Abstract
Objective
To evaluate the ability of thio-urethane oligomers to improve the properties of restorative composite resins.
Materials and methods
Oligomers were synthesized by combining 1,6-hexanediol-diissocyante (aliphatic) with pentaerythritol tetra-3-mercaptopropionate (PETMP) or 1,3-bis(1-isocyanato-1-methylethyl)benzene (aromatic) with trimethylol-tris-3-mercaptopropionate (TMP), at 1:2 isocyanate:thiol, leaving pendant thiols. Oligomers were added at 0–20 wt% to BisGMA-TEGDMA (70–30 wt%). Silanated inorganic fillers were added (70 wt%). Materials were photoactivated at 800 mW/cm2 filtered to 320–500 nm. Near-IR was used to follow degree of methacrylate conversion (DC). Mechanical properties were evaluated in three-point bending with 2 mm × 2 mm × 25 mm bars for flexural strength/modulus and toughness (FS/E, and T) according to ISO 4049, and 2 mm × 5 mm × 25 mm notched specimens for fracture toughness (KIC). Polymerization stress (PS) was measured on the Bioman. Results were analyzed with ANOVA/Tukey’s test (α = 5%).
Results
Significant increase in DC was observed in thio-urethane-containing materials especially for the group with 20 wt% of aliphatic version. Materials composed by oligomers also promoted higher FS, E, and KIC in comparison to controls irrespective of thio-urethane type. A significant increase in toughness was detected by ANOVA, but not distinguished in the groups. The PS was significantly reduced by the presence of thio-urethane for almost all groups.
Conclusions
The use of thio-urethane oligomer to compose methacrylate-based restorative composite promote increase in DC, FS, E and KIC while significant reduces PS.
Keywords: Composite resin, Thio-urethane oligomers, Polymerization stress, Mechanical properties
1. Introduction
Resin-based composites are widely used in Restorative Dentistry due to their highly esthetic appearance and the possibility of performing minimally invasive cavity preparation. Although these characteristics are advantageous, the main drawback with these materials is that they last on average less than 10 years in the mouth [1]. The most common causes of composite restoration failure are secondary caries and fractures [1,2]. It has been suggested that the gaps formed due to stress generation at the bonded interface may facilitate caries re-occurrence.
The stress development is caused by a multifactorial process that includes the degree of conversion and the consequent development of shrinkage and modulus during curing, as previously reported [3,4]. Volumetric shrinkage occurs simultaneously with the increase in the elastic modulus, as the density of cross-linked bonds among the polymeric chains increases [5,6]. Also, the polymerization reaction rate, the material’s composition and the surrounding conditions around the restoration (bonding integrity, C-factor, and deformation of the adjacent structures) are important factors regulating the development and transmission of stress to the bonded interface and to dental structures [7–9].
A series of reports pointed out that the behavior of composite resins in bending is an important predictor of the clinical performance [10–14]. Ferracane [15] has shown a direct relationship between mechanical properties such as fracture toughness, flexural strength, elastic modulus and toughness and clinical outcomes, suggesting that the improvement of these mechanical properties may contribute to better clinical performance and longevity of the restorations. Most of the advances in the formulations that have made it possible to improve mechanical properties of current composite materials stem from changes in the filler (inorganic) portion of the composite [16]. One of the weak links remains the hydrolysis-prone methacrylate organic matrix [17], which has not changed significantly since the introduction of BisGMA on the market more than six decades ago.
Based on the clear shortcomings of current materials, there is a need for formulations with the ability to develop lower stress to show less degradation and improved mechanical properties, which would indeed decrease the variability of the outcomes [15]. Earlier studies have demonstrated the ability of materials based on thiol-enes or thiol-methacrylate to increase conversion and mechanical properties such as fracture toughness, at the same time reducing stress and water sorption/solubility [18,19]. This is possible due to delayed gelation/vitrification of the thiol-modified networks, and has been demonstrated for several small molecule and oligomeric thiol species [20,21]. Very recently, thio-urethane oligomers were shown to promote more homogeneous network formation compared to simple urethane counterparts leading to increase in degree of conversion [22] and in mechanical properties, especially toughness and fracture toughness [23–25]. In the case of thio-urethane oligomeric additives, the higher molecular weight leads to lower volumetric shrinkage and absence of odor concerns [26]. Still, the chain-transfer reaction of the pendant thiols to the surrounding methacrylate matrix results in delayed gelation and vitrification, promoting reduction in polymerization stress [27].
The objectives of this study were to formulate composite materials modified with thio-urethane additives previously synthesized in our laboratory [25,26] and to assess the influence of such additives on degree of conversion and reaction kinetics, bulk mechanical properties, polymerization shrinkage and stress. The hypotheses of this study were that, depending on the type and concentration of the thio-urethane, the addition of oligomers will lead to (1) increased degree of conversion; (2) increased mechanical properties; and (3) reduced polymerization stress of methacrylate-based composites.
2. Materials and methods
2.1. Experimental materials composition
The composites formulated for the study were composed of Bisphenol A diglycidyl methacrylate (Bis-GMA; Esstech, Essington, PA, USA) and tri-ethylene glycol dimethacrylate (TEGDMA; Esstech) in a 70:30 mass ratio. Photoinitiators were added to the matrix as follows: 0.1 wt% of 2,2-Dimethoxy-2-phenylacetophenone (DMPA – Sigma-Aldrich, St. Louis, MO, USA) and 0.3 wt% inhibitor (BHT – 2,6-di-tert-butyl-4-methylphenol; Sigma–Aldrich, St. Louis, MO, USA).
Oligomers were synthesized in solution by combining 1,6-hexanediol-diissocyante (aliphatic) with pentaerythritol tetra-3-mercaptopropionate (PETMP) or 1,3-bis(1-isocyanato-1-methylethyl)benzene (aromatic) with trimethylol-tris-3-mercaptopropionate (TMP), at 1:2 isocyanate:thiol molar ratio, leaving pendant thiols. Oligomers were purified by precipitation in hexanes and rotaevaporation, then characterized by 1H NMR and mid-IR spectroscopy. The thiol group (SH) concentration for each oligomer was determined using a titration method with Ellman’s reagent well established in the literature [28]. Thio-urethane oligomers were added to organic matrix in proportions of 0–20 wt%; no thio-urethane group served as control.
Filler was introduced at 70wt% (7% OX-50 – 0.04 mm; 93% silica 0.7 [μm, density 3.0g/ml, refractive index 1.553 – V117 4107, Esstech), with the aid of a mechanical mixer (DAC 150 Speed mixer, Flacktek, Landrum, SC, USA) for 5min at 2400 rpm. All procedures were carried out under safe yellow light.
2.2. Degree of conversion
Degree of conversion based on the methacrylate =CH2 absorption at 6165 cm−1 [29] was calculated based on near–infrared (NIR) spectroscopy at 2 scans per spectrum with 4 cm−1 resolution, which provides a greater than 2 Hz data acquisition rate. Samples (n = 3) were irradiated during the course of the analysis (5 min) to account for thermal effects during the kinetics experiment, at an incident irradiance of 800 mW/cm2 filtered to 320–500 nm with a 2 cm distance to specimen. This light source was used for convenience and stability of the irradiance through long exposure times. Specimens were 10 mm in diameter and 0.8 mm thick laminated between two glass slides.
2.3. Flexural strength, elastic modulus and toughness
Flexural strength of the samples was measured according to the 3-point bending method carried out with a universal test machine (Q-test, MTS, Eden Prairie, WI) at a cross-head speed of 0.5mm min−1. The bar specimens (n=10) were prepared in dimensions of 2 mm × 2 mm × 25 mm according to ISO 4049 [30]. The specimens were fabricated between glass slides and photopolymerized with 300 s exposures as mentioned above. Specimens were stored for 1 week in dark containers at room temperature. The flexural strength (FS) in MPa was then calculated as:
where F stands for load at fracture (N), l is the span length(20 mm), and b and h are the width and thickness of the specimens in mm, respectively.
The elastic modulus was determined from the slope of theinitial linear part of stress–strain curve.
F=the load at some point on the linear region of the stress-strain curve; d = the slack compensated deflection at load F; l, b, and h are as defined above.
Toughness was calculated in MPa from the integration of the stress × strain curve using software (Origin 9.1, OriginLab Corporation, Northampton, MA, USA).
2.4. Polymerization stress
Polymerization stress development was followed in real-time using the Bioman, described previously [9]. This system consists of a cantilever load cell whose extremity is fitted to a rigid integral clamp on its free end. The clamp holds a 10 mm diameter and 22 mm tall steel rod vertically and perpendicular to the load cell axis. A 5-mm diameter, 1-mm tall steel rod was fixed at the center of the lower face of the standard rod with a cyanoacrylate adhesive to produce a rod substrate with a reduced surface area to be consistent with a C-factor of 3. The surface of the rod was treated with a thin layer of metal primer (Z-prime plus, Bisco, Schaumburg, IL). The opposite surface was a rigid fused silica glass plate of 3 mm thickness, treated with a thin layer of silane ceramic primer (3M ESPE, St. Paul, MN, USA). The composite was then inserted into the 0.5-mm gap between the upper rod and the lower glass slide and shaped into a cylinder. The specimens were photoactivated through the glass during 60 s at an incident irradiance of 800 mW/cm2 (UV curing light) and the stress followed for 500 s. The load signal from the cantilever cell was amplified and acquired by a computer.
2.5. Fracture toughness
The method utilized to determine fracture toughness (KIC) was based on the evaluation of pre-cracked specimens under fatigue in linear-elastic, plane-strain conditions [31]. Single-edge notch beam (SENB) specimens were fabricated according to ASTM Standard E399-90 [31] in a 5 mm × 2 mm × 25 mm split steel mold with a razor blade providing a 2.5 mm notch in the middle of the specimens. Specimens were cured with an UV light during 300 s at 800mW/cm2 irradiance filtered to 320–500 nm. The bending fracture test was performed using a universal test machine (Q-test) at a cross-head speed of 0.5mm min−1 and KIC was calculated according the following equation:
where P is load at fracture (N), L is the length, W is the width, B is the thickness, and a is the notch length (all in mm) [32].
2.6. Statistical analysis
Statistical analysis was carried out using two-way ANOVA (thio-urethane concentration and thio-urethane type). Multiple comparisons were done using Tukey’s test. All tests were carried out at a global level of significance of 95%.
3. Results
3.1. Degree of conversion
The thiol group concentration determined with the Ellman’s reagent was 57.7 ±5.1 and 37.0 ± 3.6 mM/g for the aliphatic and aromatic versions of the thio-urethanes, respectively. The degree of conversion (DC, Table 1) in the groups modified with the aliphatic version of thio-urethanes increased significantly from 70.0 ± 2.3% (control) to up to 88.2 ± 3.0% (20wt% thiourethane). A significant increase was also observed in DC for the 20 wt% group modified with aromatic thio-urethane compared to the control (from 70.0 ± 2.3% to 80.4 ± 0.4%). Aliphatic thio-urethanes were statistically superior to aromatics for all groups within the same concentration. The interaction between the two factors (thio-urethane type and concentration) was significant, meaning that the ability of each oligomer type to improve the DC was dependent of its concentration.
Table 1.
Mean and standard deviation for degree of conversion, polymerization stress and fracture toughness. Values followed by the same letter within the same test are statistically similar (α = 5%).
| Degree of conversion (%)
|
Polymerization stress (MPa)
|
Fracture toughness (MPa m1/2)
|
||||
|---|---|---|---|---|---|---|
| Aromatic | Aliphatic | Aromatic | Aliphatic | Aromatic | Aliphatic | |
| Control | 70.0 (2.3)c | 20.7 (0.6)c | 0.96 (0.1)c | |||
| 10wt% | 68.3 (1.7)c | 80.6 (4.4)b | 15.3 (2.3)ab | 14.7 (1.0)ab | 1.7 (0.2)b | 1.4 (0.1)b |
| 15 wt% | 69.4 (1.0)c | 77.6 (1.9)b | 16.8 (2.0)bc | 13.6 (0.9)ab | 1.7 (0.2)b | 1.7 (0.2)b |
| 20wt% | 80.4 (0.4)b | 88.2 (3.9)a | 14.3 (0.5)ab | 11.6 (1.8)a | 2.2 (0.1)a | 2.3 (0.3)a |
| p-value | TU type: p = 0.000 | TU type: p = 0.013 | TU type: p = 0.563 | |||
| TU concentration: p = 0.000 | TU concentration: p = 0.000 | TU concentration: p = 0.000 | ||||
| Interaction: p = 0.007 | Interaction: p = 0.181 | Interaction: p = 0.187 | ||||
TU, thio-urethane
3.2. Polymerization stress
Polymerization stress was statistically reduced in thio-urethane groups (except for 15 wt% of aromatic version). The group with 20wt% of aliphatic material showed the lowest stress values, being 44% lower than control (Table 1). Even though the analysis of variance showed statistical differences between some experimental groups in respect to thio-urethane concentration, the same were not identified by Tukey’s test. The interaction between the two factors (thio-urethane type and concentration) was not significant, meaning that the stress reduction shown by each type of thio-urethane was not dependent on its concentration. Curves of polymerization stress as a function of time are shown in Fig. 1.
Fig. 1.
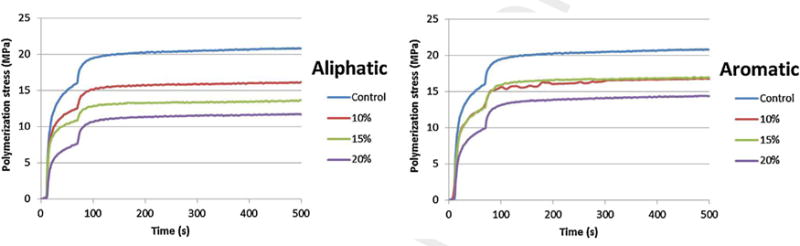
Polymerization stress in respect of the time for aliphatic and aromatic groups in comparison to control.
3.3. Fracture toughness
Fracture toughness results for both groups modified by thio-urethanes were statistically higher than the control. Materials with 20wt% of oligomers showed significantly higher values among TU groups (Table 1). Statistical significance was not observed in regard to thio-urethane type. The interaction between factors was not significant. The addition of 20 wt% of thio-urethanes led to increases of 129.1% and 139.5% in fracture toughness for the aromatic and aliphatic versions, respectively (Table 1).
3.4. Flexural strength, elastic modulus and toughness
Both thio-urethanes in the concentration of 15 and 20wt% caused an increase in the flexural strength in comparison to the control (Table 2). Flexural strength increased from 90.0 ± 10.3 MPa in the control group to up to 134.8 ± 8.8 MPa in the group with 20 wt% of aliphatic version, leading to an increase of 49.7%. Statistical significance was not observed in regard to thio-urethane type.
Table 2.
Mean and standard deviation for flexural strength, flexural modulus and toughness. Values followed by the same letter within the same test are statistically similar (α = 5%).
| Flexural strength (MPa)
|
Elastic modulus (GPa)
|
Toughness (MPa)
|
||||
|---|---|---|---|---|---|---|
| Aromatic | Aliphatic | Aromatic | Aliphatic | Aromatic | Aliphatic | |
| Control | 90.0 (10.3)b | 3.3 (0.4)b | 1.4 (0.2)a | |||
| 10wt% | 112.7 (9.4)ab | 119.0 (16.5)ab | 4.6 (0.3)a | 4.5 (0.3)a | 1.55 (0.2)a | 1.87 (0.6)a |
| 15 wt% | 120.9 (13.3)a | 130.4 (30.9)a | 4.7 (0.2)a | 4.9 (0.4)a | 1.88 (0.6)a | 2.22 (1.1)a |
| 20wt% | 121.1 (15.4)a | 134.8 (8.8)a | 4.6 (0.5)a | 4.9 (0.4)a | 1.91 (0.5)a | 2.08 (0.8)a |
| p-value | TU type: p = 0.133 | TU type: p = 0.359 | TU type: p = 0.108 | |||
| TU concentration: p = 0.000 | TU concentration: p = 0.000 | TU concentration: p = 0.015 | ||||
| Interaction: p = 0.770 | Interaction: p = 0.768 | Interaction: p = 0.764 | ||||
TU, thio-urethane
All thio-urethane groups were shown to increase the elastic modulus in regards to the control, being statistical similar among them (Table 2). An increase in the elastic modulus was observed from 3.3 ± 0.4GPa in the control group to up to 4.9 ± 0.4 GPa in the group with 20 wt% of aliphatic version, reaching an increase of 48.4%. Thio-urethane type did not affect elastic modulus.
For toughness values, although the statistical analysis showed significance in respect to thio-urethane concentration (p = 0.015), the difference among the groups was not distinguished after comparison by Tukey’s test (Table 2). An increase of up to 48.5% in toughness values could be observed with the use of the oligomer – from 1.4 ± 0.2 MPa in the control group to 2.08 ±0.8 MPa for the group with 20wt% of aliphatic thio-urethane. The thio-urethane type was not significant. For all mechanical properties, the interaction between factors was not significant, which means the increase in properties for each type of oligomer was independent of its concentration.
4. Discussion
The use of thio-urethanes in the polymer coatings and molded parts industry has been widely reported [33,36]. Those networks have demonstrated the capacity of improving mechanical properties, more specifically toughness, due to a more homogeneous polymer network formation in comparison to simple urethane counterparts [36]. Recently, work by our group has demonstrated that this technology can be translated into dental materials applications through the relatively simple approach of including pre-polymerized thio-urethane oligomers into the methacrylate matrix [25,26]. The covalent interaction of the thio-urethane with the methacrylate occurs through chain-transfer to the pendant thiol functionalities on the backbone of the oligomer. Those chain-transfer reactions delay the gelation/vitrification to higher degrees of conversion [37,38] while at the same time reducing stress development and improving network homogeneity and fracture toughness [25–27]. For all of those reasons, the incorporation of thio-urethane oligomers into methacrylate networks used for dental materials applications is very promising.
The results of this study have further demonstrated that the formulation of methacrylate-based restorative composite containing thio-urethane oligomers leads to improvement on important properties of dental composite materials. In summary, degree of conversion significantly increased by up to 18% with the use of the aliphatic version of the oligomer, and by up to 10% with the use of the aromatic one. Most importantly, this was accompanied by a significant increase of up to 50% in mechanical properties and reductions of up to 43% in polymerization stress. These results are very encouraging in terms of the application of these oligomers as restorative materials. It needs to be pointed out that the high molecular weight (around 5 kDa) [25] and the absence of free small molecule thiols (completely removed with the thorough purification procedure) eliminates odor and toxicity concerns. The thiol functionalities pendant from the thio-urethane backbone are available to react with the methacrylate on the secondary matrix through chain-transfer reactions, which in turn are able to delay network formation and the build-up of diffusional limitations to propagation [22]. In practical terms, this means that the vitrification is delayed to later stages of conversion [39]. Based on these considerations, the first hypothesis was accepted.
The aliphatic version of the oligomer has shown greater potential to increase DC, even at lower concentrations than the aromatic, showing that the effect on conversion and network formation was dependent on the thio-urethane structure. This was attributed to the greater flexibility of the aliphatic oligomer network given not only by the thio-urethane bonds but also by the absence of aromatic rings or other rigid substitutions [40]. The increase in DC was also observed for aromatic thio-urethanes, but that only happened at higher concentrations (20 wt%), likely because the presence of the aromatic core impaired diffusion of macro-radicals and the chain-transfer reactions within the methacrylate matrix [40], until a certain concentration of thio-urethane bonds was present to improve mobility.
Evaluation of flexural properties by means of ISO 4049 Standard has shown a relevant improvement with the use of thio-urethane oligomers to formulate restorative composites, irrespective of the oligomer type being analyzed. Significant increase of up to 50% of flexural strength was observed in materials formulated with 15 and 20 wt% of thio-urethanes. Elastic modulus was significantly increased for all thio-urethane concentrations, by up to 50%. The increase in mechanical properties might be partially explained by the generally increased conversion of thio-urethane groups (except for the 15wt% aromatic group). In addition to the increased conversion, the more homogenous network expected to be formed in the presence of the oligomers when compared to simple methacrylates is another factor for the increase in mechanical properties [23]. The lack of statistical difference in the results obtained with the aliphatic vs. aromatic versions of the oligomers was not expected, but can be partially explained by the conversion results. It was expected that the oligomer with the rigid aromatic core would be better able to reinforce the network. However, because the rigidity also likely decreased conversion, the effects on mechanical properties were overshadowed. In addition, for these highly filled materials, the overall concentration of the oligomer is only 6 wt%, even for the highest oligomer concentration formulations. That way, it is not that surprising that the small differences caused by the aliphatic vs. aromatic structure and thiol group concentration were not as marked. It is noteworthy, however, that in comparison with the control, even at an overall concentration of only 6 wt%, the mechanical properties in flexure were statistically higher. This result is even more impressive when toughness results are considered, since an increase of up to 49% in that property was observed with the addition of the thio-urethanes. This is consistent with previously reported data [23].
Fracture toughness measured in notched specimens has already been demonstrated to be an important predictor of clinical behavior of composites restorations [15]. All groups modified by thio-urethanes led to a significant increase in fracture toughness. This was a function of oligomer concentration, with groups containing 20wt% of oligomer showing higher fracture toughness values than all other experimental groups, and, more importantly, showing more than a two-fold increase in that property in relation to the control. As already mentioned, factors contributing to these KIC results include a more homogenous network formation, with overall greater flexibility [23]. Still, as will be discussed later, thio-urethane based composites produce lower volumetric shrinkage and polymerization stress [25,26]. The fracture toughness is affected by residual tensile stresses built up around inorganic filler particles due to polymerization shrinkage [41]. In this way, the higher interfacial stress in the control group amplifies the stress intensity as the crack tip reaches the filler particle, reducing the toughness of the composite [2,42]. Based on these considerations, our second hypothesis that the use of oligomers would promote better mechanical properties is accepted.
The benefits of increased conversion and mechanical properties observed in this study were accompanied by a significant reduction in polymerization stress for all but one group of thio-urethane-modified materials (15wt% of aromatic oligomer; Table 1; Fig. 1). The thio-urethane structure did not influence polymerization stress development, probably because the measured thiol concentration on either type of oligomer was very similar as determined by the titration with Ellman’s reagent, especially considering the low overall thiol concentration on the fully formulated composite. As previously demonstrated, the chain-transfer reactions from the pendant thiols on the thio-urethane backbone to the surrounding methacrylate matrix likely delayed gelation/vitrification to a high degree of conversion, avoiding early-stage diffusion limitations to polymerization delaying stress development [27,39], as well as reducing overall stress values, [20]. Similarly high stress reductions in methacrylate networks modified with small molecule thiols have already been demonstrated at a relatively low thiol concentration of 5 wt% [27]. Moreover, our previous studies with these materials have also demonstrated a reduction in volumetric shrinkage with the addition of thio-urethane oligomers [26], as expected based on the decrease in the concentration of methacrylate double-bonds per unit volume of the material [43], which also help explain the reduction observed on the stress values. Thus, our third hypothesis, that the use of thio-urethane promotes lower polymerization stress in methacrylate-based restorative composites, is accepted.
5. Conclusion
Based on the results of this study, it can be concluded that the use of thio-urethane oligomers as part of organic matrix of methacrylate-based restorative composites leads to increased degree of conversion and general mechanical properties, especially fracture toughness, while significant reducing the polymerization stress in highly filled materials. Importantly, the use of this additive does not require any change in the operatory techniques currently used in dental practice, facilitating the translation of this technology to commercially available materials.
References
- 1.Opdam NJ, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, et al. Longevity of posterior omposite restorations: a systematic review and eta-analysis. J Dent Res. 2014;93:943–9. doi: 10.1177/0022034514544217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohbauer U, Belli R, Ferracane JL. Factors involved in mechanical fatigue degradation of dental composites. J Dent Res. 2013;92:584–91. doi: 10.1177/0022034513490734. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL. Developing a more complete understanding of stress produced in dental composites during polymerization. Dent Mater. 2005;21:36–42. doi: 10.1016/j.dental.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Pfeifer CS, Ferracane JL, Sakaguchi RL, Braga RR. Factors affecting photopolymerization stress in dental composites. J Dent Res. 2008;87:1043–7. doi: 10.1177/154405910808701114. [DOI] [PubMed] [Google Scholar]
- 5.Peutzfeld A. Resin composite in dentistry: the monomer system. Eur J Oral Sci. 1997;105:97–116. doi: 10.1111/j.1600-0722.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 6.Dauvillier BS, Feizer AJ, De Gee AJ, Davidson CL. Visco-elastic parameters of dental restorative materials during setting. J Dent Res. 2000;79:818–23. doi: 10.1177/00220345000790030601. [DOI] [PubMed] [Google Scholar]
- 7.Feilzer AJ, De Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dent Res. 1987;66:1636–9. doi: 10.1177/00220345870660110601. [DOI] [PubMed] [Google Scholar]
- 8.Miguel A, de la Macorra JC. A predictive formula of the contraction stress in restorative and luting materials attending to free and adhered surfaces, volume and deformation. Dent Mater. 2001;17:241–6. doi: 10.1016/s0109-5641(00)00077-4. [DOI] [PubMed] [Google Scholar]
- 9.Watts DC, Satterthwaite JD. Axial shrinkage-stress depends upon both C-factor and composite mass. Dent Mater. 2008;24:1–8. doi: 10.1016/j.dental.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Brunthaler A, König F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clin Oral Investig. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- 11.Pallesen U, Qvist V. Composite resin fillings and inlays. An 11-year evaluation. Clin Oral Investig. 2003;7:71–9. doi: 10.1007/s00784-003-0201-z. [DOI] [PubMed] [Google Scholar]
- 12.Van Neiuwenhuysen JP, D’Hoore W, Carvalho J, Qvist V. Long-term evaluation of extensive restorations in permanent teeth. J Dent. 2003;31:395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 13.Opdam NJ, Loomans BA, Roeters FJ, Bronkhorst EM. Five-year clinical performance of posterior resin composite restorations placed by dental students. J Dent. 2004;32:379–83. doi: 10.1016/j.jdent.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Opdam NJ, Bronkhorst EM, Roeters JM, Loomans BA. Longevity and reasons for failure of sandwich and total-etch posterior composite resin restorations. J Adhes Dent. 2007;9:469–75. [PubMed] [Google Scholar]
- 15.Ferracane JL. Resin-based composite performance: are there some things we can’t predict. Dent Mater. 2013;29:51–8. doi: 10.1016/j.dental.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–16. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin omposites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 18.Boulden JE, Cramer NB, Schreck KM, Couch CL, Bracho-Troconis C, Stansbury JW, et al. Thiol-ene-methacrylate composites as dental restorative materials. Dent Mater. 2011;27:267–72. doi: 10.1016/j.dental.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beigi S, Yeganeh H, Atai M. Evaluation of fracture toughness and mechanical properties of ternary thiol-ene-methacrylate systems as resin matrix for dental restorative composites. Dent Mater. 2013;29:777–87. doi: 10.1016/j.dental.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Carioscia JA, Stansbury JW, Bowman N. Investigatios of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater. 2005;21:1129–36. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Hoyle CE, Bowman CN. Thiol-ene click chemistry. Angew Chem. 2010;49:1540–73. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 22.Berchtold KA, Lovestead TM, Bowman CN. Coupling chain length dependent and reaction diffusion controlled termination in the free radical polymerization of multivinyl (meth)acrylates. Macromolecules. 2002;35:7968–75. [Google Scholar]
- 23.Senyurt AF, Hoyle CE. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules. 2007;40:3174–82. [Google Scholar]
- 24.Li Q, Wicks DA, Hoyle CE. Thiouretane-based thiol-ene high Tg networks: preparation, thermal, mechanical, and physical properties. J Polym Sci A: Polym chem. 2007;45:5103–11. [Google Scholar]
- 25.Bacchi A, Dobson A, Ferracane JL, Consani RL, Pfeifer CS. Thio-urethanes improve properties of dual-cured composite cements. J Dent Res. 2014;93:1320–5. doi: 10.1177/0022034514551768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacchi A, Consani RC, Martim GC, Pfeifer CS. Thio-urethane oligomers improve the properties of light-cured resin cements. Dent Mater. 2015;31:565–74. doi: 10.1016/j.dental.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Delayed gelation through chain-transfer reactions: mechanism for stress reduction in methacrylate. Polymer. 2011;52:3295–303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riener CK, Kada G, Gruber HJ. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal Bioanal Chem. 2002;373:266–76. doi: 10.1007/s00216-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 29.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–9. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 30.Standard I. ISO 4049 polymer based filling, restorative and luting materials. International Organization for Standardization. 2000:1–27. [Google Scholar]
- 31.Designation A. E399-90. Standard test method for plane-strain fracture toughness of metallic materials. Philadelphia, PA: American Society for Testing Materials; 1997. p. t990. [Google Scholar]
- 32.Musanje L, Ferracane JL. Effects of resin formulation and nanofiller surface treatment on the properties of experimental hybrid resin composite. Biomaterials. 2004;25:4065–71. doi: 10.1016/j.biomaterials.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Matsushima H, Shin J, Bowman CN, Hoyle CE. Thiol-isocyanate-acrylate ternary networks by selective thiol-click chemistry. J Polym Sci A: Polym Chem. 2010;48:3255–64. [Google Scholar]
- 34.Shin J, Matsushima H, Comer CM, Bowman CN, Hoyle CE. Thiol-isocyanate-ene ternary networks by sequential and simultaneous thiol click reactions. Chem Mater. 2010;22:2616–25. [Google Scholar]
- 35.Shin J, Matsushima H, Chan JW, Hoyle CE. Segmented polythiourethane elastomers through sequential thiol-ene and thiol-isocyanate reactions. Macromolecules. 2009;42:3294–301. [Google Scholar]
- 36.Li Q, Zhou H, Wicks DA, Hoyle CE, Magers DH, McAlexander HR. Comparison of small molecule and polymeric urethanes, thiourethanes, and dithiourethanes: hydrogen bonding and thermal, physical, and mechanical properties. Macromolecules. 2009;42:1824–33. [Google Scholar]
- 37.Cramer NB, Couch CL, Schreck KM, Boulden JE, Wydra R, Stansbury JW, et al. Properties of methacrylate-thiol-ene formulations as dental restorative materials. Dent Mater. 2010;26:799–806. doi: 10.1016/j.dental.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy SK, Cramer NB, Bowman CN. Thio-vinyl mechanisms. 2. Kinetic modeling of ternary thiol-vinyl photopolymerizations. Macromolecules. 2006;39:3681–7. [Google Scholar]
- 39.Lecamp L, Houllier F, Youssef B, Bunel C. Photoinitiated cross-linking of a thiol methacrylate system. Polymer. 2001;42:2727–36. [Google Scholar]
- 40.Zhang Y, Zhan F, Shi W. Photopolymerization behavior and properties of highly branched poly(thioether-urethane) acrylates used for UV-curing coatings. Prog Org Coat. 2011;71:399–405. [Google Scholar]
- 41.Feng L, Suh BI, Shortall AC. Formation of gaps at the filler-resin interface induced by polymerization contraction stress: gaps at the interface. Dent Mater. 2010;26:719–29. doi: 10.1016/j.dental.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Sun CJ, Wu W, Mahanti K, Sadeghipour K, Baran G, Debnath S. Finite element analysis of toughening for a particle-resin forced composite. Polym Compos. 2006;27:360–7. [Google Scholar]
- 43.Patel MP, Braden M, Davy KW. Polymerization shrinkage of methacrylate esters. Biomaterials. 1987;8:53–6. doi: 10.1016/0142-9612(87)90030-5. [DOI] [PubMed] [Google Scholar]


