.89 (−1.65 to −0.39) | ||
| WHZ | 0.07 (−0.72 to 0.72) | ||
| Anthropometry (24 months)† | LAZ | −1.99 (−2.60 to −1.30) | |
| Stunting | 104 (49.5) | ||
| WAZ | −1.62 (−2.31 to −0.99) | ||
| WHZ | −0.87 (−1.35 to −0.10) | ||
| Breastfeeding | Exclusively breastfed until (day) | 102.5 (58 to 150) | |
| Diarrhea (days) | Days in year 1* | 11 (5 to 22) | |
| Days in year 2† | 8 (4 to 15) | ||
| Mother | Age (years) | 25 (21 to 28) | |
| Anthropometry at baseline | Height (cm) | 149 (146 to 153) | |
| Weight (kg) | 48.5 (43.1 to 55.8) | ||
| BMI | 21.8 (19.6 to 24.6) | ||
| BMI < 18.5 | 32 (13.0) | ||
| Marital status | Married—only wife | 212 (86.2) | |
| Married—polygamous | 34 (13.8) | ||
| Age at first marriage | 17 (16 to 19) | ||
| Maternal education | Never attended school | 46 (18.7) | |
| Schooling completed (years) | 5 (2 to 7) | ||
| Pregnancy information | Age at first pregnancy | 18 (17 to 20) | |
| Lifetime gravidity | 2 (1 to 3) | ||
| Number of live births | 2 (1 to 2) |
BMI = body mass index; IQR = interquartile range; LAZ = length-for-age z-score; WAZ = weight-for-age z-score; WHZ = weight-for-height z-score.
232 children with 12 months follow-up.
210 children with 24 months follow-up.
Figure 1.
One thousand one hundred and ninety-five samples from 246 children were evaluated for fecal levels of neopterin, myeloperoxidase, and alpha-1-antitrypsin. Samples used in the growth analysis were restricted to stools from children with no history of diarrhea in the last 7 days or history of lactulose administration on the day of or before stool collection and for which complete anthropometric data were available.
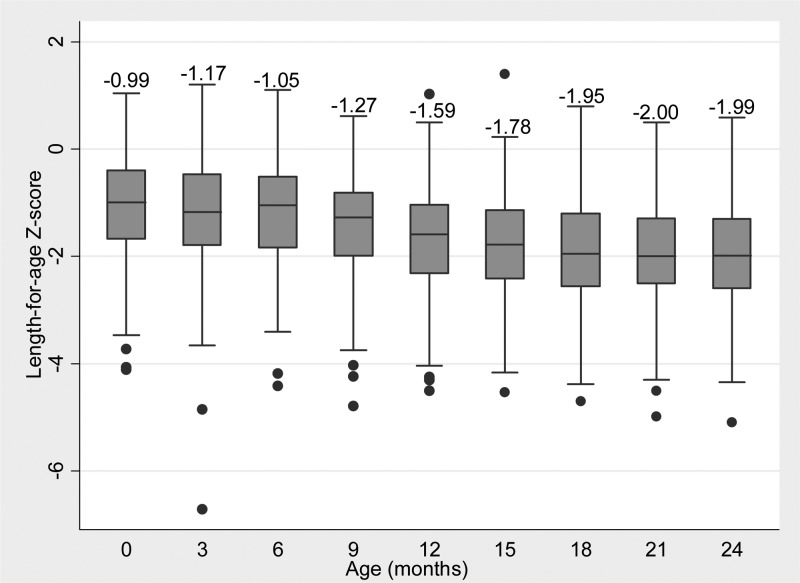
At birth, nearly 16% of children were stunted (median LAZ, weight-for-age z-score [WAZ], and weight-for-height z-score [WHZ] were −1, −1.3, and −0.9, respectively). Median LAZ declined by 3 months of age to −1.2; however, WAZ and WHZ improved to −0.9, and 0.1 respectively. Of the children stunted at birth, 78% were stunted at 24 months of age, compared with 44% of those not stunted at birth. Median LAZ was relatively stable in the first 6 months then declined steadily between months 6 and 18, before stabilizing at −2.0 (Figure 2 ).
Figure 2.
Quarterly length-for-age z-score (LAZ) among children contributing one or more stools unassociated with acute or recent diarrhea or lactulose-mannitol testing (N = 246, median value displayed).
Maternal characteristics.
Enrolled mothers had a median age of 25 years, with a median height and weight of 149 cm and 49 kg, respectively. More than 10% of mothers had a body mass index (BMI) less than 18.5 kg/m2. Most mothers were married and monogamous (86.2%), and had married in their late teens. More than 80% of mothers had received some schooling, with most completing at least 5 years. The majority of mothers had been pregnant previously, having become pregnant for the first time at a median age of 18.
Fecal marker distribution and categorization.
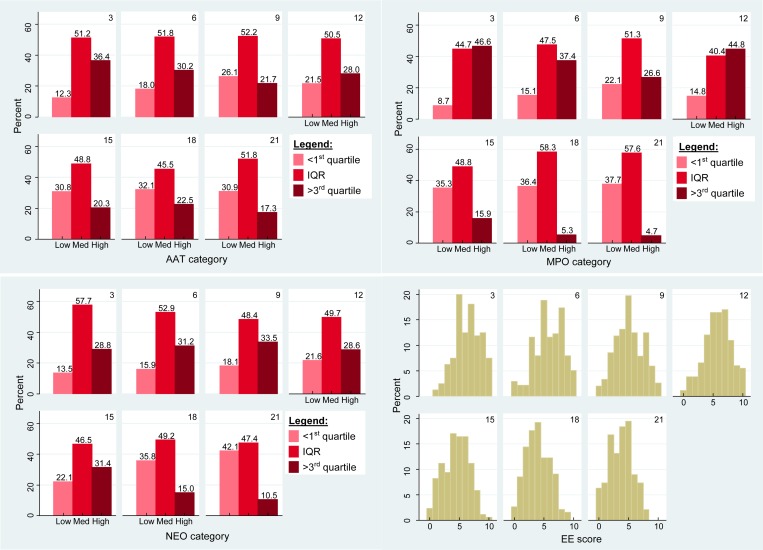
The distribution of AAT, MPO, and NEO from the 1,195 samples (N = 246) is presented in Table 2. The marker category distribution and composite EE score distribution by month of age is presented in Figure 3 . Approximately 63%, 68%, and 95% of samples were above values considered normal in nontropical settings for AAT (< 0.27 mg/g), MPO (< 2,000 ng/mL), and NEO (< 70 nmol/L), respectively.30–32
Table 2.
Fecal marker distribution in stool collected from 3 to 21 months of age (N = 246 children)
| AAT (mg/g) | MPO (ng/mL) | NEO (nmol/L) | EE score (0–10) | |
|---|---|---|---|---|
| First quartile | 0.1900 | 1,594.9 | 366.2 | 3 |
| Median | 0.3800 | 3,354.9 | 1,017.6 | 5 |
| Third quartile | 0.7175 | 7,430.1 | 2,210.8 | 7 |
| n (samples) | 1,194 | 1,185 | 1,190 | 1,179 |
AAT = alpha-1-antitrypsin; EE = environmental enteropathy; MPO = myeloperoxidase; NEO = neopterin.
Figure 3.
Fecal marker category distribution by month of age (N = 246 children).
Fecal markers and short-term linear growth.
Fecal levels of MPO in the full period (months 3–21) and the early period (months 3–9) were not associated with subsequent 3-month linear growth (Table 3). However, in the model restricted to the second year of life, children with high MPO levels at month 12, 15, 18, or 21 lost an average of 0.100 more LAZ in the subsequent 3-month period than children with low levels (95% confidence interval [CI] = −0.167 to −0.032) after adjustment for LAZ at time of marker assessment and diarrhea experienced. The crude estimate without adjustment for diarrhea was of similar magnitude (crude estimate = −0.094, 95% CI = −0.162 to −0.025). A sensitivity analysis which omitted quarterly MPO data in a stepwise fashion from the year 2 model did not substantially alter the magnitude of the estimate (−0.08 to −0.12 LAZ per 3-month period).
Table 3.
Fecal markers and subsequent 3-month change in LAZ
| Months 3–21 (N = 237) | Months 3–9 (N = 221§) | Months 12–21 (N = 221) | ||
|---|---|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | Coefficient (95% CI) | ||
| AAT | Low | Ref | Ref | Ref |
| Medium | −0.005 (−0.053 to 0.043) | 0.003 (−0.102 to 0.107) | −0.013 (−0.061 to 0.034) | |
| High | −0.042 (−0.098 to 0.014) | −0.037 (−0.153 to 0.078) | −0.042 (−0.100 to 0.017) | |
| Diarrhea proportion | −0.408* (−0.765 to −0.052) | −0.184 (−0.815 to 0.447) | −0.698† (−1.113 to −0.283) | |
| LAZ | −0.065‡ (−0.085 to −0.045) | −0.099‡ (−0.140 to −0.057) | −0.047‡ (−0.067 to −0.027) | |
| Constant | −0.143‡ (−0.215 to −0.071) | −0.193† (−0.313 to −0.074) | −0.198‡ (−0.263 to −0.132) | |
| N (samples) | 1,141 | 436 | 705 | |
| MPO | Low | Ref | Ref | Ref |
| Medium | 0.003 (−0.045 to 0.051) | 0.062 (−0.051 to 0.175) | −0.017 (−0.063 to 0.030) | |
| High | −0.047 (−0.107 to 0.013) | 0.030 (−0.089 to 0.149) | −0.100† (−0.167 to −0.032) | |
| Diarrhea proportion | −0.418* (−0.775 to −0.062) | −0.207 (−0.839 to 0.425) | −0.704† (−1.116 to −0.292) | |
| LAZ | −0.066‡ (−0.086 to −0.046) | −0.103‡ (−0.145 to −0.061) | −0.047‡ (−0.067 to −0.028) | |
| Constant | −0.148‡ (−0.222 to −0.073) | −0.258‡ (−0.388 to −0.129) | −0.165‡ (−0.233 to −0.096) | |
| N (samples) | 1,133 | 432 | 701 | |
| NEO | Low | Ref | Ref | Ref |
| Medium | 0.006 (−0.042 to 0.055) | 0.065 (−0.047 to 0.177) | −0.013 (−0.061 to 0.034) | |
| High | −0.019 (−0.076 to 0.038) | 0.019 (−0.101 to 0.140) | −0.024 (−0.084 to 0.035) | |
| Diarrhea proportion | −0.402* (−0.758 to −0.046) | −0.199 (−0.830 to 0.432) | −0.672† (−1.085 to −0.258) | |
| LAZ | −0.064‡ (−0.084 to −0.045) | −0.101‡ (−0.143 to −0.059) | −0.045‡ (−0.065 to −0.026) | |
| Constant | −0.161‡ (−0.233 to −0.089) | −0.253‡ (−0.379 to −0.127) | −0.200‡ (−0.265 to −0.135) | |
| N (samples) | 1,137 | 434 | 703 | |
| EE score | Score (0–10) | −0.009* (−0.018 to 0.000) | −0.002 (−0.019 to 0.015) | −0.013† (−0.023 to −0.004) |
| Diarrhea proportion | −0.433* (−0.790 to −0.076) | −0.209 (−0.844 to 0.425) | −0.740‡ (−1.155 to −0.324) | |
| LAZ | −0.064‡ (−0.084 to −0.044) | −0.100‡ (−0.143 to −0.058) | −0.048‡ (−0.068 to −0.028) | |
| Constant | −0.107* (−0.190 to −0.023) | −0.199† (−0.338 to −0.059) | −0.136† (−0.215 to −0.058) | |
| N (samples) | 1,127 | 428 | 699 |
AAT = alpha-1-antitrypsin; CI = confidence interval; EE = environmental enteropathy; LAZ = length-for-age z-score; MPO = myeloperoxidase; NEO = neopterin; Ref = reference.
P < 0.05.
P < 0.01.
P < 0.001.
N = 219 for MPO and composite EE score.
Fecal levels of AAT and NEO in months 3–21 were not associated with subsequent 3-month linear growth, nor were they associated with linear growth when marker data from months 3–9 and months 12–21 were examined separately (Table 3).
The composite EE score was negatively associated with subsequent 3-month LAZ change in the full period and when models were restricted to stool data from months 12–21, but not in months 3–9. A single unit increase in composite EE score in months 3–21 was associated with a loss of 0.009 LAZ per 3-month period (95% CI = −0.018 to 0.000), after adjustment for LAZ at time of marker assessment and diarrhea experienced. In the second year, each unit of composite EE score was associated with a loss of 0.013 LAZ per 3-month period (95% CI = −0.023 to −0.004). Therefore, a child with the highest composite EE score (10) lost 0.13 LAZ more than a child with a composite EE score of zero over the subsequent 3-month period. This corresponds to 0.36 cm less length gained between children in the highest versus the lowest composite EE score. When all models were reparameterized with marker concentrations categorized into tertiles, the results did not change substantially (data not shown). When the significance level was adjusted for multiple comparisons (using either the Benjamini–Hochberg or Bonferroni method), none of the marker coefficients remained significant.
In mixed-effects models of 6-month growth, none of the fecal markers tested, nor the composite EE score, were significantly associated with subsequent growth (Supplemental Table 1).
Discussion
High fecal MPO levels in Bangladeshi children were associated with decreases in 3-month linear growth in the second year of life. However, neither AAT nor NEO were associated with subsequent growth during any observed period in this analysis. In the present study, the composite EE score was associated with decreases in short-term linear growth. Prior MAL-ED multisite analyses also demonstrated an association between MPO and growth.18 In addition, a previous study in Bangladesh observed an association between MPO (but not AAT or NEO) at age 12 weeks, and change in LAZ over the first year of age among 700 infants living in the same area of Mirpur.33 However, our observations contrast with prior multisite analyses of MAL-ED data (which included some of the data presented here) which have reported an association between all three of these fecal markers and subsequent 6-month growth. Prior analyses have suggested that the composite EE score predicts such growth better than any single marker. In the present study, which was restricted to individuals in the Bangladesh MAL-ED site, none of the fecal markers tested, nor the composite EE score, were significantly associated with subsequent 6-month growth (Supplemental Table 1).
The etiologies of and risk factors for stunting differ in different populations. In this setting in an urban slum in Bangladesh, major contributors to stunting include low birth weight (LBW) as a result of intrauterine growth restriction and/or prematurity], inappropriate infant and young child feeding practices, food insecurity, recurrent infections, and EE. Consistent with other reports from south Asia, prenatal growth deficits were common in this cohort. In all, 27.6% of children had low birthweight (LBW; < 2,500 g), even though children with very low birth weight (< 1,500 g) were excluded.34 Nearly 17% of children had stunting at birth, and the mean birth LAZ was −1.0, well below the mean of −0.5 among newborns in developing settings.35 These prenatal growth deficits may be related in part to poor maternal nutrition and the small average size of mothers, 13% of whom had a BMI under 18.5 kg/m2. Prenatal factors may play a relatively more important role in Bangladesh as compared with other settings; 78% of the children stunted at birth were stunted at 24 months of age, compared with 44% of those who were not stunted at birth. Nevertheless, it appears that EE, as indicated by fecal MPO levels, contributed to growth faltering in this setting between ages 12 and 21 months.
Overall, AAT, MPO, and NEO levels among the children in this cohort from Mirpur were highly elevated in comparison to populations in high-income countries, suggesting widespread intestinal inflammation and increased intestinal permeability. Such levels, however, were lower than those reported in the multisite MAL-ED study (Supplemental Table 2).18 MPO levels in particular were much lower; the third quartile (7,430.1 ng/mL) was less than half of that observed in the previous study (20,526.3 ng/mL) and over 3,000 ng/mL lower than the multisite median (11,118.9 ng/mL). The present study includes marker data from children in a wider age range (3–21 months) than the previous multisite study (3–9 months), and because most marker levels declined during the second year (see median marker levels by age in Supplemental Table 3), this may have reduced the predictive ability of marker categories in the first year. The finding that MPO was predictive of linear growth despite lower levels observed in this setting suggests that the inflammation measured by MPO may significantly contribute to growth shortfalls in Bangladesh at levels lower than previously observed.
Because of the complex interplay among the many contributors to chronic malnutrition, nutritional interventions do not effectively normalize linear growth and reduce morbidity in stunted children less than 2 years of age. A systematic review of complementary feeding interventions targeting malnutrition among children 6–24 months of age reported that while several interventions strategies effectively improve the weight growth of children, the majority of interventions have modest effects on linear growth.36 It remains critical to improve the identification of children at risk of linear growth failure, so that corrective approaches may be tested and validated. EE is an appealing target in this regard, as the complex physiologic mechanisms by which unhygienic environmental exposures impede healthy growth may provide clues far in advance of significant growth or cognitive decline. Since none of the candidate EE markers included here provide a comprehensive measure of the persistent physiological dysfunction thought to underlie the relationship of EE with chronic malnutrition, the composite EE score was created in an attempt to more accurately reflect intestinal dysfunction by incorporating uncorrelated fecal marker data.17,18 However, while the composite EE score was associated with decreases in 3-month linear growth in the present study, the magnitude of effect for an 8–10 unit difference in composite EE score was similar to that for high MPO. In this setting, fecal MPO appears to be the most important contributor to the score's association with subsequent growth. The association between fecal MPO and short-term growth should be examined in other settings to determine the generalizability of these findings and to further gauge the potential usefulness of MPO as a screening tool for linear growth failure.
Our study had several important limitations. There was loss to follow-up among the included children; nearly 15% of children were lost to follow-up before 24 months of age. In addition, a lower number of stools were fully tested at each quarterly time point from months 3–9 than in the second year. This may have been due in part to the higher incidence of diarrhea episodes in the early period. The higher amount of missing marker data in the first year reduced the power to detect associations with short-term growth in that period. About 20% (173) of the 817 stool samples included from children between 3 and 9 months were also included in the previous multisite study, constituting 164 AAT results, 152 MPO results, and 127 NEO results. These samples had a higher median MPO concentration (8,800 ng/mL) than the full set of samples from months 3–9 in the present study (5,444 ng/mL), and may have influenced the analysis of MPO and short-term growth. Median NEO (1,470 nmol/L) and AAT (0.49 mg/g) concentrations in these samples did not differ substantially from the full set of samples from months 3–9. AAT as a marker of intestinal permeability may be less sensitive to small gaps in mucosal integrity due to its large size, than smaller molecules such as urinary lactulose. Although no fecal marker P values remain significant when adjusted for multiple comparisons using the Benjamini–Hochberg or Bonferroni method, the use of unadjusted P values is acceptable in exploratory analyses. Our comparisons to the multisite study must be read with some caution, as these analyses may not be sufficiently powered to detect differences in effects, and no formal statistical comparison was run. Although inflammatory marker data was available from children at time points closer to birth, we chose to use equally spaced marker data with nonoverlapping growth periods. Fecal EE markers from samples collected at birth may not reflect postnatal environmental insults, and their inclusion may attenuate estimates of the association between EE and linear growth.
Despite these limitations, this study was well designed, benefited from high-quality laboratory facilities, skilled staff, and used appropriate statistical methods. Enrolled children were recruited from a single setting using a well-defined recruitment protocol and rigorous inclusion and exclusion criteria established by the MAL-ED consortium, facilitating comparability of results with those from other MAL-ED sites. The high population density, poor sanitation, and low socioeconomic status of the Bauniabadh area are representative of a typical urban slum in Dhaka and are similar to others in south Asia.27 A unique strength in the present study is that children were visited several times each week to collect highly detailed surveillance information on the incidence of diarrhea, respiratory disease, and other morbidity events. The detailed morbidity data facilitated precise adjustment for diarrhea in analyses and enabled the exclusion of stool samples whose close proximity to diarrhea symptoms or intestinal permeability testing would have potentially diluted marker concentrations.17 The use of piecewise linear mixed effects models to examine associations between fecal markers and growth allowed for natural variation in growth rates at different ages, enabled the inclusion of time-varying covariates, and adequately accounted for correlated data coming from the same individuals. This approach also had good interpretability and comparability with the previous study.18
In summary, in this analysis of children in Mirpur, Bangladesh, only high MPO levels were associated with decreases in short-term linear growth only in the second year of life. The composite EE score was negatively associated with subsequent 3-month LAZ change, mostly driven by fecal MPO levels. In Bangladeshi children, fecal MPO appears predictive of linear growth at levels lower than in the multisite MAL-ED analysis. Our findings suggest that the overall attributable impact of EE on linear growth outcomes may differ in various settings and populations, suggesting that further efforts to improve the interpretation of fecal markers may need to be site or population specific.
Supplementary Material
ACKNOWLEDGMENTS
We thank the staff of the MAL-ED Network, the field and laboratory staff at icddr,b, and the participants of the MAL-ED Mirpur birth cohort for their important contributions.
Disclaimer: The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) study is a collaborative project supported by the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This research and publication were made possible with support from the Bill & Melinda Gates Foundation EED Planning Grant.
Authors' addresses: Michael B. Arndt, Department of Epidemiology, University of Washington, Seattle, WA, E-mail: marndt@uw.edu. Barbra A. Richardson, Department of Biostatistics, University of Washington, Seattle, WA, and Department of Global Health, University of Washington, Seattle, WA, E-mail: barbrar@uw.edu. Tahmeed Ahmed and Mustafa Mahfuz, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, E-mails: tahmeed@icddrb.org and mustafa@icddrb.org. Rashidul Haque, Parasitology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), Dhaka, Bangladesh, E-mail: rhaque@icddrb.org. Grace C. John-Stewart and Judd L. Walson, Department of Epidemiology, University of Washington, Seattle, WA, Department of Global Health, University of Washington, Seattle, WA, Department of Medicine, University of Washington, Seattle, WA, and Department of Pediatrics, University of Washington, Seattle, WA, E-mails: gjohn@uw.edu and walson@uw.edu. Donna M. Denno, Department of Global Health, University of Washington, Seattle, WA, and Department of Pediatrics, University of Washington, Seattle, WA, E-mail: ddenno@uw.edu. William A. Petri Jr., Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, VA, E-mail: wap3g@virginia.edu. Margaret Kosek, Division of Global Disease Epidemiology and Control, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, E-mail: mkosek@jhu.edu.
References
- 1.de Onis M, Blössner M, Borghi E. Prevalence and trends of stunting among pre-school children, 1990–2020. Public Health Nutr. 2012;15:142–148. doi: 10.1017/S1368980011001315. [DOI] [PubMed] [Google Scholar]
- 2.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, Caulfield LE, Danaei G. Nutrition Impact Model Study (anthropometry cohort pooling) Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. 2013;8:e64636. doi: 10.1371/journal.pone.0064636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, Shekar M. Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371:417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 5.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R. Maternal and Child Nutrition Study Group Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 7.Berkman D, Lescano A, Gilman R, Lopez S, Black M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- 8.Mendez M, Adair L. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129:1555–1562. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 9.Powell C, Walker S, Himes J, Fletcher P, Grantham-McGregor S. Relationships between physical growth, mental development and nutritional supplementation in stunted children: the Jamaican study. Acta Paediatr. 1995;84:22–29. doi: 10.1111/j.1651-2227.1995.tb13479.x. [DOI] [PubMed] [Google Scholar]
- 10.Dewey K, Begum K. Long-term consequences of stunting in early life. Matern Child Nutr. 2011;7((Suppl 3)):5–18. doi: 10.1111/j.1740-8709.2011.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozaltin E, Hill K, Subramanian S. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA. 2010;303:1507–1516. doi: 10.1001/jama.2010.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keusch GT, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, Nataro JP, Rosenberg IH, Ryan ET, Tarr PI, Ward H, Bhutta ZA, Coovadia H, Lima A, Ramakrishna B, Zaidi AKM, Hay Burgess DC, Brewer T. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis. 2014;59:S207–S212. doi: 10.1093/cid/ciu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–1035. doi: 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 14.Korpe PS, Petri WA. Environmental enteropathy: critical implications of a poorly understood condition. Trends Mol Med. 2012;18:328–336. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59:S213–S219. doi: 10.1093/cid/ciu541. [DOI] [PubMed] [Google Scholar]
- 17.Kosek M, Guerrant RL, Kang G, Bhutta Z, Yori PP, Gratz J, Gottlieb M, Lang D, Lee G, Haque R, Mason CJ, Ahmed T, Lima A, Petri WA, Houpt E, Olortegui MP, Seidman JC, Mduma E, Samie A, Babji S. MAL-ED Network Investigators Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Dis. 2014;59:S239–S247. doi: 10.1093/cid/ciu457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RL, Bhutta Z, Mason C, Kang G, Kabir M, Amour C, Bessong P, Turab A, Seidman J, Olortegui MP, Quetz J, Lang D, Gratz J, Miller M, Gottlieb M. MAL-ED Network Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson KM, Buss J, Easley R, Yang Z, Korpe PS, Niu F, Ma JZ, Olortegui MP, Haque R, Kosek MN, Petri WA. REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am J Clin Nutr. 2013;97:1129–1133. doi: 10.3945/ajcn.112.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widner B, Wirleitner B, Baier-Bitterlich G, Weiss G, Fuchs D. Cellular immune activation, neopterin production, tryptophan degradation and the development of immunodeficiency. Arch Immunol Ther Exp (Warsz) 2000;48:251–258. [PubMed] [Google Scholar]
- 21.Wagner M, Peterson CG, Ridefelt P, Sangfelt P, Carlson M. Fecal markers of inflammation used as surrogate markers for treatment outcome in relapsing inflammatory bowel disease. World J Gastroenterol. 2008;14:5584–5589. doi: 10.3748/wjg.14.5584. discussion 5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson CG, Eklund E, Taha Y, Raab Y, Carlson M. A new method for the quantification of neutrophil and eosinophil cationic proteins in feces: establishment of normal levels and clinical application in patients with inflammatory bowel disease. Am J Gastroenterol. 2002;97:1755–1762. doi: 10.1111/j.1572-0241.2002.05837.x. [DOI] [PubMed] [Google Scholar]
- 23.Hill RE, Hercz A, Corey ML, Gilday DL, Hamilton JR. Fecal clearance of alpha 1-antitrypsin: a reliable measure of enteric protein loss in children. J Pediatr. 1981;99:416–418. doi: 10.1016/s0022-3476(81)80332-0. [DOI] [PubMed] [Google Scholar]
- 24.Bernier JJ, Florent C, Desmazures C, Aymes C, L'Hirondel C. Diagnosis of protein-losing enteropathy by gastrointestinal clearance of alpha1-antitrypsin. Lancet. 1978;2:763–764. doi: 10.1016/s0140-6736(78)92650-8. [DOI] [PubMed] [Google Scholar]
- 25.Karbach U, Ewe K, Bodenstein H. Alpha 1-antitrypsin, a reliable endogenous marker for intestinal protein loss and its application in patients with Crohn's disease. Gut. 1983;24:718–723. doi: 10.1136/gut.24.8.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, Ariawan I, Uh HW, Wibowo H, Djuardi Y, Wahyuni S, Sutanto I, May L, Luty AJ, Verweij JJ, Sartono E, Yazdanbakhsh M, Supali T. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study) BMC Infect Dis. 2010;10:77. doi: 10.1186/1471-2334-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmed T, Mahfuz M, Islam MM, Mondal D, Hossain MI, Ahmed AS, Tofail F, Gaffar SA, Haque R, Guerrant RL, Petri WA. The MAL-ED cohort study in Mirpur, Bangladesh. Clin Infect Dis. 2014;59:S280–S286. doi: 10.1093/cid/ciu458. [DOI] [PubMed] [Google Scholar]
- 28.Investigators TM-EN The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59:S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 29.Richard SA, McCormick BJJ, Miller MA, Caulfield LE, Checkley W. Modeling environmental influences on child growth in the MAL-ED cohort study: opportunities and challenges. Clin Infect Dis. 2014;59:S255–S260. doi: 10.1093/cid/ciu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiki T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med J. 1998;45:69–73. doi: 10.2739/kurumemedj.45.69. [DOI] [PubMed] [Google Scholar]
- 31.Beckmann GT, Rüffer A. Mikroökologie des Darmes: Grundlagen, Diagnostik, Therapie [Microbiology of the Intestines: Basics, Diagnostics, Therapy] Hannover, Germany: Schlütersche; 2000. [Google Scholar]
- 32.Ledjeff E, Artner-Dworzak E, Witasek A, Fuchs D, Hausen A. Neopterin concentrations in colon dialysate. Pteridines. 2001;12:155–160. [Google Scholar]
- 33.Naylor C, Lu M, Haque R, Mondal D, Buonomo E, Nayak U, Mychaleckyj JC, Kirkpatrick B, Colgate R, Carmolli M, Dickson D, van der Klis F, Weldon W, Steven Oberste M, Ma JZ, Petri WA., Jr Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2:1759–1766. doi: 10.1016/j.ebiom.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wardlaw TM. Low Birthweight: Country, Regional and Global Estimates. New York, NY: UNICEF; 2004. [Google Scholar]
- 35.Victora C, Onis M, Hallal P, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 36.Dewey K, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4((Suppl 1)):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.