Abstract
Rapid immunostaining of frozen sections within a tolerable time span would be very helpful for intraoperative diagnosis. A protocol was therefore established using the enhanced polymer one-step staining (EPOS) system (Dako) with antibodies against leucocyte common antigen (LCA), cytokeratin (CK), and anti-melanoma (MEL). Best results with reliable and specific immunostaining and a labelling intensity comparable to standard immunostaining protocols were achieved with fixation of samples in 100% acetone for 20 seconds (CK, LCA) or two minutes (MEL), followed by incubation of the primary antibody and development of the chromogen reaction with 3,3'diaminobenzidine (DAB) for three and five minutes at 37 degrees C, respectively. The total procedure takes only 12 minutes, thus enabling rapid immunostaining on intraoperative frozen sections. Apart from its use in tumour classification, this method is especially useful in detecting tumour cells in sentinel lymph nodes.
Full text
PDF
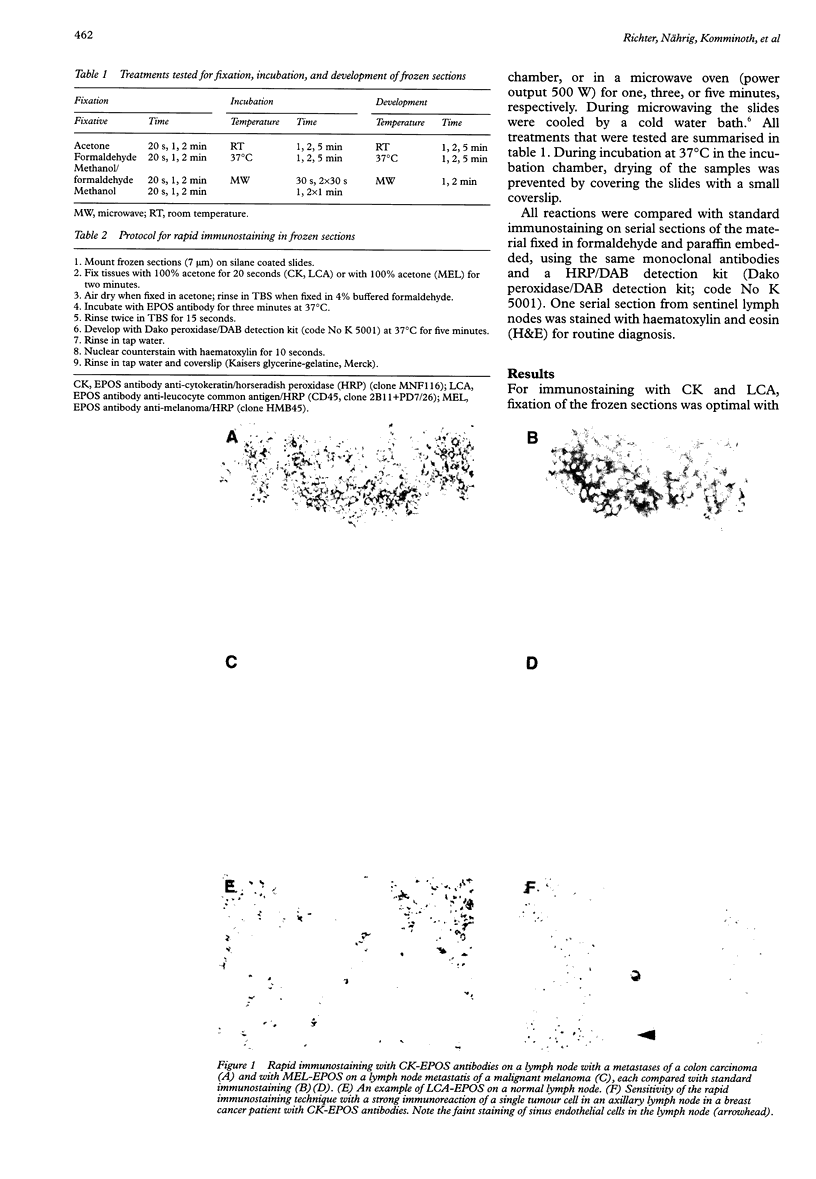

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chilosi M., Lestani M., Pedron S., Montagna L., Benedetti A., Pizzolo G., Menestrina F. A rapid immunostaining method for frozen sections. Biotech Histochem. 1994 Jul;69(4):235–239. doi: 10.3109/10520299409106292. [DOI] [PubMed] [Google Scholar]
- Heyderman E., Neville A. M. A shorter immunoperoxidase technique for the demonstration of carcinoembryonic antigen and other cell products. J Clin Pathol. 1977 Feb;30(2):138–140. doi: 10.1136/jcp.30.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong A. S., Milios J. Accelerated immunohistochemical staining by microwaves. J Pathol. 1990 Aug;161(4):327–334. doi: 10.1002/path.1711610409. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Schmidt K., Blaivas L., McCoy J. P., Wilson B. S. A rapid immunostaining method utilizing preformed antibody-avidin-biotin-peroxidase complexes. Am J Clin Pathol. 1985 May;83(5):636–639. doi: 10.1093/ajcp/83.5.636. [DOI] [PubMed] [Google Scholar]
- Morton D. L., Wen D. R., Wong J. H., Economou J. S., Cagle L. A., Storm F. K., Foshag L. J., Cochran A. J. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992 Apr;127(4):392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y., Serizawa A., Kawai K. Enhanced polymer one-step staining (EPOS) for proliferating cell nuclear antigen (PCNA) and Ki-67 antigen: application to intra-operative frozen diagnosis. Pathol Int. 1995 Feb;45(2):108–115. doi: 10.1111/j.1440-1827.1995.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Veronesi U., Paganelli G., Galimberti V., Viale G., Zurrida S., Bedoni M., Costa A., de Cicco C., Geraghty J. G., Luini A. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997 Jun 28;349(9069):1864–1867. doi: 10.1016/S0140-6736(97)01004-0. [DOI] [PubMed] [Google Scholar]
- Vitarelli E., Sippelli G., Tuccari G., Barresi G. The use of microwave irradiation for immunohistochemistry: a new methodological proposal. Histol Histopathol. 1995 Jan;10(1):35–38. [PubMed] [Google Scholar]
- Werner M., von Wasielewski R., Georgii A. Immunodetection of a tumor associated antigen (TAG-12): comparison of microwave accelerated and conventional method. Biotech Histochem. 1991;1(2):79–81. doi: 10.3109/10520299109110554. [DOI] [PubMed] [Google Scholar]




