Abstract
Objective
Studies evaluating the relationship between body mass index (BMI) and mortality demonstrate a U-shaped association. To expand, this study evaluated the relationship between adiposity indices, a body shape index (ABSI) and body adiposity index (BAI), and mortality in 77,505 postmenopausal women.
Methods
A prospective cohort analysis was conducted in the Women’s Health Initiative to ascertain the independent relationships between adiposity indices and mortality in order to inform on the clinical usefulness of alternate measures of mortality risk. ABSI (waist circumference (cm)/[BMI2/3 × height (cm)1/2]), BAI (hip circumference (cm)/[height (m)1.5] − 18), weight, BMI, and waist circumference (WC) were evaluated in relation to mortality risk using adjusted Cox proportional hazards regression models.
Results
ABSI showed a linear association with mortality (HR, 1.37; 95% CI, 1.28–1.47 for quintile 5 vs. 1) while BMI and BAI had U-shaped relationships with HR of 1.30; 95% CI, 1.20–1.40 for obesity II/III BMI and 1.06, 95% CI, 0.99–1.13 for BAI. Higher WC (HR, 1.21; 95% CI, 1.13–1.29 for quintile 5 vs. 1) showed relationships similar to BMI.
Conclusions
ABSI appears to be a clinically useful measure for estimating mortality risk, perhaps more so than BAI and BMI in postmenopausal women.
Introduction
Despite evidence that overweight status is associated with higher risk for several of the leading causes of death in the United States, studies evaluating the relationship between body mass index (BMI) and mortality have suggested a U-shaped association exists in which overweight status may be protective in older adults (1,2), although evidence is inconsistent (3,4). Primary explanations include confounding by smoking and reverse causality related to pre-mortality weight loss. Others have recommended using maximum lifetime weight to evaluate the relationship between weight or BMI and mortality risk (5). Alternatively, it has been suggested that waist circumference (WC) be evaluated as a predictor of mortality, as reported in a pooled analysis of over 650,000 adults (6). A 5-cm increase in WC, among those with BMI between 20 and 50 kg/m2, was associated with higher mortality in both men and women. Interpreting findings that suggest a protective association between BMI and mortality leaves many unanswered questions. In particular, why would high BMI, usually associated with cardiovascular disease, stroke, and even select cancers (7), be associated with a lower risk of mortality? Included among the hypotheses postulated to explain this relationship is the possibility of a survival benefit in overweight individuals who achieve greater cardiorespiratory fitness as a result of weight bearing (8), a reserve energy source during acute illness/infection, and/or higher lean mass in overweight as compared to normal-weight, non-exercising individuals, a characteristic that has also been associated with greater functional status (9).
BMI, which is an assessment of weight relative to height, does not robustly integrate information on adiposity, distribution of body fat, or body shape, factors known to be associated with obesity-related chronic disease risk in numerous studies (10–12). In fact, for the majority of studies published, analysis has focused solely on BMI and only occasionally been expanded to include WC (13). WC correlates with BMI, thus limiting its clinical predictive value in mortality analyses aimed to improve on BMI estimates of risk (14). These issues have led investigators to explore new composite measures of adiposity to describe risk estimates with an emphasis on integrating body shape and adiposity. Among measures under consideration are a body shape index (ABSI) (15) and body adiposity index (BAI) (16). ABSI has demonstrated stronger associations with total mortality than BMI in select studies (17). Originally developed by Bergman et al. (16), BAI has demonstrated relative clinical value in familial obesity syndromes (18) and has also been associated with insulin resistance among adults (19). However, a few studies have reported that BMI showed stronger associations with adiposity than BAI (19,20).
To expand on these important issues, here we evaluate the role of these newer measures of body habitus, ABSI and BAI, in relation to mortality among older women. The intent is to identify measures of stronger predictive value in relation to mortality and in turn enhance the clinical usefulness beyond what BMI provides, understanding that these newer measures may capture similar biology, but do so by more robustly integrating central adiposity. ABSI has recently been validated for use in NHANES 1999–2004 and applied in the context of mortality risk among 7,011 adults enrolled in the British Health and Lifestyle Survey (13), in which mortality rates were 61% higher among those in the highest ABSI quintile than the lowest quintile. In addition, BAI characterizes body habitus by integrating both hip circumference and height to inform on mortality and obesity-associated disease risk, with mixed results to date (21–25). Both ABSI and BAI hold potential to inform on mortality risk if associations with mortality are upheld across a variety of population samples.
The Women’s Health Initiative Observational Study (WHI OS) provides a well-characterized sample of over 93,000 postmenopausal women available to evaluate the relationship between adiposity indices and mortality. Here we test the hypotheses that ABSI and BAI are associated with mortality risk in WHI OS women, with a sensitivity analysis for never-smokers. An additional objective for these analyses was to determine if these body adiposity measures result in similar mortality risk as is shown for BMI and to explore the relationship between maximum adult BMI and mortality risk in this cohort.
Methods
Study population
This analysis uses data from the largest prospective cohort of postmenopausal women in the United States. Details of the WHI OS, with 93,676 enrolled women, have been previously described (26). In this study, women without anthropometric measurements (height, weight, WC, and hip circumference) at baseline were excluded (n = 1,468), as ABSI and BAI could not be calculated without these data; women lacking follow-up data were also excluded (n = 471). Anyone entering the study with a history of cancer (other than non-melanoma skin cancer; n = 12,075) or stroke (n = 1,415) was also excluded, as these diagnoses are associated with clinical reduction in energy intake and subsequent weight loss. Additional exclusion criteria were incident disease or death during the first 12 months on study: cancer (n = 1,061), stroke (n = 170), myocardial infarction (n = 206), congestive heart failure (n = 201), and death (n = 255). This approach was undertaken to assure exclusion of women wherein death might be imminent in the more immediate future due to some pre-existing condition not diagnosed prior to study entry. As a result of these overlapping exclusion criteria, the final sample size was 77,505. Data are from the December 2014 release (www.WHI.org) that included outcomes occurring and adjudicated on or before August 29, 2014.
Baseline demographic and lifestyle behavior data
Participants completed study-specific questionnaires that provided information on demographic characteristics including age, race/ethnicity, and education. Participants also completed a lifestyle questionnaire that included measures of physical activity (MET-h/week of moderate-strenuous activity), sedentary time, sleep, and smoking. Diet was assessed using the WHI Food Frequency Questionnaire (FFQ) (27), a 122-item instrument that was completed during the baseline clinic visit with assistance from study personnel as needed. Data from the FFQ were used to calculate alcohol intake and the overall score for diet quality using the Healthy Eating Index 2005, as previously described (28). FFQ data from women reporting < 600 kcal/day or > 5,000 kcal/day were excluded. Clinical data including hormone therapy, treatment for diabetes mellitus, and history of myocardial infarction were also collected via questionnaire. General health was assessed from the general health construct within the SF-36 (29).
Body size indices
ABSI is defined as WC (cm)/[BMI2/3 × height (cm)1/2], where BMI is weight (kg)/height (m)2. The incorporation of WC moves beyond standard BMI to account for potential metabolic abnormalities and related health consequences associated with abdominal fat (13,15). BAI is defined as hip circumference (cm)/[height (m)1.5] − 18. BAI has shown correlation with percent body fat even across sexes (30). It does not require weight measurement and as such has been proposed for use when scales are not available, as may be the case in public health settings.
Height, weight, WC, and hip circumference were measured in the study clinic at baseline (31). Measures were taken by trained study personnel using study-specific criteria based upon standard anthropometric measurement methodology. Weight and height were measured without shoes using a beam scale and a wall-mounted stadiometer, respectively, and recorded to the nearest 0.1 kg (weight) and 0.1 cm (height). WC was measured at the umbilicus, and hip circumference at the maximum circumference, to the nearest 0.1 cm. Maximum adult BMI was estimated using self-reported height at age 18 and self-reported maximum adult weight (available for 98.4% of the study population).
Mortality
Death of study participants was ascertained through semi-annual telephone contacts with WHI clinic personnel followed by mailed questionnaires (32). If clinic staff were unsuccessful reaching participants by telephone, mailed questionnaires including queries on vital and health status were forwarded to each participant’s address along with an addressed, postage-paid envelope to return the completed documents. If no response was received within the protocol-designated time frame, clinic staff contacted the designated contacts to collect the requested information. In addition, medical records and/or death certificates were collected to verify health status and mortality data. Centrally, WHI staff also collected mortality data using the National Death Index (Centers for Disease Control and Prevention, Atlanta, Georgia).
Statistical analysis
Baseline participant characteristics were compared across quintiles of ABSI and BAI using chi-squared tests (categorical variables) and ANOVA (continuous variables). Anthropometrics were compared across age categories, as weight loss is common with advancing age and generally associated with greater mortality risk (33), although significant heterogeneity in weight trajectories has been described in aging (33–35). Pearson correlations were calculated between each pair of anthropomorphic measures. Associations between baseline ABSI, BAI, and BMI as well as adiposity components within these scores (weight, WC, hip circumference, and waist-to-hip ratio [WHR]) and mortality were tested using Cox proportional hazards regression. All body measures were analyzed in quintiles, and BMI was additionally analyzed according to standard World Health Organization categories: underweight (<18.5), normal weight (18.5–24.9; reference group), overweight (25–29.9), obesity I (30–34.9), and obesity II/III (≥35). The relationship was considered U-shaped if quintile 5 was not different from quintile 1, but intermediate quintiles (2–4) were lower than quintile 1. Furthermore, the relationship was considered J-shaped if intermediate quintiles (2–4) were lower, and quintile 5 was higher, than quintile 1. Hazard ratios were adjusted for age and potential confounders, identified as variables associated with ABSI (P < 0.10) and also associated with mortality (P < 0.10), and identified from the existing literature as related to BMI and mortality. Time-to-death was defined as time from enrollment to death, censored at time of last contact for which the participant was living. Stratifying the baseline hazard of the ABSI and BAI models by BMI category had no appreciable effect on the estimates (data not shown). Models for BMI, ABSI, and BAI were also stratified by age category: 50–59, 60–69, and ≥70 years. Likelihood ratio tests were used to test for interaction between age category and BMI, ABSI, or BAI on mortality. Similar tests revealed no significant interactions between race/ethnicity and ABSI or BAI on mortality (data not shown). Given the relationship between smoking and weight, a sensitivity analysis evaluating the anthropometric exposure variables in relation to mortality that was confined to never-smokers was also conducted.
Models were compared to each other according to log-likelihood, compared to a base model without including the anthropometric measure. These model comparisons were confirmed with Akaike information criterion (AIC) and Bayesian information criterion (BIC). Additionally, since comparing higher levels of body size to a reference group of the lowest quintile has limitations, fractional polynomials (36) were used to explore nonlinear, continuous models for ABSI, BAI, and BMI as a sensitivity analysis (data not shown). All statistical analyses were conducted using Stata 14.0 (StataCorp, College Station, Texas).
Results
Women were followed for an average 13.5 ± 4.8 years, during which time 10,761 (13.9%) deaths were reported. At baseline, the study population generally comprised well-educated, non-Hispanic white women with a mean age of 63.3 ± 7.3 years. Alcohol intake was relatively low (median < 0.1 drinks/day), and over half reported never smoking. Women with higher ABSI tended to be less educated and report lower (worse) HEI scores and lower moderate-strenuous physical activity, as well as greater sedentary time (Table 1). Higher ABSI was also shown in women who reported sleeping <7 h/day and lower general health scores on the SF-36. When evaluating participant characteristics across quintiles of BAI (Supporting Information, Table S1) and BMI (Supporting Information, Table S2) compared to ABSI, notable differences were observed. In particular, higher BAI and BMI were associated with black race and lower alcohol intake as well as self-reported never-smoking (Supporting Information, Tables S1 and S2); these relationships are opposite to those shown for ABSI (Table 1).
TABLE 1.
Baseline WHI OS participant characteristics across quintiles of a body shape index (ABSI) (n = 77,505)
| ABSI quintile
|
|||||
|---|---|---|---|---|---|
| 1st (n = 15,501) |
2nd (n = 15,501) |
3rd (n = 15,501) |
4th (n = 15,501) |
5th (n = 15,501) |
|
| Age (years) | 61.3 ± 7.1 | 62.4 ± 7.2 | 63.3 ± 7.3 | 64.1 ± 7.2 | 65.4 ± 7.1 |
| Race/ethnicity | |||||
| NHW | 81.9 | 83.7 | 83.0 | 83.1 | 84.0 |
| Black | 10.5 | 8.18 | 7.59 | 6.85 | 7.00 |
| Hispanic | 3.97 | 3.70 | 4.22 | 4.06 | 3.76 |
| Asian | 1.92 | 2.86 | 3.46 | 3.90 | 3.11 |
| Native | 0.33 | 0.32 | 0.30 | 0.52 | 0.71 |
| Other | 1.37 | 1.23 | 1.42 | 1.61 | 1.38 |
| Education | |||||
| < High school | 3.97 | 3.91 | 4.69 | 5.57 | 7.11 |
| High school | 14.5 | 15.2 | 16.5 | 16.9 | 18.6 |
| Some college | 35.0 | 36.0 | 35.7 | 37.2 | 37.5 |
| ≥College | 46.5 | 44.9 | 43.2 | 40.4 | 36.8 |
| Alcohol (drink/day) | 0.38 ± 0.73 | 0.40 ± 0.76 | 0.41 ± 0.80 | 0.43 ± 0.85 | 0.43 ± 0.92 |
| < 1 | 88.2 | 86.8 | 86.2 | 85.7 | 85.9 |
| ≥ 1 | 11.8 | 13.2 | 13.8 | 14.3 | 14.1 |
| Smoking pack-years | |||||
| 0 (never smoked) | 56.6 | 54.2 | 53.8 | 51.3 | 47.6 |
| < 5 | 16.2 | 16.1 | 15.1 | 14.2 | 13.0 |
| 5 to < 20 | 14.2 | 14.7 | 14.7 | 14.4 | 14.2 |
| ≥ 20 | 13.0 | 15.1 | 16.4 | 20.2 | 25.2 |
| Energy intake (kcal/day) | 1544 ± 585 | 1553 ± 580 | 1565 ± 589 | 1587 ± 597 | 1608 ± 631 |
| HEI-2005 | 70.4 ± 9.9 | 70.1 ± 10.1 | 69.7 ± 10.3 | 68.9 ± 10.7 | 67.8 ± 11.0 |
| 1st quintile | 16.4 | 17.9 | 19.0 | 21.7 | 25.0 |
| 2nd quintile | 19.7 | 19.3 | 19.7 | 20.2 | 21.1 |
| 3rd quintile | 20.6 | 20.3 | 20.6 | 19.5 | 19.0 |
| 4th quintile | 21.5 | 20.9 | 20.5 | 19.4 | 17.7 |
| 5th quintile | 21.8 | 21.7 | 20.2 | 19.2 | 17.2 |
| Moderate-strenuous physical activity (MET-h/week) | 8.65 ± 11.9 | 7.99 ± 11.4 | 7.44 ± 10.9 | 6.71 ± 10.3 | 5.80 ± 9.7 |
| < 7.5 | 62.2 | 64.8 | 66.4 | 69.4 | 73.9 |
| ≥ 7.5 | 37.8 | 35.2 | 33.6 | 30.6 | 26.2 |
| Sedentary time (h/day) | 8.4 ± 3.9 | 8.4 ± 3.8 | 8.4 ± 3.9 | 8.5 ± 3.9 | 8.6 ± 4.0 |
| < 8 | 44.7 | 45.3 | 45.6 | 44.6 | 43.4 |
| ≥ 8 | 55.3 | 54.8 | 54.4 | 55.4 | 56.6 |
| Sleep (h/day) | 6.8 ± 1.1 | 6.9 ± 1.1 | 6.9 ± 1.1 | 6.9 ± 1.1 | 6.8 ± 1.2 |
| < 7 | 34.7 | 34.4 | 34.3 | 35.4 | 36.4 |
| 7–9 | 65.0 | 65.1 | 65.3 | 64.0 | 62.8 |
| ≥ 10 | 0.37 | 0.53 | 0.49 | 0.60 | 0.78 |
| Hormone replacement therapy | |||||
| Never | 25.1 | 26.5 | 28.5 | 31.2 | 34.6 |
| Former | 17.0 | 18.5 | 19.8 | 20.5 | 21.9 |
| Current | 57.8 | 55.0 | 51.7 | 48.3 | 43.6 |
| Treated diabetes mellitus | 1.45 | 1.78 | 2.76 | 4.26 | 8.73 |
| History of myocardial infarction | 1.19 | 1.59 | 1.67 | 2.27 | 3.62 |
| General health construct (SF-36; %) | 77.7 ± 17.1 | 76.5 ± 17.2 | 75.0 ± 17.7 | 73.5 ± 18.1 | 70.4 ± 18.9 |
| 1st tertile | 32.8 | 35.6 | 38.7 | 42.1 | 49.3 |
| 2nd tertile | 35.0 | 34.6 | 34.4 | 34.4 | 31.7 |
| 3rd tertile | 32.2 | 29.8 | 26.9 | 23.6 | 19.0 |
Values given as mean + SD or %.
All anthropometric measures were associated with age (Table 2). Weight, BMI, and hip circumference were lower in older women (age ≥ 70 years), yet ABSI and WHR were higher in older women. WC was highest in the middle age group (60–69 years), and BAI was lowest in the youngest age group (50–59 years). ABSI was positively correlated with WC (Pearson’s rho = 0.52) and WHR (rho = 0.75), but not with BMI (rho = −0.01). Of interest, BMI, but not ABSI, was strongly correlated with weight (rho = 0.92 and 0.01, respectively). BAI was strongly correlated with WC (rho = 0.71), BMI (rho = 0.84), and weight (rho = 0.67), but not with ABSI (rho = 0.05). All Pearson’s correlation coefficients showed P < 0.001.
TABLE 2.
Baseline anthropometric measures of WHI OS participants, across age groups at the time of enrollment
| Total (n = 77,505) |
Age 50–59 years (n = 25,716) |
Age 60–69 years (n = 34,176) |
Age 70 years (n = 17,613) |
|
|---|---|---|---|---|
| ABSI | 0.74 ± 0.06 | 0.73 ± 0.06 | 0.74 ± 0.06 | 0.75 ± 0.06 |
| BAI | 33.1 ± 6.2 | 32.8 ± 6.4 | 33.2 ± 6.2 | 33.2 ± 5.9 |
| BMI (kg/m2) | 27.2 ± 5.8 | 27.3 ± 6.2 | 27.3 ± 5.8 | 26.6 ± 5.2 |
| Weight (kg) | 71.1 ± 15.7 | 72.7 ± 17.0 | 71.5 ± 15.5 | 68.0 ± 13.8 |
| Waist circumference (cm) | 84.5 ± 13.5 | 83.8 ± 14.1 | 85.1 ± 13.5 | 84.4 ± 12.4 |
| Hip circumference (cm) | 104.9 ± 11.9 | 105.6 ± 12.4 | 105.2 ± 11.8 | 103.2 ± 10.9 |
| Waist-to-hip ratio | 0.80 ± 0.08 | 0.79 ± 0.08 | 0.81 ± 0.08 | 0.82 ± 0.08 |
Values given as mean ± SD. All P < 0.001 (ANOVA).
ABSI, a body shape index; BAI; body adiposity index; BMI; body mass index.
All of the body shape indices and other anthropometric measurements evaluated demonstrated associations with mortality in multivariate models (Table 3). ABSI quintiles 4 and 5 showed a 10% and 37% higher mortality risk compared to quintile 1, respectively. The top quintile for hip circumference and WHR showed higher mortality risk than the lowest quintile (13% and 20%, respectively). In contrast, results for BAI and BMI quintiles 2–4 revealed decreased mortality risk of 9–15% and 13–16%, respectively, with no relationship in quintile 5, indicating a U-shaped (nonlinear) curve. When assessing BMI according to standard categories instead of quintiles, the negative effects of underweight (58% increased risk) and obesity II/III (30% increased risk) status were highlighted, further supporting a U-shaped curve for the association between BMI and mortality. Similarly, WC also showed 5–10% decreased mortality risk in quintiles 2–4, but 21% increased risk in quintile 5, compared to quintile 1, indicating a J-shaped (nonlinear) curve. The association with weight also reflected a J-shaped curve, with 12%–15% decreased mortality risk in quintiles 2–4 and 10% increased risk in quintile 5.
TABLE 3.
All-cause mortality risk in relation to a body shape index (ABSI), body adiposity index (BAI), body mass index (BMI), and other anthropometric measures using Cox proportional hazards regression
| Anthropometric measure | Category
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| ABSI quintiles | |||||
| Median (range) | 0.68 (0.25–0.70) | 0.71 (0.70–0.72) | 0.74 (0.72–0.75) | 0.76 (0.75–0.78) | 0.81 (0.78–1.86) |
| Number of deaths (%) | 1479 (9.54) | 1734 (11.2) | 1987 (12.8) | 2308 (14.9) | 3253 (21.0) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 1.04 (0.97–1.12) | 1.15 (1.07–1.23) | 1.28 (1.20–1.37) | 1.78 (1.67–1.89) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 1.00 (0.93–1.08) | 1.05 (0.98–1.13) | 1.10 (1.03–1.18) | 1.37 (1.28–1.47) |
| BAI quintiles | |||||
| Median (range) | 26.6 (0.40–28.3) | 29.6 (28.3–30.8) | 32.1 (30.8–33.4) | 35.1 (33.4–37.3) | 40.9 (37.3–91.2) |
| Number of deaths (%) | 2047 (13.2) | 2008 (13.0) | 2023 (13.0) | 2107 (13.6) | 2576 (16.6) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 0.91 (0.86–0.97) | 0.91 (0.85–0.96) | 0.96 (0.90–1.02) | 1.34 (1.27–1.42) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.91 (0.85–0.97) | 0.85 (0.80–0.91) | 0.86 (0.81–0.92) | 1.06 (0.99–1.13) |
| BMI quintiles | |||||
| Median (range), kg/m2 | 21.2 (11.9–22.6) | 23.7 (22.6–24.9) | 26.0 (24.9–27.4) | 29.0 (27.4–31.1) | 34.6 (31.1–69.9) |
| Number of deaths (%) | 2133 (13.8) | 1998 (12.9) | 2042 (13.2) | 2129 (13.7) | 2459 (15.9) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 0.90 (0.85–0.96) | 0.93 (0.87–0.99) | 1.02 (0.96–1.08) | 1.46 (1.38–1.55) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.87 (0.82–0.93) | 0.84 (0.78–0.89) | 0.86 (0.80–0.92) | 1.05 (0.98–1.12) |
| BMI categoriesb | |||||
| Median (range), kg/m2 | 17.8 (11.9–18.5) | 22.7 (18.5–25.0) | 27.1 (25.0–30.0) | 32.0 (30.0–35.0) | 38.5 (35.0–69.9) |
| Number of deaths (%) | 200 (21.4) | 4066 (13.8) | 3549 (13.4) | 1720 (14.4) | 1226 (17.4) |
| Age-adjusted HR (95% CI) | 1.70 (1.48–1.96) | 1.00 (ref.) | 1.03 (0.99–1.08) | 1.27 (1.20–1.34) | 1.93 (1.81–2.06) |
| Multivariate HR (95% CI)a | 1.58 (1.35–1.84) | 1.00 (ref.) | 0.93 (0.88–0.97) | 1.01 (0.95–1.07) | 1.30 (1.20–1.40) |
| Weight quintiles | |||||
| Median (range), kg | 54.5 (31.0–58.7) | 62.0 (58.8–65.0) | 68.2 (65.1–71.8) | 76.0 (71.9–81.9) | 91.0 (82.0–195) |
| Number of deaths (%) | 2295 (14.7) | 2025 (13.0) | 2021 (13.2) | 2012 (13.0) | 2408 (15.6) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 0.91 (0.85–0.96) | 0.96 (0.90–1.02) | 1.01 (0.95–1.07) | 1.53 (1.44–1.62) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.85 (0.80–0.91) | 0.88 (0.82–0.93) | 0.85 (0.79–0.91) | 1.10 (1.03–1.17) |
| Waist circumference quintiles | |||||
| Median (range), cm | 69.5 (35.5–73.0) | 76.1 (73.1–79.0) | 82.5 (79.1–86.0) | 90.0 (86.1–95.0) | 103 (95.1–197) |
| Number of deaths (%) | 1852 (11.7) | 1852 (12.0) | 2182 (13.3) | 2176 (14.6) | 2699 (18.1) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 0.95 (0.89–1.01) | 1.05 (0.98–1.11) | 1.18 (1.11–1.26) | 1.77 (1.67–1.88) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.90 (0.84–0.96) | 0.93 (0.87–0.99) | 0.95 (0.89–1.02) | 1.21 (1.13–1.29) |
| Hip circumference quintiles | |||||
| Median (range), cm | 92.0 (40.0–95.5) | 98.0 (95.6–101) | 103 (101–106) | 109 (106–113) | 120 (113–200) |
| Number of deaths (%) | 2213 (14.2) | 2104 (13.3) | 1964 (12.8) | 2105 (13.2) | 2375 (16.0) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 0.95 (0.89–1.01) | 0.94 (0.88–1.00) | 1.03 (0.97–1.10) | 1.47 (1.39–1.56) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.92 (0.87–0.99) | 0.90 (0.84–0.96) | 0.90 (0.85–0.97) | 1.13 (1.05–1.20) |
| Waist-to-hip ratio | |||||
| Median (range) | 0.72 (0.28–0.74) | 0.76 (0.74–0.78) | 0.80 (0.78–0.82) | 0.84 (0.82–0.86) | 0.90 (0.86–2.88) |
| Number of deaths (%) | 1596 (10.3) | 1808 (11.7) | 2064 (13.3) | 2341 (15.1) | 2952 (19.1) |
| Age-adjusted HR (95% CI) | 1.00 (ref.) | 1.02 (0.96–1.09) | 1.13 (1.06–1.21) | 1.30 (1.22–1.38) | 1.70 (1.60–1.81) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.96 (0.89–1.03) | 1.00 (0.93–1.07) | 1.04 (0.97–1.12) | 1.20 (1.13–1.29) |
Adjusted for age (continuous), race/ethnicity (NHW, black, Hispanic, Asian, native, other/unknown), education (< high school, high school, some college, ≥ college), alcohol (< or ≥ 1 drink/day), smoking pack-years (0, < 5, 5 to < 20, ≥ 20), energy intake (continuous), HEI-2005 (continuous), moderate-strenuous physical activity (continuous), sedentary time (continuous), sleep (< 7, 7–9, ≥ 10 h/day), hormone replacement therapy (never, former, current), diabetes (yes/no), MI (ever/never), and general health construct (continuous).
Standard World Health Organization categories: underweight (< 18.5), normal weight (18.5–24.9; reference group), overweight (25–29.9), obesity I (30–34.9), and obesity II/III (≥ 35).
Comparing the log-likelihood of each multivariate model (in Table 3) to a base model (one without any anthropometric term) revealed that ABSI improved the model’s mortality risk prediction more than any of the other measurements. Overall, the strongest associations were seen for ABSI quintile 5 versus 1 (HR, 1.37; 95% CI, 1.28–1.47) and BMI underweight versus normal-weight categories (HR, 1.58; 95% CI, 1.35–1.84), followed by BMI obesity II/III (HR, 1.30; 95% CI, 1.20–1.40). Additionally, model comparisons according to AIC and BIC confirmed that the ABSI model fit the data better than any of the other measurements (data not shown). Further, exploratory analyses using fractional polynomials to generate nonlinear models for continuous versions of these anthropometric measurements largely confirmed our findings (data not shown).
Results were largely consistent and often strengthened after restricting the analysis to never-smokers (Table 4). Estimates for quintile 5 of BAI and BMI showed increased mortality risk in this sensitivity analysis (16% and 15%, respectively). The strongest associations were seen for standard BMI categories: underweight (HR, 1.69; 95% CI, 1.34–2.13) and obese II/III (HR, 1.47; 95% CI, 1.32–1.64) never-smoking women had substantially increased risk of death compared to never-smoking, normal-weight women. In an exploratory analysis, maximum adult BMI was associated with 36% (95% CI, 23–50%) and 65% (95% CI, 48–82%) increased risk of mortality for quintile 5 versus 1 and obesity II/III versus normal weight, respectively, among never-smokers. Risk among underweight women was non-significantly elevated (HR, 1.10; 95% CI, 0.72–1.70). Of note, comparison of the log-likelihood multivariate model to the base model in never-smokers suggested WC, not ABSI, was associated with the highest improvement in the risk prediction model (data not shown).
TABLE 4.
All-cause mortality risk in relation to a body shape index (ABSI), body adiposity index (BAI), body mass index (BMI), and other anthropometric measures in never-smokers only using Cox proportional hazards regression
| Anthropometric measure | Category
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| ABSI quintiles | |||||
| Number of deaths (%) | 657 (8.58) | 711 (9.73) | 810 (11.1) | 884 (12.9) | 1136 (17.8) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.98 (0.88–1.09) | 1.06 (0.95–1.17) | 1.11 (1.01–1.23) | 1.35 (1.22–1.49) |
| BAI quintiles | |||||
| Number of deaths (%) | 730 (10.6) | 781 (11.0) | 801 (11.1) | 818 (11.6) | 1068 (14.8) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.94 (0.85–1.04) | 0.89 (0.80–0.98) | 0.90 (0.82–1.00) | 1.16 (1.05–1.28) |
| BMI quintiles | |||||
| Number of deaths (%) | 854 (11.5) | 744 (10.4) | 788 (11.3) | 848 (11.9) | 964 (14.1) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.85 (0.77–0.94) | 0.88 (0.80–0.97) | 0.91 (0.82–1.00) | 1.15 (1.04–1.27) |
| BMI categoriesb | |||||
| Number of deaths (%) | 76 (16.1) | 1572 (10.8) | 1399 (11.6) | 680 (12.5) | 471 (15.5) |
| Multivariate HR (95% CI)a | 1.69 (1.34–2.13) | 1.00 (ref.) | 0.99 (0.92–1.07) | 1.08 (0.98–1.18) | 1.47 (1.32–1.64) |
| Weight quintiles | |||||
| Number of deaths (%) | 924 (12.1) | 787 (10.8) | 799 (11.5) | 780 (11.1) | 908 (13.7) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.89 (0.81–0.98) | 0.96 (0.87–1.06) | 0.92 (0.83–1.01) | 1.24 (1.12–1.37) |
| Waist circumference quintiles | |||||
| Number of deaths (%) | 786 (10.1) | 739 (10.1) | 812 (10.9) | 866 (13.1) | 995 (15.8) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.88 (0.79–0.97) | 0.91 (0.82–1.00) | 1.03 (0.94–1.14) | 1.31 (1.18–1.45) |
| Hip circumference quintiles | |||||
| Number of deaths (%) | 832 (11.3) | 835 (11.5) | 782 (11.1) | 800 (11.1) | 949 (14.4) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 1.01 (0.91–1.11) | 0.99 (0.90–1.11) | 0.98 (0.89–1.08) | 1.30 (1.17–1.43) |
| Waist-to-hip ratio | |||||
| Number of deaths (%) | 750 (9.55) | 726 (9.90) | 794 (11.2) | 900 (13.3) | 1028 (15.9) |
| Multivariate HR (95% CI)a | 1.00 (ref.) | 0.90 (0.81–1.00) | 0.96 (0.86–1.06) | 1.06 (0.96–1.16) | 1.16 (1.06–1.29) |
Adjusted for age (continuous), race/ethnicity (NHW, black, Hispanic, Asian, native, other/unknown), education (< high school, high school, some college, ≥ college), alcohol (< or ≥ 1 drink/day), energy intake (continuous), HEI-2005 (continuous), moderate-strenuous physical activity (continuous), sedentary time (continuous), sleep (< 7, 7–9, ≥ 10 h/day), hormone replacement therapy (never, former, current), diabetes (yes/no), MI (ever/never), and general health construct (continuous).
Standard World Health Organization categories: underweight (< 18.5), normal weight (18.5–24.9; reference group), overweight (25–29.9), obesity I (30–34.9), and obesity II/III (≥ 35).
Owing to published evidence that the relationship between adiposity and mortality may vary with advancing age (33–35), we evaluated the relationship between composite anthropometric measures (ABSI, BAI, and BMI) and mortality within each decade of life (50–59, 60–69, and ≥70 years; Figure 1). The aforementioned U-shaped curves for BAI and BMI appear restricted to women age ≥60 years, whereas the association between ABSI and mortality seems unmodified by age group (likelihood ratio tests for interaction between age group and BMI, ABSI, or BAI; P = 0.001, 0.117, and 0.002, respectively).
Figure 1.
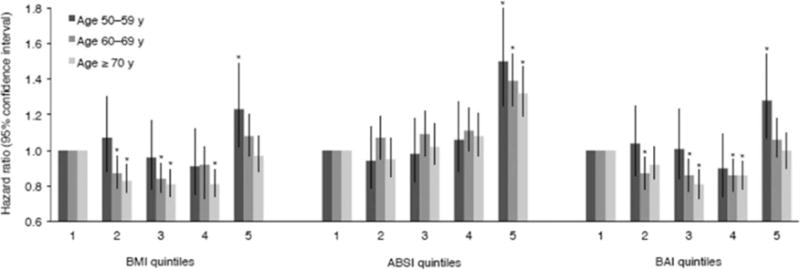
Evaluation of the relationship between body mass index (BMI), a body shape index (ABSI), or body adiposity index (BAI) and mortality, stratified by age. Hazard ratios and 95% confidence intervals were calculated using the multivariate model shown in Table 3. Likelihood ratio tests for interaction between age group and BMI, ABSI, or BAI: P = 0.001, 0.117, and 0.002, respectively. *indicates significant difference from quintile 1 (P < 0.05). The number (%) of deaths in each age group, by quintile, for BMI, ABSI, and BAI, separately, are as follows: BMI quintile 1–5, respectively, for age 50–59 years: 201 (4.2), 218 (4.8), 196 (4.6), 198 (4.8), 357 (7.5). BMI quintile 1–5, respectively, for age 60–69 years: 706 (12.4), 686 (11.4), 692 (11.4), 785 (12.7), 1004 (16.3). BMI quintile 1–5, respectively, for age more than 70 years: 950 (29.9), 826 (26.4), 852 (26.1), 871 (27.2), 758 (31.1). ABSI quintile 1–5, respectively, for age 50–59 years: 251 (4.2), 218 (4.3), 206 (4.6), 214 (5.6), 281 (9.2). ABSI quintile 1–5, respectively, for age 60–69 years: 554 (10.0), 666 (11.2), 720 (11.8), 808 (13.0), 1125 (17.9). ABSI quintile 1–5, respectively, for age more than 70 years: 481 (24.0), 623 (24.4), 806 (26.4), 947 (27.5), 1400 (33.6). BAI quintile 1–5, respectively, for age 50–59 years: 228 (4.4), 223 (4.8), 207 (4.8), 195 (4.7), 317 (7.6). BAI quintile 1–5, respectively, for age 60–69 years: 745 (12.8), 707 (11.7), 709 (11.6), 719 (12.0), 993 (16.1). BAI quintile 1–5, respectively, for age more than 70 years: 804 (29.6), 829 (27.3), 810 (25.6), 889 (27.1), 925 (30.6).
Discussion
For some time clinicians and public health professionals have relied on BMI to screen for obesity-related disease risk as well as to evaluate morbidity and mortality risk worldwide. Yet, frequently many have challenged its validity, primarily owing to the fact that it may not sufficiently account for body composition including visceral fat and/or its distribution. Stronger predictors of premature mortality are needed. To address this issue, several investigators have proposed revised measures of body habitus to evaluate mortality risk, predominantly ABSI and BAI. The WHI OS, with long-term follow-up of a large cohort of postmenopausal women that included several anthropometric measurements as well as adjudicated deaths, affords a unique opportunity to evaluate these relationships and to determine whether measures that integrate distribution of adiposity provide a more robust prediction of mortality than BMI.
In the largest analytical sample to date, data from the WHI OS suggest that the majority of measures evaluated in this study (weight, BMI, ABSI, BAI, WC, hip circumference, and WHR) demonstrated higher mortality risk for postmenopausal women in the highest versus lowest quintile, and all measures showed such increased risk in a subsample restricted to never-smokers. In the whole cohort, the highest risk estimates were seen for ABSI quintile 5 versus 1 (37% increased mortality risk) and BMI underweight versus normal-weight categories (58%), followed by BMI obesity II/III (30%). Using model comparison techniques (log-likelihood and AIC/BIC), ABSI was deemed the best at predicting mortality risk among the anthropometric measurements evaluated.
The results for ABSI and BMI corroborate earlier work by Krakauer and Krakauer (15) using data from NHANES 1999–2004, wherein ABSI showed a positive relationship with mortality risk. The estimate was 93% higher risk (HR, 1.93; 95% CI, 1.39–2.68) when comparing quintile 5 to quintile 3. Our estimate was only 37% higher risk for quintile 5 versus 1. Although the reference groups were not the same across studies, the lowest quintile in NHANES had nearly the same estimate as its reference group (HR=0.97 vs. 1.0), and our quintile 3 also had nearly the same estimate as the reference group (HR=1.05 vs. 1.0). Further, ABSI in the NHANES sample was associated with greater mortality risk than BMI, whereas our estimates for ABSI (quintile 5; 37%) and BMI (obesity II/III; 30%) are comparable. The differential degree of risk may reflect an overall healthier status of women enrolled in WHI OS or may support earlier work noting a weaker association between obesity and mortality with advancing age, even with adjustment for smoking or pre-existing illness, as was explored here (37). Additional differences between the two analytical samples include participants’ age (NHANES, ≥ 18 years; WHI, 59–79 years), sex (NHANES, men and women; WHI, only women), and menopausal status (NHANES, pre- and postmenopausal women; WHI, postmenopausal women only). Given the demonstrated reduction in weight and BMI after age 70 years in WHI OS (38), some have suggested maximum adult BMI may be a better predictor of mortality risk. In fact, highest adult BMI in never-smokers age 50–84 years was associated with 33% higher mortality risk in NHANES, a risk that was only 5% higher when current, age-specific BMI was evaluated (5). Similarly, maximum BMI in our sample of never-smokers was associated with 37% and 65% increased risk of mortality for quintile 5 versus 1 and obesity II/III versus normal weight, respectively.
ABSI was developed to integrate central adiposity with the health risk assessment equation, knowing that there is a subgroup within the population who maintain a healthy BMI but disproportionately carry weight in the form of central adiposity. Independent of other anthropometric measures, WC has been tested as a more-sensitive indicator of central adiposity. Evidence linking WC to mortality is somewhat limited, but a 2014 pooled report of over 650,000 individuals age 20–83 years enrolled in 11 prospective cohorts suggested that for every 5-cm higher WC there was a 9% higher risk of death (5). Here we show a 21% higher mortality risk for WC quintile 5 (>95 cm) versus 1 (≤73 cm). Our data, along with the results of the pooled analysis, suggest that WC should be considered a indicator of premature death. Notably, earlier work in a subgroup of WHI participants for whom body composition was assessed using dual-energy x-ray absorptiometry (DXA), showed that body composition, particularly low lean mass and high fat mass, were associated with higher mortality, but only prior to age 70 years (39).
Strengths of this study include the large, well-characterized study sample of over 76,000 women. In addition, we were able to apply clinic-measured weight, height, and waist and hip circumferences rather than rely on self-reported data, and mortality risk was derived from adjudicated deaths. Further, the dataset had several anthropometric measurements available, affording a unique opportunity to compare and contrast several clinically relevant assessments in relation to mortality risk.
Our analysis focused on women after menopause, for whom obesity-related disease and mortality risk are known to increase (40). Given this focus, we were not able to evaluate these measures in terms of mortality risk at earlier ages (or for men). Further, increasing evidence suggests that anthropometric measures (particularly BMI) and mortality may weaken with advancing age or that heterogeneity in weight trajectories may differentially associate with mortality risk (33–35). Thus interpretation of these data may be limited given that 22.7% of our sample was age ≥ 70 years at the time of study enrollment and that longitudinal measures of anthropometry and adiposity indices were not used to fully characterize anthropometric trajectories. Analyses were age-adjusted, and interactions with age were evaluated. Although body composition data were not included here, a previous analysis of the WHI subcohort with available body composition measures showed excess adiposity was associated with higher mortality for women age 50–69.9 years, but not in women age 70 years and older (39). Importantly, this is a female cohort, and while the women are all postmenopausal, sexual dimorphism exists in body shape that further limits generalization of these findings to males. Finally, the clinical usefulness of ABSI and BAI may be limited by the lack of evidence for valid and reliable self-report of these measures.
Conclusion
In the first analysis of a sizeable cohort of postmenopausal women, varying approaches to anthropometry demonstrated clinical usefulness in relation to mortality risk estimation. While direct measures (i.e., DXA) of adiposity may be preferable, until these approaches are more portable and affordable, BMI along with WC and adiposity scores (particularly ABSI) each inform mortality risk.
Supplementary Material
Acknowledgments
Funding agencies: The Women’s Health Initiative (WHI) programs are funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Dr. Lewis reports grants from NIH, during the conduct of the study, and grants from Novo Nordisk, outside the submitted work.
Footnotes
Disclosure: The other authors declared no conflict of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter JE, Macinnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014;99:875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 3.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerging Risk FC, Wormser D, Kaptoge S, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokes A. Using maximum weight to redefine body mass index categories in studies of the mortality risks of obesity. Popul Health Metrics. 2014;12:7. doi: 10.1186/1478-7954-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89:335–345. doi: 10.1016/j.mayocp.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi-Sunyer FX. The obesity epidemic: Pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 8.Gaesser GA, Tucker WJ, Jarrett CL, Angadi SS. Fitness versus fatness: Which influences health and mortality risk the most? Curr Sports Med Rep. 2015;14:327–332. doi: 10.1249/JSR.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L, Hu FB. Optimal body weight for health and longevity: Bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400. doi: 10.1111/acel.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Katzmarzyk PT, Ross R. Body mass index is inversely related to mortality in older people after adjustment for waist circumference. J Am Geriatr Soc. 2005;53:2112–2118. doi: 10.1111/j.1532-5415.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 12.Ross R, Berentzen T, Bradshaw AJ, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2008;9:312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 13.Krakauer NY, Krakauer JC. Dynamic association of mortality hazard with body shape. PloS One. 2014;9:e88793. doi: 10.1371/journal.pone.0088793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore SC. Waist versus weight: which matters more for mortality? Am J Clin Nutr. 2009;89:1003–1004. doi: 10.3945/ajcn.2009.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PloS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergman RN, Stefanovski D, Buchanan TA, et al. A better index of body adiposity. Obesity (Silver Spring) 2011;19:1083–1089. doi: 10.1038/oby.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhana K, Kavousi M, Ikram MA, Tiemeier HW, Hofman A, Franco OH. Body shape index in comparison with other anthropometric measures in prediction of total and cause-specific mortality. J Epidemiol Community Health. 2015 Jul 9; doi: 10.1136/jech-2014-205257. in press. [DOI] [PubMed] [Google Scholar]
- 18.Godoy-Matos AF, Moreira RO, Valerio CM, Mory PB, Moises RS. A new method for body fat evaluation, body adiposity index, is useful in women with familial partial lipodystrophy. Obesity (Silver Spring) 2012;20:440–443. doi: 10.1038/oby.2011.343. [DOI] [PubMed] [Google Scholar]
- 19.Hung CS, Yang CY, Hsieh HJ, Wei JN, Ma WY, Li HY. BMI correlates better to visceral fat and insulin sensitivity than BAI. Obesity (Silver Spring) 2012;20:1141. doi: 10.1038/oby.2012.86. [DOI] [PubMed] [Google Scholar]
- 20.Freedman DS, Thornton JC, Pi-Sunyer FX, et al. The body adiposity index (hip circumference÷ height1. 5) is not a more accurate measure of adiposity than is BMI, waist circumference, or hip circumference. Obesity (Silver Spring) 2012;20:2438–2444. doi: 10.1038/oby.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhaliwal SS, Welborn TA, Goh LG, Howat PA. Obesity as assessed by body adiposity index and multivariable cardiovascular disease risk. PloS One. 2014;9:e94560. doi: 10.1371/journal.pone.0094560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moliner-Urdiales D, Artero EG, Sui X, Espana-Romero V, Lee D, Blair SN. Body adiposity index and incident hypertension: The aerobics center longitudinal study. Nutr Metab Cardiovasc Dis. 2014;24:969–975. doi: 10.1016/j.numecd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzmarzyk PT, Mire E, Bray GA, Greenway FL, Heymsfield SB, Bouchard C. Anthropometric markers of obesity and mortality in white and African American adults: the pennington center longitudinal study. Obesity (Silver Spring) 2013;21:1070–1075. doi: 10.1002/oby.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichtash CT, Cui J, Guo X, et al. Body adiposity index versus body mass index and other anthropometric traits as correlates of cardiometabolic risk factors. PloS One. 2013;8:e65954. doi: 10.1371/journal.pone.0065954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moliner-Urdiales D, Artero EG, Lee DC, Espana-Romero V, Sui X, Blair SN. Body adiposity index and all-cause and cardiovascular disease mortality in men. Obesity (Silver Spring) 2013;21:1870–1876. doi: 10.1002/oby.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003 Oct;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 27.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 28.Guenther PM, Reedy J, Krebs-Smith SM. Development of the healthy eating index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE., Jr SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Johnson W, Chumlea WC, Czerwinski SA, Demerath EW. Concordance of the recently published body adiposity index with measured body fat percent in European-American adults. Obesity (Silver Spring) 2012;20:900–903. doi: 10.1038/oby.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 32.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 33.Zajacova A, Ailshire J. Body mass trajectories and mortality among older adults: a joint growth mixture-discrete-time survival analysis. Gerontologist. 2014;54:221–231. doi: 10.1093/geront/gns164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol. 2013;178:1591–1599. doi: 10.1093/aje/kwt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botoseneanu A, Liang J. Latent heterogeneity in long-term trajectories of body mass index in older adults. J Aging Health. 2013;25:342–363. doi: 10.1177/0898264312468593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong ES, Wang BC, Garrison LP, et al. Examining the BMI-mortality relationship using fractional polynomials. BMC Med Res Methodol. 2011;11:175. doi: 10.1186/1471-2288-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z. Age-dependent decline of association between obesity and mortality: a systematic review and meta-analysis. Obes Res Clin Pract. 2015;9:1–11. doi: 10.1016/j.orcp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: The women’s health initiative dietary modification trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 39.Bea JW, Thomson CA, Wertheim BC, et al. Risk of mortality according to body mass index and body composition among postmenopausal women. Am J Epidemiol. 2015;182:585–596. doi: 10.1093/aje/kwv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennis KE. Postmenopausal women and the health consequences of obesity. J Obstet Gynecol Neonatal Nurs. 2007;36:511–517. doi: 10.1111/j.1552-6909.2007.00180.x. quiz 518–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


