Abstract
Background and Objectives
Identifying predictors of early drop out from outpatient treatment of opioid use disorder (OUD) with buprenorphine/naloxone (BN) may improve care for subgroups requiring more intensive engagement to achieve stabilization. However, previous research on predictors of dropout among this population has yielded mixed results. The aim of the present study was to elucidate these mixed findings by simultaneously evaluating a range of putative risk factors that may predict dropout in BN maintenance treatment.
Methods
Outpatient medical records and weekly supervised urine toxicology results were retrospectively reviewed for patients at two community psychiatric clinics (n=202): a private hospital clinic (n=84) and a federally qualified health center (n=118). A forward stepwise logistic regression was utilized to investigate the association between early dropout (i.e., discontinuing treatment or buprenorphine non-adherence within the first three months of clinic entry) and extracted sociodemographic, clinical, substance use, and treatment history variables.
Results
Overall, 56 of 202 participants (27.7%) dropped out of treatment. The multivariable analysis indicated that age under 25 (B=1.47, SEB=.52, p <.01) and opioid use in month 1 (B=1.50, SEB=.41, p<.001) were significantly associated with early dropout; those with a history of suicide attempt were significantly less likely to drop out (B=−1.44, SEB = .67, p<.05).
Conclusions and Scientific Significance
Consistent with previous research, younger age and use of opioids during the first month of treatment predicted early dropout. Having a history of prior suicide attempt was associated with 3-month BN treatment retention, which has not been previously reported.
1 Introduction
Outpatient buprenorphine/naloxone (BN) maintenance can play an essential role in recovery from opioid use disorder (OUD).1, 2 Longer periods of maintenance are increasingly favored over BN-assisted withdrawal, given relapse rates of over 90% following withdrawal, even following 12-weeks of treatment.3, 4 In contrast, abstinence with continuous BN treatment approaches 50% at one year.5, 6 Beyond promoting reductions in illicit opioid use, BN maintenance is associated with increases in quality of life,7, 8 and reductions in mortality,9, 10 HIV and hepatitis C risk behaviors,9, 11 criminal activity,12 and overall healthcare costs.13 Treatment program retention among those receiving BN maintenance has also been associated with reduced risk of relapse,14, 15 and lower likelihood of overdose.14 The array of positive outcomes associated with BN maintenance highlights the importance of retention in BN treatment programs. However, estimates suggest that up to 40–50% of patients will discontinue treatment prematurely (e.g., within 6 months of entering treatment), with most of this subgroup discontinuing treatment within the first month following induction.16–18 Identification of risk factors that predict treatment dropout may allow providers to promote retention among higher risk patients through proactive detection and intervention.
A number of studies have investigated predictors of treatment dropout in this population, with mixed results. Previous studies have identified younger age,16, 19 unemployment,5, 18, 20 lower BN dose,16, 21 and a criminal history20 as predictors of treatment dropout. Conversely, other investigations have found no effect of age,18, 22, 23 employment,17 criminal history,24 and BN dose22, 23 on dropout. Complicating matters further, it appears that certain patient characteristics predict both positive and negative outcomes in different studies, exemplified by comorbid substance use (particularly cocaine use and dependence), which has predicted treatment retention in certain studies,17 and early treatment dropout in others.16, 19
These mixed findings may be attributable to variability across studies in the predictors included. Given the potential overlap among putative risk factors, it is critical to control for relevant covariates in order to understand the relative contribution of these variables. Although early treatment response is thought to be a reliable predictor of reduced illicit opioid use following BN maintenance treatment,25, 26 few studies of BN retention have examined poor early treatment response as a predictor of dropout. A secondary analysis examining predictors of treatment retention in a clinical trial of BN maintenance for adolescents with OUD reported decreased dropout rates among early treatment responders (i.e., opioid-negative urine drug screens in weeks 1 and 2).27 A small (N=41) study of adults receiving BN maintenance in primary care settings found that poor early treatment response was associated with dropout from treatment; however, this study did not control for the effect of previously identified predictors, such as age.18 Taken together, these results suggest that poor early treatment response predicts treatment dropout; however, it is not clear whether this relationship remains significant when controlling for other potential risk factors.
The overarching aim of this study was to attempt to elucidate the mixed findings on predictors of dropout from BN maintenance treatment using a large, naturalistic sample of patients with OUD treated in an outpatient integrated psychiatry BN program. Specifically, our aim was to simultaneously evaluate a range of putative risk factors identified in previous studies (e.g., sociodemographic, substance use, clinical, and treatment) to understand the relative contribution of these variables as predictors of dropout. Given the mixed results in the published literature, we conducted an exploratory analysis to determine the relative contribution of these putative risk factors when controlling for the range of pertinent covariates.
2 Methods
A sequential, retrospective chart review was conducted for patients from two outpatient BN treatment programs: a private, academic psychiatric hospital and a federally-qualified health center with integrated community mental health services. At both treatment centers, program staff extracted data from medical records into a de-identified, aggregated database for the purpose of an internal quality improvement analysis. The local Institutional Review Board provided approval for the use of this quality improvement data in the current report.
2.1 Participants
Participants were: 1) 84 participants from a private academic psychiatric hospital initially hospitalized for OUD treatment between 2006–2013 and discharged within the hospital continuum of care to the outpatient BN treatment program, which pairs BN stabilization/maintenance with psychiatric treatment in a weekly integrated group therapy model of care, and 2) 118 participants treated between 2008 – 2014 at a federally-qualified health center serving an outpatient community mental health population treated in a community adaptation of the previously described model.
At both sites, participants completed structured clinical interviews including a comprehensive mental health/substance use disorder evaluation at the time of inpatient admission and at the point of entry into the outpatient BN treatment program. Both programs are set up for clinic-based buprenorphine treatment only and do not provide methadone maintenance services. The majority of patients (79.7%) had opioid use disorder and at least one co-occurring psychiatric disorder, while 20.3% had opioid use disorder with or without co-occurring other substance use disorder but no other psychiatric disorder. At both treatment settings, the stabilization period (month 1–3) required weekly, supervised urine toxicology with quantitative results and expanded narcotic panel, including buprenorphine/naloxone. Patients also attended weekly group therapy for 3–6 months with monthly urine toxicology and continuing group therapy for stabilized patients thereafter. Group leaders also met individually with patients to prescribe BN and other psychiatric medications. BN prescription refills were provided weekly during the initial 3 month stabilization period, then biweekly for 3 months, then monthly thereafter, and the frequency of refills was reset to the stabilization phase based on clinician judgement. Per clinic protocol at both sites, BN was flexibly dosed with the majority of patients receiving 16mg, and the remainder receiving between 8–24mg based on the need for adequate control of opioid withdrawal symptoms and extent of craving among individual patients.
2.2 Measures
Chart review included assessment of the intake evaluation supplemented by the electronic medical record (EMR), as well as assessment of progress notes to obtain information about reason for dropout (successful transfer to another treatment program was not considered to be a dropout). Medical records lacking complete outpatient intake assessments were excluded if they could not be supplemented by the EMR for the same treatment episode. We extracted sociodemographic, clinical, and treatment history variables from the chart, as well as substance use during the first 3 months of treatment. History of suicide attempt was extracted from the initial evaluation; a positive response was generated by a self-report of a prior suicide attempt, understood by the evaluator to mean at least one prior nonfatal self-injury behavior with intent to die.
Directly supervised urine collection for toxicology was obtained in clinic weekly with quantitative (GC/MS confirmed) drug panel (amphetamine, benzodiazepine, cannabinoid, cocaine, MDMA) and opioid panel (fentanyl, meperidine, opiates, oxycodone, methadone, tramadol, 6-acetylmorphine, buprenorphine, norbuprenorphine) testing.
2.3 Data analysis
Early dropout was defined as discontinuing treatment within the first 3 months of clinic entry, or failing to provide at least two BN positive urine samples per month during the first 3 months of treatment. There were 2 cases of patients unable to produce sufficient urine samples but were clinically documented as retained in treatment. In 8 cases, a well-documented, arranged transfer of care to another outpatient provider in the community during the initial 3-month treatment period, in the absence of relapse, was not considered to be early dropout.
Potential predictors of dropout were selected based on a review of other studies of buprenorphine-treated OUD reporting treatment dropout variables. All variables were evaluated for skewness and univariate outliers to determine the appropriate statistical approach. The association between these variables and early dropout were first examined in a series of bivariate analyses, using independent samples t-tests and chi-square analyses. Then, all of the predictors of interest were entered into a logistic regression with early dropout as the dependent variable. The multivariable model allowed for the examination of predictors of dropout while controlling for potential confounding variables (e.g., age and duration of opioid use). Due to the large number of potential predictors we utilized a forward stepwise regression to identify significant predictors of dropout, while mitigating the risk of collinearity.
3 Results
The sample included in this analysis reported a mean age of 41 years (SD=12.4), with 17% of the sample under the age of 25; 37% of participants were female, and 60% were currently unemployed. The sample was highly diverse with respect to race and ethnicity, with 38% self-reporting race as Caucasian, 24% as African American, and 38% as Latino. See Table 1.
Table 1.
Sample Characteristics by Dropout Status at Month-3
| Variable | Full sample | Retained | Early drop-out | t/χ2* | p |
|---|---|---|---|---|---|
| (N=202) | (n=146) | (n=56) | |||
| Demographics | |||||
| Age, mean (SD) | 40.8 (12.4) | 42.2 (11.6) | 37.3 (13.5) | 2.43 | 0.02 |
| Caucasian, % | 37.1% | 36.3% | 39.3% | 5.09 | 0.08 |
| Female, % | 37.1% | 34.2% | 44.6% | 1.87 | 0.17 |
| Employed, % | 37.4% | 41.7% | 25.9% | 4.66 | 0.03 |
| Married, % | 32.7% | 33.3% | 30.9% | 0.11 | 0.74 |
| Parental status, % with children | 70.5% | 71.2% | 68.2% | 0.15 | 0.70 |
| Housing status, % domiciled | 90.1% | 89.7% | 91.1% | 0.08 | 0.77 |
| Education, % high school or more | 53.5% | 52.7% | 55.4% | 0.11 | 0.74 |
| Legal problems, % | 65.6% | 63.0% | 72.5% | 1.49 | 0.22 |
| Substance use characteristics | |||||
| Prescription opioid use only, % | 16.7% | 16.7% | 16.7% | 0.00 | 1.00 |
| IV drug use, % | 78.3% | 79.2% | 75.9% | 0.24 | 0.62 |
| Prior opioid agonist treatment, % | 69.1% | 75.0% | 53.8% | 7.89 | <0.01 |
| 12-step participation, % | 54.6% | 58.9% | 41.0% | 3.81 | 0.05 |
| 12-step sponsor, % | 13.9% | 13.9% | 13.9% | 0.00 | 1.00 |
| Prior detoxification, % | 83.6% | 84.9% | 80.0% | 0.71 | 0.40 |
| Other co-occurring substance use disorder, % | 68.3% | 66.4% | 73.2% | 0.86 | 0.35 |
| Clinical characteristics | |||||
| Other co-occurring psychiatric disorder, % | 79.7% | 78.8% | 82.1% | 0.29 | 0.59 |
| Chronic pain, % | 20.0% | 23.8% | 9.6% | 4.78 | 0.03 |
| History of suicide attempt, % | 22.4% | 28.2% | 7.4% | 9.69 | <0.01 |
Chi-squared and t-tests compared those that were retained vs. those that dropped out at Month-3
Note: Percentages reported in the table do not include missing values, missing data points ranged from 0–28.7% for the variables reported.
There were 28 dropouts at the hospital site (33.3%) and 28 at the community site (23.7%) for a total dropout rate of 56 of 202 participants (27.7%). This included 23 dropouts prior to Month 2, and 35 dropouts prior to the end of Month 3. The mean duration of OUD was 18.7 years (SD=12.0); this did not significantly differ between early dropouts (19.2 years; SD=11.6) and retained patients (17.5 years; SD=13.1). Among those older than 25, the mean duration of opioid use was 21.5 years (SD=11.1), whereas those under 25 had a shorter duration of opioid use (4.4 years; SD=2.4).
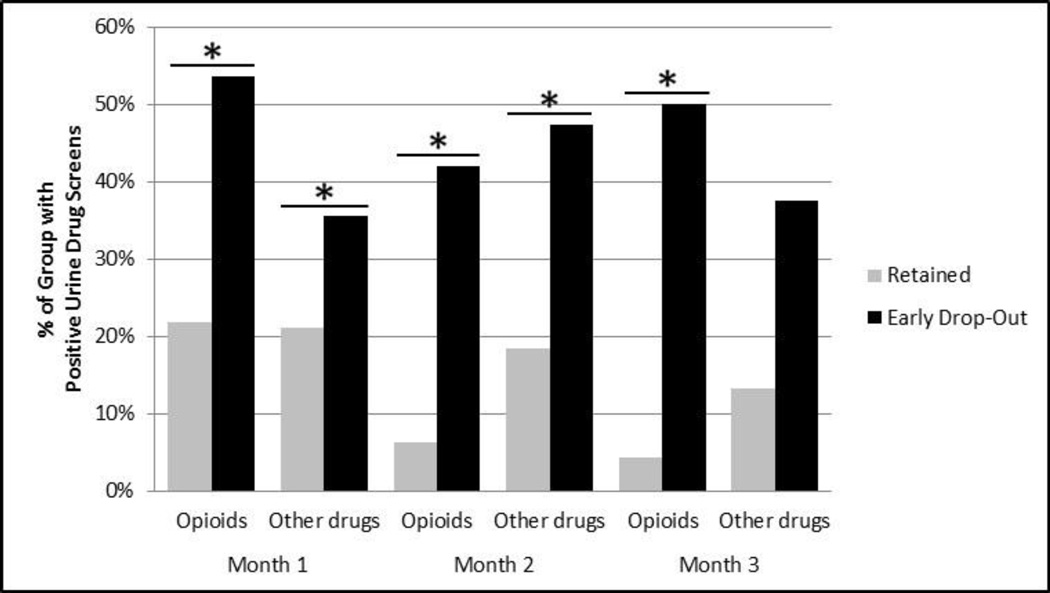
Early dropouts were significantly more likely to test positive for both opioids and other drugs at Month 1 (χ2=19.06, p<.001 for opioids; χ2=4.50, p<.05 for other substances), and were more likely to test positive for opioids at Month 2 (χ2=31.79, p<.001 for opioids) relative to those who did not drop-out. See Figure 1. Early treatment response was evenly distributed between age groups with 32% of those under 25 and 30% of those 25 and older using opioids in the first month of treatment.
Figure 1. Percent Positive Urine Drug Screens by Dropout Status.
* Results are statistically significant at p <.05.
Note: % Positive for opioids for a given month represents percent of subjects with at least one urine positive for opioids. % Positive for other drugs for a given month represents percent of subjects with at least one urine positive for cocaine, cannabis or non-prescribed benzodiazepine or amphetamine. Percentages reported in the table do not include missing urine drug screens.
In preliminary bivariate analyses, the following variables were associated with early dropout: younger age, unemployment, absence of chronic pain, absence of prior suicide attempt, first time receiving opioid agonist treatment, and opioid use at month 1 (see Table 1). In the community cohort, where data on mutual-help involvement were consistently recorded, 12-Step participation was associated with lower likelihood of dropout (χ2 = 6.51, p<.05).
The multivariable analysis indicated that age under 25 (B=1.47, SEB=.52, p <.01; OR=4.33, 95% CI=1.56, 12.00) and opioid use in month 1 (B=1.50, SEB=.41, p<.001, OR=4.48, 95% CI=2.12, 9.96) were significantly associated with early dropout; those with a history of suicide attempt were significantly less likely to dropout (B=−1.44, SEB = .67, p<.05, OR=0.23, 95% CI=0.06, 0.86). No other variables were significantly associated with early dropout in the model. See Table 2.
Table 2.
Forward Stepwise Logistic Regression Predicting Treatment Dropout at or before Month-3
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Under 25 years old | 4.33 | (1.56–12.00) | 0.005 |
| History of a suicide attempt | 0.24 | (0.06–0.86) | 0.029 |
| Opioid use in Month-1* | 4.48 | (2.01–9.96) | <0.001 |
Determined by at least one urine drug screen positive for opioids in Month 1
3 Discussion
Consistent with prior studies, a range of variables were associated with early dropout from buprenorphine maintenance treatment in this study. When considering all putative risk factors together in a multivariable analysis, age under 25 years, and opioid use in the first month of treatment were each independently associated with early dropout from treatment; history of suicide attempt was associated with reduced likelihood of early dropout. In addition to these variables, being unemployed, absence of chronic pain, and first time receiving opioid agonist treatment (and lack of 12-Step participation in the community cohort) were associated with early dropout in the bivariate analysis. It seems that the effects of these latter variables were modest, and better accounted for by the contribution of age, early treatment response, and suicide attempt history. This highlights the importance of considering key confounding variables and covariates when evaluating risk factors for dropout. Variability across studies in the use of bivariate vs. multivariate approaches and in the breadth of potential risk factors considered may account for inconsistent signals in the literature on dropout from BN treatment.
Lack of early treatment response, operationalized as opioid use in month 1, was strongly associated with early dropout. Participants who used opioids in the first month of treatment were nearly 4.5 times more likely to drop out of treatment. This finding is consistent with the literature that has linked early opioid-positive urine toxicology to lack of BN treatment success. In the largest clinical trial of prescription opioid dependence conducted to date, opioid use in weeks 1 and 2 had a 94% negative predictive value for a successful outcome (defined as total abstinence in weeks 9–12 of treatment).25 Those who do not respond quickly to buprenorphine stabilization may require additional monitoring and intervention to facilitate treatment retention and good clinical outcomes. Specifically, for those who do continue to use opioids in the first month of treatment, additional intervention and monitoring is indicated, because a delayed response to treatment is unlikely for the majority of those patients who continue to use while on buprenorphine.
Younger age (i.e. age under 25 years) was a strong predictor of early dropout in this study, quadrupling the risk of early dropout. This finding is consistent with other studies that found an association between older age and treatment success, including reduced illicit opioid use5, 28, 29 and retention.16 Although, some studies have not found an association between age and either retention or abstinence,17 when subjects were grouped dichotomously by emerging adult status (age 18–25) versus older adult status (>25) in one cohort, younger age was a robust predictor of dropout at both 3 and 12 months.19 In sum, targeting emerging adults and those who do not respond early to BN treatment for increased supervision and intervention is increasingly supported by the literature, and our study supports this trend.
Our finding that a history of suicide attempt was associated with a lower rate of dropout is noteworthy given the enduring vulnerability of the OUD population to suicidal thoughts and behavior. Estimates of lifetime prevalence of suicide attempts among those with OUD range from 17.8% to 48%,30, 31 and despite treatment enrollment, suicidal thoughts and behaviors (i.e., suicidal ideation, suicide planning and suicide attempts) among those with OUD persist out to 11 years following treatment.31, 32 One important implication of this finding is that patients reporting a clear history of prior suicidal behavior and seeking BN treatment for OUD do not appear to require a different medical approach to stabilization (e.g. methadone maintenance or BN in an opioid treatment program setting), if all other patient factors suggest sufficient stability to engage well in an appropriate outpatient BN treatment setting.
Despite the need for replication, it is interesting to speculate about a theoretical basis for the association between history of suicidal behavior and lower dropout, especially given previous findings that depression may be associated with BN treatment success,22, 28 and treatment retention.22 Several authors have suggested that the putative antidepressant effects of buprenorphine could partially explain these findings.28, 33 Consistent with this hypothesis, two recent randomized trials found an acute mood improvement with low-to-medium dose and ultra-low-dose buprenorphine in small cohorts of patients with treatment-resistant depression and severe suicidal ideation, respectively.34, 35 Of note, neither cohort included patients with substance use disorders. Alternatively, patients with prior suicidal behavior may be more experienced with mental health treatment, and thus more comfortable adhering to BN treatment therapies.
The primary limitations of this naturalistic, retrospective chart review are the absence of structured diagnostic interviews, the lack of information on specific BN dose at given time points, and some missing data from individual charts. As noted, BN was flexibly dosed with the majority of patients receiving 16mg, and the remainder receiving 8–24mg based on the need for adequate control of opioid withdrawal syndrome and craving among individual patients. This data may have impacted the multivariate analysis and is a significant limitation of our study. Prior studies have yielded mixed results with respect to the association between BN dose and treatment retention, suggesting that dose is not consistently related to retention in BN treatment.16, 21–23 Nonetheless, inclusion of BN dose will be important in future studies. One potential reason for mixed findings with respect to BN dose is the potential confound of disorder severity or early treatment response. Particularly in naturalistic settings with flexible dosing procedures--such as those in the current study--prescribers may dose more aggressively in response to poorer interim outcomes, which may then result in the confound of severity/treatment response impacting and obscuring correlations between dose and outcome. This potential confound further highlights the importance of considering early treatment response as a covariate in these analyses.
Data were extracted from charts at two different BN treatment programs, and although both programs follow a similar treatment model, the possibility of different institutional treatment effects cannot be ruled out. The absence of a main effect of site in the multivariable model somewhat mitigates this concern. Finally, the inclusion of more detailed information on co-occurring psychiatric disorders and symptoms, and on the role of therapeutic alliance, are important future directions for this line of research. The strengths of this study include demographic heterogeneity, high-quality toxicology providing objective BN adherence and outcome data, and the effectiveness of retention in two very different real-world treatment settings.
The overall retention rate in our sample of 72.3% (66.7% at the academic site and 76.3% at the community site) compared favorably with previously reported 3-month retention rates.19, 22, 26 This may be partly attributable to program efforts to optimize retention, including integrated mental health and SUD care, outreach to patients who had missed group meetings, social services support within the federally-qualified health center model, and an abstinence-based model of care that encourages group members to support each other’s efforts in achieving full abstinence.
The results of this study suggest that despite use of such strategies to maximize retention, younger patients and those who do not respond quickly to BN treatment remain at substantially higher risk of early dropout. The variables that emerged in the multivariable analysis include both pre-treatment (age and history of suicide attempt) and treatment-related (early treatment response) factors. As such, these data allow for the identification of those at risk of drop-out prior to beginning treatment, as well as those who are at risk due to poor early treatment response. Interventions targeted to these subgroups – e.g., intensive case management to assist with housing and vocational difficulties, supportive employment, contingency reinforcers, more intensive family education and support, and enhanced access to residential treatment and/or sober housing supports – may be needed to improve retention in treatment and successful recovery from OUD.
Acknowledgments
K23DA035297: National Institute on Drug Abuse, Bethesda, MD R. Kathryn McHugh, PhD.
In appreciation of support provided by The Eleanor and Miles Shore Fellowship award (Harvard Medical School) to HSC which contributed to the development of this integrated treatment model.
Patricia Murphy and Renee Swan, McLean Medical Records
Footnotes
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Fudala PJ, Bridge TP, Herbert S, et al. Office-Based Treatment of Opiate Addiction with a Sublingual-Tablet Formulation of Buprenorphine and Naloxone. N Engl J Med. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 2.Ling W, Amass L, Shoptaw S, et al. A multi-center randomized trial of buprenorphine–naloxone versus clonidine for opioid, detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woody GE, Poole SA, Subramaniam G, et al. Extended vs Short-term Buprenorphine-Naloxone for Treatment of Opioid-Addicted Youth: A Randomized Trial. JAMA. 2008;300(17):2003–2011. doi: 10.1001/jama.2008.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiellin DA, Moore BA, Sullivan LE, et al. Long-term treatment with buprenorphine/naloxone in primary care: results at 2–5 years. Am J Addict. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 7.Ponizovsky AM, Grinshpoon A. Quality of life among heroin users on buprenorphine versus methadone maintenance. Am J Drug Alcohol Abuse. 2007;33(5):631–642. doi: 10.1080/00952990701523698. [DOI] [PubMed] [Google Scholar]
- 8.Raisch DW, Campbell HM, Garnand DA, et al. Health-related quality of life changes associated with buprenorphine treatment for opioid dependence. Qual Life Res. 2012;21(7):1177–1183. doi: 10.1007/s11136-011-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 10.Kimber J, Larney S, Hickman M, Randall D, Degenhardt L. Mortality risk of opioid substitution therapy with methadone versus buprenorphine: a retrospective cohort study. Lancet Psychiatry. 2015;2(10):901–908. doi: 10.1016/S2215-0366(15)00366-1. [DOI] [PubMed] [Google Scholar]
- 11.Edelman EJ, Chantarat T, Caffrey S, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug Alcohol Depend. 2014;139:79–85. doi: 10.1016/j.drugalcdep.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon MS, Kinlock TW, Schwartz RP, Fitzgerald TT, O’Grady KE, Vocci FJ. A randomized controlled trial of prison-initiated buprenorphine: prison outcomes and community treatment entry. Drug Alcohol Depend. 2014;142:33–40. doi: 10.1016/j.drugalcdep.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch FL, McCarty D, Mertens J, et al. Costs of care for persons with opioid dependence in commercial integrated health systems. Addict Sci Clin Pract. 2014;9(1):1. doi: 10.1186/1940-0640-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clausen T, Anchersen K, Waal H. Mortality prior to, during and after opioid maintenance treatment (OMT): a national prospective cross-registry study. Drug Alcohol Depend. 2008;94(1):151–157. doi: 10.1016/j.drugalcdep.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz R, Zelenev A, Bruce RD, Altice FL. Retention on buprenorphine treatment reduces emergency department utilization, but not hospitalization, among treatment-seeking patients with opioid dependence. J Subst Abuse Treat. 2012;43(4):451–457. doi: 10.1016/j.jsat.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hser Y-I, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: Outcomes at 1 year. J Subst Abuse Treat. 2009;37(4):426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Stein MD, Cioe P, Friedmann PD. Buprenorphine retention in primary care. J Gen Intern Med. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuman-Olivier Z, Weiss RD, Hoeppner BB, Borodovsky J, Albanese MJ. Emerging adult age status predicts poor buprenorphine treatment retention. J Subst Abuse Treat. 2014;47(3):202–212. doi: 10.1016/j.jsat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillhouse M, Canamar CP, Ling W. Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat. 2013;44(3):336–342. doi: 10.1016/j.jsat.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Accurso AJ, Rastegar DA. The Effect of a Payer-Mandated Decrease in Buprenorphine Dose on Aberrant Drug Tests and Treatment Retention Among Patients with Opioid Dependence. J Subst Abuse Treat. 2016;61:74–79. doi: 10.1016/j.jsat.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Gerra G, Borella F, Zaimovic A, et al. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75(1):37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- 24.Wang EA, Moore BA, Sullivan LE, Fiellin DA. Effect of incarceration history on outcomes of primary care office-based buprenorphine/naloxone. J Gen Intern Med. 2010;25(7):670–674. doi: 10.1007/s11606-010-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott KA, Griffin ML, Connery HS, et al. Initial response as a predictor of 12-week buprenorphine-naloxone treatment response in a prescription opioid–dependent population. J Clin Psychiatry. 2014;76(2):1–478. doi: 10.4088/JCP.14m09096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam GA, Warden D, Minhajuddin A, et al. Predictors of abstinence: National Institute of Drug Abuse multisite buprenorphine/naloxone treatment trial in opioid-dependent youth. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1120–1128. doi: 10.1016/j.jaac.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warden D, Subramaniam GA, Carmody T, et al. Predictors of attrition with buprenorphine/naloxone treatment in opioid dependent youth. Addict Behav. 2012;37(9):1046–1053. doi: 10.1016/j.addbeh.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreifuss JA, Griffin ML, Frost K, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug Alcohol Depend. 2013;131(1):112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating opioid addiction with buprenorphine-naloxone in community-based primary care settings. Ann Fam Med. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen VC-H, Lin T-Y, Lee CT-C, et al. Suicide attempts prior to starting methadone maintenance treatment in Taiwan. Drug Alcohol Depend. 2010;109(1):139–143. doi: 10.1016/j.drugalcdep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Darke S, Ross J, Marel C, et al. Patterns and correlates of attempted suicide amongst heroin users: 11-year follow-up of the Australian treatment outcome study cohort. Psychiatry Res. 2015;227(2):166–170. doi: 10.1016/j.psychres.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Teesson M, Ross J, Darke S, et al. One year outcomes for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS) Drug Alcohol Depend. 2006;83(2):174–180. doi: 10.1016/j.drugalcdep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Fava M, Memisoglu A, Thase ME, et al. Opioid Modulation With Buprenorphine/Samidorphan as Adjunctive Treatment for Inadequate Response to Antidepressants: A Randomized Double-Blind Placebo-Controlled Trial. [Accessed March 13, 2016];Am J Psychiatry. 2016 doi: 10.1176/appi.ajp.2015.15070921. http://ajp.psychiatryonline.org.ezp-prod1.hul.harvard.edu/doi/abs/10.1176/appi.ajp.2015.15070921. [DOI] [PubMed] [Google Scholar]
- 35.Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. [Accessed February 21, 2016];Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15040535. http://ajp.psychiatryonline.org.ezp-prod1.hul.harvard.edu/doi/pdf/10.1176/appi.ajp.2015.15040535. [DOI] [PubMed] [Google Scholar]