Abstract
Background
With over 3,500 species encompassing a diverse range of morphologies and ecologies, snakes make up 36% of squamate diversity. Despite several attempts at estimating higher-level snake relationships and numerous assessments of generic- or species-level phylogenies, a large-scale species-level phylogeny solely focusing on snakes has not been completed. Here, we provide the largest-yet estimate of the snake tree of life using maximum likelihood on a supermatrix of 1745 taxa (1652 snake species + 7 outgroup taxa) and 9,523 base pairs from 10 loci (5 nuclear, 5 mitochondrial), including previously unsequenced genera (2) and species (61).
Results
Increased taxon sampling resulted in a phylogeny with a new higher-level topology and corroborate many lower-level relationships, strengthened by high nodal support values (> 85%) down to the species level (73.69% of nodes). Although the majority of families and subfamilies were strongly supported as monophyletic with > 88% support values, some families and numerous genera were paraphyletic, primarily due to limited taxon and loci sampling leading to a sparse supermatrix and minimal sequence overlap between some closely-related taxa. With all rogue taxa and incertae sedis species eliminated, higher-level relationships and support values remained relatively unchanged, except in five problematic clades.
Conclusion
Our analyses resulted in new topologies at higher- and lower-levels; resolved several previous topological issues; established novel paraphyletic affiliations; designated a new subfamily, Ahaetuliinae, for the genera Ahaetulla, Chrysopelea, Dendrelaphis, and Dryophiops; and appointed Hemerophis (Coluber) zebrinus to a new genus, Mopanveldophis. Although we provide insight into some distinguished problematic nodes, at the deeper phylogenetic scale, resolution of these nodes may require sampling of more slowly-evolving nuclear genes.
Introduction
Phylogenies form the cornerstone of our understanding of evolutionary relationships between organisms and provide a historical basis for testing and inferring ecological and evolutionary processes [1–4]. Although phylogenetic methodologies have witnessed an explosion of advancements, estimating large trees remains costly, time-intensive, and computationally difficult. Thus, most analyses have concentrated on resolving the relationships of smaller taxonomic groups, culminating in the accumulation of published sequences available for compiling into larger datasets, or "super-matrices" [5,6]. Coalescent-based species-trees methods are currently favored over concatenated approaches owing to their greater accuracy, but their use for large datasets is still impractical [7,8]. Consequently, many researchers rely on the supermatrix approach [9] or on shortcut coalescence methods [10]. The supermatrix uses concatenated sequences to estimate large-scale phylogenies with branch lengths [11–17]. This technique has earned criticism because large amounts of missing data may obscure phylogenetic signal, leading to uncertainty in topology and branch lengths [18–21], but shortcut coalescence methods are also prone to these same shortcomings [10]. However, several studies have shown that concatenated procedures may nonetheless produce similar results to species-trees [8,22], particularly when there is no agreement among gene trees, and between gene and species trees [7]. This is also the case for deep divergences because shortcut coalescence has difficulty integrating gene-tree incongruity at this level [10]. Our goal for this study was to estimate a species-level phylogeny for snakes using the supermatrix technique.
To date, only two studies have estimated a species-level phylogeny of snakes [15,23], with the latter adding more independent loci to the dataset of the former. These studies featured 1262 known snake species, integrated as part of a larger phylogeny focusing on Squamata, accounting for merely 39% of the total snake diversity at the time. At greater than 3,500 species [24], over a thousand more than the estimate provided by Heise et al [25] two decades earlier, and with the recent recognition of new families and subfamilies [26–31], phylogenetic estimates of the snake tree of life are markedly underrepresented. Indeed, the first phylogenetic analysis including all families and subfamilies was only recently completed [32], and only included one representative from each rank. Over the years, researchers have emphasized resolving higher-level snake relationships [15,22,23,25,27,32–49], and topology within families: typhlopids [26,29,31,50]; boids [30,51–53]; acrochordids [54]; xenodermatids [55]; homalopsids [56,57]; pareatids [58]; viperids [59–61]; elapids and lamprophiids [28,62–64]; dipsads [65,66]; pseudoxendontids [67]; natricines [68]; sibynophiids [27]; and colubrids [39,40]. Despite these efforts, many unresolved nodes remain scattered throughout the entire snake tree, such as the monophyly of Scolecophidia [15], topology of Typhlopinae [29], monophyly of Cylindrophiidae and Anomochilidae [35], topology of Booidea [30,53], placement of Xenophidiidae and Bolyeridae [53], and several issues within Caenophidia [22,39,40]. With higher-level relationships of snakes still not settled, our understanding of the snake tree of life remains incomplete.
Although snakes have received a great deal of attention from biologists [69–71], studies of snake biology from comparative and evolutionary perspectives are scarce relative to other reptile taxa such as lizards, in part because of the lack of comprehensive and well-supported snake phylogenies. Estimating a clade-wide species-level phylogeny for snakes with utility for testing evolutionary hypotheses will greatly augment our knowledge of snake biology. Here, we present an updated hypothesis on extant snake phylogeny with increased sampling using the supermatrix approach comprising 1745 taxa (1652 snake species + 7 outgroup taxa), representing 46.33% of the currently known snake species from all known families and subfamilies (Table 1), an increase of 7.24% from Pyron et al [15] and Zheng and Wiens [23]. Accepting this tree, we discuss higher-level relationships and highlight taxonomic issues at the genus-level.
Table 1. Number of taxa sampled per family or subfamily.
Families are listed in order according to Fig 1. For the taxonomy of families and subfamilies, we use Adalsteinsson et al, [26] for Anomalepididae and Leptotyphlopidae, Pyron and Wallach [29] for Gerrhopilidae, Typhlopidae, and Xenotyphlopidae, Pyron et al [30] for Booidea, and Pyron et al [15] for Alethinophidia. The number of species per clade was taken from The Reptile Database (http://www.reptile-database.org/) on 10/01/2015. Percentages of the number of species sampled do not include taxa not assigned to species status. Paraphyletic taxa are included under their traditional family and/or subfamily. In the Total cell for total number of species, the number not in parentheses equals the sum of the values in the table and the number in the parentheses equals the number returned when a search for Serpentes is conducted in The Reptile Database. Percentage for total number of species sampled is based on 3566 species.
| Clade | Number of Species Sampled (% Sampled) | Total Number of Species |
|---|---|---|
| Scolecophidia | ||
| Anomalepididae | 2 (11%) | 18 |
| Leptotyphlopidae | — | — |
| Epictinae | 17 (23%)– 2 sp. | 64 |
| Leptotyphlopinae | 18 (36%) | 50 |
| Gerrhopilidae | 2 (11%) | 18 |
| Xenotyphlopidae | 2 (100%)– 1 sp. | 1 |
| Typhlopidae | ||
| Typhlopinae | 52 (52%)– 19 sp. | 64 |
| Afrotyphlopinae | 19 (26%)– 3 sp. | 61 |
| Madatyphlopinae | 2 (15%) | 13 |
| Asiatyphlopinae* | 49 (33%)– 8 sp. | 124 |
| Alethinophidia | ||
| Aniliidae | 1 (100%) | 1 |
| Tropidophiidae | 10 (29%) | 34 |
| Calabariidae | 1 (100%) | 1 |
| Candoiidae | 3 (60%) | 5 |
| Sanziniidae | 3 (75%) | 4 |
| Charinidae | ||
| Charininae | 3 (75%) | 4 |
| Ungaliophiinae | 3 (100%) | 3 |
| Erycidae | 9 (75%) | 12 |
| Boidae | 24 (80%) | 30 |
| Cylindrophiidae | 2 (15%) | 13 |
| Anomochilidae | 1 (33%) | 3 |
| Uropeltidae | 15 (28%)– 1 sp. | 54 |
| Xenopeltidae | 1 (50%) | 2 |
| Loxocemidae | 1 (100%) | 1 |
| Pythonidae | 32 (80%) | 40 |
| Bolyeridae | 1 (50%) | 2 |
| Xenophidiidae | 1 (50%) | 2 |
| Acrochordidae | 3 (100%) | 3 |
| Xenodermatidae | 4 (22%) | 18 |
| Pareatidae | 16 (80%) | 20 |
| Viperidae | ||
| Viperinae | 66 (67%) | 98 |
| Azemiopinae | 1 (50%) | 2 |
| Crotalinae | 190 (82%)– 1 sp. | 231 |
| Homalopsidae | 26 (47%)– 1 sp. | 53 |
| Lamprophiidae | ||
| Psammophiinae | 45 (87%)– 3 sp. | 52 |
| Prosymninae | 5 (31%) | 16 |
| Pseudaspidinae | 2 (100%) | 2 |
| Atractaspidinae | 7 (30%) | 23 |
| Aparallactinae | 11 (23%) | 47 |
| Lamprophiinae | 31 (43%) | 72 |
| Pseudoxyrhophiinae | 61 (64%)– 4 sp. | 89 |
| Elapidae | 195 (54%)– 1 sp. | 358 |
| Colubridae | ||
| Sibynophiinae | 6 (55%) | 11 |
| Natricinae | 110 (47%)– 3 sp. | 226 |
| Pseudoxenodontinae | 5 (36%)– 1 sp. | 11 |
| Dipsadinae | 242 (32%)– 2 sp. | 754 |
| Grayiinae | 3 (75%) | 4 |
| Calamariinae | 4 (5%) | 87 |
| Ahaetullinae subfam. nov. | 27 (48%) | 56 |
| Colubrinae | 315 (47%)– 3 sp. | 670 |
| Incertae Sedis | 4† | 22 |
| TOTAL | 1652 (46.33%) | 3549 (3566) |
*Number of species of Xerotyphlops is included in Asiatyphlopinae.
†Buhoma depressiceps, Buhoma procterae, and Oxyrhabdium leporinum are all listed as incertae sedis on The Reptile Database, but Micrelaps bicoloratus is not. We list these four species as incertae sedis because of their variable topological history (see Fig 1).
Materials and Methods
Tissue data collection and sequence acquisition
We constructed a dataset of 1745 taxa (1659 species), of which the following seven species represent outgroups: Calotes versicolor, Chamaeleo calyptratus, Elgaria multicarinata, Heloderma suspectum, Liolaemus darwinii, Plica plica, and Varanus salvator. The dataset consisted of 9,523 bp from the following 10 genes: three mitochondrial protein-coding genes, cytochrome b (cyt-b; 1,107 bp; 1,398 taxa), NADH subunit 2 (ND2; 1,042 bp; 334 taxa), and NADH subunit 4 (ND4; 802 bp; 986 taxa); two non-coding ribosomal genes (12S; 790 bp; 1,023 taxa) and (16S; 649 bp; 1,167 taxa); and five nuclear protein-coding genes, brain-derived neurotrophic factor precursor (BDNF; 675 bp; 314 taxa), neurotrophin-3 (NT3; 669 bp; 449 taxa), oocyte maturation factor Mos (c-mos; 753 bp; 957 taxa), and two recombination-activating genes (RAG-1.1; 926 bp; 209 taxa, RAG-1.2; 880 bp; 166 taxa; RAG-1.3; 517 bp; 153 taxa), and (RAG-2; 716 bp; 153 taxa). We split RAG-1 into three separate alignments because the majority of sequences did not overlap, but instead formed three separate segments of overlapping sequences. Sequences for seven outgroups and 1591 snake species were downloaded from GenBank (S1 Table). To maximize gene coverage for each species, we combined sequences from multiple individuals of the same species. We sequenced an additional 150 tissue samples from 88 species, of which 61 were not previously sequenced (S2 Table). Eighteen we field collected and 132 we obtained from museum vouchers. For field collected samples, we obtained tissue from tail clips or ventral scale clips using sterilized scissors, from snakes collected in Costa Rica and Singapore. We placed all tissue samples in 90% ethanol under the Alexander D. McKelvy Field Series (ADM). Methods for tissue collection were approved by the University of New Orleans Animal Welfare Committee and by both permitting agencies for each country: Costa Rica, Ministerio del Ambiente y Energía Sistema Nacional de Areas de Conservación, permit ACTo-GASP-PIN-023-2010, and; Singapore, NParks, permit NP/RP11-030. Museum tissue samples represent a combination of liver, muscle, and heart tissue and were gathered from the following museums: AMNH, CAS, FMNH, KU, LSUHC, LSUMNS, MVZ, and YPM (refer to S2 Table for museum codes). Species we sequenced are identified by species name and voucher number (S2 Table). For taxonomic classification, we consulted The Reptile Database (http://www.reptile-database.org/). As of October 2015, the database recognizes 3566 species of snakes. Our dataset accounted for approximately 46.33% of currently recognized snake species.
DNA extraction, amplification, sequencing, and alignment
We extracted genomic DNA from tissue samples following the standard protocol provided for Qiagen® DNeasy kits. We sequenced six genes: 16S, c-mos, cyt-b, ND4, NT3, and RAG-1. A list of the primers used, their source, and annealing temperatures are provided in S3 Table. We aliquoted a 2 μl portion of each purified DNA extract and combined it with GoTaq Green MasterMix (Promega Corp.), primers from respective gene, and deionized water to create a 10 μl reaction to be used in the Polymerase Chain Reaction (PCR). We placed all PCR reactions on a thermal cycler under the following protocol: 95°C for 2 min; 95°C for 30 s; 50°C for 30 s for 40 cycles; 72°C for 1:15 min; 72°C for 3–5 min; and chilled at 4°C until taken off cycler. Next, we cleaned the PCR products using 1 μL of ExoSap-IT (USB Corp.) per 10 μL of PCR product. We performed cycle sequencing on purified PCR products using 1 μL primer (10 μM), 2 μL template, and 5 μL deionized water along with a Big Dye Terminator 3.1 (Amersham Pharmacia Biotech) reaction premix for 50 cycles of 96°C for 10 s; 45°C for 5 s; and 60°C for 4 min and purified using a Sephdex column, then used an ABI 3130XL Genetic Analyzer to determine nucleotide sequences of each sample.
We aligned all sequences using the default parameters of the Geneious alignment, and refined alignments using the default parameters of the MUSCLE alignment [72] in the program Geneious v4.8.4 (http://www.genious.com; [73]). We then edited alignments by eye and trimmed ambiguous end regions. For some genes, a few species had identical sequences with other taxa so we retained the first taxon in alphabetical order ([15]; S1 Table). Finally, we used Geneious to concatenate all genes to create a supermatrix. This matrix contained 71.41% of missing data; however, previous studies have shown that missing data does not negatively influence topology, branch length estimates, and node support [15,23,40,41]. We deposited all sequences generated from this study in GenBank (S2 Table). The final alignment is available in Phylip format in S1 File.
Phylogenetic inference
We performed phylogenetic analyses on the 10-gene concatenated matrix using the maximum likelihood (ML) criterion in the program RAxML HpC-2 v8 [74] on the CIPRES portal (http://www.phylo.org; [75]). First, we analyzed each gene separately to check topological congruence by performing rapid bootstrap analyses and pruned misplaced taxa with suspect placement out of the alignment, before concatenating them into the final alignment. The following five species were removed from the alignment due to poor placement for all genes: Boiga siamensis FMNH267726, Chrysopelea ornata LSUHC7158, Dipsadoboa werneri, Emydocephalus ijmae, and Psammodynastes pictus FMNH267940. We conducted analyses by generating starting trees under the default parsimony model and obtained node support from 100 non-parametric bootstrap replicates using the GTRGAMMA model for all genes and codon partitions since the GTRGAMMA model is recommended over GTR + Γ + I as the 25 rate categories implemented with GTRGAMMA accounts for potentially invariant sites [76]. After concatenating the genes, we performed a rapid bootstrap analysis on the data partitioned by gene and codon position and obtained node support from 1000 non-parametric bootstrap replicates using the GTRGAMMA model.
Rogue taxa can present themselves in phylogenetic estimates due to ambiguous or insufficient phylogenetic signal [77]. These taxa decrease resolution and support in any best tree estimate because they cannot be placed with any confidence anywhere in the tree due to occupying numerous different phylogenetic positions in a set of trees [78]. Thus to produce a more informative best tree estimate with improved clade support, we identified and eliminated rogue taxa with the webserver version of RogueNaRok at http://rnr.h-its.org/submit [79] using the support on best tree estimate threshold, optimizing support, and maximum dropset size of 1. To avoid pruning a large number of taxa, we only pruned 22 taxa that had a random improvement score (i.e., fraction of improvement in bootstrap support values throughout the tree when the selected taxon is pruned and all rogue taxa above it are also pruned) above 0.8 (S4 Table). We acknowledge that excluding additional rogue taxa will improve clade support values, but we wanted to include a maximum number of taxa to estimate a more comprehensive phylogeny. After pruning rogue taxa, the final dataset resulted in 1745 taxa (1659 species). We then performed 10 ML searches on 10 random stepwise addition parsimony-based starting trees using the GTRGAMMA model. Next, we executed a final topology optimization on the best scoring ML tree to produce a nearest-neighbor interchange (NNI)-optimized estimate of the ML tree also using the GTRGAMMA model. Finally, we assessed node support using the non-parametric Shimodaira-Hasegawa-Like (SHL) implementation of the approximate likelihood-ratio test (aLRT; [80]) based on several advantages over other support methods and considered SHL values of 85% or greater as strong support [15]. We also estimated the tree with all rogue taxa from the first analysis and species classified as incertae sedis, all within the family Lamprophiidae (Buhoma depressiceps, Buhoma procterae, Micrelaps bicoloratus, and Oxyrhabdium leporinum), eliminated to scrutinize their influence on higher-level relationships.
Nomenclatural acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is: urn:lsid:zoobank.org:pub: 3966804E-D532-4C52-92AC-BECAE776E434. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
Results and Discussion
Higher-level phylogeny
As in previous studies, we find very strong support (SHL = 100) for the clade Serpentes [15,23,36,42,48,81]. In Fig 1 we display a summary of the full ML tree (lnL = -919390.188) to exhibit relationships above the genus-level and present the full species-level tree in Figs 2–10, made available in Newick format in S2 File. Overall, more than half of the nodes in the full species-tree received strong support (73.45% of nodes with SHL values > 85). In the following section we largely compare our tree to Pyron et al [15], since they provide a recent detailed comparison to preceding publications and because theirs is the only other clade-wide species-level tree (but see [23]). In general, we substantiate many of the higher-level relationships reported in Pyron et al [15]; however, several differences also exist. Support for monophyly for each family and subfamily was above 88%, except for Gerrhopilidae (SHL = 48), and Cylindrophiidae was paraphyletic with Anomochilidae [23,35,53].
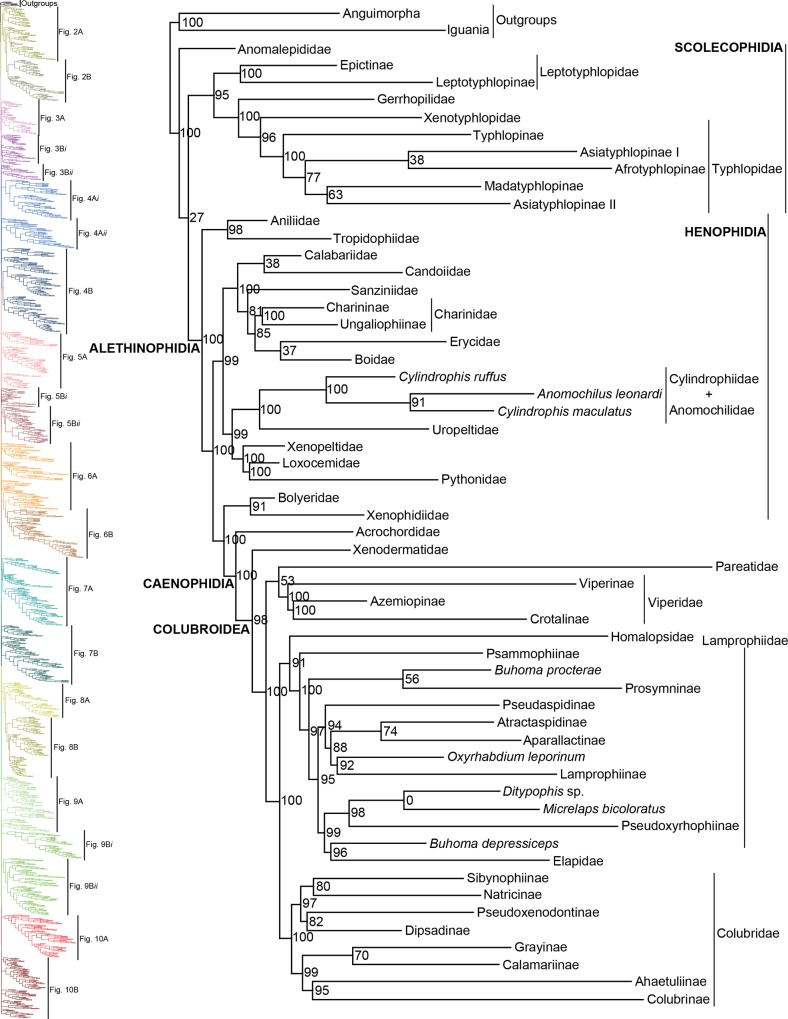
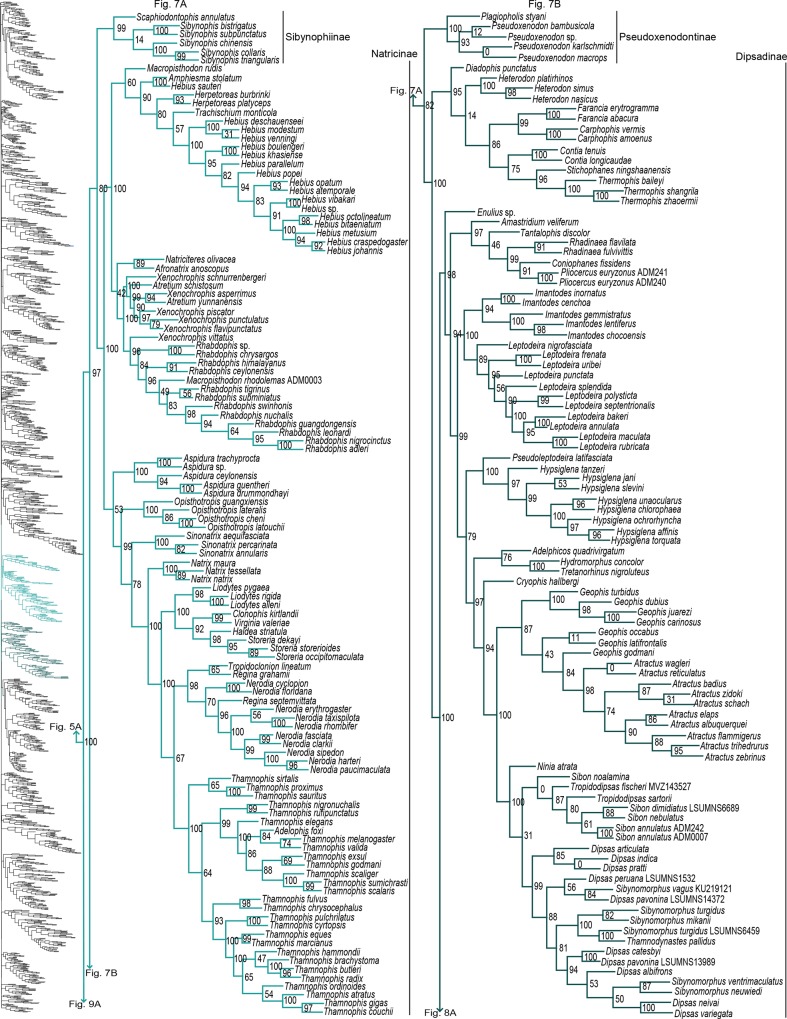
Fig 1. Abridged phylogeny on final dataset of 1652 snake species and seven outgroup taxa displaying higher-level relationships.
Maximum-likelihood phylogenetic estimate based on 10 concatenated genes. Tips represent families and sub-families. Commonly recognized higher-level clades are labeled in all caps and bold. Species classified as Lamprophiidae incertae sedis are also shown since they did not place within a subfamily. Node values represent SHL support values. Skeleton of the species tree is displayed on the left, colored and labeled as they appear in Figs 2–10.
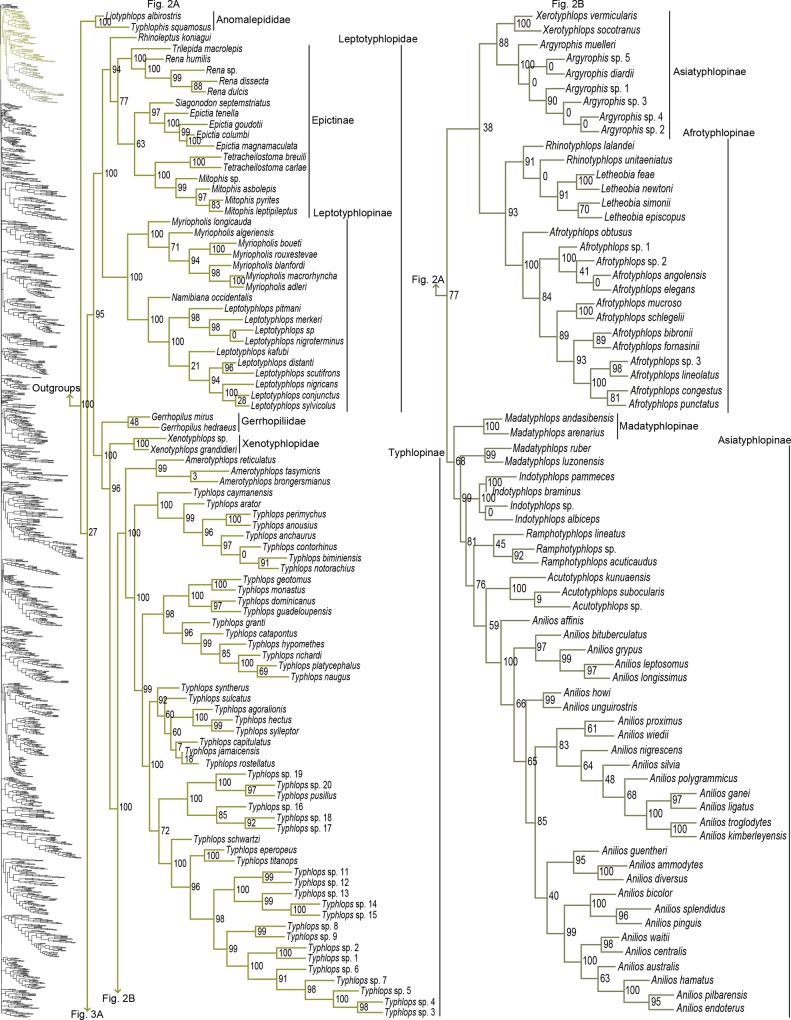
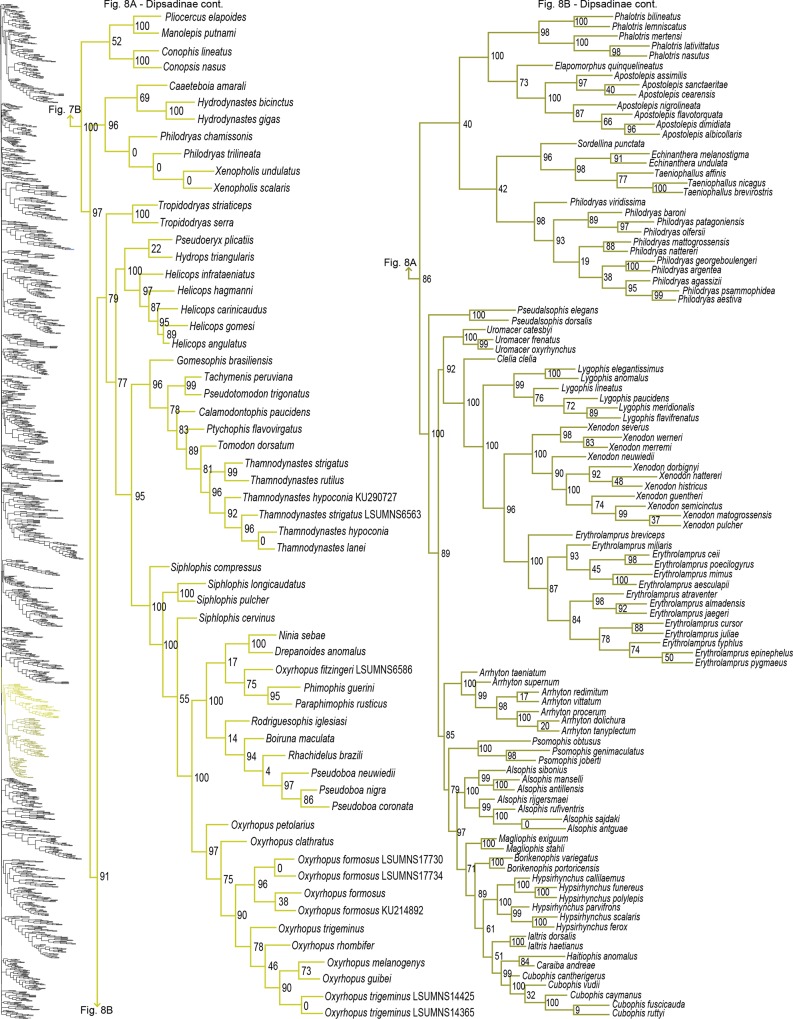
Fig 2. Species-level phylogeny on final dataset of 1652 snake species.
Maximum-likelihood phylogenetic estimate based on 10 concatenated genes. Node values represent SHL support values. Seven outgroup taxa are not shown. Colors of clades indicate their position in the overall tree, shown at left. Newly sequenced taxa are highlighted in bold. Skeleton of the species tree is displayed on the left with displayed subfamilies/families highlighted. Letters denoted by i and ii represent parts of the tree where external branches do not connect to the part of the tree immediately preceding it. A) Anomalepididae, Epictinae, Leptotyphlopinae, Gerrhopilidae, Xenotyphlopidae, and Typhlopinae. B) Asiatyphlopinae I, Afrotyphlopinae; Madatyphlopinae, and Asiatyphlopinae II.
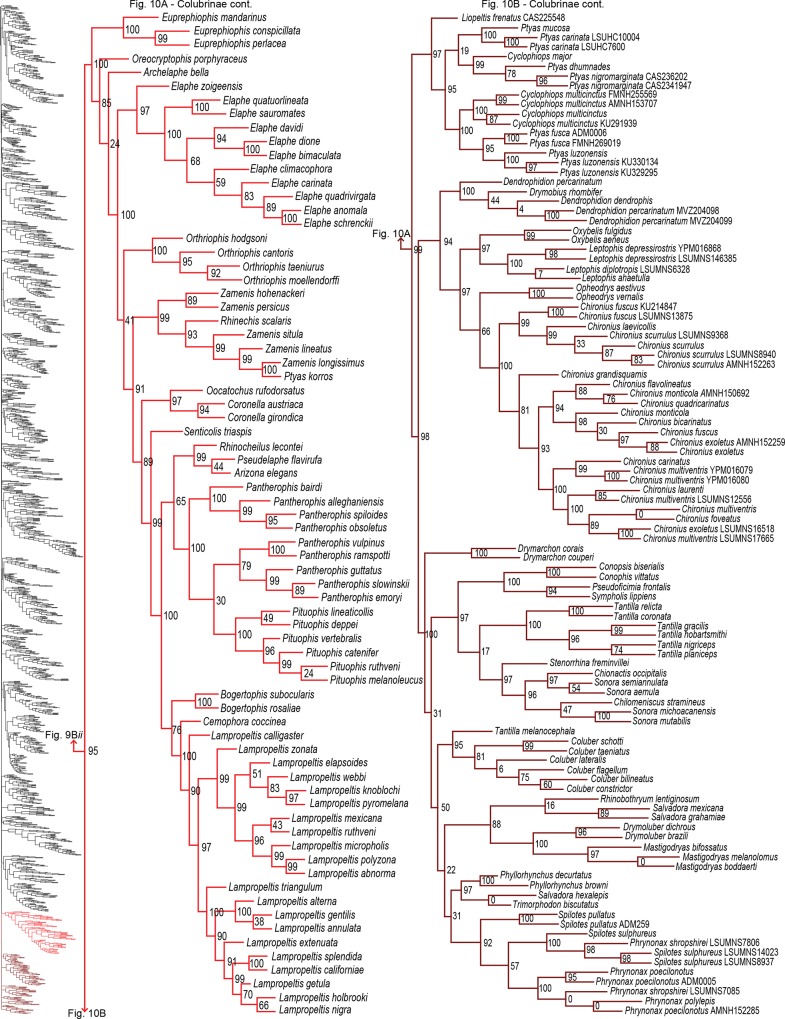
Fig 10. Phylogenetic tree of Serpentes continued.
A) Colubrinae continued. B) Colubrinae continued.
Scolecophidia
Similar to many prior examinations, we find relationships within Scolecophidia unresolved [15,23,25,31,32,41,42,46–48,82–85], with studies showing either Scolecophidia [25,31,84,85], Anomalepididae [15,41] or Leptotyphlopidae + Typhlopoidea [23,42,46,47,48] as sister to all snakes. Morphology also reveals uncertainty surrounding Scolecophidia (reviewed in [84]), but based on the presence of vestigial supratemporal and ectopterygoid bones, absent in other scolecophidians, Anomalepididae may be the most basal scolecophidian [85]. We believe future work will lead to a reclassification of Scolecophidia, but until then relationships within the infraorder remain problematic. In addition, we find weak support for the placement of Asiatyphlopinae, Afrotyphlopinae, and Madatyphlopinae within Typhlopidae as in previous studies [15,23,29,31,50,86]. The issue appears to lie primarily with the placement of Argyrophis [50] and Xerotyphlops [15,23,50], which together formed Asiatyphlopinae I. Xerotyphlops is represented by two species, one occurring in the eastern Mediterranean and the other on Socotra Island [86], and Argyrophis is distributed from western Asia to Southeast Asia [29,86]. Discordance in topology therefore appears associated with these two genera being intermediate in distribution between African and Asian typhlopids, which may show affinities to clades from both regions.
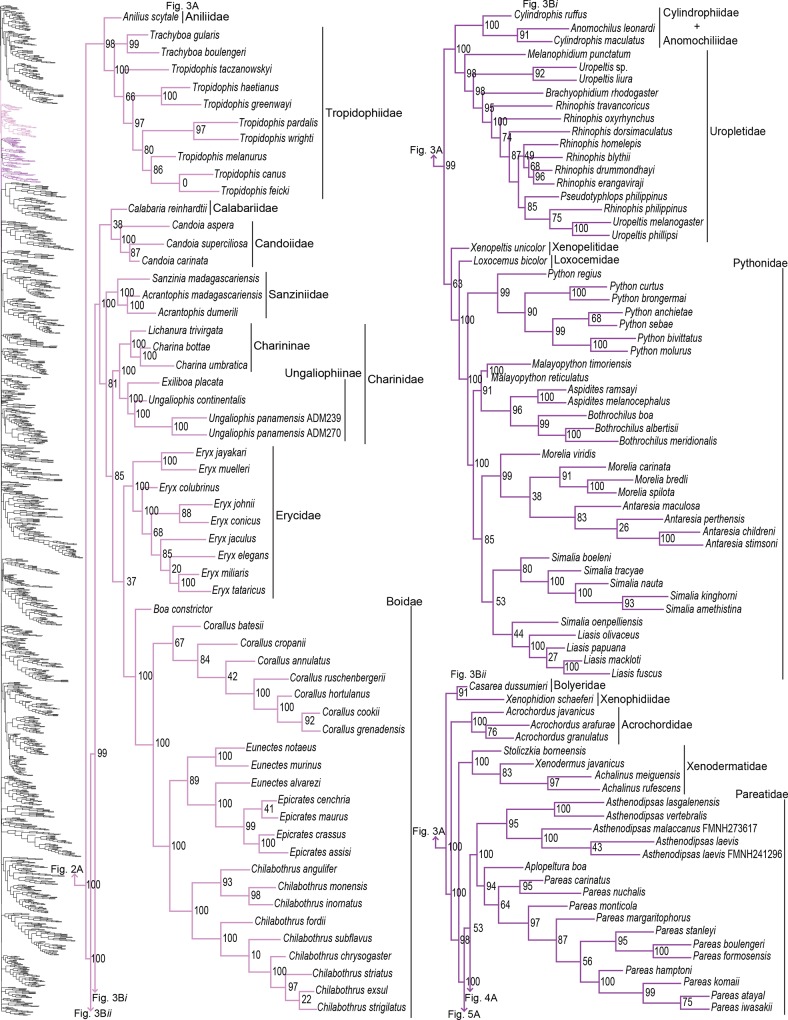
Henophidia
As mentioned above, Cylindrophiidae is paraphyletic with Anomochilidae. Difficulty in resolving this relationship is likely due to the representation of Anomochilus by one species and two genes (12S and 16S), and Cylindrophis by two species with greater gene coverage. Both of these families were formerly shown as part of or paraphyletic with Uropeltidae [41,42,47,48]. Based on the history of paraphyly between these families, Burbrink and Crother [84] recommended synonymizing Cylindrophiidae and Anomochilidae with Uropeltidae to resolve these families. However, we recommend retaining the current classification until more species are sampled (Table 1) on the grounds that Cylindrophiidae + Anomochilidae share morphological features not present in Uropeltidae [35,84] and since strong support has been shown distinguishing them from Uropeltidae [15,23,32,41]. For boids, our analysis validates the taxonomic changes made in Pyron et al [30], but differs in topology from previous assessments in the placement of Calabariidae, Candoiidae, and Sanziniidae [15,23,53]. Although the relationship Erycidae + Boidae is recovered in all studies [15,23], except one [53], support for this relationship is low. Thus, the only node we can have confidence in is the one joining Charininae and Ungaliophiinae [15,23,53].
Xenophidiidae and Bolyeridae
Perhaps the most notable difference from the topology of Pyron et al [15] was the placement we recovered for Xenophidiidae + Bolyeridae (SHL = 91). Earlier studies showed them as sister to various clades within Henophidia [23,32,38,41,42], but we found very strong support (SHL = 100) for them as sister to Caenophidia (SHL = 100), as also shown in other studies [53,85]. In addition, these snakes possess morphological characters, particularly within the palate, bolstering their close relationship with Caenophidia and not to Henophidia [85]. Pyron et al [15] is the only study showing a disassociation between these families placing Xenophidiidae as sister to Alethinophidia, with the exception for Aniliidae + Tropidophiidae, and Bolyeridae as sister to Booidea. Currently, both clades are represented by one species and Xenophidiidae by only one gene (cyt-b). Both clades contain two species; for Xenophidion, both species are known only from one specimen each, and for Bolyeridae, Bolyeria is extinct, and Casarea is rare [38], so obtaining additional sequences for either clade is unlikely. If this placement is retained, then Caenophidia should be redefined to include Xenophidiidae and Bolyeridae, or they should be given their own taxonomic grouping.
Caenophidia
Pyron et al [22] recently reviewed and attempted to resolve several problematic issues within Caenophidia. The major problems hindering resolution of this clade are 1) placement of Xenodermatidae inside or outside of Colubroidea; 2) placement of Homalopsidae; 3) topology of Lamprophiidae; and 4) topology of Colubridae. Previous studies have placed Xenodermatidae as sister to Acrochordidae [15,37] or as basal in Colubroidea [23,27,40,42,47,87], have placed Homalopsidae as sister to Lamprophiidae + Elapidae [15,27,40] or as sister to (Lamprophiidae + Elapidae) + Colubridae [23,32,39,42,45,47], and have shown conflicting topologies for the subfamilies within Lamprophiidae and Colubridae [15,23,27,28,37,40,45,47]. Pyron et al [22] used seven methods to examine these relationships showing Xenodermatidae as basal in Colubroidea with varying support and Homalopsidae as sister to (Lamprophiidae + Elapidae) + Colubridae with strong support. However, they expressed little confidence in resolving the topology within Lamprophiidae and Colubridae since several divergences were defined by low support. We confirm their findings that Xenodermatidae is sister to the rest of Colubroidea (SHL = 100) and that relationships within Lamprophiidae and Colubridae remain unresolved, but our findings for the placement of Homalopsidae contradicted theirs, as we recovered strong support (SHL = 91) for Homalopsidae + Lamprophiidae, and found Elapidae to be nested within Lamprophiidae. Typically, Lamprophiidae and Elapidae are recovered as distinct clades [15,22,28,39,40,41,64], but we found strong support (SHL = 96) for Elapidae + Buhoma depressiceps as sister to Pseudoxyrhophiinae (SHL = 99), shown previously only in Pyron and Burbrink [32]. The topology of Lamprophiidae is complicated by the presence of several incertae sedis taxa (see Lamprophiidae [28,32,39,41]), but Elapidae remains nested within Lamprophiidae even when these taxa are removed (S1 Fig). In addition, we found the placement of Pareatidae and Viperidae within Colubroidea unresolved. Pareatidae is consistently placed as sister to Viperidae, which is sister to Colubridae, Elapidae, Homalopsidae, and Lamprophiidae [15,22,23,27,32,41,42]. A possible explanation for this is that our dataset includes the greatest sampling of pareatids, adding seven additional species previously not included in higher-level relationships, two we sequenced and five from You et al [58].
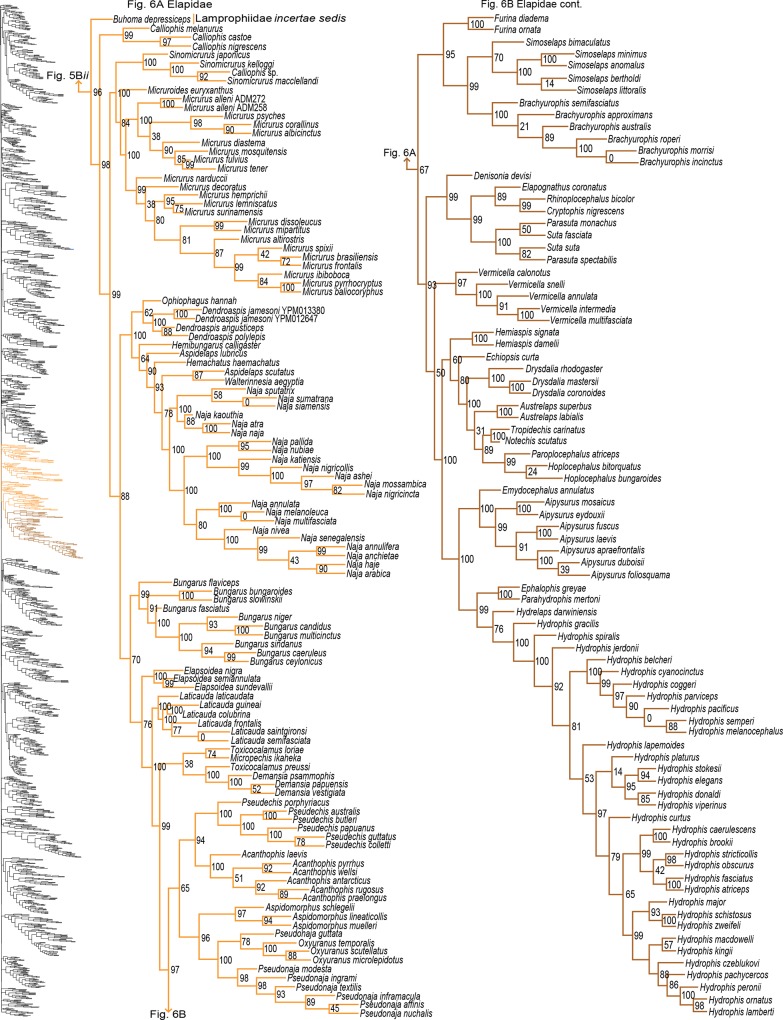
Lamprophiidae
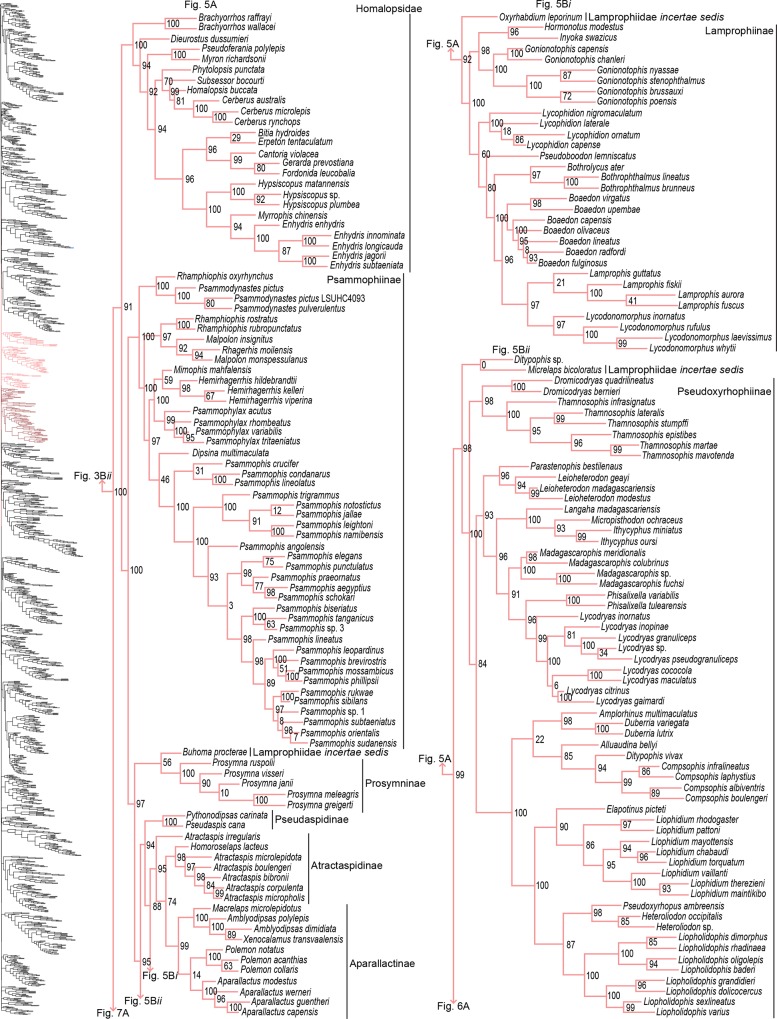
Part of the issue with resolving the topologies within Lamprophiidae, and within Colubridae, is that they exemplify rapid radiations manifested by the presence of short internodes [22]. Yet another major issue hindering progress within Lamprophiidae is the presence of several incertae sedis taxa, not identified as rogue taxa by RogueNaRok. These taxa constantly show contrasting phylogenetic placement between studies [15,23,28,39,40,64,87]. We are reluctant in placing any confidence in the topology between subfamilies recovered for Lamprophiidae, despite high support values. However, the topology after all rogues and incertae sedis taxa were pruned remained essentially the same (S1 Fig) adding supplementary support for this topology. Nonetheless, our topology differs from earlier studies. Previous studies have consistently recovered the sister relationship between Aparallactinae + Atractaspidinae [15,22,28,32,39,40,41,64]; however, we found this relationship unresolved, likely due to the strong placement (SHL = 95) of Atractaspis irregularis as sister to these two clades, and this taxon is represented by only one gene. The topology recovered here was Psammophiinae + ((B. procterae + Prosymninae) + (Pseudaspidinae + (Atractaspidinae + Aparallactinae) + (O. leporinum + Lamprophiinae)) + (((Ditypophis sp. + M. bicoloratus) + Pseudoxyrhophiinae) + (B. depressiceps + Elapidae)))). All nodes received strong support (SHL > 88), except for subclades B. procterae + Prosymninae and Ditypophis sp. + M. bicoloratus. Pyron et al [15] had augmented the definition of Pseudaspidinae to include Buhoma and Psammodynastes. With added sampling of Psammodynastes, we recovered this genus as paraphyletic with Rhamphiophis oxyrhynchus (SHL = 100) within Psammophiinae, making Rhamphiophis paraphyletic (Fig 5A). Buhoma, on the other hand, was split with B. procterae sister to Prosymninae and B. depressiceps sister to Elapidae. Oxyrhabdium leporinum was sister to Lamprophiinae and Micrelaps bicoloratus was placed within Pseudoxyrhophiinae. In all preliminary and final analyses, Psammodynastes constantly occupied the same phylogenetic position; however, placement of the other four species was erratic and always differed. Therefore, we tentatively include Psammodynastes as part of Psammophiinae. Due to their perpetual variable placement, we continue recognizing Buhoma, M. bicoloratus, and O. leporinum as Lamprophiidae incertae sedis.
Fig 5. Phylogenetic tree of Serpentes continued.
A) Homalopsidae, Psammophiinae, Buhoma procterae, Prosymninae, Pseudaspidinae, Atractaspidinae, and Aparallactinae. Bi) Oxyrhabdium leporinum and Lamprophiinae. Bii) Ditypophis sp. + Micrelaps bicoloratus and Pseudoxyrhophiinae.
Colubridae
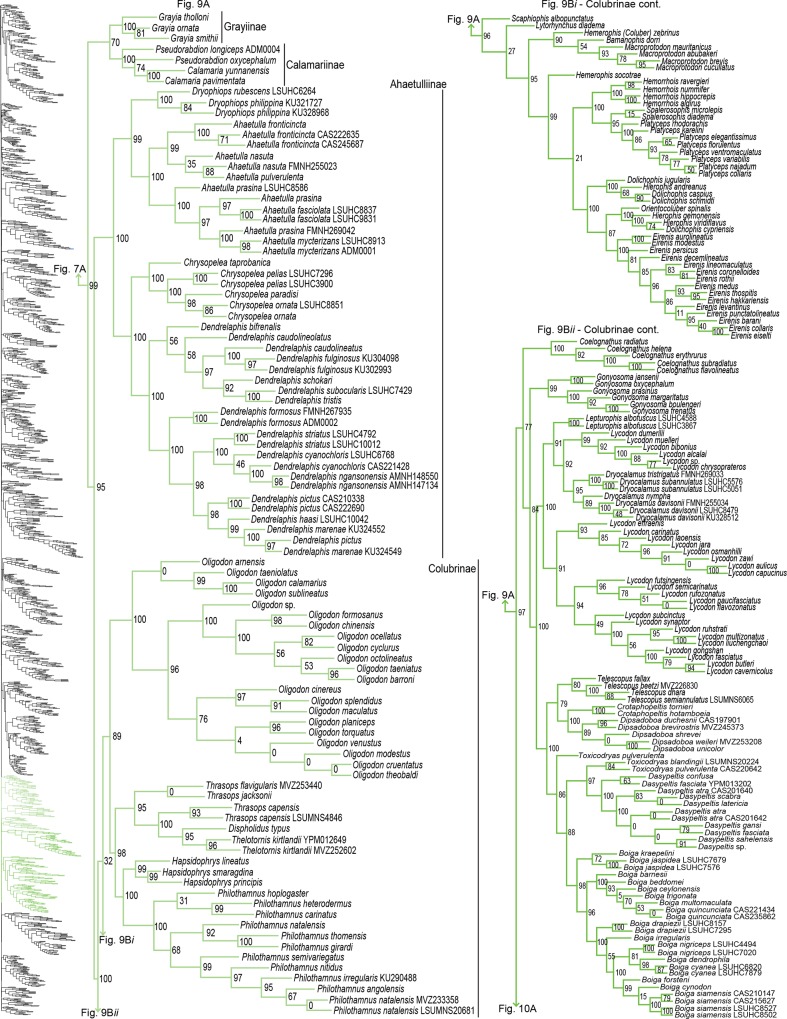
For Colubridae, we recovered the following four subclades: i) Sibynophiinae + Natricinae (SHL = 80); ii) Pseudoxenodontinae + Dipsadinae (SHL = 82); iii) Grayiinae + Calamariinae (SHL = 70); and iv) Ahaetuliinae subfam. nov. + Colubrinae (SHL = 95). The nodes between these subclades all received very strong support (SHL > 97). The only consistently recovered clade among these is subclade ii [22,27,32,40,41]; although other studies do not recover this subclade [15,23,65]. Several studies also regularly recovered the subclade Natricinae + (Pseudoxenodontinae + Dipsadinae) [22,27,32,40], but we do not uncover that relationship here. Instead, Natricinae formed a subclade with Sibynophiinae, also reported in [41]. The subfamily Sibynophiinae was only recently included in molecular analyses, originally grouped with Calamariinae [27], then subsequently placed as sister to Grayiinae + Colubrinae [15,23], and to Calamariinae + (Colubrinae + Grayiinae) [22]. The subfamily Grayiinae was also recently described [45] and grouped with Calamariinae in that study, also recovered in Pyron and Burbrink [32]. However, Grayiinae has most frequently been grouped with Colubrinae [15,22,23,27,39–41]. Dipsadinae is exclusively a New World family, but recent placement of Stichophanes and Thermophis as sister to Dipsadinae [15,88,89] expanded its distribution into the Old World. Pyron et al [15] did not include Stichophanes, and they mentioned that Thermophis may even warrant its own subfamily. However, our results do not uphold this view since we show Stichophanes + Thermophis (SHL = 96; Fig 7B) as placed within Dipsadinae. Wang et al [89], on the other hand, supported Stichophanes + Thermophis as sister to Dipsadinae, but their dataset was not as extensive and did not include T. zhaoermii. Until now, the basal node of Colubrinae has remained ambiguous. Pyron et al [15] suggested that monophyly of Ahaetulla, Chrysopelea, and Dendrelaphis at the base of Colubrinae, may warrant recognition as a distinct subfamily, but support for division of these taxa in their study was low. Due to increased sampling, and the inclusion of Dryophiops, we established strong support for recognizing these taxa as a new subfamily, using the name proposed by Pyron et al [15], Ahaetuliinae subfam. nov.
Fig 7. Phylogenetic tree of Serpentes continued.
A) Sibynophiinae and Natricinae. B) Pseudoxenodontinae and Dipsadinae.
Higher-level phylogeny with all rogue taxa eliminated
With all rogue taxa (101) and incertae sedis species (4) eliminated, higher-level relationships and support values remained relatively unchanged (S1 Fig). Where changes in topology or support values occurred, it was in the problematic clades discussed above, specifically Typhlopidae, Booidea, Pareatidae + Viperidae, Lamprophiidae, and Colubridae. For Typhlopidae, Xerotyphlops formed a clade by itself, sister to all other typhlopids. Madatyphlopinae formed a moderately supported (SHL = 87) clade with Typhlopinae. However, the placements of Afrotyphlopinae and Asiatyphlopinae remained unresolved. In Booidea, the placement of Calabariidae + Candoiidae swapped with Sanziniidae, greatly altering support values throughout Booidea, except in Charininae + Ungaliophiinae. Within Colubroidea, the placement of Pareatidae and Viperidae remains unresolved. Interestingly, with incertae sedis species removed from Lamprophiidae, topology of the subfamilies and of Elapidae within Lamprophiidae remained the same and the relationship between Atractaspidinae and Aparallactinae was strongly resolved, providing compelling support for the topology recovered. However, the node joining Prosymninae to all other lamprophiids became ambiguous. Relationships within Colubridae remained stable, except that Pseudoxenodontinae placed as sister to all other colubrids. In addition, we note that the sister relationship of Xenopeltidae to Loxocemidae + Pythonidae became ambiguous, and that with the exclusion of Xenophidiidae as a rogue taxon, Bolyeridae still placed as sister to Caenophidia with high support (SHL = 99), upholding its position outside of Henophidia.
Genus- and species-level phylogeny
Of the 147 samples we sequenced, two genera (Dryophiops, and Liopeltis) and 61 species were not previously incorporated in any phylogenetic analyses. Dryophiops placed within Ahaetullinae subfam. nov. as sister to Ahaetulla (SHL = 99), and Liopeltis fell within Colubrinae as sister taxon (SHL = 97) to Ptyas + Cyclophiops. We recovered strong support for the phylogenetic placement of 105 of our samples (SHL > 85). For taxa where our sequences resulted in multiple terminals of the same species, the following species were not monophyletic: Ahaetulla nasuta, A. prasina, Chironius exoletus, C. fuscus, C. monticola, C. multiventris, Dasypeltis fasciata, Dendrelaphis cyanochloris, D. marenae, Dendrophidion percarinatum, Philothamnus natalensis, Phrynonax poecilonotus, P. shropshirei, Psammodynastes pictus, Sibynomorphus turgidus, Spilotes sulphureus, and Trimeresurus fucata. Throughout the entire tree, most genera were monophyletic with varied node support. Space does not allow for exhaustive scrutiny at the generic and species level of our tree with previous publications, although a cursory examination reveals consistency with previous publications. Instead, we focus on assessing the placement of paraphyletic genera, most of which require greater sampling of species and genes, or perhaps individuals, to provide an improved appraisal of their phylogenetic positions.
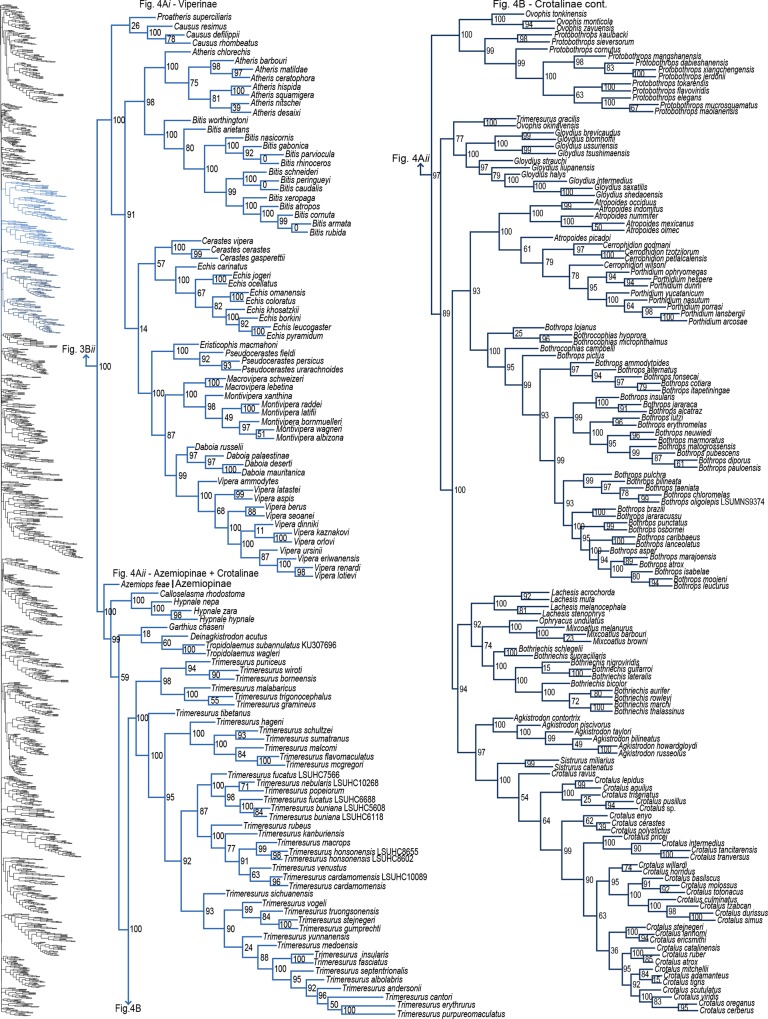
Paraphyly at the lower-level of the tree emerged due to various reasons. For some clades paraphyly is well-established and confirmed here, more notably in Brachyophidium, Pseudotyphlops, Rhinophis, and Uropeltis in Uropeltidae (Fig 3Bi) [15,41,53,90]; Ovophis and Trimeresurus in respect to Ovophis okinavensis + Trimeresurus gracilis as basal to Gloydius (Fig 4B) [61,91]; Adelophis, Amphiesma, Atretium, Nerodia, Regina, Thamnophis, Tropidoclonion, and Xenochrophis in Natricinae (Fig 7A) [15,68,92,93]; and Dipsas, Geophis, and Sibynomorphus in Dipsadinae (Fig 7B) [15,49,65,66]. Additional taxa include: variable placement of Morelia viridis (Fig 3Bi) [15,38,52,94] and Bothrocophias campbelli (Fig 4B) [95]; and Suta with Parasuta (Fig 6B) [15,96]. Clearly, these clades require further inspection. On the other hand, we were able to rectify other paraphyletic taxa with strong support, specifically within Colubrinae: Boiga, Chironius, Coronella, Crotaphopeltis, Dasypeltis, Dipsadoboa, Hapsidophrys, and Philothamnus, Rhinechis, and Scaphiophis.
Fig 3. Phylogenetic tree of Serpentes continued.
A) Aniliidae, Tropidophiidae, Calabariidae, Candoiidae, Sanziniidae, Charininae, Ungaliophiinae, Erycidae, and Boidae. Bi) Cylindrophiidae + Anomochilidae, Uropeltidae, Xenopeltidae, Loxocemidae, and Pythonidae. Bii) Bolyeridae, Xenophidiidae, Acrochordidae, Xenodermatidae, and Pareatidae.
Fig 4. Phylogenetic tree of Serpentes continued.
Ai) Viperinae. Aii) Azemiopinae and Crotalinae. B) Crotalinae continued.
Fig 6. Phylogenetic tree of Serpentes continued.
A) Buhoma depressiceps and Elapidae. B) Elapidae continued.
In some taxa, such as Cerrophidion wilsoni (Fig 4B), Atractus irregularis (Fig 5A), Ditypophis sp. (Fig 5Bii), Aspidelaps irregularis (Fig 6A), Pseudonaja guttata (Fig 6A), Geophis with Atractus (Fig 7B), Sibon noalamina (Fig 7B), Philodryas chamissonis and P. trilineata (Fig 8A), Conophis and Conopsis (Fig 8A & Fig 10B), Ptyas korros (Fig 10A), Tantilla melanocephala (Fig 10B), and Salvadora hexalepis (Fig 10B), sequence overlap with related taxa was zero or minimal. Whereas for the following taxa, their placement were unresolved: Typhlopidae, Rhinotyphlops unitaeniata (Fig 2B); Uropeltidae, Rhinophis philippinus (Fig 3B); Pythonidae, Simalia oenpelliensis (Fig 3B); Viperidae, Atropoides picadoi and Bothrops lojanus (Fig 4B); Elapidae, Toxicocalamus loriae (Fig 6A); Natricinae, Macropisthodon rhodolemas ADM0003 (Fig 7A); Dipsadinae, Oxyrhopus fitzingeri LSUMNS6586 and Siphlophis cervinus (Fig 8A); Calamariinae, Pseudorabdion oxycephalum (Fig 9A); and Colubrinae, Hierophis andreanus and Dolichophis cypriensis (Fig 9Bi), Pantherophis and Pituophis (Fig 10A), Drymobius rhombifer, Dendrophidion dendrophis, Chilomeniscus stramineus, Tantilla melanocephala, and Salvadora hexalepis (Fig 10B).We do not classify Calliophis and Sinomicrurus as paraphyletic until the identity of Calliophis sp. is known.
Fig 8. Phylogenetic tree of Serpentes continued.
A) Dipsadinae continued. B) Dipsadinae continued.
Fig 9. Phylogenetic tree of Serpentes continued.
A) Grayiinae, Calamariinae, Ahaetullinae subfam. nov., and Colubrinae. Bi) Colubrinae continued. Bii) Colubrinae continued.
For some clades, paraphyly was strongly supported allowing us to synonymize these taxa. Within Psammophiinae, we synonymize Rhagerhis moilensis with Malpolon. This species consistently forms a monophyletic clade with Malpolon [15,28,62,97] (Fig 5A), but two studies [64,98], inaccurately cite Kelly et al [62] as providing evidence for their separation. In Aparallactinae, we synonymize Xenocalamus with Amblyodipsas (Fig 5A), also recovered in Pyron et al [15], the only other study including these taxa. Within Colubrinae we synonymize several clades. First, we synonymize Lepturophis and Dryocalamus with Lycodon, which forms a strong clade (SHL = 100) with these taxa strongly embedded within [15,99] (Fig 9Bii). Next, we synonymize Rhinechis scalaris, a species with an erratic phylogenetic history [100,101], with Zamenis, but the addition of more genes shows it related to Zamenis [15,102] (Fig 10A), with which it has morphological affinities to [103]. Finally, we also synonymize Cyclophiops with Ptyas. Previously recovered as sister clades [15,104], our increased sampling for both genera shows that Ptyas forms a strong clade (SHL = 95) with the two species of Cyclophiops strongly nested within two separate subclades (Fig 10B). Conversely, in other clades paraphyly was strong, but we do not propose taxonomic changes, specifically in Hebius sauteri placing with Amphiesma (Fig 7A), Balanophis ceylonensis within Rhabdophis (Fig 7A), Thamnodynastes pallidus placing with Sibynomorphus (Fig 7B), Pliocercus split (Figs 7B & 8A), Ninia split (Figs 7B & 8A), Dispholidus typus within Thelotornis (Fig 9A), Chionactis occipitalis placing with Sonora (Fig 10B), and P. shropshirei LSUMNS7806 within Spilotes (Fig 10B), mainly because these taxa, or taxa they placed with, are presented for the first time in a phylogenetic analysis.
In the case of Hemerophis, after the genus Bamanophis was erected for Coluber dorri [105], H. zebrinus remained as the only Old World Coluber representative, until it was recently recognized as Hemerophis without justification [24,106]. Yet, the two are distantly-related within a clade of Old World racers [15,40,107,108]. H. zebrinus is typically placed in a clade sister to Bamanophis and Macroprotodon, but a very recent study incorporating new sequence data for Rhynchocalamus, not included here, places H. zebrinus as the basal lineage within this clade sister to (Bamanophis + Macroprotodon) and all other Old World racers [109]; while H. socotrae, occupies a branch away from this clade. Nagy et al [108] shows weak support for a sister relationship between the two using maximum parsimony, but shows them separated with greater support using Bayesian inference and ML. Therefore, we create a new genus for H. zebrinus, Mopanveldophis gen. nov.
Supermatrix approach
Despite the utility of the supermatrix approach, this method is also potentially responsible for uncertainty in some nodes. Compiling available molecular data from numerous studies leads to a sparse data matrix with a substantial portion of missing data unequally scattered throughout the alignment due to sampling differences between studies [11]. Our dataset consisted of 71.41% of missing data with several taxa represented by a single gene to taxa with data spanning all loci. Heterogeneity in sparse data matrices can alter topological relationships and negatively impact tree support by increasing the presence of rogue taxa [110]. Rogue taxa typically are characterized by little character data that do not overlap with closely-related taxa [21]. We identified and removed 22 rogue taxa from our data matrix, 12 of which were delineated by one gene and eight by two genes. The genes 12S, 16S, c-mos, and ND4 were most associated with rogue taxa. These genes evolve more slowly and are not adequate for delimiting species-level relationships (see methods), and several families in our tree are only represented by one or two individuals with few sequenced loci (i.e., Anomalepididae, Anomochilidae, Bolyeridae, Cylindrophiidae, and Xenophidiidae; Table 1). Many taxa in the tree with low support were also represented by a single gene. Furthermore, lack of sequence overlap between closely-related species can also lead to misplacement of taxa in the tree, sometimes with high support as mentioned above. However, many taxa with extensive missing data were placed correctly in the tree (e.g., Chironius multiventris, Pseudocerastes urarachnoides, Rhabdophis chrysargos, Trimeresurus wiroti), grouping with closely-related taxa with high support, confirming that increased taxon sampling is a favorable choice for improving phylogenetic accuracy [111], even with a high percentage of missing data [112]. This can occur when the overall number of characters in the data matrix is high [5,113–116], especially for SHL support values since they are not negatively affected by the amount of missing data in the data matrix [40].
In many cases, denser sampling influenced phylogenetic relationships and node support [117]. For example, adding 30 samples of 18 species (14 never before sequenced) to Ahaetuliinae, resolved the basal Colubrinae node and distinguished Ahaetuliinae as a new subfamily. Increased taxon sampling also resolved several paraphyletic issues at the generic level, identified new associations of paraphyly, mostly due to poor gene sampling, resulted in new phylogenetic hypotheses for some taxa such as Scaphiophis, Stichophanes + Thermophis, and Xerotyphlops, and prompted us to make some taxonomic changes. Moreover, our sequencing contribution resulted in complete or nearly complete taxonomic coverage of several genera, including Ahaetulla, Asthenodipsas, Chrysopelea, Dendroaspis, Dryocalamus, Dryophiops, Phrynonax, Ptyas, and Ungaliophis, and greatly increased representation of species of the speciose genera Boiga and Dendrelaphis. Nonetheless, many challenges exist to estimating the snake tree of life.
Taxonomic descriptions
Subfamily Ahaetuliinae subfam. nov. urn:lsid:zoobank.org:act: 22C47597-1DEF-45A4-ABAC-11C4911557AD
Type genus: Ahaetulla Link [118]
Content: Four genera containing 56 species. Ahaetulla (8 species), Chrysopelea (5 species), Dendrelaphis (41 species), and Dryophiops (2 species).
Etymology: From the Sri Lankan language Sinhala, ahaetulla/ahata gulla/as gulla, meaning “eye plucker” or “eye picker” for belief that they pluck out the eyes of humans as accounted by the Portuguese traveler João Ribeiro in 1685 (as cited in [119]).
Diagnosis and definition: Snakes of this subfamily are arboreal and are diagnosed by keeled ventral and subcaudal scales (laterally notched in some species), and enlarged posterior grooved fangs lacking in some Dendrelaphis. Support for monophyly of this clade is very strong (SHL = 100) as also reported in Pyron et al [15]. Ahaetuliinae is further split into two monophyletic groups: 1) Dryophiops and Ahaetulla (SHL = 96) and; 2) Chrysopelea and Dendrelaphis (SHL = 100). Diagnostic characteristics of the first group include, elongate and laterally-compressed bodies, elongate heads, 15 smooth mid-body dorsal scale rows, and large eyes with horizontal pupils and well-developed canthus rostralis outfitting these snakes with binocular vision [120]. Features diagnostic of the second group include, slender body, rectangular slightly compressed heads, large eyes with round pupils, 13–17 smooth to weakly-keeled mid-body dorsal scale rows. Chrysopelea are celebrated for their unique gliding behavior, whereas Dendrelaphis are capable of jumping [121].
Sister taxon: Previously placed within Colubrinae, Ahaetuliinae forms a strong (SHL = 95) sister relationship with Colubrinae, also weakly supported by Pyron et al [15].
Distribution: Members of this subfamily inhabit various habitats, but are mostly associated with forests distributed from Pakistan, Sri Lanka and India, north to Nepal and Bangladesh, eastwards all throughout Southeast Asia to southern China, Philippines, Papua New Guinea, and northeast Australia.
Remarks: The name Ahaetulla has suffered from a tumultuous nomenclatural history [122]. In addition, members of these genera have historically been grouped with unrelated taxa based on absence or presence of hypapophyses [123,124].
Genus Mopanveldophis gen. nov. urn:lsid:zoobank.org:act: 3B0CB6A0-1EEC-4512-9E77-B105C22ACABB
Type species: Mopanveldophis zebrinus.
Content: The genus is monotypic containing only the species, Mopanveldophis zebrinus.
Etymology: The generic nomen Mopanveldophis is derived from the word “mopanveld”, the name of the type of habitat the specimens were found in, and the Greek adjective ophis, meaning “snake”. This name refers to veld habitat distributed in Southern Africa, from the Afrikaans word “field”, that is dominated by the mopane tree, Colophospermum mopane, from the Sechuana word “mopani”.
Diagnosis and definition: As described in Broadley and Schätti [125] and Bauer et al [126], a snake with pale grey dorsal coloration and irregular broad, dark crossbands becoming faint in coloration posteriorly and on tail. Ventrals are uniform white with irregular lateral black spots, and subcaudals are also white with lateral grey stippling. Dorsal portion of head is uniform grey-brown with yellowish orange snout and labials, and dark markings on supralabials 2–6. Dorsal scales with two apical pits, 23 scale rows near neck, 23 at midbody, and 17–19 anterior to the vent. Approximately 195 ventrals, 90 paired subcaudals, and divided anal scute. Nine supralabials with the fifth and sixth entering the orbit, one anterior subocular smaller than the loreal shield and situated above the fourth and anterior part of the fifth supralabials, and two preoculars and two postoculars. Also, diagnosed by a single large lower anterior temporal shield above the 7th and 8th supralabials, two upper anterior temporal, three posterior temporal, and maxillary with 17 + 2 teeth separated by a diastema. Its banded pattern was suggested as Batesian mimicry of the sympatric spitting cobra, Naja nigricollis. Bamanophis differs by having 25–27 scale rows near neck, 29–33 at midbody, and 17 near vent, 229–265 ventral scale and 75–95 paired subcaudals, lacking an anterior subocular, having one posterior subocular, 10 supralabials, and 15–19 maxillary teeth with diastema [105].
Sister taxa: M. zebrinus is basal lineage to a clade including Bamanophis + Macroprotodon, placed within a larger clade of Old World racers [15,40,107,108].
Distribution: Currently recognized as endemic to northern Namibia, Africa [127], but its range may extend into Angola, Africa [126].
Remarks: First described from a dead specimen collected in 1991 [125], the species is currently known from only three specimens [126]. Upon its description it was assigned to the genus Coluber, presumably on basis of similar morphology, but then switched to Hemerophis [24,106] with no published reasoning. Schätti and Trape [105] provide an account detailing the differences of Bamanophis to other racer species, including M. zebrinus.
Conclusions
At less than half (46.33%) of the total snake diversity sampled, we provide the most comprehensive sampling effort to date, but remain far from fully estimating the snake tree of life. This sampling effort pales in comparison to larger clades such as birds that have approximately 70% of more than 10,000 species sequenced [11]. Although our results provide resolution for several higher-level nodes, these nodes may continue to prove problematic. Collectively, future analyses should target or pay special attention to the following ten issues: 1) resolving topology of Scolecophidia; 2) resolving topology of Typhlopinae; 3) resolving paraphyly of Cylindrophiidae with Anomochilidae; 4) placement of Xenophidiidae and Bolyeridae; 5) resolving topology of Booidea; 6) placement of Xenodermatidae; 7) placement of Pareatidae; 8) placement of Homalopsidae; 9) resolving topology of Lamprophiidae + Elapidae; and 10) resolving topology of Colubridae. Clearly, greater taxon and gene sampling will help better formulate a picture of snake relationships and resolve ambiguous nodes in the tree [111,117]. Taxa most lacking in representation are fossorial clades, mainly Afrotyphlopinae, Anomalepididae, Aparallactinae, Calamariinae, Cylindrophiidae, Epictinae, Gerrhopilidae, Madatyphlopinae, Uropeltidae, and Xenodermatidae at below 30% (Table 1). Similar deficiencies occur at the genus level, but are not listed here. The genes most frequently sampled for snakes are 12S, 16S, c-mos, cyt-b, and ND4, and should be considered as candidate genes in future studies. Sampling more nuclear genes will also be crucial in resolving deeper nodes [23]. Where coalescence-based methods are practiced, researchers should place emphasis on short and weakly supported branches since they are more prone to incomplete lineage sorting and thus, conflict most often with branches on species-trees [8]. This phylogeny has major implications on snake evolution such as on the evolution of gape size and the evolution of venom-delivery systems [44,46,85], and serves as a resource for formulating future studies on snake phylogenetics.
Supporting Information
Maximum-likelihood phylogenetic estimate based on 10 concatenated genes. Tips represent families and sub-families. Commonly recognized higher-level clades are labeled in all caps and bold. Node values represent SHL support values. Skeleton of the species tree is displayed on the left, colored and labeled as they appear in Fig 1.
(EPS)
(PHY)
(DOCX)
Two sequences were deleted during preliminary tree searches and 21 were identified as rogue taxa and pruned from the dataset leaving 1592 snake species from GenBank in the tree. Names represent species names as listed on The Reptile Database (http://www.reptile-database.org/) as of October 2015. Refer to S4 Table for list of rogue taxa. Taxa deleted during preliminary tree searches are highlighted in red, rogue taxa are highlighted in yellow, and sequences that were deleted because they were identical to other sequences are highlighted in green.
(DOCX)
Tissue samples for Boiga siamensis FMNH267726, Chrysopelea ornata LSUHC7158, and Psammodynastes pictus FMNH267940 were represented by clear chromatograms, but placed poorly in preliminary phylogenetic trees, so they were not included in the final data matrix. Tropidolaemus subannulatus KU327425 was identified as a rogue taxon by RogueNaRok and was pruned from the dataset and thus, is not represented in the phylogeny.
(DOCX)
(DOCX)
Each taxon is associated with a raw improvement score (R.I.S.), which represents the fraction of improvement in bootstrap support values throughout the tree when the selected taxon is pruned and all rogue taxa above it are also pruned. We performed one run and chose to sacrifice relatively lower node support values to maximize the number of taxa represented in the phylogeny. Thus we elected to only prune taxa with R.I.S. greater than 0.8, resulting in a total of 22 pruned taxa (highlighted in bold).
(DOCX)
Acknowledgments
We are indebted to the many researchers who donated tissue samples to museum collections and uploaded their sequences on GenBank. For supporting this research in the way of loaning tissue samples, we would like to acknowledge curators and staff at the Ambrose Monell Cryo Collection (AMCC) at the American Museum of Natural History, New York (AMNH), the California Academy of Sciences (CAS), the Field Museum of Natural History (FMNH), the Natural History Museum & Biodiversity Research Center at the University of Kansas (KU), and the LSU Museum of Natural Science Collection of Genetic Resources (LSUMNS) and L.J. Vitt and his NSF grants DEB-9200779 and DEB-9505518, the Museum of Vertebrate Zoology (MVZ), and the Peabody Museum of Natural History Yale University (YPM).
We thank Robin Rowe at the University of New Orleans W.M. Keck Conservation and Molecular Genetics Laboratory for sequencing molecular samples, and Caño Palma Biological Research Station in Costa Rica and David Bickford at The National University of Singapore for providing lab space during field research. We also thank several anonymous reviewers for providing useful comments that helped improve the manuscript.
Data Availability
GenBank accession numbers are contained within the paper. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Science Foundation East Asia & Pacific Summer Institute (OISE-1107819); University of New Orleans College of Sciences Graduate Student Research Grant; University of New Orleans Department of Biological Science Dissertation Enhancement Award; University of New Orleans Dissertation Improvement Grant; and University of New Orleans Latin American Studies Abroad Program Award. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology (Vol. 239). Oxford: Oxford University Press; 1991. [Google Scholar]
- 2.Huelsenbeck JP, Rannala B. Phylogenetic methods come of age: testing hypotheses in an evolutionary context.Science. 1997; 276: 227–232. [DOI] [PubMed] [Google Scholar]
- 3.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999; 401: 877–884. [DOI] [PubMed] [Google Scholar]
- 4.Whelan S, Liò P, Goldman N. Molecular phylogenetics: state-of-the-art methods for looking into the past.Trends Genet.2001; 17: 262–272. [DOI] [PubMed] [Google Scholar]
- 5.Driskell AC, Ane C, Burleigh JG, McMahon MM, O'Meara B, Sanderson MJ. Prospects for building the tree of life from large sequence databases. Science. 2004; 306: 1172–1174. [DOI] [PubMed] [Google Scholar]
- 6.McMahon MM, Sanderson MJ. Phylogenetic supermatrix analysis of GenBank sequences from 2228 papilionoid legumes. Syst. Biol. 2006; 55: 818–836. [DOI] [PubMed] [Google Scholar]
- 7.Edwards SV. Is a new and general theory of molecular systematics emerging? Evolution. 2009; 63: 1–19. 10.1111/j.1558-5646.2008.00549.x [DOI] [PubMed] [Google Scholar]
- 8.Lambert SM, Reeder TW, Wiens JJ. When do species-tree and concatenated estimates disagree? An empirical analysis with higher-level scincid lizard phylogeny. Mol. Phylogenet. Evol.2015; 82: 146–155. 10.1016/j.ympev.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 9.de Queiroz A, Gatesy J. The supermatrix approach to systematics. Trends Ecol. Evol. 2007; 22: 34–41. [DOI] [PubMed] [Google Scholar]
- 10.Gatesy J, Springer MS. Phylogenetic analysis at deep timescales: Unreliable gene trees, bypassed hidden support, and the coalescence/concatalescence conundrum. Mol. Phylogenet. Evol. 2014; 80: 231–266. 10.1016/j.ympev.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Burleigh JG, Kimball RT, Braun EL. Building the avian tree of life using a large-scale, sparse supermatrix. Mol. Phylogenet. Evol. 2015; 84: 53–63. 10.1016/j.ympev.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 12.McCormack JE, Harvey MG, Faircloth BC, Crawford NG, Glenn TC, Brumfield RT. A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS One. 2013; 8: e54848 10.1371/journal.pone.0054848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol. Phylogenet. Evol. 2011; 61: 543–583. 10.1016/j.ympev.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 14.Piwczyński M, Szpila K, Grzywacz A. Pape T. A large‐scale molecular phylogeny of flesh flies (Diptera: Sarcophagidae). Syst. Entomol. 2014; 39: 783–799. [Google Scholar]
- 15.Pyron RA, Burbrink FT, Wiens JJ. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013; 13: 93 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabosky DL, Santini F, Eastman J, Smith SA, Sidlauskas B, Chang J, et al. Rates of speciation and morphological evolution are correlated across the largest vertebrate radiation. Nat. Comm. 2013; 4: 1–8. [DOI] [PubMed] [Google Scholar]
- 17.Soltis DE, Mort ME, Latvis M, Mavrodiev EV, O’Meara BC, Soltis PS, et al. Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am. J. Bot. 2013; 100: 916–929. 10.3732/ajb.1300044 [DOI] [PubMed] [Google Scholar]
- 18.Lemmon AR, Brown JM, Stanger-Hall K, Lemmon EM. The effect of ambiguous data on phylogenetic estimates obtained by maximum likelihood and Bayesian inference. Syst. Biol. 2009; 58: 130–145. 10.1093/sysbio/syp017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemmon EM, Lemmon AR. High-throughput genomic data in systematics and phylogenetics. Annu. Rev. Ecol. Evol. Syst. 2013; 44: 99–121. [Google Scholar]
- 20.Sanderson MJ, McMahon MM, Steel M. Phylogenomics with incomplete taxon coverage: the limits to inference. BMC Evol. Biol. 2010; 10: 155 10.1186/1471-2148-10-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomson RC, Shaffer HB. Sparse supermatrices for phylogenetic inference, taxonomy, alignment, rogue taxa, and the phylogeny of living turtles. Syst. Biol. 2009; 59: 42–58. 10.1093/sysbio/syp075 [DOI] [PubMed] [Google Scholar]
- 22.Pyron RA, Hendry CR, Chou VM, Lemmon EM, Lemmon AR, Burbrink FT. Effectiveness of phylogenomic data and coalescent species-tree methods for resolving difficult nodes in the phylogeny of advanced snakes (Serpentes: Caenophidia). Mol. Phylogenet. Evol. 2014; 81: 221–231. 10.1016/j.ympev.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 23.Zheng Y, Wiens JJ. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 2016; 94: 537–547. 10.1016/j.ympev.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 24.Uetz P, Hošek J. The Reptile Database. Available: http://www.reptile-database.org/. Accessed October 2015.
- 25.Heise PJ, Maxson LR, Dowling HG, Hedges SB. Higher-level snake phylogeny inferred from mitochondrial DNA sequences of 12S rRNA and 16S rRNA genes. Mol. Biol. Evol. 1995; 12: 259–265. [DOI] [PubMed] [Google Scholar]
- 26.Adalsteinsson SA, Branch WR, Trape S, Vitt LJ, Hedges SB. Molecular phylogeny, classification, and biogeography of snakes of the family Leptotyphlopidae (Reptilia, Squamata). Zootaxa. 2009; 2244: 1–50. [Google Scholar]
- 27.Chen X, Huang S, Guo P, Colli GR, de Oca ANM., Vitt LJ, et al. Understanding the formation of ancient intertropical disjunct distributions using Asian and Neotropical hinged-teeth snakes (Sibynophis and Scaphiodontophis: Serpentes: Colubridae). Mol. Phylogenet. Evol. 2013; 66: 254–261. 10.1016/j.ympev.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 28.Kelly CMR, Barker NP, Villet MH, Broadley DG. Phylogeny, biogeography and classification of the snake superfamily Elapoidea: a rapid radiation in the late Eocene. Cladistics. 2009; 25: 38–63. [DOI] [PubMed] [Google Scholar]
- 29.Pyron RA, Wallach V. Systematics of the blindsnakes (Serpentes: Scolecophidia: Typhlopoidea) based on molecular and morphological evidence. Zootaxa. 2014; 3829: 1–81. 10.11646/zootaxa.3829.1.1 [DOI] [PubMed] [Google Scholar]
- 30.Pyron RA, Reynolds RG, Burbrink FT. A taxonomic revision of Boas (Serpentes: Boidae). Zootaxa. 2014; 3846: 249–260. 10.11646/zootaxa.3846.2.5 [DOI] [PubMed] [Google Scholar]
- 31.Vidal N, Marin J, Morini M, Donnellan S, Branch WR, Thomas R, et al. Blindsnake evolutionary tree reveals long history on Gondwana. Biol. Lett. 2010; 6: 558–561. 10.1098/rsbl.2010.0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pyron RA, Burbrink FT. Extinction, ecological opportunity, and the origins of global snake diversity. Evolution. 2012; 66: 163–178. 10.1111/j.1558-5646.2011.01437.x [DOI] [PubMed] [Google Scholar]
- 33.Cadle JE. Phylogenetic relationships among advanced snakes: A molecular perspective. Univ. of California Publ. Zool. 1988. p. 1–77. [Google Scholar]
- 34.Dowling HG, Hass CA, Hedges SB, Highton R. Snake relationships revealed by slow-evolving proteins: a preliminary survey. J. Zool., Lond. 1996; 240: 1–28. [Google Scholar]
- 35.Gower DJ, Vidal N, Spinks JN, McCarthy CJ. The phylogenetic position of Anomochilidae (Reptilia: Serpentes), first evidence from DNA sequences. J. Zool. Syst. Evol. Res. 2005; 43: 315–320. [Google Scholar]
- 36.Hsiang AY, Field DJ, Webster TH, Behlke AD, Davis MB, Racicot RA, et al. The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 2015; 15: 87 10.1186/s12862-015-0358-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly CMR, Barker NP, Villet MH. Phylogenetics of advanced snakes (Caenophidia) based on four mitochondrial genes. Syst. Biol. 2003; 52: 439–459. [DOI] [PubMed] [Google Scholar]
- 38.Lawson R, Slowinski JB, Burbrink FT. A molecular approach to discerning the phylogenetic placement of the enigmatic snake Xenophidion schaeferi among the Alethinophidia. J. Zool. 2004; 263: 285–294. [Google Scholar]
- 39.Lawson R, Slowinski JB, Crother BI, Burbrink FT. Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Mol. Phylogenet. Evol. 2005; 37: 581–601. [DOI] [PubMed] [Google Scholar]
- 40.Pyron RA, Burbrink FT, Colli GR, de Oca ANM, Vitt LJ, Kuczynski CA, et al. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol. Phylogenet. Evol. 2011; 58: 329–342. 10.1016/j.ympev.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 41.Pyron RA, Kandambi HD, Hendry CR, Pushpamal V, Burbrink FT, Somaweera R. Genus-level phylogeny of snakes reveals the origins of species richness in Sri Lanka. Mol. Phylogenet. Evol. 2013; 66: 969–978. 10.1016/j.ympev.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 42.Reeder TW, Townsend TM, Mulcahy DG, Noonan BP, Wood PL Jr, Sites JW Jr, et al. Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS One. 2015; 10: e0118199 10.1371/journal.pone.0118199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slowinski JB, Lawson R. Snake phylogeny: evidence from nuclear and mitochondrial genes. Mol. Phylogenet. Evol. 2002; 24: 194–202. [DOI] [PubMed] [Google Scholar]
- 44.Vidal N, Hedges SB. Higher-level relationships of snakes inferred from four nuclear and mitochondrial genes. C.R. Biologies 2002; 325: 977–985. [DOI] [PubMed] [Google Scholar]
- 45.Vidal N, Delmas AS, David P, Cruaud C, Couloux A, Hedges SB. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C.R. Biologies 2007; 330: 182–187. [DOI] [PubMed] [Google Scholar]
- 46.Vidal N, Rage JC, Couloux A, Hedges SB. Snakes (Serpentes) In: Hedges SB, Kumar S, editors. The Timetree of Life. New York: Oxford University Press; 2009. p. 390–397 [Google Scholar]
- 47.Wiens JJ, Kuczynski CA, Smith SA, Mulcahy DG, Sites JW, Townsend TM, et al. Branch lengths, support, and congruence: testing the phylogenomic approach with 20 nuclear loci in snakes. Syst. Biol. 2008; 57: 420–431. 10.1080/10635150802166053 [DOI] [PubMed] [Google Scholar]
- 48.Wiens JJ, Hutter CR, Mulcahy DG, Noonan BP, Townsend TM, Sites JW, et al. Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol. Lett. 2012; 8: 1043–1046. 10.1098/rsbl.2012.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaher H, Grazziotin FG, Cadle JE, Murphy RW, Moura-Leite JC, Bonatto SL. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South America xenodontines: a revised classification and descriptions of new taxa. Pap. Av. Zool. 2009; 49: 115–153. [Google Scholar]
- 50.Hedges SB, Marion AB, Lipp KM, Marin J, Vidal N. A taxonomic framework for typhlopid snakes from the Caribbean and other regions (Reptilia: Squamata). Carib. Herpetol. 2014; 49: 1–61. [Google Scholar]
- 51.Noonan BP, Chippindale PT. Dispersal and vicariance: the complex evolutionary history of boid snakes. Mol. Phylogenet. Evol. 2006; 40: 347–358. [DOI] [PubMed] [Google Scholar]
- 52.Rawlings LH, Rabosky DN, Donnellan SC, Hutchinson MN. Python phylogenetics: inference from morphology and mitochondrial DNA. Biol. J. Linn. Soc. 2008; 93: 603–619. [Google Scholar]
- 53.Reynolds RG, Niemiller ML, Revell LJ. Toward a Tree-of-Life for the boas and pythons: Multilocus species-level phylogeny with unprecedented taxon sampling. Mol. Phylogenet. Evol. 2014; 71: 201–213. 10.1016/j.ympev.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 54.Sanders KL, Hamidy A, Head JJ, Gower DJ. Phylogeny and divergence times of filesnakes (Acrochordus): inferences from morphology, fossils and three molecular loci. Mol. Phylogenet. Evol. 2010; 56: 857–867. 10.1016/j.ympev.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 55.Teynie A, David P, Lottier A, Le MD, Vidal N, Nguyen TQ. A new genus and species of xenodermatid snake (Squamata: Caenophidia: Xenodermatidae) from northern Lao People’s Democratic Republic. Zootaxa. 2015; 3926: 523–540. 10.11646/zootaxa.3926.4.4 [DOI] [PubMed] [Google Scholar]
- 56.Alfaro ME, Karns DR, Voris HK, Brock CD, Stuart BL. Phylogeny, evolutionary history, and biogeography of Oriental–Australian rear-fanged water snakes (Colubroidea: Homalopsidae) inferred from mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 2008; 46: 576–593. 10.1016/j.ympev.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 57.Murphy JC, Sanders KL. First molecular evidence for the phylogenetic placement of the enigmatic snake genus Brachyorrhos (Serpentes: Caenophidia). Mol. Phylogenet. Evol. 2011; 61: 953–957. 10.1016/j.ympev.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 58.You CW, Poyarkov NA, Lin SM. Diversity of the snail‐eating snakes Pareas (Serpentes: Pareatidae) from Taiwan. Zool. Scr. 2015; 44: 349–361. [Google Scholar]
- 59.Castoe TA, Parkinson CL. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Mol. Phylogenet. Evol. 2006; 39: 91–110. [DOI] [PubMed] [Google Scholar]
- 60.Lenk P, Kalyabina S, Wink M, Joger U. Evolutionary relationships among the true vipers (Reptilia: Viperidae) inferred from mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2001; 19: 94–104. [DOI] [PubMed] [Google Scholar]
- 61.Malhotra A, Creer S, Pook CE, Thorpe RS. Inclusion of nuclear intron sequence data helps to identify the Asian sister group of New World pitvipers. Mol. Phylogenet. Evol. 2010; 54: 172–178. 10.1016/j.ympev.2009.09.007 [DOI] [PubMed] [Google Scholar]
- 62.Kelly CMR, Barker NP, Villet MH, Broadley DG, Branch WR. The snake family Psammophiidae (Reptilia: Serpentes): phylogenetics and species delimitation in the African sand snakes (Psammophis Boie, 1825) and allied genera. Mol. Phylogenet. Evol. 2008; 47: 1045–1060. 10.1016/j.ympev.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 63.Sanders KL, Lee MSY, Mumpuni Bertozzi T, Rasmussen AR. Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae). Mol. Phylogenet. Evol. 2013; 66: 575–591. 10.1016/j.ympev.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 64.Vidal N, Branch WR, Pauwels OSG, Hedges SB, Broadley DG, Wink M, et al. Dissecting the major African snake radiation: a molecular phylogeny of the Lamprophiidae Fitzinger (Serpentes: Caenophidia). Zootaxa. 2008; 1945: 51–66. [Google Scholar]
- 65.Grazziotin FG, Zaher H, Murphy RW, Scrocchi G, Benavides MA, Zhang YP, et al. Molecular phylogeny of the new world Dipsadidae (Serpentes: Colubroidea): a reappraisal. Cladistics. 2012; 28: 437–459. [DOI] [PubMed] [Google Scholar]
- 66.Vidal N, Dewynter M, Gower DJ. Dissecting the major American snake radiation: a molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, Caenophidia). C.R. Biologies 2010; 333: 48–55. 10.1016/j.crvi.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 67.Zhang B, Huang S. Relationship of Old World Pseudoxenodon and New World Dipsadinae, with comments on underestimation of species diversity of Chinese Pseudoxenodon. Asian Herpetol. Res. 2013; 4: 155–165. [Google Scholar]
- 68.McVay JD, Flores‐Villela O, Carstens B. Diversification of North American natricine snakes. Biol. J. Linn. Soc. 2015; 116: 1–12. [Google Scholar]
- 69.Mullin SJ, Seigel RA. Snakes: Ecology and conservation. New York: Cornell University Press; 2009. [Google Scholar]
- 70.Seigel RA, Collins JT. Snakes: Ecology and Behavior. New York: McGraw-Hill; 1993. [Google Scholar]
- 71.Seigel RA, Collins JT, Novak SS. Snakes: Ecology and evolutionary biology New York: Macmillan; 1987. [Google Scholar]
- 72.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004; 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28: 1647–1649. 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stamatakis A. RAxML Version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014; 30: 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA; 2010. p. 1–8.
- 76.Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006; 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- 77.Sanderson M, Shaffer H. Troubleshooting molecular phylogenetic analyses. Ann. Rev. Ecol. Syst. 2002; 33: 49–72. [Google Scholar]
- 78.Wilkinson M. Majority-rule reduced consensus trees and their use in bootstrapping. Mol. Biol. Evol. 1996; 13: 437–444. [DOI] [PubMed] [Google Scholar]
- 79.Aberer AJ, Krompass D, Stamatakis A. Pruning rogue taxa improves phylogenetic accuracy, an efficient algorithm and webservice. Syst. Biol. 2013; 62: 162–166. 10.1093/sysbio/sys078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: A fast, accurate, and powerful alternative. Syst. Biol. 2006; 55: 539–552. [DOI] [PubMed] [Google Scholar]
- 81.Townsend TM, Larson A, Louis E, Macey JR. Molecular phylogenetics of Squamata: the position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Syst. Biol. 2004; 53: 735–757. [DOI] [PubMed] [Google Scholar]
- 82.Rieppel O. A review of the origin of snakes. Evol. Biol. 1988; 22: 37–130. [Google Scholar]
- 83.Underwood G. A contribution to the classification of snakes London: Trustees of the British Museum (Natural History); 1967. [Google Scholar]
- 84.Burbrink FT, Crother BI. Evolution and taxonomy of snakes In: Aldridge RA, Sever DM, editors. Reproductive biology and phylogeny of snakes. Boca Raton: CRC Press; 2011. p. 19–53. [Google Scholar]
- 85.Scanlon JD, Lee MSY. The major clades of living snakes: Morphological evolution, molecular phylogeny, and divergence dates In: Aldridge RD, Sever DM, editors. Reproductive biology and phylogeny of snakes. Boca Raton: CRC Press; 2011. p. 55–95. [Google Scholar]
- 86.Kornilios P, Giokas S, Lymberakis P, Sindaco R. Phylogenetic position, origin and biogeography of Palearctic and Socotran blind-snakes (Serpentes: Typhlopidae). Mol. Phylogenet. Evo. 2013; 68: 35–41. [DOI] [PubMed] [Google Scholar]
- 87.Vidal N, Hedges SB. Higher-level relationships of caenophidian snakes inferred from four nuclear and mitochondrial genes. C.R. Biologies 2002; 325: 987–995. [DOI] [PubMed] [Google Scholar]
- 88.Peng LF, Lu CH, Huang S, Guo P, Zhang YP. A new species of the genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a proposal for an eclectic rule for species delimitation. Asian Herpetol. Res. 2014; 5: 228–239. [Google Scholar]
- 89.Wang X, Messenger K, Zhao E, Zhu C. Reclassification of Oligodon ningshaanensis Yuan, 1983 (Ophidia: Colubridae) into a new genus, Stichophanes gen. nov. with description on its malacophagous behavior. Asian Herpetol. Res. 2014; 5: 137–149. [Google Scholar]
- 90.Bossuyt F, Meegaskumbura M, Beenaerts N, Gower DJ, Pethiyagoda R, Roelants K, et al. Local endemism within the Western Ghats—Sri Lanka biodiversity hotspot. Science. 2004; 306: 479–481. [DOI] [PubMed] [Google Scholar]
- 91.Malhotra A, Thorpe RS. A phylogeny of four mitochondrial gene regions suggests a revised taxonomy for Asian pitvipers (Trimeresurus and Ovophis). Mol. Phylogenet. Evol. 2004; 32: 83–100. [DOI] [PubMed] [Google Scholar]
- 92.Alfaro ME, Arnold, SJ. Molecular systematics and evolution of Regina and the thamnophiine snakes. Mol. Phylogenet. Evol. 2001; 21: 408–423. [DOI] [PubMed] [Google Scholar]
- 93.Guo P, Liu Q, Zhang L, Li JX, Huang Y, Pyron RA. A taxonomic revision of the Asian keelback snakes, genus Amphiesma (Serpentes: Colubridae: Natricinae), with description of a new species. Zootaxa. 2014; 3873: 425–440. 10.11646/zootaxa.3873.4.5 [DOI] [PubMed] [Google Scholar]
- 94.Kluge AG. Aspidites and the phylogeny of pythonine snakes. Rec. Aus. Mus. 1993; (Suppl. 19): 77. [Google Scholar]
- 95.Fenwick AM, Gutberlet RL Jr Evans JA, Parkinson CL. Morphological and molecular evidence for phylogeny and classification of South American pitvipers, genera Bothrops, Bothriopsis and Bothrocophias (Serpentes: Viperidae). Zool. J. Linn. Soc. 2009; 156: 617–640. [Google Scholar]
- 96.Sanders KL, Lee MSY, Leys R, Foster R, Scott Keogh J. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (Hydrophiinae): evidence from seven genes for rapid evolutionary radiations. J. Evol. Biol. 2008; 21: 682–695. 10.1111/j.1420-9101.2008.01525.x [DOI] [PubMed] [Google Scholar]
- 97.Carranza S, Arnold EN, Pleguezuelos JM. Phylogeny, biogeography, and evolution of two Mediterranean snakes, Malpolon monspessulanus and Hemorrhois hippocrepis (Squamata: Colubridae), using mtDNA sequences. Mol. Phylogenet. Evol. 2006; 40: 532–546. [DOI] [PubMed] [Google Scholar]
- 98.Böhme W, De Pury S. A note on the generic allocation of Coluber moilensis Reuss 1834 (Serpentes: Psammophiidae). Salamandra. 2011; 47: 120–123. [Google Scholar]
- 99.Grismer L, Quah ES, Muin MA, Wood PJL, Nor SAM. A diminutive new species of cave-dwelling Wolf Snake (Colubridae: Lycodon Boie, 1826) from Peninsular Malaysia. Zootaxa. 2014; 3815: 051–067. [DOI] [PubMed] [Google Scholar]
- 100.Utiger U, Helfenberger N, Schätti B, Schmidt C, Ruf M, Ziswiler C. Molecular systematics and phylogeny of Old World and New World ratsnakes, Elaphe Auct., and related genera (Reptilia, Squamata, Colubridae). Russ. J. Herpetol. 2002; 9: 105–124. [Google Scholar]
- 101.Lenk P, Joger U, Wink M. Phylogenetic relationships among European ratsnakes of the genus Elaphe Fitzinger based on mitochondrial DNA sequence comparisons. Amphibia-Reptilia. 2001; 22: 329–339. [Google Scholar]
- 102.Burbrink FT, Lawson R. How and when did Old World ratsnakes disperse into the New World? Mol. Phylogenet. Evol. 2007; 43: 173–189. [DOI] [PubMed] [Google Scholar]
- 103.Schulz KD. A monograph of the colubrid snakes of the genus Elaphe Fitzinger. Schulz, Königstein; 1995.
- 104.Chen X, McKelvy AD, Grismer LL, Matsui M, Nishikawa K, Burbrink FT. The phylogenetic position and taxonomic status of the Rainbow Tree Snake Gonyophis margaritatus (Peters, 1871) (Squamata: Colubridae). Zootaxa. 2014; 3881: 532–548. 10.11646/zootaxa.3881.6.3 [DOI] [PubMed] [Google Scholar]
- 105.Schätti B, Trape JF. Bamanophis, a new genus for the West African colubrid Periops dorri Lataste, 1888 (Reptilia: Squamata: Colubrinae). Rev. Suisse Zool. 2008; 115: 595–615. [Google Scholar]
- 106.Wallach V, Williams KL, Boundy J. Snakes of the world: A catalogue of living and extinct species Florida: CRC Press, Taylor and Francis Group; 2014. [Google Scholar]
- 107.Nagy ZT, Joger U, Wink M, Glaw F, Vences M. Multiple colonization of Madagascar and Socotra by colubrid snakes: evidence from nuclear and mitochondrial gene phylogenies. Proc. R. Soc. Lond. B. 2003; 270: 2613–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagy ZT, Lawson R, Joger U, Wink M. Molecular systematics of racers, whipsnakes and relatives (Reptilia: Colubridae) using mitochondrial and nuclear markers. J. Zool. Syst. Evol. Res. 2004; 42: 223–233. [Google Scholar]
- 109.Šmίd J, Martίnez G, Gebhart J, Aznar J, Gállego J, Göçmen B, et al. Phylogeny of the genus Rhynchocalamus (Reptilia; Colubridae) with a first record from the Sultanate of Oman. Zootaxa. 2015; 4033: 380–392. 10.11646/zootaxa.4033.3.4 [DOI] [PubMed] [Google Scholar]
- 110.Wilkinson M. Majority-rule reduced consensus trees and their use in bootstrapping. Mol. Biol. Evol. 1996; 13: 437–444. [DOI] [PubMed] [Google Scholar]
- 111.Hedtke SM, Townsend TM, Hillis DM. Resolution of phylogenetic conflict in large data sets by increased taxon sampling. Syst. Biol. 2006; 55: 522–529. [DOI] [PubMed] [Google Scholar]
- 112.Wiens JJ, Tiu J. Highly incomplete taxa can rescue phylogenetic analyses from the negative impacts of limited taxon sampling. PLoS One. 2012; 7: e42925 10.1371/journal.pone.0042925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Philippe H, Snell EA, Bapteste E, Lopez P, Holland PW, Casane D. Phylogenomics of eukaryotes: impact of missing data on large alignments. Mol. Biol. Evol. 2004; 21: 1740–1752. [DOI] [PubMed] [Google Scholar]
- 114.Wiens JJ, Moen D S. Missing data and the accuracy of Bayesian phylogenetics. J. Syst. Evol. 2008; 46: 307–314. [Google Scholar]
- 115.Wiens JJ, Morrill MC. Missing data in phylogenetic analysis: reconciling results from simulations and empirical data. Syst. Biol. 2011; 60: 719–731. 10.1093/sysbio/syr025 [DOI] [PubMed] [Google Scholar]
- 116.Roure B, Baurain D, Philippe H. Impact of missing data on phylogenies inferred from empirical phylogenomic datasets. Mol. Biol. Evol. 2013; 30: 197–214. 10.1093/molbev/mss208 [DOI] [PubMed] [Google Scholar]
- 117.Nabhan AR, Sarkar IN. The impact of taxon sampling on phylogenetic inference: a review of two decades of controversy. Brief. Bioinformatics. 2011; 13: 122–134. 10.1093/bib/bbr014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Link HF. Beschreibung der Naturalien-Sammlung der Universität zu Rostock, Volume 2 Rostock: Adlers Erben; 1807. [Google Scholar]
- 119.Weinstein SA, Warrell DA, White J, Keyler DE. Venomous bites from non-venomous snakes: A critical analysis of risk and management of “colubrid” snake bites London: Elsevier; 2011. [Google Scholar]
- 120.Walls GL. The vertebrate eye and its adaptive radiation Michigan: The Cranbrook Institute of Science, Bloomington Hills; 1942. [Google Scholar]
- 121.Socha JJ. Gliding flight in Chrysopelea: turning a snake into a wing. Integr. Comp. Biol. 2011; 51: 969–982. 10.1093/icb/icr092 [DOI] [PubMed] [Google Scholar]
- 122.Savage JM, Oliver JA. Proposed addition to the "Official List of Generic Names in Zoology" of "Ahaetulla" Link, 1807, with "Ahaetulla mycterizans" Link, 1807, as the type species (Class Reptilia). Bull. Zool. Nomen. 1956; 12: 147–152. [Google Scholar]
- 123.Boulenger GA. Catalogue of the snakes in the British Museum (Natural History). Vol. III. Containing the Colubridae (opisthoglyphae and proteroglyphae), Amblycephalidae, and Viperidae London: Taylor and Francis; 1896. [Google Scholar]
- 124.Brongersma LD. On the presence or absence of hypapophyses under the posterior precaudal vertebrae in some snakes. Zool. Meded. 1938; 20: 240–242. [Google Scholar]
- 125.Broadley DG, Schätti B. A new species of Coluber from northern Namibia (Reptilia, Serpentes). Madoqua. 1997; 19: 171–174. [Google Scholar]
- 126.Bauer AM, Lamb T, Branch WR, Babb RD. New records of two rare snakes from northern Namibia, with comments on the trans-Kunene distribution of mopaneveld squamates. Herpetozoa. 2001; 14: 75–79. [Google Scholar]
- 127.Herrmann HW, Branch WR. Fifty years of herpetological research in the Namib Desert and Namibia with an updated and annotated species checklist. J. Arid Environs. 2013; 93: 94–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum-likelihood phylogenetic estimate based on 10 concatenated genes. Tips represent families and sub-families. Commonly recognized higher-level clades are labeled in all caps and bold. Node values represent SHL support values. Skeleton of the species tree is displayed on the left, colored and labeled as they appear in Fig 1.
(EPS)
(PHY)
(DOCX)
Two sequences were deleted during preliminary tree searches and 21 were identified as rogue taxa and pruned from the dataset leaving 1592 snake species from GenBank in the tree. Names represent species names as listed on The Reptile Database (http://www.reptile-database.org/) as of October 2015. Refer to S4 Table for list of rogue taxa. Taxa deleted during preliminary tree searches are highlighted in red, rogue taxa are highlighted in yellow, and sequences that were deleted because they were identical to other sequences are highlighted in green.
(DOCX)
Tissue samples for Boiga siamensis FMNH267726, Chrysopelea ornata LSUHC7158, and Psammodynastes pictus FMNH267940 were represented by clear chromatograms, but placed poorly in preliminary phylogenetic trees, so they were not included in the final data matrix. Tropidolaemus subannulatus KU327425 was identified as a rogue taxon by RogueNaRok and was pruned from the dataset and thus, is not represented in the phylogeny.
(DOCX)
(DOCX)
Each taxon is associated with a raw improvement score (R.I.S.), which represents the fraction of improvement in bootstrap support values throughout the tree when the selected taxon is pruned and all rogue taxa above it are also pruned. We performed one run and chose to sacrifice relatively lower node support values to maximize the number of taxa represented in the phylogeny. Thus we elected to only prune taxa with R.I.S. greater than 0.8, resulting in a total of 22 pruned taxa (highlighted in bold).
(DOCX)
Data Availability Statement
GenBank accession numbers are contained within the paper. All other relevant data are within the paper and its Supporting Information files.