Abstract
Background
Catheter systems that permit targeted delivery of genes, molecules, ligands, and other agents represent an investigative tool critical to the development of clinically relevant animal models that facilitate the study of neurological health and disease. The development of new sustained catheter delivery systems to spinal and peripheral sites will reduce the need for repeated injections, while ensuring constant levels of drug in plasma and tissues.
New Method
Here, we introduce two novel catheter delivery systems in the mouse: the O’Buckley intrathecal catheter system for sustained delivery to the spinal region and a subcutaneous bifurcated catheter system for sustained drug delivery to both hindpaws.
Results
The O’Buckley intrathecal catheter system consistently distributed Evans Blue throughout the spinal cord, with the greatest concentration at the thoracic region, and with an 85% surgery success rate. The subcutaneous catheter system consistently distributed Evans Blue to the hindlimbs, with a 100% surgery success rate.
Comparison to existing method
The O’Buckley intrathecal catheter system accomplishes sustained drug delivery to the spinal region, with a 2-fold increase in surgery success rate, as compared to the traditional method. Our subcutaneous bifurcated catheter system accomplishes sustained drug delivery to both hindpaws, eliminating the need for repeated intraplantar injections.
Conclusions
We have developed catheter systems that improve upon traditional methods in order to achieve sustained localized drug delivery to spinal tissues and to hindpaw tissues surrounding peripheral sciatic nerve terminals. These methods have a broad reach, and can be used to enhance behavioral, physiologic and mechanistic studies in mice.
Keywords: bifurcated, continuous infusion, drug delivery, hindpaw, mouse, osmotic pump, peripheral, routes of administration, spinal cord, surgical methods
1. Introduction
Rodent models are an integral component of neuroscience research that allow us to better understand the processes underlying the function of central and peripheral nervous systems, as well as the hundreds of diseases that affect these systems. Catheter delivery systems, which consist of a catheter attached to an osmotic mini-pump, are commonly used in rodent models in order to achieve continuous drug infusion to a localized area. Continuous infusion of genes, small molecules, ligands, or other agents over extended periods of time is routinely used to study the underlying mechanisms of homeostatic processes, physiological systems, and behavioral phenotypes (de Yebenes et al. 1987; Oladosu et al. 2015) as well as to assess the therapeutic potential of various drugs (Kosumi et al. 2001; Yu et al. 2014). Sustained catheter drug delivery has been implemented via several different routes including intraperitoneal (Boudreau et al. 2010), intracavitary (Casazza et al. 2012), intrauterine (Gillio-Meina et al. 2009), intravenous (Wedel et al. 2014; Feng et al. 2015), intra-arterial (Feng et al. 2015), intraluminal (Sherwin et al. 2010), intracerebroventricular (Altaner et al. 2014), and intrathecal (i.t.) (Lindau et al. 2014). Repeated direct injection is still commonly used, however, for chronic delivery of drugs by several of these routes in mice, including localized delivery to (1) the intrathecal space in spinal tissue and (2) peripheral nerve terminals in hindpaw tissue.
Although commonly used, repeated injection protocols can add variability and error to experiments that require sustained drug administration to the spinal cord or hindpaws. Multiple injections, irrespective of site, can impact the psychology, physiology, and behavior of mice and, thus, introduce experimental variability. Compared to continuous infusion, repeated injections subject rodents to significant stress (Blenkinsopp and Blenkisopp 1967), which can contribute to a depressive-like state (Izumi et al. 1997). Another disadvantage of multiple injection protocols is the production of transient peaks in plasma levels of the delivered drug, versus catheter systems which provide consistent and enhanced efficiency of drug delivery (Ulrich et al. 1997). In summary, exposing rodents to multiple injections introduces extraneous variables, which can confound experimental results.
Although i.t. drug delivery catheter systems have been described and utilized in rat models to accomplish sustained drug delivery to the spinal cord (Sakura et al. 1996; Jasmin et al. 2003; Jimenez Hamann et al. 2003; Malkmus and Yaksh 2004; Luo et al. 2005; Ray et al. 2011), they have yet to be optimized for use in mice. In mice, i.t. catheterization via lumbar puncture is presently the most commonly used method (Hylden and Wilcox 1980; Liu et al. 2013). This procedure involves inserting and securing a catheter into the side of the L5-6 vertebrae and accessing the catheter for chronic injections. Although effective, this method for repeated drug administration is only functional for a limited period of time (~10 days) and requires locating the L5-6 vertebrae and incising muscle (Wu et al. 2004). An ideal i.t. catheter method in mice would access the i.t. space through the atlanto-occipital (A-O) membrane, similar to the i.t. catheter insertion performed in rats (Malkmus and Yaksh 2004). This method, however, commonly results in injury, paralysis, or fatality when used in mice. A continuous i.t. catheter delivery system, optimized for use in mice, would allow for the accurate study of sustained spinal drug delivery with reduced risk of injury, paralysis, and fatality.
Experiments involving multiple injections to the hindpaw possess several additional disadvantages, as the hindpaw region is a site of dense nerve bundles, containing both myelinated and unmyelinated axons. The localized innervation of the intraplantar (i.pl.) region makes it a key model system for the study of functional and morphological changes resulting from disease, or from drug administration (Navarro et al. 1995). For this reason, many behavioral assays; including Von Frey, Hargreaves, locomotor activity, object burying, elevated plus maze, rotor-rod and conditioned place preference tests; utilize the hindpaws of rodents. Hindpaw assessment in these and other behavioral assays can be affected by i.pl. injections which produce localized pain and inflammation (Kamala 2007). Sustained drug delivery to the hindpaw via a subcutaneous (s.c.) catheter system has been performed in rat (Culman et al. 1997; Haddad et al. 2005), however these previously described systems provide delivery only to one paw and have not been optimized for use in mice. A s.c. catheter delivery system that allows sustained and simultaneous delivery to both hindpaws, would enhance the accuracy and efficiency of studies involving hindpaw-delivered drugs and hindpaw-based phenotypes in mice.
Here, we report two novel catheter delivery systems in mice. The first accomplishes continuous drug delivery into the i.t. space via insertion into the condylar canal, reducing surgery and recovery times and achieving a two-fold increase in surgery success rate as compared to the more traditional A-O insertion site method. The second accomplishes simultaneous and sustained drug delivery to both hindpaws with a 100% success rate, improving upon the previously described s.c. catheter system and eliminating the need for i.pl. injections. These innovative techniques have the propensity to reduce experimental variability and mortality associated with existing delivery systems, thus, improving the quality of neuroscience studies that require sustained drug administration to the spinal cord or hindpaws in mice.
2. Methodology
2.1 Animals
For all experiments, male and female mice were grouped together, as no sex-dependent responses were observed. Mice were purchased from Jackson Laboratories (Bar Harbor, ME) and had either a 129Sv/C57BL/6J (N=47) or a CXB-7/ByJ (N=144) genetic background. All mice were 8–12 weeks old and weighed 20–30 grams. Mice were maintained under a 12-hour light/dark cycle, and were fed ad libitum. All procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC) and adhered to the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf) and the Committee for Research and Ethical Issues of the International Association of the Study of Pain (http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1217).
2.2 Osmotic Pump Preparation
2.2.1 Indicator Dye Preparation
To make the indicator dye solution, Evans Blue (Sigma, MO) was dissolved in 0.9% sterile saline (Hospira, IL) to create a 1% solution. For i.t. experiments, the Evans Blue solution was administered at a rate of 0.5uL/hr for a period of 7 days via 1007D Alzet osmotic mini-pumps (Durect, CA). For s.c. experiments, the Evans Blue solution was delivered at a rate of 0.25uL/hr for a period of 7 days via 1002 Alzet osmotic mini-pumps (Durect, CA). All pumps were placed in 15mL conical tubes containing sterile 0.9% saline and primed overnight in a dry heat bath (Lab Armor, OR) at 37°C. Pumps for s.c., but not i.t., experiments were attached to bifurcated (bif.) catheters prior to priming.
2.2.2 Bupivacaine Preparation
To examine the functional efficacy of s.c. bif. catheter administration, 2.5% solution of the local anesthetic bupivacaine (Hospira, IL) or 0.9% sterile saline (Hospira, IL) was administered to mice. Bupivacaine or saline was delivered via bif. catheter, at a rate of 0.5uL/hr for a period of 7 days via 1007D Alzet osmotic mini-pumps (Durect, CA).
2.3 Surgical Procedures
2.3.1 General surgical procedures
For all surgical procedures, mice were anesthetized by isoflurane (Eagle Eye Anesthesia, FL) inhalation (5% induction, 1.5–5% maintenance). Hair at incision sites was removed by shaving and/or depilatory cream (Nair, NJ) and disinfected with 70% ethanol and betadine. Sterile technique was employed throughout the duration of all procedures according to IACUC requirements. All surgeries were performed by researchers with several years of rodent survival surgery experience.
2.3.2 Traditional atlanto-occipital intrathecal catheter delivery in mouse
With the mouse in sternal recumbency, the neck of the mouse was gently bent towards the surgical table, creating a 60–70° angle between the head and the body. A small incision was made at the nape of the neck, between the ears. The muscle on either side of the external occipital crest was detached in order to expose the A-O membrane (Ray et al. 2011). The membrane was incised and the 6cm Alzet Mouse Intrathecal Catheter (Durect, CA) consisting of 2.5cm [0.23mm outer diameter (OD) x 0.09mm inner diameter (ID)], 1cm [0.76mm OD x 0.09mm ID], and 2.5cm [1.02mm OD x 0.09mm ID] polyurethane segments was gently guided into the intrathecal space with an 8cm Teflon-coated stylus (SAI Infusion Technologies, IL.) About 2.5cm of the catheter was inserted into the intrathecal space. The catheter was secured to surrounding tissues using 4-0 silk sutures (Oasis Medical, IL). Once the catheter is secured, the Teflon-coated stylus is removed and the exposed end of the catheter is attached to an osmotic mini-pump containing the 1% Evans Blue solution. The pump was placed s.c. over the right shoulder blade. Stainless steel wound clips (Braintree Scientific, MA) were used to close the incision site. Surgeries ranged from 19 to 72 minutes in duration. Surgeries were deemed “successful” if the mouse fully recovered without any paralysis or injury. If mice were injured, paralyzed, or died during or following the procedure, the surgery was deemed a “failure”.
2.3.3 O’Buckley intrathecal catheter delivery in mouse
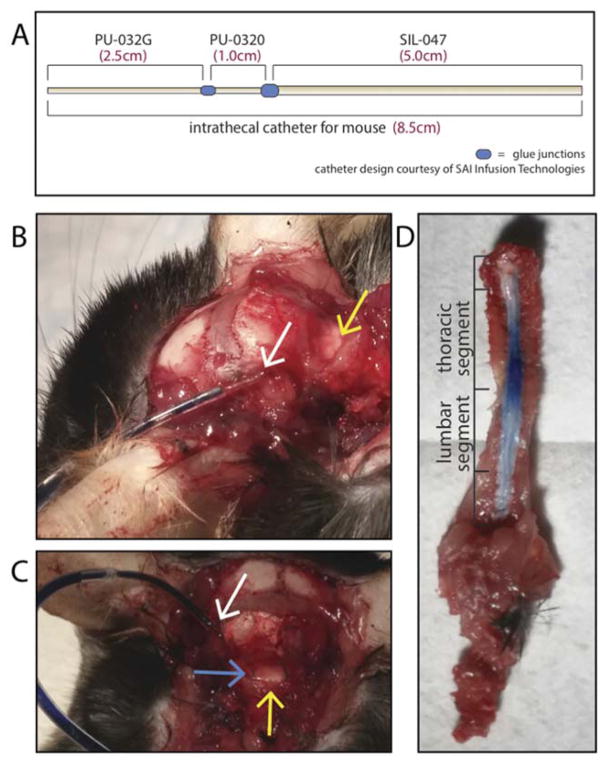
A small incision was made at the nape of the neck, left of the spinal column. Scissors and forceps were used in order to lift muscle and expose the condylar canal, giving access to the cisterna magna, located left of the spinal cord (Fig. 2). A custom-made 8.5cm polyurethane-silicone catheter, consisting of 2.5cm 32G-polyurethane, 1cm 30G-polyurethane, and 5cm 047-silicone segments (SAI Infusion Technologies, IL; Fig. 2), was gently guided into the condylar canal with a 10.5cm Teflon-coated stylus (SAI Infusion Technologies, IL.). Surgeries ranged from 16 to 52 minutes in duration. All other surgery components including surgeon, animal position, wound closure, and primary outcomes, were identical to those used for the A-O i.t. method, as described in full detail in section 2.3.2. Following behavioral testing, mice were euthanized and inspected for catheter looping around the spinal cord. Mice were also inspected for inflammation and/or fibrosis surrounding the condylar canal.
Figure 2. Modified intrathecal catheter system allows for sustained delivery of Evans Blue to the spinal cord.
The O’Buckley i.t. catheter system (A–C) is shown, along with side (B) and dorsal (C) views of catheter placement within the condylar canal. The O’Buckley i.t. catheter system successfully achieved sustained delivery of Evans Blue dye throughout the spinal cord (D). Images B–D were taken on Day 7 following catheter implantation. White arrows indicate the i.t. catheter insertion site, yellow arrows indicate the A-O membrane, and the blue arrow indicates the i.t. catheter underneath intact A-O membrane.
2.3.4 Subcutaneous bifurcated catheter delivery to the hindpaws in mouse
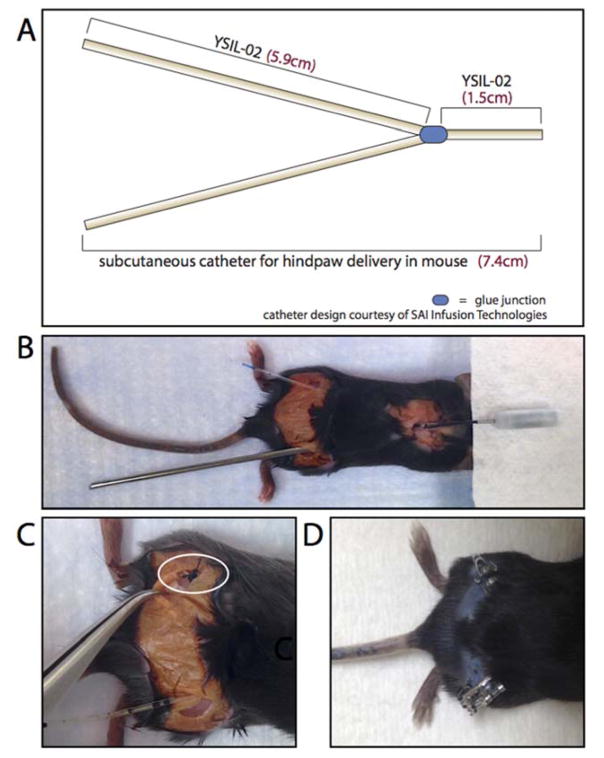
For s.c. delivery to the hindpaws, an osmotic mini-pump containing 1% Evans Blue solution was attached to a custom-made Y-shaped, bif. 7.4cm 3F-silicone catheter (SAI Infusion Technologies, IL; Fig. 4). A small incision was made parallel to and over the shoulder blades and hemostats were used to create a small s.c. pocket. The pump was implanted into the pocket, with the delivery port facing caudally. Small incisions were made over each hindlimb and a stainless steel 14G X 8.5cm semi-blunt trocar (SAI Infusion Technologies, IL) was used to route each catheter end from the incision at the shoulder blades to an incision made at either hindlimb (Fig. 4). Each side of the bif. section of the catheter was sloped similarly towards its respective hindpaw, in order to ensure equal resistance on each side. The catheter ends were attached to fascia in the hindlimbs using 4-0 silk sutures (Oasis Medical, IL). Hemostats were then used in order to create small s.c. pockets in the hindlimbs towards the hindpaws, creating resistance-free regions in which each catheter end could be placed. Catheter ends were trimmed in order to fit into their respective s.c. pockets. The shoulder blade incision was closed using stainless steel wound clips (Braintree Scientific, MA). Depending on the size of the mouse, hindlimb incisions were closed either by wound clips (Braintree Scientific, MA) or by 4-0 PDS-II sutures (Ethicon, OH). Sutures were used on smaller animals in order to prevent interference of wound clips with catheter placement or animal gait. Surgeries ranged from 20 to 40 minutes in duration. Surgeries were deemed “successful” if the mouse fully recovered without any injury, change in gait, or difficulty moving hindlimbs following surgery.
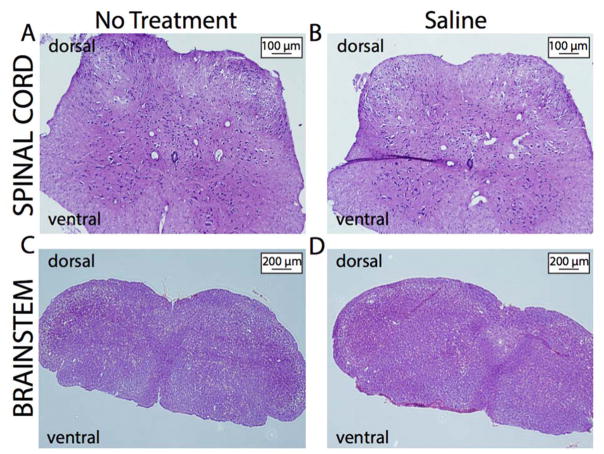
Figure 4. O’Buckley intrathecal catheter system does not produce fibrosis or inflammation in mice.
Both mice that received sustained i.t. administration of saline and their naïve controls demonstrated normal tissue composition and no structural abnormalities in the spinal cord (A–B) or brainstem (C–D).
2.4 Assessment of Behavioral Responses to Mechanical Stimuli
Mice were handled and habituated to the testing environment for 4 days prior to baseline assessments. On test days, mice were placed in plexiglass cages positioned over an elevated wire mesh platform and habituated to the environment for 20–40 minutes. Mechanical threshold was determined with the “up-down” method (Chaplan et al. 1994), using a series of 9 von Frey filaments (with bending forces of 0.03, 0.07, 0.17, 0.40, 0.70, 1.19, 1.50, 2.05, 3.63g; Stoeling, IL) and starting with a filament of 0.70g. In the absence of a paw withdrawal response, an incrementally stronger filament was presented and in the event of a paw withdrawal, an incrementally weaker filament was presented. After the initial response threshold was crossed, this procedure was repeated in order to obtain a total of six responses in the immediate vicinity of the threshold. The pattern of withdrawals and absence of withdrawals were noted together with the terminal filament used in the series of six responses. The 50% of the paw withdrawal threshold is calculated as (10[Xf+kδ])/10,000, where Xf = value (in log units) of the final von Frey hair used; k = tabular value of pattern of positive (X) and negative (O) responses, and δ = mean difference (in log units) between stimuli.
Mechanical allodynia was assessed by presenting a filament with bending force of 0.40 g to the hind paw 10 times, each time for a duration of 1s, with an inter-stimulus interval of 1s. A significant increase in the percentage frequency of paw withdrawal ([# of paw withdrawals/10] x 100) was defined as mechanical allodynia. Mechanical hyperalgesia was assessed in the same manner, using a filament with a bending force of 1.50 g.
2.5 Assessment of Functional Efficacy of Subcutaneous Bifurcated Delivery System
The sustained peripheral administration of bupivacaine to both hindpaws was used to measure the functional efficacy of the s.c. bif. catheter over time. Mice were first assessed for baseline responses to mechanical stimuli, as previously described. Following baseline assessment, mice underwent surgery to receive either sustained delivery of 2.5% bupivacaine or saline. Mice were then re-assessed for responses to mechanical stimuli on days 1, 3, 5, and 7.
2.6 Assessment of Peripheral Edema
The paw edema assay (Winter et al., 1962) was used to measure edema in the hindpaws and surrounding regions at baseline and on days 1, 3, 5 and 7 following implantation of the s.c. bif. catheter system. Edema of the left and right hindlimbs, hocks, and hindpaws were assessed using a caliper. Visual inspection of all animals was performed daily following to monitor signs of edema (swelling, redness, decreased activity and locomotion, change in gait, weight bearing).
2.7 Assessment of Bif. Catheter Clearing
In order to ensure equal distribution of drug between each side of the catheter, a catheter-clearing test was performed on Day 7 following euthanasia of the animals. Catheters were carefully removed from mice, along with any tissue that had adhered to or surrounded the catheters. Catheters were detached from pumps. Each catheter side was placed into a 2mL tube, and 2mL of saline was injected into the bif. catheter end that was originally attached to the pump. Saline that passed through the left and right sides into their respective tubes was quantified. Equal distribution was defined as a non-significant difference between the amounts of saline passed through the left and right sides.
2.8 Sample Fixation and Histological Staining
Mice that underwent no treatment or received sustained i.t. saline administration via osmotic pump were deeply anesthetized with Fatal Plus (Vortech Pharmaceuticals, MI), then perfused transcardially with 0.1M PBS followed by 4% paraformaldehyde. Whole brain and spinal cord samples were collected, post-fixed overnight in 4% paraformaldehyde at 4°C, cryoprotected for 24hr in 30% sucrose/0.1M PBS at 4°C, then sectioned using a Microm HM 550 cryostat (Thermo Fisher Scientific, MA) at 5–10μm. The following sections were placed on Superfrost Plus slides (Thermo Fisher Scientific, MA) and stained by routine hematoxylin and eosin staining in the UNC Oral and Maxillofacial Pathology Laboratory using the Leica ST5010 Autostainer XL (Leica Biosystems Inc, IL), per manufacturer’s protocol.
2.9 Statistical Analyses
Mechanical threshold, mechanical allodynia, and mechanical hyperalgesia following the O’Buckley method were analyzed by 1-way analysis of variance (ANOVA), comparing each time-point to baseline. Mechanical threshold, mechanical allodynia, mechanical hyperalgesia and paw edema assessment of the hindlimbs, hocks, and hindpaws following s.c. bif. catheter implantation were analyzed by 2-way ANOVA. Post-hoc comparisons were performed using the Bonferroni test, which corrected for multiple comparisons. The differences between the surgery and recovery times of the traditional and O’Buckley i.t. surgical methods, the differences between left and right catheter end clearance, and the differences between the average thresholds and average responses between bupivacaine treated and saline treated mice were analyzed using unpaired t-tests. Differences between surgery success rates using traditional versus O’Buckley i.t. surgical methods were analyzed using Fisher’s exact test. For all analyses, statistical significance was defined as p<0.05. All statistical analyses were performed using GraphPad Prism (GraphPad Software, La Jolla, CA).
3. Results
3.1 O’Buckley intrathecal catheter system
Traditional i.t. catheter delivery involves laceration of the posterior A-O membrane (Sakura et al. 1996; Malkmus and Yaksh 2004; Wang et al. 2011; Mattioli et al. 2012). We implemented the traditional i.t. catheter delivery surgical method and found that only 47.8% of mice successfully recovered (Table 1). To increase the survival rate, we reexamined the different variables that impact i.t. catheterization. First, we examined the structural composition of the intrathecal catheter. For our surgical purposes, we found the polyurethane catheter was inflexible enough to possibly create unnecessary strain and pressure onto the spinal cord. To address this, we consulted with Durect to create a customized polyurethane/silicone catheter. Although we found that, compared to the traditional catheter, the customized catheter provided more flexibility, it did not change the surgical success rate (Table 1).
Table 1.
Comparative surgery time, recovery time, and success rates
| Method | Surgery Range (min) | Surgery Avg ± SEM (min) | Recovery Range (min) | Recovery Avg ± SEM (min) | Success (n) | Success (%) |
|---|---|---|---|---|---|---|
| Novel s.c. bif. catheter hindpaw delivery system | --- | --- | --- | --- | 42 | 100.00 |
| Traditional i.t. catheter delivery system (original catheter) | 26–53 | 37±1.2 | 3–40 | 13±1.2 | 29 | 46.30 |
| Traditional i.t. catheter delivery system (customized catheter) | 27–43 | 35±1.9 | 9–24 | 15±1.5 | 8 | 53.33 |
| O’Buckley i.t. catheter delivery system (customized catheter) | 16–52 | 30±1.8* | 2–16 | 5±0.65* | 64 | 85.33 |
Next, we re-examined the catheter insertion site. Although the laceration of posterior A-O membrane provides easiest access to the spinal cord, it also incurs a substantial risk of injury to the spinal cord. Thus, we sought to identify an alternative insertion site that would minimize the risk of injury, but still provide access to the spinal cord. In our modified version, the O’Buckley i.t. catheter system, the catheter is inserted in the condylar canal located in the occipital bone, left of the A-O membrane (Fig. 2B–C). We hypothesized that, given its smaller circumference and diameter, the condylar canal reduced the risk of accidental lesioning of the spinal while still providing access via the cisterna magna. The O’Buckley i.t. catheter system improved upon the traditional method, reducing surgery (Table 1; p=0.0026) and recovery (p<0.0001) times and providing mice with a 85.3% chance of successful recovery (p<0.0001). Furthermore, the O’Buckley i.t. catheter system enabled consistent distribution of Evans Blue throughout the spinal cord, observed on Days 2–7, with the highest concentration appearing in the thoracic spinal region (Fig. 2D). This method has been successfully implemented in CXB7/ByJ mice in order to study the effects of i.t. delivery of small interfering RNA targeting mu opioid splice variant MOR-1K on the development of opioid-induced hyperalgesia (Oladosu et al. 2015)). Specifically, we found that sustained i.t. delivery of exon 13 MOR-1K antisense siRNA over seven days not only blocked the development of morphine-induced hyperalgesia in CXB7/ByJ mice, but also unmasked morphine analgesia previously absent within the mouse strain.
3.2 O’Buckley intrathecal catheter system does not affect responses to mechanical stimuli in mice
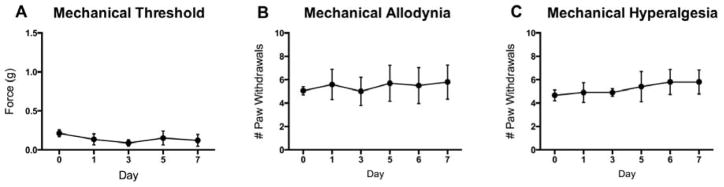
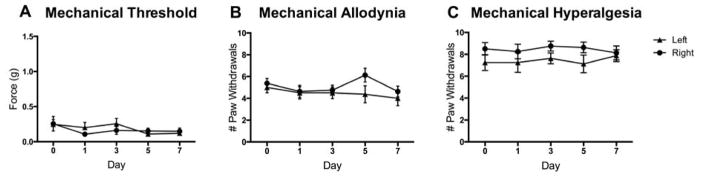
To determine if the O’Buckley i.t. catheter delivery system alters pain sensitivity, we measured responses to mechanical stimuli in mice receiving saline via the catheter system for a 1-week period following surgeries. Mice did not exhibit altered mechanical thresholds (Fig. 3A), mechanical allodynia (Fig. 3B), or mechanical hyperalgesia (Fig. 3C) on days 1–7 following surgery as compared to day 0 prior to surgery.
Figure 3. O’Buckley intrathecal catheter system does not produce mechanical pain behaviors in mice.
Surgical implantation of the O’Buckley i.t. catheter system for sustained delivery of saline to the spinal cord does not produce (A) altered mechanical thresholds, (B) mechanical allodynia or (C) mechanical hyperalgesia on Days 2, 4, 6 and 8 following surgery, as compared to baseline. N=6. Data are expressed as mean ± SEM.
3.3 O’Buckley intrathecal catheter system does not produce fibrosis or inflammation on dorsal side of the spinal cord
To determine if the O’Buckley intrathecal catheter system produces fibrosis and/or inflammation in the spinal cord, mice that underwent surgical implantation to receive sustained i.t. saline administration for a 1-week period and naïve controls were euthanized. Following PBS and paraformaldehyde transcardial perfusions, cervical spinal cord and brainstem samples were collected, processed, and stained in order to assess for fibrosis and/or inflammation, specifically on the dorsal aspect of the sections. Histological examination of the tissues showed no differences in tissue composition and an absence of structural abnormalities between the two groups, suggesting that intrathecal catheter placement does not produce fibrosis and/or inflammation in the spinal cord.
3.4 Subcutaneous bifurcated catheter delivery to the hindpaws
In order to achieve localized drug delivery to the left and right hindpaws in mice, i.pl. injections are traditionally used. Here, we eliminated the need for multiple injections by utilizing a s.c. catheter system. Evans Blue dye was delivered simultaneously to both hindpaws via a custom-made bif. catheter (Fig. 5A–C) attached to an osmotic mini-pump. Each catheter end was fastened to either the left or right hindlimb of the mouse (Fig. 5B–C), ensuring consistent delivery of Evan’s Blue to both sides beginning as early as Day 2 following catheter implantation and continuing throughout the duration of the experimental protocol to Day 7 (Fig. 5D). Catheter-clearing experiments following euthanasia demonstrated that the two catheter ends cleared equal amounts of saline (Left: 0.906mL, Right: 1.094mL; p=0.3392).
Figure 5. Subcutaneous bifurcated catheter system allows for sustained delivery of Evans Blue to both hindpaws simultaneously.
A novel s.c. bif. catheter system (A–C) was implanted in mice using a stainless steel trocar (B) for guidance. Sutures were used to fasten the catheter to surrounding fascia of the hindpaws, as denoted by the white circle (C). The s.c. bif. catheter system successfully achieved sustained delivery of Evans Blue dye to both hindlimbs (D). Evans Blue dye was observed as early as Day 2 following catheter implantation and lasted until Day 7. Image in (D) was taken on Day 7.
3.5 Subcutaneous bifurcated catheter delivery system does not affect responses to mechanical stimuli or produce paw edema in mice
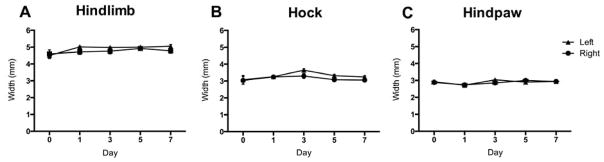
To determine if the s.c. bif. catheter delivery system alters pain sensitivity, we measured responses to mechanical stimuli in mice receiving saline via the catheter system for a 1-week period following surgeries. Mice did not exhibit altered mechanical thresholds (Fig. 6A), mechanical allodynia (Fig. 6B), or mechanical hyperalgesia (Fig. 6C) on days 1–7 following surgery as compared to day 0 prior to surgery. Further, no edema of the hindlimbs (Fig. 7A), hocks (Fig. 7B) or hindpaws (Fig. 7C) was measured.
Figure 6. Bifurcated subcutaneous catheter system does not produce mechanical pain behaviors in mice.
Surgical implantation of the s.c. bif. catheter system for sustained delivery of saline to both hindpaws does not produce (A) altered mechanical thresholds, (B) mechanical allodynia or (C) mechanical hyperalgesia on Days 1, 3, 5 and 7 following surgery, as compared to baseline. N=8. Data are expressed as mean ± SEM.
Figure 7. Bifurcated subcutaneous catheter system does not produce edema of the hindlimbs, hocks, or hindpaws.
Surgical implantation of the s.c. bif. catheter system for sustained delivery of saline to both hindpaws does not cause edema of the left or right (A) hindlimbs, (B) hocks or (C) hindpaws of mice on Days 1, 3, 5 and 7 following surgery, as compared to baseline. N=5. Data are expressed as mean ± SEM.
3.6 Subcutaneous delivery of bupivacaine reduces hindpaw responses to mechanical stimuli
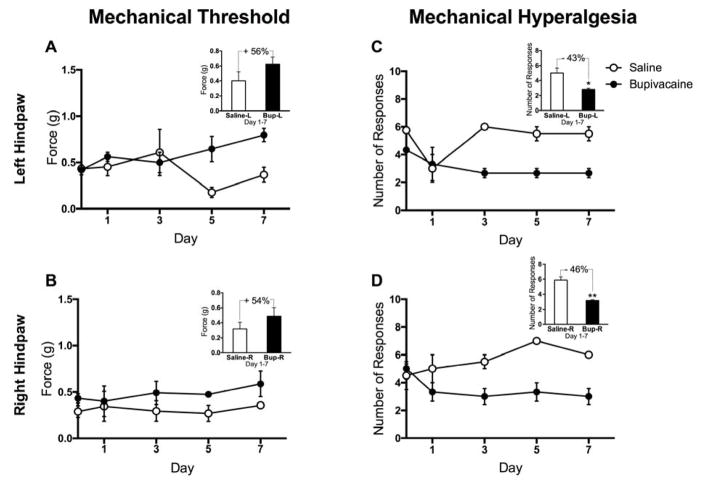
To determine if the s.c. bif. catheter system was equally effective in both hindpaws, we measured responses to mechanical stimuli in mice receiving 2.5% bupivacaine solution or saline via the delivery system for a 1-week period following surgery. Compared to the saline group, mice that received sustained bupivacaine administration exhibited increased mechanical threshold in both the left (Fig 8A; F(1,15)=6.903, p=0.0190) and right (Fig. 8B; F(1,15)=5.036, p=0.0403) hindpaws. Mice that received sustained bupivacaine administration also exhibited decreased mechanical hyperalgesia in the left (Fig 8C; F(1,15) = 26.54, p=0.0001) and right (Fig 8D; F(1,15) = 26.51, p=0.0001) hindpaws as well. Percent changes across the treatment groups were similar in both hindpaws for mechanical threshold (Left: +56%, p=0.2278; Right: +54%, p=0.3698) and mechanical hyperalgesia (Left: −43%, p=0.0290; Right: −46%, p=0.0042), indicating that the sc. bif. catheter system effectively delivers proportional amounts of drug to both hindpaws.
Figure 8. Sustained subcutaneous administration of bupivacaine decreases responses to mechanical stimuli equally in both left and right hindpaws.
Surgical implantation of the s.c. bif. catheter system for sustained delivery of bupivacaine to both hindpaws produces (A–B) increased mechanical thresholds and (C–D) decreased mechanical hyperalgesia on Days 1, 3, 5 and 7 following surgery, as compared to baseline. N=5. Data are expressed as mean ± SEM. **p<0.01, *p<0.05 indicates significant difference from saline treated mice.
4. Discussion
There are over 445 neurological disorders, the majority of which are chronic in nature (NINDS 2015). Sustained targeted delivery of genes, small molecules, ligands, or other agents in mice is critical to our understanding of the mechanisms underlying these disorders as well as to the development of new therapeutic regimens. Here, we present two novel methods for localized sustained drug delivery in the mouse: the O’Buckley i.t. catheter system that permits consistent drug delivery to the spinal cord; and a s.c. bif. catheter system that permits sustained drug delivery to the hindlimbs and surrounding peripheral nerve terminals. These catheter systems improve upon existing approaches for chronic administration of drugs and represent useful techniques for the development of clinically relevant animal models.
It is well known that neurological conditions involve and affect spinal tissues. Conditions such as amyotrophic lateral sclerosis (Kiernan et al., 2011) and spinal muscular atrophy (Kolb and Kissel, 2011) originate in the spinal cord. Others, such as multiple sclerosis, impact the entire CNS, including both the brain and spinal cord (Goldenberg, 2012). Further, the spinal cord allows for signal transduction between the CNS and the periphery during disease. Enhancing, disrupting, or modifying this communication via i.t. drug delivery could help us to better understand and treat symptoms of neurological conditions. Previous studies have shown that i.t. delivery of therapeutics improves signs of neuropathology in animal models as well as clinical and behavioral outcomes in human studies (Calias et al. 2014). Still, in mice, direct injections remain the standard method for spinal cord drug delivery. Injections are not ideal and can confound the experimental results of i.t. drug delivery studies, just as they do for peripheral injection studies.
While sustained i.t. catheter systems have been implemented in rat, they have not been optimized for use in mouse. In mouse, the traditional i.t. drug delivery has a high incidence of fatality. The traditional procedure puts rodents at risk of paralysis or death, usually as a result of accidental incision of the spinal cord while cutting the A-O membrane (Malkmus and Yaksh 2004). Further, the Alzet intrathecal catheter designed for mouse is composed of polyurethane, which is inflexible and resistant to bending. It easily slips out of the catheter insertion site, requiring repeated attempts to secure it into place. For these reasons, a novel persistent i.t. catheter delivery system, optimized for use in mice, is required.
Here, we describe a novel i.t. catheter system, the O’Buckley method, in which we use the condylar canal as an alternative catheter insertion site. When compared to the atlanto-occipital membrane, the condylar canal has a smaller circumference. The smaller size of the condylar canal reduces the amount of exposed spinal cord that can be accidentally lesioned during the surgical procedure. Thus, this alternative insertion site provides access to the cisterna magna while reducing the risk of injury to the spinal cord, thus minimizing the incidence of paralysis during surgery. Histological examination of cervical spinal cord and brainstem sections reveal no indication of structural abnormalities, suggesting that the O’Buckley method does not produce fibrosis and/or inflammation. Further, this method utilizes a customized silicone-polyurethane catheter, similar to those used for i.t. delivery in rats (Sakura et al. 1996), which possesses the flexibility required to stabilize and secure the catheter with ease. Overall, we found that these modifications in the O’Buckley method decrease surgery time and increase surgical success rate (Table 1). This novel i.t. catheter method will provide researchers with the possibility to study the spinal cord, and its role in neurological conditions, through the use of sustained i.t. drug delivery. We have also optimized this method for use in rat, using a 27.3 cm catheter.
Various neurological conditions contain peripheral components that contribute to the manifestation and identification of the disorder. For example, conditions such as peripheral neuropathy (Torpy et al. 2010), Guillain-Barre Syndrome (Hartung 1999) and sciatica (Ropper and Zafonte 2015), each originate in the peripheral nervous system. Other conditions, such as multiple sclerosis (Goldenberg 2012), and lupus (Tsokos 2011) act primarily on the central nervous system (CNS) but also affect the peripheral sensory and motor function. For this reason, peripheral drug delivery systems are an intricate component of studying neurological diseases in a rodent model. Though multiple injection protocols are commonly used to deliver drugs to peripheral regions, they cannot achieve consistent drug delivery over an extended period of time (Fara and Urquhart 1984; Perkins et al. 2004). Further, repeated injections expose the animal to excessive handling and injury, often confounding the results of physiological and behavioral experiments (Wright and Lincoln 1985; Dilsaver and Majchrzak 1990; Lafarga et al. 1998; Vinkers et al. 2009; Drude et al. 2011).
Repeated injections that are specific to the hindpaw region may add an extra element of variability to experiments, as hindpaw injury may alter the animal’s ability to bear weight and walk normally (Kamala 2007). Additionally, many behavioral assays utilize the hindpaw in order to assess phenotypes such as pain, motor function, weight-bearing, locomotion, and exploratory behaviors (Gregory et al. 2013; Pratt et al. 2013; Pertici et al. 2014; Freund et al. 2015). For these reasons, a sustained peripheral drug delivery system is required in order to accurately study the physiology and behavior of neurological diseases in a mouse model. Our s.c. bif. catheter system eliminates the need for injections, as it allows for persistent and simultaneous delivery to both hindpaws. This method will provide researchers with the possibility to accurately assess the effects of hindpaw-specific drug delivery, over an extended period of time. We have also optimized this method for use in rat, using trocar bars of 10G X 20 cm and a 15 cm bif. catheter.
When utilizing the novel catheter delivery system protocols described here, it is important to consider the effect of surgery and anesthesia on the animal. Though surgery in a rodent model results in injury and inflammation, particularly at the surgical site, significant stress caused by catheter implantation persists only for the first 24 hours following surgery (Blenkisopp 1969). To combat post-surgical stress and injury, animals should be given at least one day following surgery for recovery, prior to behavioral or phenotypic assessment. Anesthesia can also impact the behavior or physiology of animals, specifically that of which is associated with learning and memory (Callaway et al. 2012; Wang et al. 2012; Erasso et al. 2013). Again, a recovery period should be used, and should be chosen based on the duration of anesthesia and the experimental phenotypes under study, in order to negate any possible effects of surgery and anesthesia.
A potential concern with our proposed catheter systems is that they could possibly enhance inflammation due to the presence of foreign material. Such an inflammatory response could affect the behavior of the animal. Although we did not detect paw edema in animals with the bif. catheter, inflammatory responses may still occur on the molecular and cellular level. Future experiments utilizing flow cytometry, immunohistochemistry, and histology are necessary to determine if the bif. catheter ends lead to increased levels of key cell types associated with acute inflammation (i.e. neutrophils), chronic inflammation (i.e. mononuclear leukocytes), granulation (i.e. fibroblasts, endothelial cells) and fibrosis (Anderson and McNally 2011). These studies will also reveal possible enhancements of inflammatory pain produced by the bif. catheter. Experiments using protein and DNA detection techniques are needed to determine if the presence of bif. catheter ends produces this enhancement of inflammatory agents via increased levels of inflammatory mediators in the hock and hindpaw. Though neither edema nor pain was observed in our experiments, it is important to monitor inflammation at incision and catheter sites for all experiments that involve sustained implantation of foreign material. Additionally, inflammation to the hindlimbs or surrounding areas could possibly cause resistance and alter the flow of drug through one side of the catheter. Catheter-clearing experiments should be used alongside this method to ensure equal distribution to both hindlimbs. The methodology in the present study has been optimized to minimize inflammation and enhance catheter flow.
5. Conclusions
Our novel catheter systems accomplish sustained drug delivery to the i.t. space as well as to both hindpaws of mice. Compared to existing i.t. delivery methods, the O’Buckley i.t. catheter system is less likely to produce injury, paralysis and mortality. Compared to existing delivery methods, our bif. catheter system eliminates the need for i.pl. injections, which can cause stress and impact the behavior and physiology. These methods have the propensity to enhance the accuracy of persistent drug delivery experiments in rodents, which may help to inform our knowledge, as well as the treatment, of the hundreds of debilitating neurological conditions involving central and/or peripheral nervous systems.
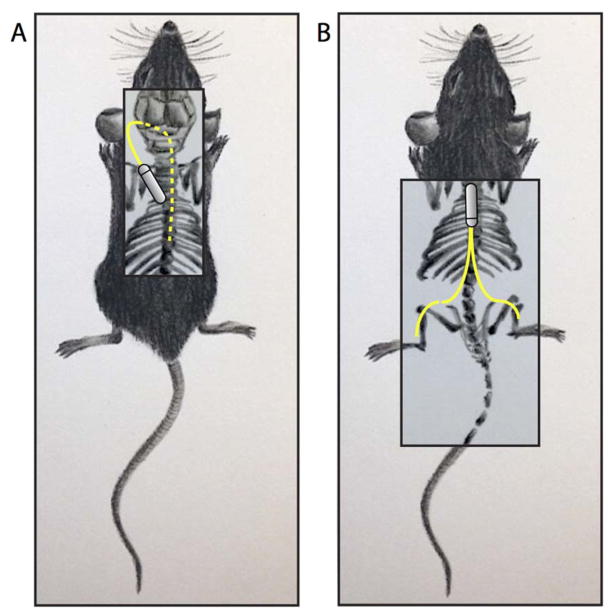
Figure 1. Novel i.t. and s.c. catheter delivery systems in mouse.
This schematic illustrates the catheter systems designed to achieve consistent delivery of drug (A) intrathecally throughout the spinal cord and (B) subcutaneously to both hindpaws. Dotted yellow lines indicate portions of the catheter underneath occipital tissue or vertebrate bone, while solid yellow lines indicate s.c. portions of the catheters.
Highlights.
We have developed two novel catheter systems that achieve sustained drug delivery in mice: a subcutaneous bifurcated system for drug administration to both hindpaws and an intrathecal system for drug administration to the spinal cord.
Compared to existing hindpaw delivery methods, our subcutaneous bifurcated catheter system eliminates the need for intraplantar injections, which can cause tissue injury and stress.
Compared to existing spinal delivery methods, our intrathecal catheter system is less likely to produce injury, paralysis, or mortality.
Acknowledgments
Financial support was provided by NIH/NINDS R01 NS072205 and NIH/NINDS P01 NS045685 funded to AGN. The authors thank Durect Corporation and SAI Infusion Technologies for their informative and hands-on assistance with the design and development of the intrathecal and bifurcated catheters respectively. The authors also thank Eric P. Gilchrist and the UNC Oral and Maxillofacial Pathology Laboratory for the hematoxylin and eosin staining of spinal cord and brainstem sections.
Abbreviations
- A-O
atlanto-occipital
- bif
bifurcated
- CNS
central nervous system
- i.t
intrathecal
- s.c
subcutaneous
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Folabomi A. Oladosu, Email: folabomi_oladosu@unc.edu.
Brittney P. Ciszek, Email: brittney_ciszek@unc.edu.
Sandra C. O’Buckley, Email: sandra_obuckley@duke.edu.
Andrea G. Nackley, Email: andrea.nackley@duke.edu.
References
- Altaner C, Altanerova V, Cihova M, Ondicova K, Rychly B, Baciak L, et al. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer. 2014 Mar 15;134(6):1458–65. doi: 10.1002/ijc.28455. [DOI] [PubMed] [Google Scholar]
- Anderson JM, McNally AK. Biocompatibility of implants: lymphocyte/macrophage interactions. Semin Immunopathol. 2011 Jan 27;33(3):221–33. doi: 10.1007/s00281-011-0244-1. [DOI] [PubMed] [Google Scholar]
- Blenkinsopp EC, Blenkisopp WK. Effects of a glucocorticoid (dexamethasone) on the eosinophils of the rat. J Endocrinol. 1967 Apr 1;37(4):463–9. doi: 10.1677/joe.0.0370463. [DOI] [PubMed] [Google Scholar]
- Blenkisopp WK. Comparison of multiple injections with continuous infusion of tritiated thymidine, and estimation of the cell cycle time. J Cell Sci. 1969 Nov;5(3):575–82. doi: 10.1242/jcs.5.3.575. [DOI] [PubMed] [Google Scholar]
- Boudreau E, Chen G, Li X, Buck K, Hitzemann R, Hickman D. Intraperitoneal catheter placement for pharmacological imaging studies in conscious mice. Lab Anim. 2010 Jan;39(1):23–5. doi: 10.1038/laban0110-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calias P, Banks WA, Begley D, Scarpa M, Dickson P. Intrathecal delivery of protein therapeutics to the brain: A critical reassessment. Pharmacol Therapeut. 2014 Nov;144(2):114–22. doi: 10.1016/j.pharmthera.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Callaway JK, Jones NC, Royse CF. Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesth. 2012 May;29(5):239–45. doi: 10.1097/EJA.0b013e32835103c1. [DOI] [PubMed] [Google Scholar]
- Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, et al. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant Semaphorin 3E isoform. EMBO Mol Med. 2012 Mar;4(3):234–50. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994 Jul;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Culman J, Ritter S, Ohlendorf C, Haass M, Maser-Gluth C, Spitznagel H, et al. A new formalin test allowing simultaneous evaluation of cardiovascular and nociceptive responses. Can J Physiol Pharmacol. 1997 Oct;75(10–11):1203–11. [PubMed] [Google Scholar]
- de Yebenes JG, Fahn S, Lovelle S, Jackson-Lewis V, Jorge P, Mena MA, et al. Continuous intracerebroventricular infusion of dopamine and dopamine agonists through a totally implanted drug delivery system in animal models of Parkinson’s disease. Mov Disord. 1987;2(3):143–58. doi: 10.1002/mds.870020302. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Majchrzak MJ. Effects of placebo (saline) injections on core temperature in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(3):417–22. doi: 10.1016/0278-5846(90)90029-g. [DOI] [PubMed] [Google Scholar]
- Drude S, Geissler A, Olfe J, Starke A, Domanska G, Schuett C, et al. Side effects of control treatment can conceal experimental data when studying stress responses to injection and psychological stress in mice. Lab Anim. 2011 Apr;40(4):119–28. doi: 10.1038/laban0411-119. [DOI] [PubMed] [Google Scholar]
- Erasso DM, Camporesi EM, Mangar D, Saporta S. Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Res. 2013 Sep 12;1530(C):1–12. doi: 10.1016/j.brainres.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Fara J, Urquhart J. The value of infusion and injection regimens in assessing efficacy and toxicity of drugs. Trends Pharmacol Sci. 1984;5:21–5. [Google Scholar]
- Feng J, Fitz Y, Li Y, Fernandez M, Cortes Puch I, Wang D, et al. Catheterization of the carotid artery and jugular vein to perform hemodynamic measures, infusions and blood sampling in a conscious rat model. J Vis Exp. 2015;95:e51881–1. doi: 10.3791/51881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, et al. Association between exploratory activity and social individuality in genetically identical mice living in the same enriched environment. NSC. 2015 Jun 8;:1–13. doi: 10.1016/j.neuroscience.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Gillio-Meina C, Phang SH, Mather JP, Knight BS, Kennedy TG. Expression patterns and role of prostaglandin-endoperoxide synthases, prostaglandin E synthases, prostacyclin synthase, prostacyclin receptor, peroxisome proliferator-activated receptor delta and retinoid x receptor alpha in rat endometrium during artificially-induced decidualization. Reproduction. 2009 Mar;137(3):537–52. doi: 10.1530/REP-08-0294. [DOI] [PubMed] [Google Scholar]
- Goldenberg MM. Multiple sclerosis review. P T. 2012 Mar;37(3):175–84. [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. J Pain. 2013 Nov 1;14(11):1255–69. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005 Mar;98(3):911–7. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- Hartung HP. Infections and the Guillain-Barré syndrome. J Neurol Neurosurg Psychiatr. 1999 Mar;66(3):277. doi: 10.1136/jnnp.66.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylden J, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67(2–3):313–6. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Izumi J, Washizuka M, Hayashi-Kuwabara Y, Yoshinaga K, Tanaka Y, Ikeda Y, et al. Evidence for a Depressive-like State Induced by Repeated Saline Injections in Fischer 344 Rats. Pharmacol Biochem Behav. 1997 Aug;57(4):883–8. doi: 10.1016/s0091-3057(96)00455-8. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Boudah A, Ohara PT. Long-term effects of decreased noradrenergic central nervous system innervation on pain behavior and opioid antinociception. J Compar Neurol W. 2003 May 19;460(1):38–55. doi: 10.1002/cne.10633. [DOI] [PubMed] [Google Scholar]
- Jimenez Hamann MC, Tsai EC, Tator CH, Shoichet MS. Novel intrathecal delivery system for treatment of spinal cord injury. Exp Neurol. 2003 Aug;182(2):300–9. doi: 10.1016/s0014-4886(03)00040-2. [DOI] [PubMed] [Google Scholar]
- Kamala T. Hock immunization: a humane alternative to mouse footpad injections. J Immunol Methods. 2007 Dec 1;328(1–2):204–14. doi: 10.1016/j.jim.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosumi T, Usui N, Kubota A, Hoki H, Yamauchi K, Nogami T, et al. Application of a drug delivery system in a novel rat model of chronic hyperendotoxemia. Pediatr Surg Int. 2001 May;17(4):321–5. doi: 10.1007/s003830100603. [DOI] [PubMed] [Google Scholar]
- Lafarga M, Berciano MT, Garcia-Segura LM, Andres MA, Carmo-Fonseca M. Acute osmotic/stress stimuli induce a transient decrease of transcriptional activity in the neurosecretory neurons of supraoptic nuclei. J Neurocytol. 1998 Apr;27(4):205–17. doi: 10.1023/a:1006937032068. [DOI] [PubMed] [Google Scholar]
- Lindau NT, Bänninger BJ, Gullo M, Good NA, Bachmann LC, Starkey ML, et al. Rewiring of the corticospinal tract in the adult rat after unilateral stroke and anti-Nogo-A therapy. Brain. 2014 Mar;137(Pt 3):739–56. doi: 10.1093/brain/awt336. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peng M, Zang D, Zhang B. Leukemia inhibitory factor promotes nestin-positive cells, and increases gp130 levels in the Parkinson disease mouse model of 6-hydroxydopamine. Neurosciences (Riyadh) 2013 Oct;18(4):363–70. [PubMed] [Google Scholar]
- Luo M-C, Zhang D-Q, Ma S-W, Huang Y-Y, Shuster SJ, Porreca F, et al. An efficient intrathecal delivery of small interfering RNA to the spinal cord and peripheral neurons. Mol Pain. 2005;1(1):29. doi: 10.1186/1744-8069-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med. 2004;99:109–21. doi: 10.1385/1-59259-770-X:011. [DOI] [PubMed] [Google Scholar]
- Mattioli TA, Sutak M, Milne B, Jhamandas K, Cahill CM. Intrathecal Catheterization Influences Tolerance to Chronic Morphine in Rats. Anesth Analg. 2012 Mar;114(3):690–3. doi: 10.1213/ANE.0b013e31823fad94. [DOI] [PubMed] [Google Scholar]
- Navarro X, Verdú E, Wendelscafer-Crabb G, Kennedy WR. Innervation of cutaneous structures in the mouse hind paw: a confocal microscopy immunohistochemical study. J Neurosci Res. 1995 May 1;41(1):111–20. doi: 10.1002/jnr.490410113. [DOI] [PubMed] [Google Scholar]
- NINDS. Disorders Index: National Institutes of Neurological Disorders and Stroke [Internet] 2015 Available from: http://www.ninds.nih.gov/disorders/disorder_index.htm.
- Oladosu FA, Conrad MS, OBuckley SC, Rashid NU, Slade GD, Nackley AG. Mu Opioid Splice Variant MOR-1K Contributes to the Development of Opioid-Induced Hyperalgesia. PLoS ONE. 2015;10(8):e0135711. doi: 10.1371/journal.pone.0135711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L, Peer C, Murphy-Hackley P. The Use of Mini-Osmotic Pumps in Continuous Infusion Studies. In: Healing G, Smith D, editors. Handbook of Pre-clinical Continuous Intravenous Infusion. 2004. pp. 1–34. [Google Scholar]
- Pertici V, Pin-Barre C, Felix M-S, Laurin J, Brisswalter J, Decherchi P. A new method to assess weight-bearing distribution after central nervous system lesions in rats. Behav Brain Res. 2014 Feb 1;259:78–84. doi: 10.1016/j.bbr.2013.10.043. [DOI] [PubMed] [Google Scholar]
- Pratt D, Fuchs PN, Sluka KA. Assessment of avoidance behaviors in mouse models of muscle pain. NSC. 2013 Sep 17;248(C):54–60. doi: 10.1016/j.neuroscience.2013.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SB, Saini R, Kumar R. Intrathecal catheterization and drug delivery in rats to compare the analgesic effects of morphine with ketorolac. J Anaesthesiol Clin Pharmacol. 2011 Jan;27(1):84–6. [PMC free article] [PubMed] [Google Scholar]
- Ropper AH, Zafonte RD. Sciatica. N Engl J Med. 2015 Mar 26;372(13):1240–8. doi: 10.1056/NEJMra1410151. [DOI] [PubMed] [Google Scholar]
- Sakura S, Hashimoto K, Bollen AW, Ciriales R, Drasner K. Intrathecal catheterization in the rat. Improved technique for morphologic analysis of drug-induced injury. Anesthesiology. 1996 Nov;85(5):1184–9. doi: 10.1097/00000542-199611000-00028. [DOI] [PubMed] [Google Scholar]
- Sherwin JRA, Hastings JM, Jackson KS, Mavrogianis PA, Sharkey AM, Fazleabas AT. The endometrial response to chorionic gonadotropin is blunted in a baboon model of endometriosis. Endocrinology. 2010 Oct;151(10):4982–93. doi: 10.1210/en.2010-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpy JM, Kincaid JL, Glass RM. JAMA patient page. Peripheral neuropathy. JAMA. 2010:1556–6. doi: 10.1001/jama.303.15.1556. [DOI] [PubMed] [Google Scholar]
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011 Dec 1;365(22):2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- Ulrich YM, Hargreaves KM, Flores CM. A comparison of multiple injections versus continuous infusion of nicotine for producing up-regulation of neuronal [3H]-epibatidine binding sites. Neuropharmacology. 1997 Aug;36(8):1119–25. doi: 10.1016/s0028-3908(97)00107-x. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Groenink L, van Bogaert MJV, Westphal KGC, Kalkman CJ, van Oorschot R, et al. Stress-induced hyperthermia and infection-induced fever: two of a kind? Physiol Behav. 2009 Aug 4;98(1–2):37–43. doi: 10.1016/j.physbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wang F, Xu S, Shen X, Guo X, Peng Y, Yang J. Spinal macrophage migration inhibitory factor is a major contributor to rodent neuropathic pain-like hypersensitivity. Anesthesiology. 2011 Mar;114(3):643–59. doi: 10.1097/ALN.0b013e31820a4bf3. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu Z, Feng C, Wang Y, Jia X, Wu A, et al. Changes of learning and memory in aged rats after isoflurane inhalational anaesthesia correlated with hippocampal acetylcholine level. Ann Fr Anesth. 2012 Mar 1;31(3):e61–6. doi: 10.1016/j.annfar.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Wedel J, Weij M, Oosten AS-V, Hillebrands J-L. Simultaneous subcutaneous implantation of two osmotic minipumps connected to a jugular vein catheter in the rat. Lab Anim. 2014 Oct;48(4):338–41. doi: 10.1177/0023677214543089. [DOI] [PubMed] [Google Scholar]
- Wright DM, Lincoln DW. Stress-induced analgesia evoked by intraperitoneal injection of hypertonic saline: Evidence for its occurrence in vasopressin deficient rats. Physiol Behav. 1985 May;34(5):691–5. doi: 10.1016/0031-9384(85)90366-x. [DOI] [PubMed] [Google Scholar]
- Wu W-P, Xu X-J, Hao J-X. Chronic lumbar catheterization of the spinal subarachnoid space in mice. J Neurosci Methods. 2004 Feb;133(1–2):65–9. doi: 10.1016/j.jneumeth.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Yu G, Zhang F-Q, Tang S-E, Lai M-J, Su R-B, Gong Z-H. Continuous infusion versus intermittent bolus dosing of morphine: A comparison of analgesia, tolerance, and subsequent voluntary morphine intake. J Psychiat Res. 2014 Dec 1;59(C):161–6. doi: 10.1016/j.jpsychires.2014.08.009. [DOI] [PubMed] [Google Scholar]