Abstract
Objective
To evaluate how limited English proficiency affects treatment outcome in head and neck cancer (HNC) patients treated with curative intent radiation therapy (RT).
Methods
From 2004 to 2010, 131 patients with HNC underwent RT. Patient's self-reported primary language and race/ethnicity were obtained at hospital registration. English proficiency was categorized as being English proficient (EP) or limited English proficient (LEP). Race/ethnicity was categorized as white, black and other (Hispanics and Asians). Patients were evaluated for locoregional (LRC), distant control (DC), overall (OS) and disease-free (DFS) survival.
Results
Fewer LEP patients (60.0%) underwent chemoradiation compared to EP (83.8%), P = 0.028. The three-year actuarial LRC for EP and LEP patients was 82.2% and 58.3%, respectively, P = 0.038. LEP patients had an increased risk of locoregional failure on univariate Cox regression analysis (hazard ratio, HR 2.4, 95% CI, 1.0–5.8). No differences by English proficiency were seen for DC, OS and DFS. Race/ethnicity was not associated LRC, DC, OS and DFS.
Conclusion
Inferior locoregional control was observed in LEP patients receiving RT for HNC. Potential health disparities as a result of limited English proficiency require further investigation.
Practice implications
Patient education, use of culturally sensitive interpreter and patient navigation services, and improved patient compliance should be considered in head and neck cancer patients receiving complex multidisciplinary care.
Keywords: Head and neck cancer, Limited English proficiency, Race, Health disparities, Language disparities, Radiotherapy
1. Introduction
Head and neck cancers (HNC) in the United States accounted for an estimated 53,640 new cancer cases and approximately 11,520 deaths in 2013 [1]. In spite of treatment advances over the years including use of multimodal treatment approaches, HNC patients are at high risk of locoregional failure and poor long-term survival [2]. National studies on racial/ethnic health disparities in cancer suggest that differences in patient survival exists by race/ethnicity which are not entirely explained by cancer biology and factors such as disease stage at presentation, mode of treatment received, and existence of co-morbidities have a substantial role [3]. Studies specific to HNC patient population have also identified these factors as potential contributors to observed disparities [4].
In the urban hospital setting an additional challenge facing the delivery of health care is optimizing communication between health care professionals and patients from diverse racial, cultural and linguistic backgrounds. In this regard the data on role of spoken language of communication on HNC outcomes such as disease control and overall survival are lacking. There is concern that HNC patients with limited English proficiency have difficulty with understanding and navigating complex treatments and multidisciplinary care. This can potentially affect patient satisfaction with their health care, and influence management decisions and execution of complex therapy and outcomes.
The Agency for Healthcare Research and Quality defines limited English proficiency in the health care context as the ability to speak English “less than very well” [5]. According to the 2005–2009 American Community Survey nearly 20% of Americans ages five years and above speak a language other than English at home [6]. There is growing evidence that limited English proficiency contributes to health disparities [7–9]. Limited English proficient (LEP) patients are less likely to have a regular source of care [10], less likely to receive standard care for chronic medical illnesses [11], more likely to report medical comprehension issues [12], report longer length of hospital stay [13,14] and report a greater dissatisfaction in acute medical care [15]. In cancer care, studies examining the role of English proficiency are largely restricted to cancer screening where speaking a language other than English is negatively associated with receipt of cancer screening services [16,17].
There is increasing evidence that spoken English proficiency is an important determinant of an individual's access to and utilization of health care services. In a study including Hispanic patients with similar income distribution, the use of English as a primary language was associated with greater utilization of health care services with the authors reporting a stronger correlation with patient's primary language than with their annual income [7]. Spanish only speaking Hispanics report fewer routine check-ups [18] and decreased utilization of preventive services [19] compared to English speaking Hispanics. In a study evaluating the language used for interview, Hispanics adults who were interviewed in Spanish reported lower health status and inferior access to care than Hispanics who were interviewed in English, suggesting that access to health care is more likely a function of EP rather than ethnicity/race alone [10]. Similar findings have been reported for other non-English speaking population groups. The lack of access and underutilization of health care services could potentially affect time to diagnosis of the disease and therefore result in a more advance disease presentation at the time of diagnosis leading to a poor prognosis for the patient. Furthermore, navigating the complexities of multimodality head and neck cancer treatment and potential delays in starting therapy or early detection of recurrence may adversely affect survival.
While most studies have examined English proficiency with respect to health care access, satisfaction with medical care and logistic issues such as length of hospital stay and medical comprehension in the general medical setting, such factors are also potentially important in the delivery of complex multidisciplinary HNC treatment. Limited data exists regarding the potential effect of English Proficiency on treatment outcome in terms of locoregional failure or survival in HNC patients undergoing curative intent radiation treatment. Furthermore, head and neck cancer represent a distinct entity whereby the complex multi-modality treatment consisting of surgery, radiation and chemo-therapy may have significant impact on speech function and communication long term. For patients who have LEP, diagnosed with head and neck cancer, the interplay of the primary language spoken to interface with the health care system combined with potential functional deficits of speech before and after therapy have not been studied. Hence, the purpose of this study was to evaluate the effect of limited English proficiency on treatment outcomes in HNC patients treated with curative intent radiation therapy (RT) in a private, non-profit, academic urban medical center, in which patients are offered treatment regardless of race/ethnicity, language spoken, gender, insurance status, or the ability to pay.
2. Methods
2.1. Hypothesis
The hypothesis is that patients with LEP have worse outcome (as measured by disease control and survival) compared to EP patients in an urban academic setting, whereby all patients regardless of race/ethnicity, language spoken, gender, insurance status, or the ability to pay are able to receive access to the same high quality multidisciplinary head and neck cancer care treatment.
2.2. Patient selection
This retrospective review was approved by Institution Review Board with waiver of informed consent. From August 2004 to May 2010, 168 patients with biopsy proven HNC completed curative intent RT at our institution. Eligibility included patients with non-metastatic, non-recurrent HNC, without previous malignancies, receiving RT to at least 58 Gy, and with at least 3 months follow-up. Thirty-seven patients were excluded from the present study for the following reasons: synchronous primary cancers (patients, n = 14), recurrent HNC (n = 11), previous malignancies (n = 4), death before any post-treatment follow-up (n = 4), and 4 were lost to follow-up. The final study population consisted of 131 non-metastatic and non-recurrent HNC patients.
2.3. Data collection
Electronic medical and hospital registration records were reviewed. The data review and collection was performed by a research associate and entered into an excel database. Data included patient demographics, including age at diagnosis, gender, smoking history, race/ethnicity at hospital registration. Treatment data including radiation dose, technique, treatment dates, use of concurrent chemotherapy, missed treatment breaks. Tumor characteristics including tumor, nodal staging, and histology were recorded. At hospital registration the patient's self-reported information on their primary language spoken, race/ethnicity and marital status was collected. Patients who reported their primary language spoken other than English to prompt the need for hospital interpreter services were classified as LEP, while patients reporting their primary language as English were defined as proficient (EP). The information is obtained on all patients at the time of hospital registration in order to assess the patient's preferred language for communication in the hospital setting and to determine the need for employing interpreter services to ensure the optimal communication between patients and providers. Bilingual/multilingual patients who were proficient in the English language and who did not require interpreter services were classified as EP. Race/ethnicity was recorded as white, black, Hispanic, or Asian. Due to sample size limitation, Hispanic and Asians were analyzed together as ‘Other’ and marital status was categorized as married or unmarried (single, divorced or widowed). All data was stored in an anonymized database.
2.4. Pre-treatment work-up
Pre-treatment work-up included history and physical examination with a focused head and neck evaluation, panendoscopy and biopsy, computed tomography (CT) scan, with or without [18F]-fluorodeoxyglucose positron emission tomography–computed tomography (FDG-PET/CT) and/or magnetic resonance imaging (MRI). Patients were staged according to the 2002 American Joint Committee on Cancer (AJCC) classification [20]. Locally advanced patients (stages III and IV disease) compromised 86.2% (n = 113) of the patient cohort. Histology consisted of 117 (89.3%) patients with squamous cell carcinoma and 14 patients (10.7%) with non-squamous carcinomas; adenocarcinoma (n = 4), neuroendocrine (n = 1), spindle cell carcinoma (n = 1), basal cell carcinoma (n = 1), adenocystic (n = 3), mucoepidermoid (n = 3) and 1 acinic cell carcinoma. All cases were presented at a multidisciplinary HNC tumor board consisting of a multidisciplinary review of each patient case by head and neck surgeons, medical oncologists, a radiation oncologist, radiologists and allied health professionals prior to the initiation of treatment.
2.5. Treatment
The median total radiation dose was 70 Gy (range 58–72 Gy) delivered in 33 fractions (range 29–42) over 48 elapsed days (range 38–72). The median time from diagnosis to start of treatment (surgery, induction chemotherapy, radiotherapy alone or concurrent radiotherapy) was 41 days (range 6–249 days). Intensity modulated radiotherapy (IMRT) was used in 78.6% (n = 103) and 3-dimensional conformal radiation therapy (3D-CRT) in 21.4% (n = 28) of patients. Concurrent chemotherapy, with or without induction chemotherapy, was administered in 80.2% (n = 105) of patients. Eighty-six patients (65.7%) received definitive (primary) RT and 45 patients (34.4%) received surgery followed by post-operative RT.
2.6. Follow-up and treatment outcome
Patients were monitored for disease recurrence from the conclusion of RT until last available follow-up or patient death. Follow-up consisted of serial clinical examinations every 3 months, including fiberoptic examination, a PET/CT at 8–12 weeks after the completion of RT. Disease recurrence was defined as first site of failure including local failure, nodal failure or distant failure. All recurrences were confirmed by biopsy. Patients were evaluated for locoregional control (LRC, local and/or nodal failure), distant control (DC, distant failure), overall survival (OS, death due to any cause) and disease-free survival (DFS, death and/or disease relapse). The overall median study follow-up (conclusion of RT until last available follow-up or patient death) was 39.8 months (range: 2–91 months), while surviving patients had a median follow-up (conclusion of RT until last available follow-up) of 47.9 months (range: 5–91 months).
2.7. Statistical analysis
Descriptive statistics were used to describe patient, tumor and treatment characteristics. Analysis of variance and Chi-square tests (Fisher's exact test for small samples) were performed to assess differences in continuous and categorical variables, respectively. Logistic regression models were run and odds ratio (OR) were calculated to estimate association between categorical variables. Three year actuarial control and survival rates were estimated for LRC, DC, OS, and DFS using the Kaplan–Meier product-limit method [21]. The comparison of rates among the groups was performed using the two tailed log rank test [22].
Bivariate Cox regression analyses were performed to investigate following potential confounding variables: race/ethnicity (white, black, other) marital status (married versus unmarried), smoking history (smokers versus non-smokers), treatment intent (definitive RT versus post-operative RT), chemotherapy status (no chemotherapy versus concurrent or induction chemotherapy) and AJCC 2002 stage (stages I–III versus stage IV). Crude and adjusted hazard ratios (HR) with 95% confidence intervals (CI) were computed using Cox regression modeling. A probability value of less than 0.05 was considered statistically significant for all analyses. All statistical computations were performed on SAS 9.1 system (SAS Institute, Cary, NC) or GraphPad prism software (version 3.0, GraphPad Software).
3. Results
3.1. Patient, tumor and treatment characteristics
Patient, tumor and treatment characteristics for the whole patient cohort and by EP are described in Table 1. Limited English proficiency was identified in 20 patients (15.3%). Primary language spoken included Spanish (n = 10), Portuguese (n = 2), Russian (n = 2), Vietnamese (n = 2), Arabic (n = 1), Chinese-Mandarin (n = 1), Haitian Creole (n = 1), and Hindi (n = 1). Non-white race/ethnicity was reported in 45.0% (n = 59) of the patient cohort; of whom 27.5% (n = 36), 13.0% (n = 17), and 4.6% (n = 6) were black, Hispanic and Asian, respectively. Approximately 68% (n = 89) of patient cohort was unmarried at the time of treatment while 86% (n = 112) of patients had a current or past history of smoking. The distribution of unmarried was as follows: single (n = 58), divorced (n = 22) and widowed (n = 9).
Table 1.
Patient, tumor and treatment characteristics.
| All patients (n=131) | English proficient (n=111) | Limited English proficient (n=20) | P value‡ | |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age (years) | 58.0 (9.7) | 57.8 (9.2) | 59.3 (12.3) | 0.526 |
| Smoking (pack-years)a | 40.5 (25.2) | 41.4 (25.4) | 33.2 (22.9) | 0.286 |
| Diagnosis to treatment (days) | 47.9 (34.1) | 46.2 (35.5) | 57.0 (23.9) | 0.197 |
| Treatment duration (days) | 49.8 (7.0) | 50.0 (7.2) | 48.6 (5.7) | 0.418 |
| n (column percent) | ||||
| Gender | 0.178 | |||
| Male | 89 (67.9%) | 78 (70.3%) | 11 (55.0%) | |
| Female | 42 (32.1%) | 33 (29.7%) | 9 (45.0%) | |
| Race/ethnicity | <0.001 | |||
| White | 72 (55.0%) | 69 (62.2%) | 3 (15.0%) | |
| Black | 36 (27.5%) | 35 (31.5%) | 1 (5.0%) | |
| Otherb | 23 (17.6%) | 7 (6.3%) | 16 (80.0%) | |
| Marital status | 0.760 | |||
| Married | 42 (32.1%) | 35 (31.5%) | 7 (35.0%) | |
| Unmarriedb | 89 (67.9%) | 76 (68.5%) | 13 (65.0%) | |
| Smoking history | 0.003 | |||
| Non-smokers | 19 (14.5%) | 11 (10.8%) | 8 (40.0%) | |
| Smokers | 112 (85.5%) | 100 (89.2%) | 12 (60.0%) | |
| Pathology | 0.228 | |||
| Squamous cell carcinoma | 117 (89.3%) | 101 (91.0%) | 16 (80.0%) | |
| Other | 14 (10.7%) | 10 (9.0%) | 4 (20.0%) | |
| AJCC stage | 39 (29.8%) | 0.106 | ||
| I–III | 92 (70.2%) | 30 (27.0%) | 9 (45.0%) | |
| IV | 81 (73.0%) | 11 (55.0%) | ||
| Treatment intent | 0.276 | |||
| Definitive RT (primary) | 86 (65.7%) | 75 (67.6%) | 11 (55.0%) | |
| Post-operative RT | 45 (34.4%) | 36 (32.4%) | 9 (45.0%) | |
| Chemotherapy | 0.028 | |||
| No chemotherapy | 26 (19.8%) | 18 (16.2%) | 8 (40.0%) | |
| Chemotherapy | 105 (80.2%) | 93 (83.8%) | 12 (60.0%) | |
Abbreviations: SD, standard deviation; n, number of patients; RT, radiation therapy; AJCC, American Joint Committee on Cancer.
Pack-year information was available for 110 smokers.
Other race/ethnicity includes 17 Hispanics and 6 Asians. Unmarried patients include 58 single, 22 divorced and 9 widowers.
P values are for the comparison between English proficient and limited English proficient patients.
3.2. Race/ethnicity
No statistically significant differences between race/ethnicity groups were noted for age (P = 0.978), gender (P = 0.973), marital status (P = 0.660), time from diagnosis to start of treatment (P = 0.996), treatment duration (P = 0.052), treatment intent (P = 0.173), histopathology (P = 0.264) and AJCC stage (P = 0.809). Differences between whites, blacks, and other were noted for smoking pack-years: 36, 40, and 22, respectively, P = 0.043. Eighty-six percent (n = 62) of whites, 94.4% (n = 34) blacks, and 69.6% (n = 16) other were smokers, P = 0.029. Fewer patients in other group (Hispanics/Asians) (60.9%, n = 14) received chemoradiation compared to whites (84.7%, n = 61) and blacks (83.3%, n = 30), P = 0.038.
3.3. English proficiency
By race/ethnicity, 95.8% (n = 62) of Whites, 97.2% (n = 35) of Blacks, 30.4% (n = 7) of Other were EP, P < 0.001. Compared to EP, fewer LEP patients were smokers (60.0% versus and 89.2%, P = 0.003) though amount of pack-years smoked was not different (33 versus 41, P = 0.29). The percentage of EP and LEP patients presenting with stage AJCC stage IV disease was 73.0% (n = 81) vs. 55.0% (n = 11), respectively, P = 0.106. Corresponding rates of chemotherapy administration were 83.8% (n = 93) versus 60.0% (n = 12) for EP and LEP, respectively, P = 0.028. The LEP patients had a lower odds of receiving chemotherapy (OR, 0.29; 95% CI, 0.10– 0.81) although this lost significance after accounting for AJCC stage presentation (OR, 0.34; 95% CI, 0.10–1.1).
No significant differences in age, gender, marital status, tumor and treatment characteristics were noted between EP and LEP patients. The LEP patients reported longer time from diagnosis to start of treatment (57 versus 46 days) however the difference between the two groups was statistically insignificant, P = 0.197. Complete results are presented in Table 1.
3.4. Treatment outcome
Locoregional failure and distant recurrences for the whole patient cohort occurred in 26 (19.9%) and 28 (21.4%) patients, respectively. The median time to recurrence for LRF was 2.9 months (range, 0.3–35.4) and distant recurrence was 7.3 months (range, 1.5–37.6). The estimated 3 year actuarial LRC, DC, OS and DFS were 79.1%, 78.0%, 70.6% and 58.0%, respectively.
The 3 year LRC rates by race/ethnicity for whites, blacks, and others were 85.8%, 70.9%, and 69.6%, respectively, P = 0.136, the corresponding 3 year actuarial OS rates were 73.8%, 66.4%, 63.9% and 74.9%, (P = 0.416). Race/ethnicity was also not associated DC (P = 0.902) and DFS (P = 0.535).
3.5. English proficiency, and disease control and survival
English proficiency was associated with improved three-year actuarial LRC observed among EP compared to LEP patients (82.2% and 58.3%, P = 0.038), Fig. 1. Language was not associated with DC, OS and DFS, Table 2. LEP patients who received chemoradiation (n = 12) had inferior 3 year LRC (29.2%) compared to LEP patients who received radiation alone (n = 8) (87.5%), EP patients who received chemoradiation (n = 93) (79.7%) and EP patients who received radiation alone (n = 18) (94.4%), log-rank P = 0.007, Fig. 2. In a restricted analysis of HNC patients with squamous cell carcinoma histology (n = 117), an improved LRC was still noted among EP compared to LEP patients (82.8% and 41.7%, P = 0.005).
Fig. 1.
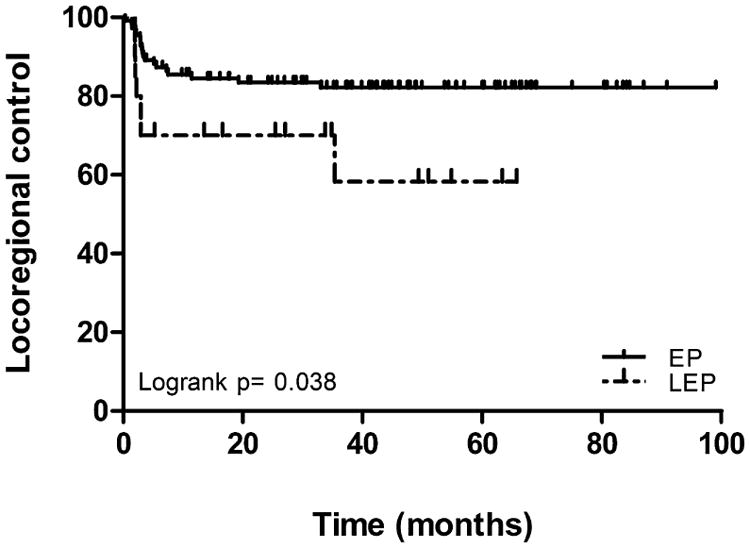
Locoregional control according to English proficiency.
Table 2.
Control and survival outcome.
| All patients (n=131) | English proficient (n=111) | Limited English proficient (n=20) | P value* | |
|---|---|---|---|---|
| Events (3 year actuarial control and survival rates) | ||||
| Locoregional control | 26 (79.1%) | 19 (82.2%) | 7 (58.3%) | 0.038 |
| Distant control | 28 (78.0%) | 24 (78.2%) | 4 (77.9%) | 0.884 |
| Overall survival | 49 (70.6%) | 42 (71.3%) | 7 (66.6%) | 0.559 |
| Disease-free survival | 61 (58.0%) | 51 (60.0%) | 10 (44.1%) | 0.320 |
P values are for the comparison between English proficient and limited English proficient patients.
Fig. 2.
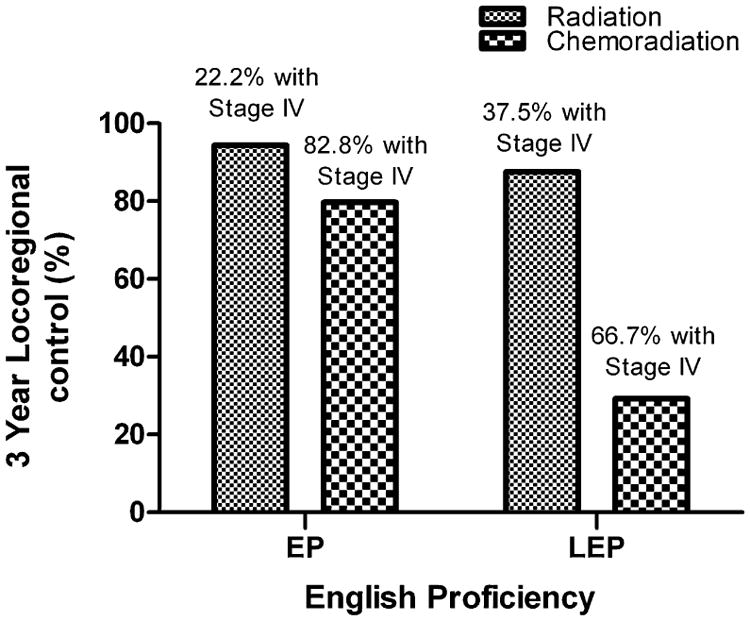
Locoregional control at 3 years according to English proficiency and treatment regimen.
3.6. Univariate and bivariate Cox regression analyses
LEP patients had an increased risk of LRF (un-adjusted HR 2.4, 95% CI, 1.0–5.8). On bivariate analyses, LEP remained a significant predictor of increased risk of LRF after adjusting individually for marital status, smoking history, RT intent, chemotherapy and AJCC stage, Fig. 3. After adjusting for race/ethnicity the risk of LRF among LEP patients increased however the analysis failed to attain statistical significance (adjusted HR 3.3, 95% CI, 0.91–11.7).
Fig. 3.
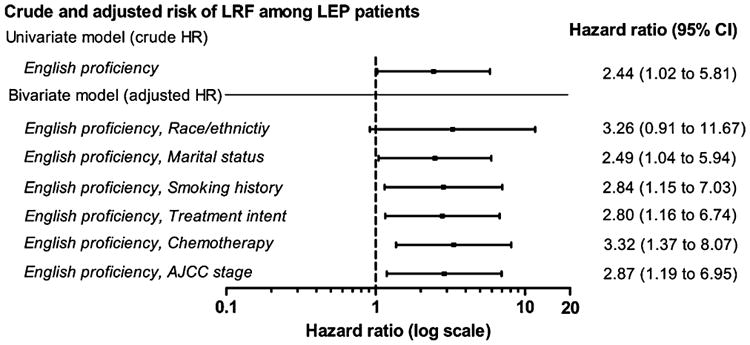
Univariate and bivariate analysis of locoregional failure (LRF) by English proficiency. The adjusted hazard ratios are for risk of LRF adjusting individually for patient, tumor and treatment characteristics. Both univariate and bivariate models are based on 111 English Proficient and 20 limited English proficient patients.
4. Discussion and conclusion
4.1. Discussion
In head and neck cancer, LRC is an important endpoint, as locoregional recurrence after radiotherapy, has limited salvage options, which often leads to significant morbidity and functional limitations. Herein we report that HNC patients with limited English proficiency have an inferior LRC compared to English proficient patients after radiotherapy with or without concurrent chemotherapy in an urban academic medical center setting. All care was provided in a specialized head and neck multi-disciplinary setting with a long-standing and well-documented history of comprehensive management of an ethnic and linguistically diverse patient population.
Interestingly, LEP patients were noted to have a lower receipt of chemotherapy, were more likely to be non-smokers and had a lower percentage of stage IV disease presentation. Also, despite having robust in hospital translation services with live in-person interpreters in 21 spoken languages and additional telephone and video translation services since 2002 in more than 150 different languages, disparities were noted in LEP HNC patients compared to the EP patients in this study. In our study, fewer LEP patients (55%) presented with AJCC stage IV disease compared to EP patients (73%). LEP patients did report a longer time from cancer diagnosis to the start of treatment regimen and adjusting for this difference did not explain the high risk of LRF noted among LEP patients. Potential delays to starting definitive therapy pose a significant risk for recurrence in head and neck cancer as it has been shown that prolonged radiotherapy duration, or total package time from initial treatment (such as surgery) to the end of radiotherapy adversely affect disease control and survival. It is possible that patients with LEP are at increased risk for having delays in starting and completing therapy compared to EP, due to the multiple appointments and duration of cancer therapy, particularly for patients who are initially treated with surgery, potential delays in starting adjuvant radiotherapy with or without chemotherapy need to be considered. It is also noted that LEP patients had a greater percentage receiving initial surgery compared to EP which may have affected outcome.
The study also examined the impact of race/ethnicity on English proficiency and treatment outcome. Race/ethnicity of a patient does not necessarily equate with English proficiency in all race/ethnic groups in the United States. In the current study 95.8% of Whites and 97.2% of Blacks were EP. Compared to that only 30.4% of “Others” were EP. Among the “Other” category, the distribution of EP Hispanics and Asians was similar (29.4% and 33.3%). Therefore race/ethnicity was associated with EP with Whites and Blacks most likely to be EP compared to Hispanic and Asians. This was further reflected in bivariate model where after adjusting for race/ethnicity, the hazard ratio for locoregional failure increased from 2.44 to 3.26 among LEP patients. Hence we found a greater independent effect of limited language proficiency as a determinant of LRF. In a report by Ponce et al., EP speakers (42% Whites) and English Only speakers (86% Whites) had comparable access to health care and general health status [8]. In a study including Hispanics and non-Hispanic Whites, Cheng et al. reported that Hispanics who speak English at home were found to receive the recommended health care services in similar proportions to non-Hispanic Whites [9]. These studies point toward an independent role of English proficiency on outcomes such has access to health care and general health status of the patient which could possibly explain the differences observed in this study.
The lack of English proficiency may also pose major barriers to the patient and their families in fully understanding all the treatment options available and complying to post-treatment follow-up instructions. Major communication challenges exist in LEP cancer patients in discussing treatment options and explaining the complexities of multidisciplinary cancer care, instructions for medications and multiple appointments [23,24] despite the availability of translation services. Furthermore, LEP patients have greater difficulty with compliance with medications and report greater drug complications, understanding instructions for dispensing prescriptions [12,25]. These are likely to be important factors in setting of complex cancer treatments such as head and neck surgery, RT and chemotherapy. During follow up, multiple issues may arise related to the management of treatment related short term and long term side effects such as speech and swallowing dysfunction, management of multiple medications, long term pain control and the early detection of recurrence or second primary cancers. Understanding and adherence to such intensive follow up schedules may be potentially more difficult in LEP patients. In HNC patients with speech difficulties after treatment, for example after surgery for oral tongue cancer or in total laryngectomy patients, LEP poses yet an additional barrier as they are unable to rely on spoken communication and may be primarily dependent on literacy proficiency in their primary language and written communication.
In this study the inferior prognosis for LEP patients was found primarily from the patients receiving chemoradiation with a 3 year LRC of 29.2% compared to 79.7% among EP patients receiving chemoradiation, which indicates the potential role of chemotherapy use and compliance other than disease stage as a reason for poor prognosis among LEP patients. The added toxicity of concurrent chemoradiation requires more intensive medical care, unplanned hospital admissions which require frequent allied health care support, interpreter services and frequent interface between the patient's home and health care facilities.
One of the limitations of this study is that we did not investigate percent utilization of interpreter services. Since head and neck cancer patients undergo a series of complex hospital and outpatient visits, determining the utilization of interpreter services for each visit could elucidate barriers to receiving health care in LEP HNC patients. Testing the language proficiency of interpreter services or language proficiency of health care staff who speak another language other than English was also not evaluated. There is a growing concern among language specialists that using health care staff with limited foreign language proficiency used in lieu of professional interpreters or using family members without medical training may result in miscommunication particularly in explaining complex treatments, instructions for taking medications, and also navigating appointments.
Effective patient–physician communication is critical for improving patient's health outcome [26]. The language concordance between patient and providers is considered the most optimal solution for communication and understanding of complex treatments being delivered to the patient [27,28] and is also associated with better treatment compliance [24]. With the increase in ethnic diversity among the urban patient population the provision of interpreter and language translation services to patients with LEP is an important step in eliminating health care barriers [29], however translation services alone maybe insufficient to fully address the barriers facing LEP patients [30]. Baker et al. reported lower satisfaction among patients who used interpreter services, majority of whom were ad-hoc interpreters, as compared to patients who communicated adequately with their provider without the need of an interpreter [31]. Similarly, Karliner et al. showed that the use of professional interpreters resulted in improved clinical outcomes, utilization of services, patient satisfaction and patient comprehension compared to ad-hoc interpreters [32]. In setting of cancer management, it is therefore essential that medical interpreters are professionally trained in cancer related terminology so that LEP patients receive accurate interpretations [33].
Potential solutions include improving the overall health literacy of patient which is defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions” [34]. In this regard number of steps can be undertaken by health care providers and health groups to close the communication gap between patient–physician [34]. More specifically, providing culturally sensitive education materials in written or video format regarding cancer care delivery of radiation and chemotherapy for LEP patients in their primary language, patient support groups that are language based may also facilitate LEP understanding of the complexity and expectations of cancer treatment and community outreach for LEP patients to educate on potential risks factors, cancer detection and diagnosis and access to health care. Patient navigation services employing navigators that target a particular LEP community who are proficient in their primary language may also facilitate bridging the communication gap between LEP communities and patients with hospital based care.
The present study has several other limitations; education level, health insurance status and HPV-status were not available for analyses. Consideration of patient comorbidities, psychosocial factors and lack of social support systems may also contribute to inferior treatment outcomes, especially among LEP patients. However, it is possible that these factors are not confounders, but rather part of the causal pathway. In this study, we used self-reported use of language at home which is a less sensitive than measuring the degree of a patient's English proficiency [8]. The language differences among patients may also be a surrogate for significant cultural differences between LEP and EP patients and their ability to navigate the US health care system. Since despite having robust translation services within the hospital system, simple translation of language does not equate understanding and conceptualization of cancer diagnosis and potential treatment options. This study does not attempt to assess the degree of the patient's English proficiency and level of understanding of their disease and treatment when navigating health care services. Finally, our study cohort is comprised of patients treated at one medical center in an urban academic setting, thus limiting generalizability of the results. However, it is noted that at the current institution, patients are treated regardless of their U.S. immigration, insurance or employment status.
4.2. Conclusion
In this cohort of advanced head and neck cancer patients receiving definitive radiation-based treatment, limited English proficient patients reported inferior treatment outcome compared to English proficient patients. Therefore, the effects of English proficiency and methods to address this barrier such as optimizing translation/interpreter services and providing culturally sensitive patient navigation services should be considered in the care of HNC patients receiving complex multidisciplinary care.
4.3. Practice implications
We recommend (a) improving community outreach and patient education tailored to the needs of LEP patients on potential risks factors, cancer detection and diagnosis and access to health care, (b) use of culturally sensitive translation/interpreter services to address communications barriers in the care of these patients, (c) provision of culturally sensitive and easily accessible patient navigation services for LEP patients, (d) improved monitoring of patient compliance in LEP patients following treatment.
Acknowledgments
Funding: No financial disclosures.
Footnotes
Presented in part at the 53rd Annual Meeting of the American Society for Radiation Oncology, October 2nd–6th, 2011, Miami Beach, FL, USA.
References
- 1.American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.McDonald MW, Lawson J, Garg MK, Quon H, Ridge JA, Saba N, et al. ACR Appropriateness Criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1292–8. doi: 10.1016/j.ijrobp.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. J Amer Med Assoc. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 4.Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22:25–38. doi: 10.1023/a:1022255800411. [DOI] [PubMed] [Google Scholar]
- 5.Race Ethnicity Language Data: Standardization for Health Care Quality Improvement. AHRQ Publication No 10-0058-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Mar, http://www.ahrq.gov/research/iomracereport/ [Google Scholar]
- 6.FILES: 2005-2009 American Community Survey [United States]/prepared by the U.S. Census Bureau; 2010
- 7.Hu DJ, Covell RM. Health care usage by Hispanic outpatients as function of primary language. West J Med. 1986;144:490–3. [PMC free article] [PubMed] [Google Scholar]
- 8.Ponce NA, Hays RD, Cunningham WE. Linguistic disparities in health care access and health status among older adults. J Gen Intern Med. 2006;21:786–91. doi: 10.1111/j.1525-1497.2006.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng EM, Chen A, Cunningham W. Primary language and receipt of recommended health care among Hispanics in the United States. J Gen Intern Med. 2007;22:283–8. doi: 10.1007/s11606-007-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkman-Liff B, Mondragon D. Language of Interview: relevance for research of southwest Hispanics. Am J Public Health. 1991;81:1399–404. doi: 10.2105/ajph.81.11.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S, Lee JA, Rush E. Ethnic and language disparities in diabetes care among California residents. Ethn Dis. 2011;21:183–9. [PubMed] [Google Scholar]
- 12.Wilson E, Chen AH, Grumbach K, Wang F, Fernandez A. Effects of limited English proficiency and physician language on health care comprehension. J Gen Intern Med. 2005;20:800–6. doi: 10.1111/j.1525-1497.2005.0174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindholm M, Hargraves JL, Ferguson WJ, Reed G. Professional language interpretation and inpatient length of stay and readmission rates. J Gen Intern Med. 2012;27:1294–9. doi: 10.1007/s11606-012-2041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John-Baptiste A, Naglie G, Tomlinson G, Alibhai SM, Etchells E, Cheung A, et al. The effect of English language proficiency on length of stay and in-hospital mortality. J Gen Intern Med. 2004;19:221–8. doi: 10.1111/j.1525-1497.2004.21205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez D, Engel KG, Tang TS. Language interpreter utilization in the emergency department setting: a clinical review. J Health Care Poor Underserved. 2008;19:352–62. doi: 10.1353/hpu.0.0019. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs EA, Karavolos K, Rathouz PJ, Ferris TG, Powell LH. Limited English proficiency and breast and cervical cancer screening in a multiethnic population. Am J Public Health. 2005;95:1410–6. doi: 10.2105/AJPH.2004.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W, Wang JH, Chen MY, Mandelblatt JS. Language use and the receipt of cancer screening recommendations by immigrant Chinese American women. J Womens Health (Larchmt) 2009;18:201–7. doi: 10.1089/jwh.2007.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson WS, Ahluwalia IB, Ford ES, Mokdad AH. Language preference as a predictor of access to and use of healthcare services among Hispanics in the United States. Ethn Dis. 2008;18:93–7. [PubMed] [Google Scholar]
- 19.DuBard CA, Gizlice Z. Language spoken and differences in health status, access to care, and receipt of preventive services among US Hispanics. Am J Public Health. 2008;98:2021–8. doi: 10.2105/AJPH.2007.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AJCC. Cancer staging manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 22.Cox DR. Regression models and life tables. J R Stat Soc: Ser B (Stat Methodol) 1972;34:187–220. [Google Scholar]
- 23.Karliner LS, Hwang ES, Nickleach D, Kaplan CP. Language barriers and patient-centered breast cancer care. Patient Educ Couns. 2011;84:223–8. doi: 10.1016/j.pec.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Manson A. Language concordance as a determinant of patient compliance and emergency room use in patients with asthma. Med Care. 1988;26:1119–28. doi: 10.1097/00005650-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Gandhi TK, Burstin HR, Cook EF, Puopolo AL, Haas JS, Brennan TA, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15:149–54. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travaline JM, Ruchinskas R, D'Alonzo GE., Jr Patient–physician communication: why and how. J Am Osteopath Assoc. 2005;105:13–8. [PubMed] [Google Scholar]
- 27.Green AR, Ngo-Metzger Q, Legedza AT, Massagli MP, Phillips RS, Iezzoni LI. Interpreter services, language concordance, and health care quality. Experiences of Asian Americans with limited English proficiency J Gen Intern Med. 2005;20:1050–6. doi: 10.1111/j.1525-1497.2005.0223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngo-Metzger Q, Sorkin DH, Phillips RS, Greenfield S, Massagli MP, Clarridge B, et al. Providing high-quality care for limited English proficient patients: the importance of language concordance and interpreter use. J Gen Intern Med. 2007;22:324–30. doi: 10.1007/s11606-007-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coren JS, Filipetto FA, Weiss LB. Eliminating barriers for patients with limited English proficiency. J Am Osteopath Assoc. 2009;109:634–40. [PubMed] [Google Scholar]
- 30.Abbe M, Simon C, Angiolillo A, Ruccione K, Kodish ED. A survey of language barriers from the perspective of pediatric oncologists, interpreters, and parents. Pediatr Blood Cancer. 2006;47:819–24. doi: 10.1002/pbc.20841. [DOI] [PubMed] [Google Scholar]
- 31.Baker DW, Hayes R, Fortier JP. Interpreter use and satisfaction with inter-personal aspects of care for Spanish-speaking patients. Med Care. 1998;36:1461–1470. doi: 10.1097/00005650-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Karliner LS, Jacobs EA, Chen AH, Mutha S. Do professional interpreters improve clinical care for patients with limited English proficiency? A systematic review of the literature Health Serv Res. 2007;42:727–54. doi: 10.1111/j.1475-6773.2006.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donelan K, Hobrecker K, Schapira L, Mailhot JR, Goulart BH, Chabner BA. Medical interpreter knowledge of cancer and cancer clinical trials. Cancer. 2009;115:3283–92. doi: 10.1002/cncr.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis TC, Williams MV, Marin E, Parker RM, Glass J. Health literacy and cancer communication. CA Cancer J Clin. 2002;52:134–49. doi: 10.3322/canjclin.52.3.134. [DOI] [PubMed] [Google Scholar]


