Abstract
Adipocytes promote progression of multiple cancers, but their role in pancreatic intraepithelial neoplasia (PanIN) and ductal adenocarcinoma (PDAC) is poorly defined. Nutrient transfer is a mechanism underlying stromal cell-cancer crosstalk. We studied the role of adipocytes in regulating in vitro PanIN and PDAC cell proliferation with a focus on glutamine metabolism. Murine 3T3L1 adipocytes were used to model adipocytes. Cell lines derived from PKCY mice were used to model PanIN and PDAC. Co-culture was used to study the effect of adipocytes on PanIN and PDAC cell proliferation in response to manipulation of glutamine metabolism. Glutamine secretion was measured with a bioanalyzer. Western blotting was used to study the effect of PanIN and PDAC cells on expression of glutamine-related enzymes in adipocytes. Adipocytes promote proliferation of PanIN and PDAC cells, an effect that was amplified in nutrient-poor conditions. Adipocytes secrete glutamine and rescue PanIN and PDAC cell proliferation in the absence of glutamine, an effect that was glutamine synthetase-dependent and involved PDAC cell-induced down-regulation of glutaminase expression in adipocytes. These findings suggest glutamine transfer as a potential mechanism underlying adipocyte-induced PanIN and PDAC cell proliferation.
Abbreviations: FCS, fetal calf serum; GPNA, l-glutamic acid-γ-p-nitroanilide-hydrochloride; Gln, glutamine; GSI, glutamine synthetase inhibitor; GLS, glutaminase; GDH, glutamate dehydrogenase; GS, glutamine synthetase; PanIN, pancreatic intraepithelial neoplasia; PDAC, pancreatic ductal adenocarcinoma; QRTPCR, quantitative real-time polymerase chain reaction
Keywords: Adipocyte, Pancreatic cancer, Pancreatic intraepithelial neoplasia, 3T3L1, Glutamine
Highlights
-
•
Adipocytes promote pancreatic cancer cell proliferation in nutrient-poor conditions.
-
•
Adipocytes secrete glutamine.
-
•
Adipocytes rescue pancreatic cancer cell proliferation in the absence of glutamine.
-
•
Glutamine transfer may underlie adipocyte-induced pancreatic cancer cell proliferation.
1. Introduction
Stromal cell-tumor crosstalk contributes to carcinogenesis. Study of tumor microenvironment stromal cells has focused on fibroblasts, but a growing literature implicates adipocytes. Peri-tumor adipocytes predict poor prognosis in multiple cancers [1], [2], and adipocytes promote proliferation and invasion of multiple types of cancer cells in in vitro and in vivo models [3], [4], [5], [6], [7], [8], [9], [10]. Similar data support a role for adipocytes in PDAC: pancreatic steatosis in humans predisposes to PanIN, PDAC, and to more advanced disease [11], [12], while human adipose tissue stem cells promote pancreatic cell proliferation and invasion in vitro [13]. Finally, pancreatic adipocytes are associated with PDAC progression in murine models [14], [15], [16].
The mechanisms by which adipocytes promote cancer are unknown. An important candidate mechanism is reciprocal metabolic programming that promotes energy transfer from stromal cells to cancer cells. Such metabolic crosstalk has been demonstrated in fibroblast-tumor lactate shuttling in breast and prostate cancers [17], [18], while reciprocal changes in lipolysis and fatty acid oxidation promote fatty acid delivery from adipocytes to ovarian cancer [8].
Many cancers are glutamine-dependent, including PDAC, which undergoes metabolic reprogramming towards a non-canonical glutamine catabolic pathway to produce NADPH for reducing capacity [19]. Transfer of glutamine from cancer-associated fibroblasts to breast cancer cells drives breast cancer cell proliferation, with reciprocal metabolic reprogramming that promotes glutamine synthesis in fibroblasts and glutamine catabolism in breast cancer cells [20]. A single report implicates adipocytes as a source of glutamine for cancer cells in the context of leukemia, with reciprocal induction of glutamine synthetase (GS) in adipocytes that promotes glutamine transfer to leukemia cells [21]. The specific effects of adipocytes on PDAC cells in vitro are poorly defined, and whether adipocytes provide glutamine to PDAC is unknown. The goal of this study was to define the effects of adipocytes on PanIN and PDAC cell proliferation and determine if adipocytes influence PanIN and PDAC cell proliferation via glutamine-dependent metabolic crosstalk, utilizing murine PanIN and PDAC cell lines and the murine 3T3L1 adipocyte line.
2. Methods
2.1. Cell culture
All cell culture was performed in standard tissue culture conditions of 37 °C, 5% CO2. Mature 3T3L1 adipocytes were generated by culturing 3T3L1 preadipocytes (American Type Culture Collection, Manassas, VA, USA) in DMEM, 10% FCS, 500 μM IBMX, 1 μg/ml insulin, 0.25 μM dexamethasone, 2 μM rosiglitazone for 3 days, followed by culture in DMEM, 10%FCS, 1 μg/ml insulin for 3 days, followed by culture in DMEM, 10% FCS for 7 days. Trypan Blue staining was used to determine adipocyte viability.
Murine PI34 and PD7591 cell lines are derived from a PanIN lesion (PI34) and a PDAC tumor (PD7591) from PKCY mice. The PKCY strain contains a codon-12 K-ras gene mutation, present in 95% of human PDAC tumors and a floxed p53 allele, expression of which is targeted to pancreatic epithelial cells via a Pdx1 promoter-driven Cre-recombinase gene, thus generating a pancreas-specific heterozygous p53 knockout on a mutant K-ras background. PKCY mice develop autochthonous PDAC by 18–20 weeks of age with histopathologic progression similar to human disease [22], [23] Monoculture and co-culture experiments with mature adipocytes, preadipocytes, and cancer cells were performed in DMEM, 0.5% FCS with indicated glucose and glutamine concentrations. Conditioned media was generated by culturing mature adipocytes or PI34/PD7591 cells for 72 h in substrate conditions matching the corresponding experiment, after which media was harvested and used in a 50:50 mixture with fresh media containing the same substrates as the conditioned media. The irreversible glutamine synthetase inhibitor l-methionine sulfoximine (Sigma-Aldrich Inc., St. Louis MO, USA) was used at 10 mM to pre-treat adipocytes for 24 h followed by thorough washing of adipocytes 3× with PBS prior to their use in co-culture. The glutamine exporter inhibitor l-glutamic acid γ-p-nitroanilide-hydrochloride (GPNA) (Sigma-Aldrich Inc.) was used at 1 mM and added directly to co-culture media, given that its effect is not irreversible, thus precluding pre-treatment of adipocytes prior to co-culture.
2.2. Oil Red-O staining
Differentiated adipocytes were washed with 500 μL 1X PBS, then 200 μL 4% formalin was added and cells fixed for 15 min, then formalin was removed, and cells washed twice with 1X PBS, then 200 μL of 60% isopropanol added and cells incubated 5 min, then isopropanol removed and 200 μL Oil Red-O solution (American Master Tech Scientific Inc., Lodi, CA, USA) added, then cells incubated 15 min, then Oil Red-O solution removed and cells washed with 500 μL 1X PBS 3 times and imaged with light microscopy.
2.3. QRTPCR
Equal amounts of input RNA prepared from cells using RNeasy kit (Qiagen, Inc., Germantown, MD, USA) were used for quantitative real-time polymerase chain reaction (QRTPCR). RNA was reverse-transcribed using a high capacity cDNA kit (Applied Biosystems, Inc., Foster City, CA, USA), and QRTPCR performed using transcript-specific Taqman primer-probes using actin as an endogenous control on an AB StepOnePlus Thermocycler (Applied Biosystems, Inc., Foster City, CA, USA). The 2−ddCT quantification method was used to calculate fold-difference in transcript levels. The following murine-specific primer-probes were used (ThermoFisher Scientific Inc., Rockford, IL, USA): peroxisome proliferator-activated receptor gamma (PPARg:Mm01184322_m1), fatty acid synthase (FASN:Mm00662319_m1), adipose triglyceride lipase (AGTL:Mm00503040_m1), sterol regulatory element-binding transcription factor 1c (SREBP1c, Mm00550338_m1), CCAAT/Enhancer Binding Protein-alpha (CEBPa, Mm00514283_s1).
2.4. Western blotting
Adipocyte protein lysates in RIPA buffer (25–50 µg) underwent 7.5% SDS-PAGE gel electrophoresis, were transferred to PVDF membrane, blocked in TBST+5%BSA for one hour at 25 °C, washed 3 times in TBST, incubated in TBST+1%BSA overnight at 4 °C with primary antibodies specific for glutamine synthetase (rabbit polyclonal, 1:5000, ThermoFisher Scientific Inc., Rockford, IL, USA, Cat#PA1-46165), glutaminase (rabbit polyclonal, 1:1000, Abcam Inc., Cambridge, MA, USA, Cat#ab93434), or glutamate dehydrogenase (rabbit monoclonal, 1:1000, Abcam Inc., Cambridge, MA, USA, Cat#ab166618), then washed in TBST and incubated with IRDye800-conjugated goat anti-rabbit IgG (1:10,000, Rockland Immunochemicals Inc., Gilbertsville, PA, USA, Cat#611-145-122) in TBST/1%BSA for one hour at 25 °C. Parallel blots loaded with identical amounts of the same lysates were probed with actin-specific primary antibody (mouse monoclonal, 1:1000, ThermoFisher Scientific Inc., Rockford, IL, USA, Cat#MA5-15739) and Alexa Fluor 700-conjugated secondary antibody (goat anti-mouse IgG, 1:1000, ThermoFisher Scientific Inc., Rockford, IL, USA, Cat#A-20136). Densitometry was performed using an Odyssey Infrared Imaging System and software (LI-COR Biosciences Inc., Lincoln, NE, USA); densitometry signals for glutamine synthetase, glutaminase, and glutamate dehydrogenase were normalized to actin densitometry levels.
2.5. Proliferation assay
Semipermeable transwell membrane inserts (0.4 µm, Corning Costar Inc., Tewksbury, MA, USA) containing 15,000 3T3L1 preadipocytes or mature adipocytes were placed above 2000 PanIN/PDAC cells in 24-well culture plates in 500 μl DMEM, 0.5%FCS with glucose/glutamine concentrations as indicated, cultured 72 h, transwells removed, and 50 μl XTT reagent (Biotium Inc., Hayward, CA, USA) added, cells incubated overnight, then 100 μl of media was removed and added to a 96-well assay plate (Corning Costar Inc., Tewksbury MA, USA, Cat#3795) and absorbance read at 450 nm with background subtraction reading at 650 nm on an Biotek Epoch plate reader (BioTek Inc., Winooski, VT, USA).
2.6. Glutamine measurements
Glutamine levels were measured in cell culture media using an immobilized enzyme electrode-based YSI 2950 Biochemistry Analyzer (YSI Life Sciences, Inc., Yellow Springs, OH, USA) and normalized to levels in control medium not exposed to cells but treated identically (to control for evaporation) and to cell number. Cells numbers used matched those in the proliferation assay. Final media volume was raised to 1 ml per manufacturer's requirements for analysis on the bioanalyzer.
2.7. Statistical analysis
All statistical tests were two-tailed. All data were normally distributed. Independent t-test was used to compare outcomes of in vitro assays between mono- and co-culture arms and between media and treatment arms. Delta CT values were compared for statistical analysis of QRTPCR data. Error bars in figures are standard error of the mean.
3. Results
3.1. PanIN and PDAC cell proliferation is glutamine-dependent
3T3L1 adipocytes accumulate cytoplasmic lipid and up-regulate adipogenic genes (Fig. 1A), and maintain >90% viability over 5–7 days in in vitro culture based on Trypan Blue staining (data not shown). We confirmed glutamine dependency of PanIN and PDAC cell (PI34 and PD7591 respectively) proliferation in monoculture in DMEM/0.5% FCS with 0 mM glutamine and variable glucose concentrations, or 0 mM glucose and variable glutamine concentrations, followed by XTT assay. Time-course experiments demonstrated peak proliferation at 72 h, the time-point used for subsequent experiments. In the absence of glutamine with glucose present, PanIN/PDAC cell proliferation was low, similar to proliferation in nutrient-poor media (DMEM/0 mM glucose/0 mM glutamine, 0.5% FCS as sole energy source); in the absence of glucose, in contrast, glutamine rescued PanIN/PDAC cell proliferation at levels as low as 0.05–0.1 mM (Fig. 1B). Time-course experiments confirmed a positive proliferative trajectory in nutrient-poor media, albeit at lower levels than in nutrient-rich media (Fig. 1C). Glutamine concentration in cell-free nutrient-poor media (DMEM, 0.5% FCS, 0 mM glucose, 0 mM glutamine) measured with a YSI bioanalyzer was <0.01 mM, demonstrating that 0.5% FCS is not a significant source of glutamine.
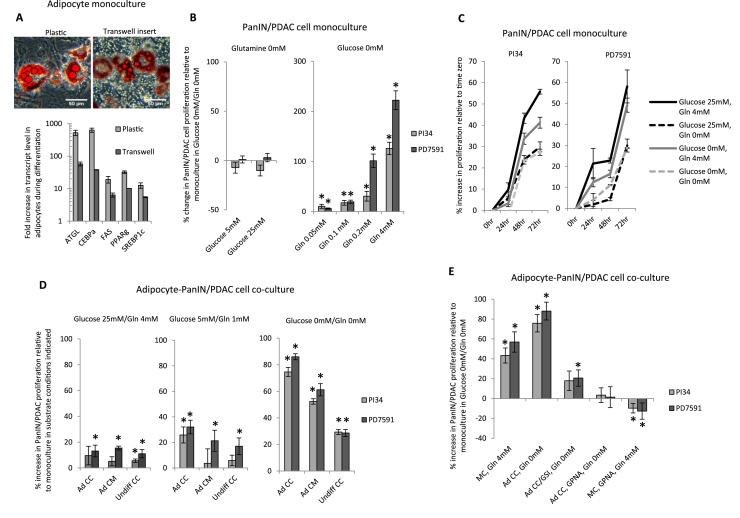
Fig. 1.
Adipocytes promote PanIN and PDAC cell proliferation in a glutamine-dependent manner: (A) 3T3L1 adipocytes: Top: Photomicrograph of Oil Red-O-stained mature 3T3L1 adipocytes on standard plastic (left) and on a transwell insert (right). Bottom: Upregulation of adipogenic gene transcripts (PPAR-γ, FASN, ATGL, SREBP1c, CEBP-α) based on QRTPCR in mature 3T3L1 adipocytes relative to undifferentiated 3T3L1 preadipocytes cultured on standard plastic or on a transwell insert. Ordinate: fold increase in transcript levels in mature adipocytes relative to transcript levels in undifferentiated 3T3L1 cells as a referent=1; *: p<0.001 comparing transcript levels in mature adipocytes to undifferentiated 3T3L1 cell referent; n=5 experiments. (B) PanIN/PDAC cell proliferation is glutamine-dependent: PanIN/PDAC cell proliferation in monoculture for 72 h in low serum (0.5%FCS) glutamine-free media with the indicated glucose concentration (left), or in glucose-free media with the indicated glutamine (Gln) concentration (right). Ordinates: percent change in XTT signal relative to PanIN/PDAC cells in monoculture in nutrient-poor (glucose-free, glutamine-free) media as a referent set at zero; *: p<0.050 comparing experimental condition to referent PanIN/PDAC cell proliferation n=6 experiments. (C) Positive PanIN/PDAC cell proliferation trajectories are maintained in nutrient-poor media: PanIN/PDAC cell proliferation in monoculture for 72 h in low serum media with the indicated glucose and glutamine concentrations. Ordinates: percent change in XTT signal relative to PanIN/PDAC cells in monoculture at time-zero in the corresponding culture conditions as a referent=zero; *: p<0.050 comparing experimental condition to referent time-zero PanIN/PDAC cell proliferation n=7 experiments. (D) Adipocytes promote PDAC cell proliferation: PanIN/PDAC cell proliferation after 72 h in low serum media with the indicated glucose and glutamine concentrations with one of the following in an overlying transwell insert: mature differentiated adipocytes (Ad CC); mature adipocyte-conditioned media (Ad CM); or undifferentiated pre-adipocytes (Undiff CC). Ordinates: percent increase in XTT signal relative to PanIN/PDAC cells in monoculture in the same substrate conditions indicated in each graph as a referent=zero; *: p<0.050 comparing experimental condition to referent PanIN/PDAC cell proliferation n=6 experiments. (E) Adipocytes rescue PDAC cell proliferation in a glutamine-dependent manner: PanIN/PDAC cell proliferation after 72 h culture in low serum glucose-free media and (left to right): monoculture with 4 mM glutamine (MC, Gln 4 mM), co-culture with mature adipocytes in 0 mM glutamine (Ad CC, Gln 0 mM), co-culture with mature adipocytes pre-treated for 24 h with l-methionine sulfoximine in 0 mM glutamine (Ad CC/GSI, Gln 0 mM), co-culture with adipocytes and GPNA with 0 mM glutamine (Ad CC, GPNA, Gln 0 mM), or monoculture with GPNA in 4 mM glutamine (MC, GPNA, Gln 4 mM). Ordinate: percent change in XTT signal in experimental arm relative to PanIN/PDAC cells in monoculture in low serum glucose-free, glutamine-free media as a referent=0; *: p<0.050 comparing experimental condition to referent PanIN/PDAC cell proliferation n=7 experiments.
3.2. Adipocytes promote PanIN and PDAC cell proliferation
We studied the effect of undifferentiated 3T3L1 preadipocytes, mature 3T3L1 adipocytes, and 3T3L1 adipocyte-conditioned media on PanIN/PDAC cell proliferation in variable substrate conditions. Preadipocytes, adipocytes, and adipocyte-conditioned media induced modest PanIN/PDAC cell proliferation in nutrient-rich media. This effect of adipocytes and adipocyte-conditioned media (but not preadipocytes) was significantly amplified in nutrient-poor media (Fig. 1D). These data demonstrate that while both preadipocytes and adipocytes promote modest PanIN/PDAC cell proliferation in nutrient-rich conditions, adipocytes predominate in nutrient-poor conditions.
3.3. Adipocyte-induced PDAC cell proliferation is glutamine-dependent
We studied the role of glutamine in adipocyte-induced PanIN/PDAC cell proliferation in nutrient-poor conditions using l-methionine sulfoximine, an irreversible inhibitor of glutamine synthetase, or GPNA, an inhibitor of cellular glutamine export. PDAC cells in monoculture in DMEM/0.5%FCS/0 mM glucose/4 mM glutamine were studied as a positive control. Adipocytes rescued PanIN/PDAC cell proliferation in the absence of glutamine to a significantly greater degree than preadipocytes. Inhibition of adipocyte glutamine synthetase with l-methionine sulfoximine abrogated adipocyte-induced PanIN/PDAC cell proliferation in the absence of glutamine, as did inhibition of glutamine export with GPNA (Fig. 1E). These data demonstrate that adipocyte-induced PanIN/PDAC cell proliferation is glutamine-dependent, and that inhibition of glutamine synthetase or glutamine exporter function in adipocytes abrogates adipocyte-mediated rescue of PDAC cell proliferation in the absence of glutamine.
3.4. Adipocytes secrete glutamine; PDAC cells down-regulate adipocyte glutaminase expression
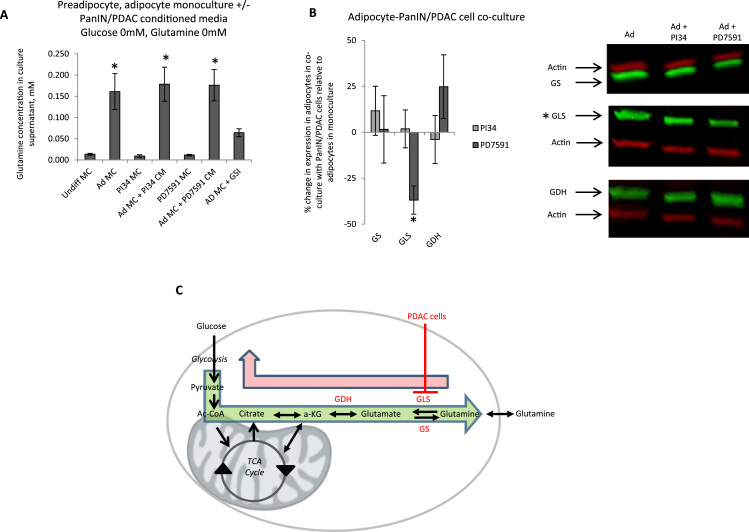
We confirmed glutamine secretion by adipocytes by directly measuring glutamine with a YSI bioanalyzer. Adipocytes in DMEM/0.5%FCS/0 mM glucose/0 mM glutamine secreted glutamine at levels of ~0.2 mM; preadipocytes secreted nominal levels of glutamine in identical culture conditions; a trend towards modest increased glutamine secretion by mature adipocytes was observed in response to culture with PanIN/PDAC cell-conditioned media (Fig. 2A). To determine if PDAC cells regulate adipocyte glutamine metabolism, we studied adipocyte expression of glutamine-related enzymes in response to PanIN/PDAC co-culture with Western blotting. Relative to adipocytes in monoculture, glutaminase expression was reduced in adipocytes co-cultured with PDAC cells, but not PanIN cells. Adipocyte glutamine synthetase or glutamate dehydrogenase expression was not regulated by PanIN or PDAC cells (Fig. 2B).
Fig. 2.
Adipocytes secrete glutamine and PDAC cells regulate adipocyte glutamine metabolism: (A) 3T3L1 adipocytes secrete glutamine: Glutamine secretion in low serum glucose-free, glutamine-free media at 72 h with a YSI Bioanalyzer in: preadipocyte and mature adipocyte monocultures (Undiff MC, Ad MC), PI34 and PD7591 cell monocultures (PI34 MC, PD7591 MC), mature adipocyte monocultures with PI34 or PD7591 cell-conditioned media (Ad MC+PI34 CM, Ad MC+PD7591 CM), and mature adipocyte monocultures with l-methionine sulfoximine and 0 mM glutamine (Ad MC+ GSI). Ordinate: glutamine concentration (mM) in culture supernatant; *: p<0.050 comparing glutamine levels in mature adipocyte monoculture to preadipocyte monoculture (Undiff MC); n=16 experiments. (B) PDAC cells down-regulate glutaminase expression in adipocytes: Left: Adipocyte metabolic enzyme expression in response to PanIN/PDAC cell co-culture; ordinate: % change in fluorescent intensity readings from Western blots of protein lysates from adipocytes cultured with PDAC cells in low serum glucose-free, glutamine-free media, relative to lysates from adipocytes in monoculture in identical media as a referent=zero probed with antibodies to glutamine synthetase (GS), glutaminase (GLS), or glutamate dehydrogenase (GDH), and normalized to actin densitometry. *: p<0.050 for densitometry reading comparing adipocytes in PanIN/PDAC cell co-culture to adipocytes in monoculture; n=6 experiments. Right: Representative Western blot of protein lysates from adipocytes in monoculture (Ad) or in co-culture with indicated PanIN/PDAC cell line. (C) Model for PDAC cell-mediated regulation of adipocyte glutamine metabolism to promote glutamine shuttling: Inhibition of glutaminase (GLS) in adipocytes by PDAC cells inhibits glutamine catabolism by adipocytes (red arrow), predisposing to glutamine secretion (green arrow).
4. Discussion
Adipocytes promote in vitro and in vivo progression of multiple cancers [3], [7], [10], [24]. Only a few studies implicate adipocytes in PDAC [11], [13], [15]. Our data add to this sparse literature. PanIN/PDAC cell proliferation was glutamine-dependent in the murine cell lines we studied, observations that led us to explore whether adipocytes promoted proliferation in the cells and whether glutamine transfer contributed to this effect. We demonstrate that 3T3L1 adipocytes promote murine PanIN and PDAC cell proliferation in nutrient-poor conditions via glutamine shuttling. Adipocytes promoted modest PanIN/PDAC cell proliferation in nutrient-rich conditions, but this effect was markedly amplified in nutrient-poor conditions, simulated in these experiments with low serum (0.5%FCS), 0 mM glucose, 0 mM glutamine media. While in vitro culture does not perfectly model in vivo conditions, the in vivo PDAC tumor microenvironment has been shown to be characterized by low energy substrate availability [25], and we demonstrate positive proliferation trajectories of PanIN/PDAC cells in nutrient-poor media. Taken together these observations suggest that the effects we observed in the nutrient-poor culture conditions we studied are clinically relevant, and that adipocytes act as an energy source for PDAC cells primarily in conditions of nutrient scarcity. Of interest, proliferation in the PI34 and PD7591 cell lines was glutamine-dependent but not glucose dependent, suggesting that glutamine plays a more important role than glucose in proliferation of PanIN and PDAC cells in such conditions. Identification of the source of glutamine secreted by adipocytes will require further study, but glutamine may derive from catabolism of lipid stores, an explanation consistent with the observed secretion of glutamine by adipocytes but not preadipocytes, the latter lacking substantial lipid stores.
Adipocyte co-culture promoted PanIN/PDAC proliferation to a greater extent than adipocyte-conditioned media, suggesting that reciprocal crosstalk underlies at least some of the proliferative effects of adipocytes. Prostate and breast cancer cells regulate fibroblast lactate exporter expression to promote lactate shuttling [26]. Reciprocal regulation of lipid metabolism promotes fatty acid shuttling between adipocytes and ovarian cancer cells [8]. Fibroblasts and breast cancer cells engage in glutamine shuttling [20], and a single report demonstrates glutamine shuttling between adipocytes and leukemia cells via regulation of adipocyte glutamine synthetase [21]. Glutamine is an important energy substrate for PDAC [19]. We demonstrate that 3T3L1 adipocytes secrete glutamine and that inhibition of glutamine cell export or glutamine synthetase abrogates adipocyte-induced PanIN/PDAC cell proliferation, confirming glutamine as an underlying mediator of this effect. Furthermore, PDAC cells decreased glutaminase expression in adipocytes, which would be expected to inhibit adipocyte glutamine catabolism and increase glutamine secretion, suggesting a mechanism of reciprocal crosstalk (Fig. 2C). No such effect was observed with PanIN cells, suggesting that PDAC influences adipocyte glutamine metabolism to a greater degree than PanIN, and that the growth-promoting effects of adipocytes on PanIN cells, unlike PDAC cells, appear to be glutaminase-independent. Adipocytes constitutively secreted glutamine in monoculture, suggesting that glutamine secretion is not completely dependent on regulation by PanIN or PDAC cells. Furthermore and of importance, adipocytes induced PDAC cell proliferation in glutamine-free conditions to a greater extent than in PDAC cell monoculture in the presence of glutamine, suggesting that mechanisms in addition to glutamine transfer underlie at least some component of adipocyte-induced PDAC cell proliferation. Further research will be required to elucidate such mechanisms, which may include other nutrients, adipokines, and other adipocyte-derived mediators. This focused study does not rule out regulation of glutamine or other metabolic pathways at posttranslational or functional levels, nor does it rule out other energy substrates (e.g. lipids) or mechanisms (e.g. adipokines) as mediators of adipocyte-PanIN/PDAC crosstalk, nor activation of oncogene gene expression (for example glutamine-mediated activation of k-Ras, for which precedent exists [27]), issues that will require further research. Nonetheless, taken together, our observations suggest that glutamine shuttling underlies adipocyte-induced PanIN/PDAC cell proliferation in nutrient-poor conditions and that reciprocal metabolic crosstalk contributes to this effect in adipocyte-PDAC interactions, with down-regulation of adipocyte glutaminase expression in response to PDAC cells.
Mature adipocytes, preadipocytes, or both promote breast, colon, and prostate cancer cell proliferation [3], [5], [7], [28], [29]. A single report demonstrates that human preadipocytes induce PDAC cell proliferation; these investigators did not study mature adipocytes [13]. We demonstrate that both 3T3L1 preadipocytes and mature 3T3L1 adipocytes promote modest PanIN/PDAC cell proliferation in nutrient-rich media, but that proliferation in response to mature adipocytes but not preadipocytes increased significantly in nutrient-poor media. These findings suggest that nutrient transfer underlies the proliferative effects of mature adipocytes in nutrient-poor conditions, but other mechanisms, including possible preadipocyte effects, may be active in nutrient-rich conditions.
This is one of only a few reports demonstrating a role for adipocytes in promoting PanIN/PDAC cell proliferation and the first to demonstrate glutamine shuttling between adipocytes and PanIN/PDAC cells. These data suggest glutamine metabolism as a target for further research into adipocyte-PDAC interactions.
Funding
This work was supported by: National Institutes of Health grants DK097449 (RWO), DK090262 (CNL), DK088945 (ADR), CA177857 (ADR), a Michigan Metabolomics-Obesity Center/Nutrition-Obesity Research Center Pilot Grant (RWO); a Pancreatic Cancer Action Network Pathway to Leadership Award (CAL); a James S. McDonnell Foundation Grant (MLW); a Department of Defense CDMRP Award (ADR); a Pancreatic Cancer Action Network Career Development Award (ADR); and an American Gastroenterological Association Augustyn Award (ADR). The funding agencies had no role in any aspects of study design, implementation, or interpretation. Authors have no relevant financial or non-financial competing interests.
Acknowledgments
We thank Sofia Merajver, MD, PhD for access to the YSI Bioanalyzer, and Charles Burant MD, PhD for advice and discussion.
Footnotes
Transparency Document associated with this article can be found in the online version at 10.1016/j.bbrep.2016.06.004.
Contributor Information
Kevin A. Meyer, Email: meyerkee@umich.edu.
Christopher K. Neeley, Email: ckneeley@med.umich.edu.
Nicki A. Baker, Email: nickiba@med.umich.edu.
Alexandra R. Washabaugh, Email: alexwash@med.umich.edu.
Carmen G. Flesher, Email: cflesher@umich.edu.
Barbara S. Nelson, Email: barbnels@umich.edu.
Timothy L. Frankel, Email: timofran@med.umich.edu.
Carey N. Lumeng, Email: clumeng@med.umich.edu.
Costas A. Lyssiotis, Email: clyssiot@med.umich.edu.
Michelle L. Wynn, Email: mlwynn@umich.edu.
Andrew D. Rhim, Email: arhim@med.umich.edu.
Robert W. O’Rourke, Email: rorourke@umich.edu.
Appendix ATransparency Document
Supplementary material
.
References
- 1.Hasebe T., Mukai K., Tsuda H., Ochiai A. New prognostic histological parameter of invasive ductal carcinoma of the breast: clinicopathological significance of fibrotic focus. Pathol. Int. 2000;50:263–272. doi: 10.1046/j.1440-1827.2000.01035.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi J., Ohtani H., Nakamura K., Shimokawa I., Kanematsu T. Prognostic impact of marginal adipose tissue invasion in ductal carcinoma of the breast. Am. J. Clin. Pathol. 2008;130:382–388. doi: 10.1309/MX6KKA1UNJ1YG8VN. [DOI] [PubMed] [Google Scholar]
- 3.Amemori S., Ootani A., Aoki S., Fujise T., Shimoda R., Kakimoto T. Adipocytes and preadipocytes promote the proliferation of colon cancer cells in vitro. Am. J. Phys. Gastro. Liv. Phys. 2007;292(3):G923–G929. doi: 10.1152/ajpgi.00145.2006. [DOI] [PubMed] [Google Scholar]
- 4.Carter J.C., Church F.C. Mature breast adipocytes promote breast cancer cell motility. Exp. Mol. Pathol. 2012;92(3):312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Dirat B., Bochet L., Dabek M., Daviaud D., Dauvillier S., Majed B. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 6.Liu E., Samad F., Mueller B.M. Local adipocytes enable estrogen-dependent breast cancer growth: role of leptin and aromatase. Adipocyte. 2013;2(3):165–169. doi: 10.4161/adip.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manabe Y., Toda S., Miyazaki K., Sugihara H. Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J. Pathol. 2003;201(2):221–228. doi: 10.1002/path.1430. [DOI] [PubMed] [Google Scholar]
- 8.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokuda Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int. 2003;91(7):716–720. doi: 10.1046/j.1464-410x.2003.04218.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Daquinag A., Traktuev D.O., Amaya-Manzanares F., Simmons P.J., March K.L. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69(12):5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathur A., Zyromski N.J., Pitt H.A., Al-Azzawi H., Walker J.J., Saxena R. Pancreatic steatosis promotes dissemination and lethality of pancreatic cancer. J. Am. Coll. Surg. 2009;208(5):989–994. doi: 10.1016/j.jamcollsurg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Rebours V., Gaujoux S., d’Assignies G., Sauvanet A., Ruszniewski P., Levy P. Obesity and fatty pancreatic infiltration are risk factors for pancreatic precancerous lesions (PanIN) Clin. Cancer Res. 2015 19;2385:2014. doi: 10.1158/1078-0432.CCR-14-2385. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Ji S.Q., Cao J., Zhang Q.Y., Li Y.Y., Yan Y.Q., Yu F.X. Adipose tissue-derived stem cells promote pancreatic cancer cell proliferation and invasion. Braz. J. Med. Biol. Res. 2013;46(9):758–764. doi: 10.1590/1414-431X20132907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grippo P.J., Fitchev P.S., Bentrem D.J., Melstrom L.G., Dangi-Garimella S., Krantz S.B., Heiferman M.J., Chung C. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61(10):1454–1464. doi: 10.1136/gutjnl-2011-300821. [DOI] [PubMed] [Google Scholar]
- 15.White P.B., True E.M., Ziegler K.M., Wang S.S., Swartz-Basile D.A., Pitt H.A. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J. Gastrointest. Surg. 2010;12:1888–1893. doi: 10.1007/s11605-010-1349-x. [DOI] [PubMed] [Google Scholar]
- 16.Zyromski N.J., Mathur A., Pitt H.A., Wade T.E., Wang S., Nakshatri P., Swartz-Basile D.A., Nakshatri H. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146(2):258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Whitaker-Menezes D., Martinez-Outschoorn U.E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A.K., Birbe R.C., Howell A. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10(11):1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiaschi T., Marini A., Giannoni E., Taddei M.L., Gandellini P., De Donatis A., Lanciotti M., Serni S. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72(19):5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 19.Son J., Lyssiotis C.A., Ying H., Wang X., Hua S., Ligorio M. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko Y.H., Lin Z., Flomenberg N., Pestell R.G., Howell A., Sotgia F. Glutamine fuels a vicious cycle of autophagy in the tumor stroma and oxidative mitochondrial metabolism in epithelial cancer cells: implications for preventing chemotherapy resistance. Cancer Biol. Ther. 2011;12(12):1085–1097. doi: 10.4161/cbt.12.12.18671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehsanipour E.A., Sheng X., Behan J.W., Wang X., Butturini A., Avramis V.I. Adipocytes cause leukemia cell resistance to l-asparaginase via release of glutamine. Cancer Res. 2013;73(10):2998–3006. doi: 10.1158/0008-5472.CAN-12-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhim A.D., Mirek E.T., Aiello N.M., Maitra A., Bailey J.M., McAllister F. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreekumar B.K., Belinsky G.S., Einwachter H., Rhim A.D., Schmid R., Chung C. Polarization of the vacuolar adenosine triphosphatase delineates a transition to high-grade pancreatic intraepithelial neoplasm lesions. Pancreas. 2014;43(8):1256–1263. doi: 10.1097/MPA.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneko A., Satoh Y., Tokuda Y., Fujiyama C., Udo K., Uozumi J. Effects of adipocytes on the proliferation and differentiation of prostate cancer cells in a 3-D culture model. Int. J. Urol. 2010;17(4):369–376. doi: 10.1111/j.1442-2042.2010.02472.x. [DOI] [PubMed] [Google Scholar]
- 25.Kamphorst J.J., Nofal M., Commisso C., Hackett S.R., Lu W., Grabocka E. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonveaux P., Végran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 2008;118(12):3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M.H., Kim H. Oncogenes and tumor suppressors regulate glutamine metabolism in cancer cells. J. Cancer Prev. 2013;18(3):221–226. doi: 10.15430/JCP.2013.18.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowicka A., Marini F.C., Solley T.N., Elizondo P.B., Zhang Y., Sharp H.J. Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLOS One. 2013;8(12):e81859. doi: 10.1371/journal.pone.0081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orecchioni S., Gregato G., Martin-Padura I., Reggiani F., Braidotti P., Mancuso P. Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res. 2013;73(19):5880–5891. doi: 10.1158/0008-5472.CAN-13-0821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material