Purpose
To measure changes in perfusion of the pharyngeal constrictor muscles (PCM) using CT perfusion (CTP) imaging during a course of definitive radiotherapy (RT) in head-and-neck cancer (HNC) patients and correlate with dysphagia outcome after RT.
Methods and Materials: Fifteen HNC patients underwent CTP imaging of the PCM at baseline and Weeks 2,4, and 6 during RT and 6 weeks after RT. Blood flow and blood volume were measured in the PCM, and percentage change from baseline scan was determined. A single physician-based assessment of dysphagia was performed every 3 months after RT using the Common Terminology Criteria for Adverse Events, version 3.0 grading system.
Results: With a median follow-up of 28 months (range, 6–44 months), Grade 3 dysphagia was present in 7 of 15 patients, and 8 patients experienced Grade 0–2 dysphagia. The CTP parameters at Week 2 of RT demonstrated an increase in mean PCM blood flow of 161.9% vs. 12.3% (p = 0.007) and an increase in mean PCM blood volume of 96.6% vs. 8.7% (p = 0.039) in patients with 6-month post-RT Grade 3 dysphagia and Grade 0–2 dysphagia, respectively. On multivariate analysis, when adjusting for smoking history, tumor volume, and baseline dysphagia status, an increase in blood flow in the second week of RT was significant for 3- and 6-month Grade 3 dysphagia (p < 0.05).
Conclusions: Perfusion changes in the PCM during Week 2 of RT in the PCM may predict the severity of dysphagia after HNC RT.
Keywords: Head-and-neck cancer, Dysphagia, Pharyngeal constrictor muscles, Functional imaging, CT perfusion imaging, Radiotherapy, Intensity-modulated radiotherapy
Introduction
Dysphagia after radiotherapy (RT) for head-and-neck cancer (HNC) is a significant toxicity that adversely impacts the quality of life in patients with HNC (1–3). Methods to improve swallowing outcome and reduce dysphagia severity after RT include optimizing RT planning by sparing muscles involved in swallowing using intensity-modulated radiotherapy (IMRT) (4–7) coupled with speech and swallow rehabilitation (7, 8). Although the underlying mechanism of dysphagia seems to be multifactorial, radiation injury to the pharyngeal constrictor muscles (PCM) has been proposed as an underlying contributor to long-term swallowing dysfunction (6, 9). Computed tomography perfusion (CTP) imaging allows a noninvasive method of measuring regional blood perfusion through a tissue, which can provide functional data about the microvascular environment by measuring physiologic parameters, including regional blood flow (BF), blood volume (BV), mean transit time (MTT), and capillary permeability surface product (CP). Although various studies have demonstrated the ability to predict tumor response to RT in HNC with CTP imaging (10–12), none have investigated the predictive role of CTP in normal tissue injury.
Although the therapeutic doses of RT used in HNC are generally within the range of 66–72 Gy, dysphagia outcome is highly variable among individuals. Factors contributing to RT-related dysphagia have primarily focused on dosimetric parameters to swallowing muscles, although the underlying mechanism of injury to swallowing structures after RT remains elusive.
Studies using CTP imaging before RT have shown altered perfusion parameters within tumors of the head and neck; however, normal tissue perfusion changes before, during, and after RT have not been fully investigated (10–12). Therefore, we conducted a pilot prospective study examining CTP imaging as a noninvasive method of measuring regional perfusion parameters in the PCM in patients with HNC undergoing definitive conformal RT at baseline, during, and after RT to correlate dysphagia outcome. The hypothesis was that PCM may demonstrate a different pattern of perfusion changes in patients with severe dysphagia compared with patients without severe dysphagia.
Methods and Materials
Patient population
The study was conducted as a prospective, single-arm study, with approval by the institutional review board. Written informed consent was obtained from the patient before entering the study. Fifteen HNC patients were treated with curative intent RT to a total median dose of 70 Gy (range, 70–72 Gy) in 33 (range, 33–42) fractions, between 2007 and 2008, of whom 13 received concurrent chemotherapy with weekly cisplatin (30 mg/m2), 1 patient received concurrent weekly cetuximab (250 mg/m2), and 1 patient received RT alone. Twelve patients were treated with a full-length IMRT field (the larynx and the inferior constrictors and upper esophagus contoured as an avoidance structure in nonlaryngeal and nonhypopharyngeal cancer patients), 1 patient was treated with IMRT matched to a low anterior neck field matched at the level of the hyoid, and 2 patients were treated with three-dimensional conformal RT with superior fields matched with a low anterior neck field using a midline block to spare the larynx, postcricoid space, and upper esophagus. The point dose to the PCM was within the 70-Gy isodose in the region of measurement of the CT perfusion changes. Gross tumor volumes (GTVs) were contoured by the radiation oncologist with volumetric expansions to create clinical and planning target volumes. Gross tumor volumes for the primary site and nodal volumes were obtained from the dose–volume histograms from the RT plans. All patients were treated by a single board-certified radiation oncologist. Patients underwent CTP imaging at CT simulation at Weeks 2, 4, and 6 during RT (corresponding to 12.72 Gy, 33.92 Gy, and 53.12 Gy, respectively) and 6 weeks after completion of RT. Tumor control and tumor perfusion characteristics have been previously published (13).
CTP image acquisition and perfusion measurements
A 64-multidetector-row CT (Lightspeed VCT; GE Medical System, Milwaukee, WI) was used for all CTP studies with initial non-contrast CT (5-mm-thick, 120 kV, 125 mA) through the known primary and nodal site, which served as a localizer for the CTP scan. Eight contiguous 5-mm-thick slices, 4-cm craniocaudal coverage, were selected to cover the largest axial diameter of the primary and nodal tumor mass. Patients received a power injection of 40 mL nonionic iodinated contrast agent (350 mgI/mL) (ioversol [Optiray 350]; Mallinckrodt, St. Louis, MO) at 4 mL/s. At 5 s after the start of the i.v. contrast injection, a cine (continuous) acquisition was initiated using the following parameters: 120 kV, 50 mA, 5 mm × 8 slice coverage, 1-s rotation for a 50-s duration, followed by another 20-s acquisition starting at 3 min after the start of the i.v. contrast injection.
All patients completed the baseline and Week 2 of RT CTP studies. Fourteen of 15 patients completed CTP imaging at Week 4 of RT, 11 of 15 patients completed the CTP scan at Week 6 of RT (CP parameter was not measurable for 1 patient at Week 6 owing to motion artifact; n = 10), and 12 of 15 patients completed the CTP scan at 6 weeks after RT. Over the course of the protocol, a total of 5 patients could not complete all CTP scans owing to difficult i.v. access in 2 patients, contrast injection failure in 1 patient, and the development of renal insufficiency during chemoradiotherapy in 2 patients.
Perfusion measurements of the PCM were performed at the axial level of the tumor by a single neuroradiologist, who placed the region of interest over the PCM, adjacent to the primary tumor and within the 70-Gy isodose line (Fig. 1). The CTP data were postprocessed using a commercially available software package based on a deconvolution technique (Advantage Windows workstation [GE Medical Systems, Milwaukee, WI] with CT Perfusion 3 software package) (14). The data were processed into maps that corresponded to BF (milliliters per 100 g per minute), BV (milliliters per 100 g), MTT (seconds), and CP (milliliters per 100 g per minute) (14).
Fig. 1.
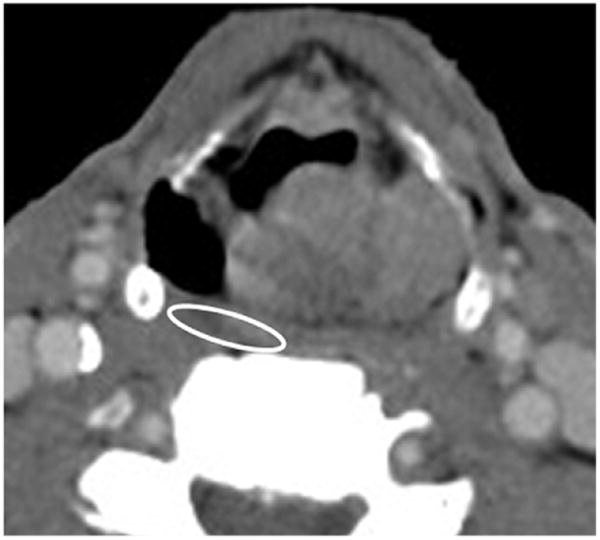
Axial CT slice of a head-and-neck cancer patient. Circle overlying pharyngeal constrictor muscle indicates place of measurement for each CT perfusion imaging parameter.
Evaluation of swallowing function
Swallowing function was assessed by history and physical examination at 3, 6, 9, and 12 months after RT and at last follow-up. Grading of swallowing function was determined by review of patient records by a single physician using the Common Terminology Criteria for Adverse Events version 3 grading system (CTCAE v3) (Appendix 1) (15).
Statistical analysis
Descriptive statistics were used to obtain patient and tumor characteristics. We calculated the percentage change of each CTP parameter from the baseline values in the PCM at Weeks 2, 4, and 6 during RT and 6 weeks after RT scan (corresponding to median radiation doses of 12.72 Gy, 33.92 Gy, 55.12 Gy, and 69.96 Gy, respectively). The percentage change in CTP parameters were not normally distributed and were log-transformed before analysis. Severe dysphagia was defined as having difficulty in swallowing of Grade 3 or above according to CTCAE v 3.0 (15). Correlation between dysphagia severity at 3, 6, 9, and 12 months' follow-up and perfusion parameters obtained at each CTP scan were performed using independent-samples t tests.
Multivariate statistical analyses were conducted using the general linear model (Proc GLM) of SAS 9.1 (SAS Institute, Cary, NC). The following potential confounding variables were explored in these analyses: smoking (number of pack-years), GTV of the primary, and GTV max (largest primary or nodal volume). A two-sided hypothesis was used for all tests, and a probability value of <0.05 was considered statistically significant.
Results
Patient characteristics and treatment
Patient and tumor characteristics are described in Table 1. All patients were staged according to the 2002 American Joint Committee on Cancer classification (16). All patients were smokers, with a median pack-year history of 36 years (range, 10–100 pack-years). No significant differences were noted between patients with Grade 3 dysphagia and patients with Grade 0–2 dysphagia with respect to GTV, smoking pack-years, or age. The mean GTV was 39.4 cm3 vs. 39.5 cm3 (p = 0.999), the mean GTV max (of nodal or primary disease) was 60.7 cm3 vs. 59.0 cm3 (p = 0.956); mean age was 59 years vs. 52 years (p = 0.065); mean pack-year smoking history was 53 vs. 43 (p = 0.538) in patients with 6-month Grades 0–2 dysphagia and Grade 3 dysphagia, respectively.
Table 1. Patient and tumor characteristics of 15 head-and-neck cancer patients.
| Age (y)* | 15, 57.0, 55.8 ± 7.5 (45.0–68.0) |
| Smoking (pack-years) | 15, 36.0, 47.4 ± 29.0 (10.0–100.0) |
| Treatment elapsed (d) | 15, 47.0, 50.4 ± 7.0 (42.0–66.0) |
| GTV-tumor – primary (cm3) | 14, 15.7, 39.5 ± 44.4 (2.9–162.5) |
| GTV-tumor – nodal (cm3) | 10, 21.0, 47.0 ± 58.7 (2.4–179.8) |
| GTV-tumor – max (cm3) | 15, 39.5, 60.1 ± 55.2 (8.8–179.8) |
| Sex | |
| Male | 12 (80.0) |
| Female | 3 (20.0) |
| Race | |
| White | 8 (53.3) |
| Black | 5 (33.3) |
| Other | 2 (13.3) |
| T stage | |
| T0 | 1 (6.7) |
| T1 | 1 (6.7) |
| T2 | 6 (40.0) |
| T3 | 3 (20.0) |
| T4 | 4 (26.7) |
| Nodal stage | |
| N0 | 5 (33.3) |
| N1 | 1 (6.7) |
| N2 | 8 (53.3) |
| N3 | 1 (6.7) |
| M stage | |
| M0 | 14 (93.3) |
| M1 | 1 (6.7) |
| AJCC stage | |
| I | 0 |
| II | 2 (13.3) |
| III | 3 (20.0) |
| IV | 10 (66.7) |
| Total dose (Gy)/fractions | |
| 69.96/33 | 13 (86.7) |
| 70.00/35 | 1 (6.7) |
| 72.00/42 | 1 (6.7) |
| Primary site | |
| Nasopharynx | 2 (13.3) |
| Oropharynx/oral | 6 (40.0) |
| Hypopharynx/larynx | 5 (33.3) |
| Other | 2 (13.3) |
Abbreviations: AJCC = American Joint Committee on Cancer; GTV = gross tumor volume; max = maximum of primary or nodal GTV.
Data in first six rows presented as n, median, mean ± SD (range). Other data presented as n (%).
Dysphagia outcome
Over a median follow-up of 28 months (range, 6–44 months), Grade 3 dysphagia was present in 7 of the 15 patients (6 patients receiving chemoradiation with cisplatin and 1 patient with radiation alone), and 8 patients (7 patients receiving chemoradiation with cisplatin and 1 patient with concurrent cetuximab and radiation) developed Grade 0–2 dysphagia during the study time period. None developed Grade 4 dysphagia. Baseline Grade 1, 2, and 3 dysphagia (before initiation of RT) was present in 2, 2, and 3 patients, respectively. Three patients with Grade 3 dysphagia before starting RT had percutaneous gastrostomy tubes placed because of significant weight loss at diagnosis. The incidence of dysphagia by grade at 3, 6, 9, and 12 months and last follow-up are described in Table 2.
Table 2. Dysphagia incidence at each 3-month follow-up.
| Follow-up period | Total patient no. | Dysphagia Grade | |||
|---|---|---|---|---|---|
|
| |||||
| 0 | 1 | 2 | 3 | ||
| Baseline (before radiotherapy) | 15 | 8 | 2 | 2 | 3 |
| 3 mo | 15 | 4 | 4 | 2 | 5 |
| 6 mo | 15 | 1 | 5 | 3 | 6 |
| 9 mo* | 14 | 2 | 4 | 4 | 4 |
| 12 mo* | 14 | 4 | 4 | 2 | 4 |
| Last follow-up† | 15 | 8 | 2 | 2 | 3 |
One patient deceased.
Includes whole patient cohort, with a median follow-up range of 6–44 months.
Perfusion characteristics of the PCM
The mean perfusion values for BF, BV, MTT, and CP of the PCM at baseline, Weeks 2, 4, and 6 of RT, and after RT are described in Table 3. The mean BF, BV, and CP of the pharyngeal constrictors increased for the whole patient cohort during and after RT compared with baseline. When comparing patients' CTP parameters according to the patients' baseline dysphagia status, no significant differences were found between the two groups (Table 4).
Table 3. Mean tumor perfusion parameters for the whole patient cohort before, during, and after RT.
| Parameter (mean value) | Baseline before RT (n = 15) | During RT | 6 wk after RT (n = 12) | ||
|---|---|---|---|---|---|
|
| |||||
| Week 2 (n = 15) | Week 4 (n = 14) | Week 6 (n = 11) | |||
| BF (mL/100 g/min) | 16.9 (6.6) | 25.7 (16.7) | 46.0 (25.9) | 36.0 (23.0) | 32.7 (12.7) |
| BV (mL/100 g) | 1.6 (0.68) | 2.0 (0.70) | 2.5 (0.86) | 2.8 (1.5) | 3.6 (1.6) |
| MTT (s) | 8.3 (3.5) | 9.4 (4.1) | 6.6 (4.1) | 7.9 (3.7) | 8.3 (2.7) |
| CP (mL/100 g/min) | 6.0 (4.8) | 6.6 (7.3) | 8.5 (5.9) | 13.7 (12.3) | 13.2 (15.5) |
Abbreviations: RT = radiotherapy; BF = blood flow; BV = blood volume; MTT = mean transit time; CP = capillary permeability. Data are presented as mean (SD). Capillary permeability was not recorded for 2 patients at Week 2 (n = 13) and 1 patient at Week 6 (n = 10).
Table 4. Computed tomography perfusion parameters at baseline correlated to dysphagia severity at baseline.
| Parameter | No. of scans | Baseline dysphagia status | p | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Grade 0, 1, 2 | Grade 3 | |||||||
|
|
|
|||||||
| n | Mean | SE | n | Mean | SE | |||
| Blood flow | 15 | 12 | 17.8 | 2.0 | 3 | 13.0 | 2.3 | 0.206 |
| Blood volume | 15 | 12 | 1.8 | 0.18 | 3 | 1.1 | 0.44 | 0.344 |
| Capillary permeability | 15 | 12 | 5.7 | 1.4 | 3 | 7.0 | 3.2 | 0.844 |
| Mean transition time | 15 | 12 | 8.4 | 1.0 | 3 | 7.6 | 2.6 | 0.536 |
Changes in CTP parameters during RT
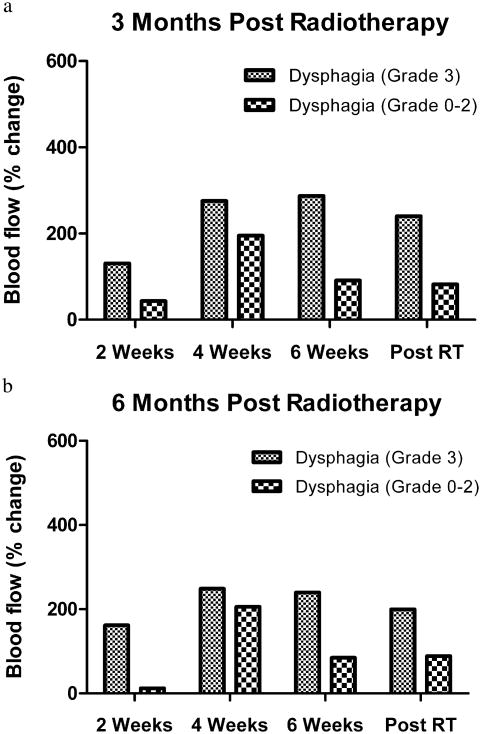
Blood flow. On evaluation of dysphagia at 3-month follow-up, percentage change in mean BF at 2 weeks of RT was increased to 130.5% of the baseline BF for patients with Grade 3 dysphagia, compared with 43.0% of baseline BF in patients with Grade 0–2 dysphagia (p = 0.083). Similarly, for dysphagia status at 6-month follow up, the percentage change in mean BF at 2 weeks of RT was increased to 161.9% of the baseline BF for patients with Grade 3 dysphagia, compared with 12.3% of baseline BF in patients with Grade 0–2 dysphagia (p = 0.007).
When correlating dysphagia status at 3 and 6 months, the percentage change in mean BF at Weeks 4 and 6 during RT and after RT was higher in patients with Grade 3 dysphagia compared with patients with Grade 0–2 dysphagia (Fig. 2), although the difference was not statistically significant. The percentage change in mean BF at Week 2 was increased to 80.4 % in patients with Grade 0–2 dysphagia compared with an increase of 66.9% from baseline with Grade 3 dysphagia (p = 0.984) at 9- and 12-month follow up. The percentage change in mean BF from baseline at Weeks 4 and 6 during RT and 6 weeks after RT was not significant for dysphagia outcome at 9- and 12-month follow-up.
Fig. 2.
Blood flow measurements in the pharyngeal constrictor muscles correlated with Grade 3 dysphagia outcome at (a) 3 months and (b) 6 months.
Mean percentage changes in BF stratified by dysphagia status at 3 and 6 months of follow-up are described in Table 5.
Table 5. Computed tomography perfusion parameters (percentage change from baseline) correlated to dysphagia severity at 3- and 6-month follow-up.
| Parameter | No. of scans | Dysphagia Grade 0–2 | Dysphagia Grade 3 | p | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | Mean | SE | n | Mean | SE | |||
| Blood flow | ||||||||
| 3-mo follow-up | ||||||||
| Week 2 | 15 | 10 | 43.0 | 38.8 | 5 | 130.5 | 54.8 | 0.083 |
| Week 4 | 14 | 9 | 195.1 | 70.6 | 5 | 275.8 | 94.8 | 0.366 |
| Week 6 | 11 | 9 | 91.2 | 39.5 | 2 | 287.2 | 83.7 | 0.141 |
| 6 wk after RT | 12 | 8 | 82.2 | 58.5 | 4 | 240.4 | 82.7 | 0.132 |
| 6-mo follow-up | ||||||||
| Week 2 | 15 | 9 | 12.3 | 34.6 | 6 | 161.9 | 42.4 | 0.007 |
| Week 4 | 14 | 8 | 205.4 | 75.9 | 6 | 248.6 | 87.7 | 0.417 |
| Week 6 | 11 | 8 | 84.5 | 43.5 | 3 | 239.9 | 71.0 | 0.121 |
| 6 wk after RT | 12 | 7 | 88.6 | 65.9 | 5 | 199.9 | 78.0 | 0.265 |
| Blood volume | ||||||||
| 3-mo follow-up | ||||||||
| Week 2 | 15 | 10 | 12.0 | 27.2 | 5 | 107.7 | 38.5 | 0.044 |
| Week 4 | 14 | 9 | 58.3 | 45.4 | 5 | 162.8 | 60.9 | 0.189 |
| Week 6 | 11 | 9 | 80.9 | 91.3 | 2 | 552.6 | 193.8 | 0.217 |
| 6 wk after RT | 12 | 8 | 105.9 | 98.8 | 4 | 414.3 | 139.7 | 0.096 |
| 6-mo follow-up | ||||||||
| Week 2 | 15 | 9 | 8.7 | 29.1 | 6 | 96.6 | 35.6 | 0.039 |
| Week 4 | 14 | 8 | 54.6 | 48.5 | 6 | 150.4 | 56.0 | 0.163 |
| Week 6 | 11 | 8 | 80.0 | 106.7 | 3 | 397.8 | 174.2 | 0.280 |
| 6 wk after RT | 12 | 7 | 82.4 | 104.6 | 5 | 385.6 | 123.8 | 0.040 |
| Capillary permeability | ||||||||
| 3-mo follow-up | ||||||||
| Week 2 | 13 | 9 | 31.6 | 33.8 | 4 | -48.3 | 50.6 | 0.090 |
| Week 4 | 14 | 9 | 320.3 | 154.8 | 5 | 205.7 | 207.7 | 0.299 |
| Week 6 | 10 | 8 | 589.7 | 482.3 | 2 | 1907.4 | 964.5 | 0.357 |
| 6 wk after RT | 12 | 8 | 603.8 | 244.0 | 4 | 243.3 | 345.1 | 0.640 |
| 6-mo follow-up | ||||||||
| Week 2 | 13 | 8 | 43.1 | 34.3 | 5 | -50.7 | 43.4 | 0.062 |
| Week 4 | 14 | 8 | 372.6 | 160.3 | 6 | 155.2 | 185.2 | 0.848 |
| Week 6 | 10 | 7 | 652.4 | 546.4 | 3 | 1322.0 | 834.7 | 0.371 |
| 6 wk after RT | 12 | 7 | 694.0 | 249.7 | 5 | 189.1 | 295.5 | 0.350 |
| Mean transition time | ||||||||
| 3-mo follow-up | ||||||||
| Week 2 | 15 | 10 | 29.0 | 20.6 | 5 | 25.0 | 29.2 | 0.944 |
| Week 4 | 14 | 9 | 6.9 | 28.8 | 5 | -13.3 | 38.7 | 0.802 |
| Week 6 | 11 | 9 | 8.7 | 24.4 | 2 | 65.4 | 51.7 | 0.988 |
| 6 wk after RT | 12 | 8 | 20.9 | 29.4 | 4 | 68.9 | 41.5 | 0.297 |
| 6-mo follow-up | ||||||||
| Week 2 | 15 | 9 | 38.4 | 21.3 | 6 | 11.6 | 26.0 | 0.447 |
| Week 4 | 14 | 8 | 9.5 | 30.5 | 6 | -13.4 | 35.2 | 0.871 |
| Week 6 | 11 | 8 | 15.5 | 27.2 | 3 | 28.4 | 44.3 | 0.524 |
| 6 wk after RT | 12 | 7 | 28.7 | 32.5 | 5 | 48.5 | 38.4 | 0.578 |
Abbreviation as in Table 3.
Blood volume
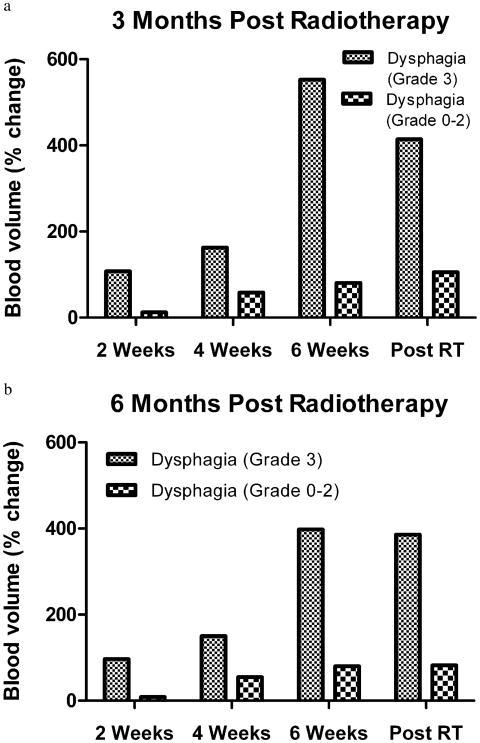
On evaluation of dysphagia at 3 months' follow-up, the percentage change in mean BV at 2 weeks of RT was increased to 107.7% from the baseline BV for patients with Grade 3 dysphagia, compared with a 12.0% increase from baseline BF in patients with Grade 0–2 dysphagia (p = 0.044). At 6 months' follow-up, the percentage change in mean BV at 2 weeks of RT was increased to 96.6% from the baseline BV for patients with Grade 3 dysphagia, compared with 8.7% from the baseline BV in patients with Grade 0–2 dysphagia (p = 0.039).
When correlating dysphagia status at 3 and 6 months, the percentage change in mean BV at Weeks 4 and 6 during RT was higher in patients with Grade 3 dysphagia compared with patients with Grade 0–2 dysphagia (Fig. 3 and Table 5), although the difference was not statistically significant. The percentage change in mean BV 6 weeks after RT was significantly higher in patients with Grade 3 dysphagia, 385.6%, as compared with those with Grade 0–2 dysphagia, 82.4% (p = 0.040) (Table 5).
Fig. 3.
Blood volume measurements in the pharyngeal constrictor muscles correlated with Grade 3 dysphagia outcome at (a) 3 months and (b) 6 months.
The percentage change in mean BV at Week 2 was increased 58.1% in patients with Grade 0–2 dysphagia, compared with an increase of 9.4% from baseline with Grade 3 dysphagia (p = 0.334) at 9- and 12-month follow-up. The percentage change in mean BV from baseline at Weeks 4 and 6 during RT and 6 weeks after RT was not significant for dysphagia outcome.
Mean transit time
No significant patterns were identified in MTT compared with baseline MTT values in patients with Grade 3 dysphagia compared with patients with Grade 0–2 dysphagia at all follow-up time points (Table 5).
Capillary permeability
The percentage change in mean CP at Week 2 from baseline was an increase of 31.6% in patients with Grade 0–2 dysphagia, compared with a decrease of 48% from baseline with Grade 3 dysphagia (p = 0.090) at 3-month follow-up, and was increased 43.1% in patients with Grade 0–2 dysphagia, compared with a decrease of 50.7% from baseline with Grade 3 dysphagia (p = 0.062) at 6-month follow-up (Table 5). The percentage change in mean CP at Week 2 was a 40.1% increase in patients with Grade 0–2 dysphagia, compared with a decrease of 68.1% from baseline with Grade 3 dysphagia (p = 0.047) at 9- and 12-month follow-up. The percentage change in mean CP from baseline at Weeks 4 and 6 during RT and 6 weeks after RT was not significant for dysphagia outcome.
Multivariate analysis of PCM perfusion parameters and dysphagia
On multivariate analysis adjusting for the following factors individually: smoking history by the number of pack-years, GTVof the primary or nodal tumor, and baseline dysphagia status, we observed a similar trend in percentage change in CTP parameters from baseline to Week 2 of RT (Table 6).
Table 6. Computed tomography perfusion parameters (percentage change from baseline to Week 2) correlated to dysphagia severity at 3- and 6-month follow-up: Multivariate analysis.
| Parameter | Dysphagia Grade 0–2 | Dysphagia Grade 3 | Δ Mean | p | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | Mean | SE | n | Mean | SE | |||
| Blood flow | ||||||||
| 3-mo follow-up | ||||||||
| Crude | 10 | 43.0 | 38.8 | 5 | 130.5 | 54.8 | −87.5 | 0.083 |
| Adjusted for pack-years | 10 | 40.2 | 40.4 | 5 | 136.1 | 57.7 | −95.8 | 0.098 |
| Adjusted for GTV-tumor – max | 10 | 43.3 | 40.4 | 5 | 129.9 | 57.2 | −86.5 | 0.096 |
| Adjusted for baseline dysphagia | 10 | 31.4 | 38.1 | 5 | 153.7 | 55.1 | −122.2 | 0.043 |
| 6-mo follow-up | ||||||||
| Crude | 9 | 12.3 | 34.6 | 6 | 161.9 | 42.4 | −149.6 | 0.007 |
| Adjusted for pack-years | 9 | 9.7 | 35.6 | 6 | 165.9 | 43.7 | −156.1 | 0.010 |
| Adjusted for GTV-tumor – max | 9 | 12.4 | 35.9 | 6 | 161.8 | 44.0 | −149.4 | 0.010 |
| Adjusted for baseline dysphagia | 9 | 4.0 | 32.4 | 6 | 174.4 | 39.9 | −170.4 | 0.004 |
| Blood volume | ||||||||
| 3-mo follow-up | ||||||||
| Crude | 10 | 12.0 | 27.2 | 5 | 107.7 | 38.5 | −95.7 | 0.044 |
| Adjusted for pack-years | 10 | 12.1 | 28.6 | 5 | 107.4 | 40.9 | −95.3 | 0.065 |
| Adjusted for GTV-tumor – max | 10 | 11.0 | 28.0 | 5 | 109.7 | 39.7 | −98.7 | 0.039 |
| Adjusted for baseline dysphagia | 10 | 19.7 | 27.0 | 5 | 92.2 | 39.0 | −72.4 | 0.120 |
| 6-mo follow-up | ||||||||
| Crude | 9 | 8.7 | 29.1 | 6 | 96.6 | 35.6 | −87.8 | 0.039 |
| Adjusted for pack-years | 9 | 9.4 | 30.4 | 6 | 95.7 | 37.4 | −86.3 | 0.055 |
| Adjusted for GTV-tumor – max | 9 | 8.6 | 30.0 | 6 | 96.8 | 36.9 | −88.1 | 0.042 |
| Adjusted for baseline dysphagia | 9 | 15.2 | 27.7 | 6 | 86.9 | 34.1 | −71.6 | 0.067 |
| Capillary permeability | ||||||||
| 3-mo follow-up | ||||||||
| Crude | 9 | 31.6 | 33.8 | 4 | −48.3 | 50.6 | 79.9 | 0.090 |
| Adjusted for pack-years | 9 | 33.0 | 35.6 | 4 | −51.4 | 54.0 | 84.4 | 0.072 |
| Adjusted for GTV-tumor – max | 9 | 27.0 | 32.0 | 4 | −38.0 | 48.3 | 64.9 | 0.126 |
| Adjusted for baseline dysphagia | 9 | 31.6 | 35.6 | 4 | −48.2 | 53.8 | 79.8 | 0.132 |
| 6-mo follow-up | ||||||||
| Crude | 8 | 43.1 | 34.3 | 5 | −50.7 | 43.4 | 93.8 | 0.062 |
| Adjusted for pack-years | 8 | 44.0 | 36.0 | 5 | −52.1 | 45.7 | 96.0 | 0.058 |
| Adjusted for GTV-tumor – max | 8 | 40.8 | 31.5 | 5 | −47.0 | 39.8 | 87.8 | 0.066 |
| Adjusted for baseline dysphagia | 8 | 42.9 | 35.9 | 5 | −50.4 | 45.5 | 93.3 | 0.068 |
GTV-tumor – max is defined in Table 1 footnote. Δ Mean is the difference between mean percentage change from baseline to Week 2 of radiotherapy for Grade 0–2 and Grade 3 dysphagia patients.
Discussion
To our knowledge, the present study is the first to show a correlation between CTP changes during RT in the PCM in HNC patients and posttreatment dysphagia severity. Serial CTP imaging of the PCM demonstrated increases in BF of 130% to 160% of baseline values and increase in BV of 96% to 108% of baseline values in the second week of RT, which correlated with Grade 3 dysphagia, especially at 3 and 6 months, whereas decreases in CP during RT relative to baseline correlates with Grade 3 dysphagia at 9–12 months.
Multiple studies have demonstrated a dose–response relationship between radiation exposure to the PCM and the severity of radiation-induced dysphagia (6, 17, 18). Variations in individual sensitivity make it difficult to determine which patients will experience severe dysphagia, even when efforts are made to optimize reduction in normal tissue exposure with radiation techniques including pharyngeal-sparing IMRT.
Radiation-induced dysphagia is thought to be related to radiation-induced free radical damage to the PCM and the subsequent development of fibrosis of the PCM, with stricture formation and loss of pliability of the muscle. The variation in perfusion changes in PCM between Grade 3 dysphagia patients and Grade 0–2 dysphagia patients may represent differences in sensitivity of the PCM microvasculature to radiation-induced injury.
Progressive increases in perfusion changes in the PCM during RT support currently proposed hypotheses that oxidative stressors and microvascular injury to the endothelium are etiologic factors of radiation toxicity in normal tissues (19, 20). Extrapolating from these studies, it may be possible that endothelial injury to the PCM may be reflected in BV and BF changes on CTP imaging. Patients who do not demonstrate significant increases in BF and BV in the PCM early during RT may be more radioresistant to underlying endothelial injury of the normal tissue. This may be part of the mechanism contributing to the development of PCM injury resulting in severe dysphagia after treatment.
The relationship between CP changes and dysphagia was unclear in our study. Decrease in CP seemed to correlate with dysphagia at longer follow-up, and the pattern of change in CP did not follow changes in BF and BV. These findings are in contrast to published tumor responses to RT, which generally shows that increases in BF and BV are often coupled with increased CP (13, 21).
The perfusion changes in the PCM and severity of dysphagia may also be influenced by patient- and tumor-related factors, such as a greater number of pack-years of smoking, advanced stage, a larger GTV, and baseline dysphagia status resulting from the tumor mass. A larger GTV implies that a larger volume of normal tissue (encompassed by the PTV) surrounding the tumor receives the full prescribed RT dose and should predict a greater probability of toxicity and severity from a dose and volumetric standpoint. Therefore, in our study, when examining changes in perfusion of the PCM, we adjusted for size of the tumor volume of the primary and nodal volumes.
In the present study, the value of CTP imaging of the PCM at Weeks 4 and 6 during RT and 6 weeks after RT did not show a statistical correlation with predicting the likelihood of Grade 3 dysphagia. It is possible that we were unable to find a pattern owing to small sample size and the decreasing number of scans performed at the later time points. However, increases in BF and BV were higher at all CTP time points in the Grade 3 dysphagia group when compared with the Grade 0–2 dysphagia group (Figs. 2 and 3).
Studies examining CTP imaging have focused on measuring perfusion parameters of the tumor as a predictor of local control of the tumor, and the value of perfusion parameters in the normal tissue has not been widely studied (21, 22). Studies examining radiation-induced dysphagia have focused on posttreatment anatomic changes using videofluoroscopic (2) or endoscopic evaluation and MR imaging (23); or radiation-induced dysphagia has been studied as a function of planned radiation dose using dose–volume histogram analysis of CT-contoured normal tissue volumes, including the larynx (24) and inferior, middle, and superior pharyngeal constrictor muscles (4, 5).
Herein, we demonstrate acute perfusion changes occurring in the PCM during the course of RT and that these observed changes, particularly increase in BF and BV during Week 2 of RT, correlate with severity of RT-induced dysphagia at 3–6 months after RT. However, the ability of intratreatment CT perfusion changes to predict dysphagia beyond 6 months was not demonstrated in the present study. Reasons for lack of correlation at longer follow-up may be that other interventions after RT, such as swallowing rehabilitation, may alter the incidence of long-term toxicity (8). Longterm dysphagia after RT may be worsened by other patient factors, such as age, gender, race, medical comorbidities (e.g., diabetes, stroke, neurological conditions), or patient habits (e.g., continued smoking or alcohol consumption) and not solely dependent upon RT-related factors. To address some of these factors, we performed an adjusted analysis to account for potential individual confounders, including baseline dysphagia status, smoking history, and tumor volume; this analysis largely showed a similar statistical trend in changes in BF, BV, and CP of the PCM and dysphagia status at 3 and 6 months. Limitations of this study include small sample size, which did not permit a more complex multivariate analysis to test multiple potential confounders.
Although there are no other confirmatory studies examining perfusion imaging for pharyngeal constrictor muscles, Dornfeld et al. (25) used fluorodeoxyglucose–positron emission tomography to study posttreatment uptake in the supra-glottis and glottis larynx and found a correlation with decreased quality of life after RT using a head-and-neck cancer inventory questionnaire. In a study by Popovtzer et al. (23), anatomic changes using MRI were compared with the radiation dose plans to determine a dose–effect relationship; in this study, thickening of the PCM and overlying mucosa was observed at 3 months after RT, and the proposed etiology was acute mucositis and underlying inflammation and edema of the PCM. The inflammatory changes in the PCM seen on MRI seem consistent with the changes in BF and BV during RT seen in our study.
A limitation of CTP measurements of the PCM is that only a limited portion of the PCM (within the region of interest, at the same axial level of the tumor) could be measured in our scan owing to the 4-cm limit of the scan. The pharyngeal constrictors represent a long craniocaudal anatomic structure, and perfusion measurements of different areas of the PCM were not done in our study.
In our study, a single measurement of the PCM was made at the same axial level adjacent to tumor; the dose received by the PCM was assumed to be the prescription dose. We were not able to determine a relationship between dose and volume statistics of the PCM and perfusion parameters to dysphagia.
Different studies implicate different regions of the PCM in dysphagia severity. In a study by Eisbruch et al. (5), injury to the middle and superior constrictors, as well as the larynx and supraglottic larynx, were hypothesized to be the main underlying etiology for severe dysphagia, whereas in other studies the inferior constrictors and laryngeal structures have been studied as the main target for injury (24). Despite different anatomic targets being implicated in dysphagia secondary to RT, most studies recognize that there are multiple etiologic factors, including dosimetric (4, 6), patient-, and tumor-related factors, that contribute to the incidence and severity of swallowing complications after treatment.
A limitation of using the CTCAE v 3.0 grading system to measure severity of dysphagia after treatment (in which Grade 3 dysphagia is measured by inadequate oral intake, the use of i.v. fluids, or nutrition through tube feedings) is that all endpoints are secondary endpoints as a consequence of swallowing dysfunction rather than direct measurements of dysphagia. When using the CTCAE v. 3.0 to grade toxicity, other factors may confuse dysphagia grading, such as mucositis, dental extractions before starting RT, and dysgeusia. Hence, evaluation of dysphagia during RT was not studied, to exclude acute effects such as mucositis during treatment.
In conclusion, serial CTP imaging of the PCM demonstrates increases in BF and BV during the course of RT that correlate with Grade 3 dysphagia at 6 months. Evaluating CTP changes in PCM early during the course of RT may predict the likelihood of RT-related dysphagia and severity after treatment. Larger studies are needed to confirm our findings.
Acknowledgments
This study was supported by Grant No. IRG-72-001-32-IRG from the American Cancer Society.
Appendix 1.
Common Terminology Criteria for Adverse Events version 3, dysphagia grading system (15).
| Dysphagia Grade | Clinical description |
|---|---|
| 0 | No change from baseline |
| 1 | Symptomatic, able to eat regular diet |
| 2 | Symptomatic and altered eating/swallowing (e.g., altered dietary habits, oral supplements); i.v. fluids indicated <24 h |
| 3 | Symptomatic and severely altered eating/swallowing (e.g., inadequate oral caloric or fluid intake); i.v. fluids, tube feedings, or Total Parental Nutrition indicated ≥24 h |
| 4 | Life-threatening consequences (e.g., obstruction, perforation) |
| 5 | Death |
Footnotes
Conflict of interest: none.
References
- 1.Nguyen NP, Sallah S, Karlsson U, et al. Combined chemotherapy and radiation therapy for head and neck malignancies: Quality of life issues. Cancer. 2002;94:1131–1141. doi: 10.1002/cncr.10257. [DOI] [PubMed] [Google Scholar]
- 2.Lazarus CL, Logemann JA, Pauloski BR, et al. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106:1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen NP, Moltz CC, Frank C, et al. Dysphagia following chemoradiation for locally advanced head and neck cancer. Ann Oncol. 2004;15:383–388. doi: 10.1093/annonc/mdh101. [DOI] [PubMed] [Google Scholar]
- 4.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 6.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: A dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Pauloski BR. Rehabilitation of dysphagia following head and neck cancer. Phys Med Rehabil Clin N Am. 2008;19:889–928. x. doi: 10.1016/j.pmr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulbersh BD, Rosenthal EL, McGrew BM, et al. Pretreatment, preoperative swallowing exercises may improve dysphagia quality of life. Laryngoscope. 2006;116:883–886. doi: 10.1097/01.mlg.0000217278.96901.fc. [DOI] [PubMed] [Google Scholar]
- 9.Bhide SA, Gulliford S, Kazi R, et al. Correlation between dose to the pharyngeal constrictors and patient quality of life and late dysphagia following chemo-IMRT for head and neck cancer. Radiother Oncol. 2009;93:539–544. doi: 10.1016/j.radonc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi D, Hoeffner EG, Carlos RC, et al. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results J Comput Assist Tomogr. 2003;27:687–693. doi: 10.1097/00004728-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Hermans R, Meijerink M, Van den Bogaert W, et al. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–1356. doi: 10.1016/s0360-3016(03)00764-8. [DOI] [PubMed] [Google Scholar]
- 12.Rumboldt Z, Al-Okaili R, Deveikis JP. Perfusion CT for head and neck tumors: Pilot study. AJNR Am J Neuroradiol. 2005;26:1178–1185. [PMC free article] [PubMed] [Google Scholar]
- 13.Truong MT, Saito N, Wang JW, et al. Prediction of locoregional control in head and neck squamous cell carcinoma with serial CT perfusion during radiotherapy. Am J Neuroradiol. doi: 10.3174/ajnr.A2501. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miles KA. Perfusion CT for the assessment of tumour vascularity: Which protocol? Br J Radiol. 2003;76(Spec. No 1):S36–S42. doi: 10.1259/bjr/18486642. [DOI] [PubMed] [Google Scholar]
- 15.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 16.American Joint Committee on Cancer Cancer staging manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 17.Caudell JJ, Schaner PE, Desmond RA, et al. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2010;76:403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Eisbruch A, Levendag PC, Feng FY, et al. Can IMRT or brachytherapy reduce dysphagia associated with chemoradiotherapy of head and neck cancer? The Michigan and Rotterdam experiences. Int J Radiat Oncol Biol Phys. 2007;69:S40–S42. doi: 10.1016/j.ijrobp.2007.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec. No 1):S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 20.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 21.Surlan-Popovic K, Bisdas S, Rumboldt Z, et al. Changes in perfusion CT of advanced squamous cell carcinoma of the head and neck treated during the course of concomitant chemoradiotherapy. AJNR Am J Neuroradiol. 2010;31:570–575. doi: 10.3174/ajnr.A1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bisdas S, Rumboldt Z, Surlan-Popovic K, et al. Perfusion CT in squamous cell carcinoma of the upper aerodigestive tract: Long-term predictive value of baseline perfusion CT measurements. AJNR Am J Neuroradiol. 2010;31:576–581. doi: 10.3174/ajnr.A1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popovtzer A, Cao Y, Feng FY, et al. Anatomical changes in the pharyngeal constrictors after chemo-irradiation of head and neck cancer and their dose-effect relationships: MRI-based study. Radiother Oncol. 2009;93:510–515. doi: 10.1016/j.radonc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caglar HB, Tishler RB, Othus M, et al. Dose to larynx predicts for swallowing complications after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1110–1118. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 25.Dornfeld K, Hopkins S, Simmons J, et al. Posttreatment FDGPET uptake in the supraglottic and glottic larynx correlates with decreased quality of life after chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:386–392. doi: 10.1016/j.ijrobp.2007.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]