Abstract
Sudden cardiac death (SCD) is an important public-health problem with multiple etiologies, risk factors, and changing temporal trends. Substantial progress has been made over the past few decades in identifying markers that confer increased SCD risk at the population level. However, the quest for predicting the high-risk individual who could be a candidate for an implantable cardioverter-defibrillator, or other therapy, continues. In this article, we review the incidence, temporal trends, and triggers of SCD, and its demographic, clinical, and genetic risk factors. We also discuss the available evidence supporting the use of public-access defibrillators.
Introduction
Sudden cardiac death (SCD) is a common and devastating event, often occurring in the prime of life and having profound consequences for surviving members of the individual’s family. Out-of-hospital SCD is the cause of more than 60% of all deaths from cardiovascular disease, which is the leading cause of death worldwide.1–4 In the past few decades, substantial progress has been made in our understanding of SCD and in its prevention and management. Multiple clinical, structural, autonomic, and genetic risk factors have been identified and the use of automated external defibrillators (AEDs) and implantable cardioverter-defibrillators (ICDs) has increased. However, SCD continues to be an important public-health problem, largely because the majority of SCDs occur in individuals without previously diagnosed heart disease who do not meet the high-risk criteria defined by clinical trials and cohort studies.5,6 Moreover, risk stratification of individuals lacks specificity and research into the genetics of sudden cardiac death is still at a very early stage. Thus, prevention of SCD hinges upon public-health interventions focused on the primary and secondary prevention of cardiovascular diseases.
The epidemiological, or population, approach to the investigation of cardiovascular disease was established in the 1940s and 1950s by Dawber and coworkers in the Framingham Heart Study and by Keys and colleagues in the Seven Countries Study.7 These investigations provided community-based data on the incidence, course, and prognosis of cardiovascular disease and helped to identify risk factors and gain insights into pathogenesis.7 Much of the established knowledge on SCD, including its genetic origins, comes from epidemiological studies. Moreover, owing to temporal trends in the incidence and prognosis of coronary heart disease (CHD), diabetes mellitus, hyperlipidemia, and obesity, the epidemiology of SCD has also seen substantial fluctuation. In this article, we review the incidence, temporal trends, triggers, and time-dependency of SCD. We will also summarize the demographic, clinical, and genetic risk factors for SCD and the evidence supporting the use of public-access defibrillators.
Definition and incidence
The widely-accepted definition of SCD is unexpected death that occurs within 1 h from the start of symptoms when death is witnessed, and within 24 h of being seen alive and well when it is unwitnessed.8 Most deaths that meet this definition are caused by cardiac arrhythmias, including those resulting from acute myocardial infarction. However, other potential causes, such as stroke, pulmonary embolism, aortic rupture, and drug or alcohol intoxication, need to be considered, although excluding these noncardiac causes of death is often difficult. The majority of SCDs are not witnessed and, if they are observed, the accounts obtained from witnesses may be unreliable. Furthermore, in many cases, medical records are unavailable, autopsy is not performed, and the cause of death given on the death certificate is speculative of the underlying event.2,9,10
The incidence of SCD has been estimated to be between 300,000 and 450,000 annually in the US.1,3,11 These retrospective assessments are based on the assumption that all out-of-hospital deaths, for which CHD is given as the primary cause of death on the death certificate, are SCDs. This approach has been shown to be sensitive, but not specific, for identifying SCD.11–14 Thus, these retrospective figures are likely to be an overestimate of the true SCD incidence in the community. Conversely, limiting the definition of SCD to deaths that occur within 1 h of symptom onset might be too restrictive and exclude many unwitnessed cases. The incidence figures obtained from studies of sudden cardiac arrest (range 40–90 SCDs per 100,000 individuals), which are based on data collected from first responders, could also be an underestimate because these statistics do not include unwitnessed SCDs or deaths not attended by emergency medical personnel.15–17 Thus, multiple sources of ascertainment are needed in order to determine the true incidence of SCD.5 Indeed, in two prospective community studies that used multiple sources to identify SCD, the annual incidence of SCD was lower than previously reported estimates—100 deaths per 100,000 among 20–75 year-old residents of Maastricht, the Netherlands,18 and 53 deaths per 100,000 among residents of Multnomah County, OR, USA.11 Data from community studies in China and Ireland, which also used multiple sources to identify SCD, indicate that SCD incidence is 40–50 per 100,000 persons annually.19,20 Furthermore, SCD occurred in 6.8% of the ~5,000 individuals in the Framingham Heart Study over ~50 years of follow-up13,21 and in 4.4% of the ~7,000 individuals in the Paris Prospective Study over 23 years of follow-up.22 On the basis of these figures, the annual incidence of sudden cardiac death in the US (total population ~320 million) would range between 180,000 and 250,000 cases per year.2
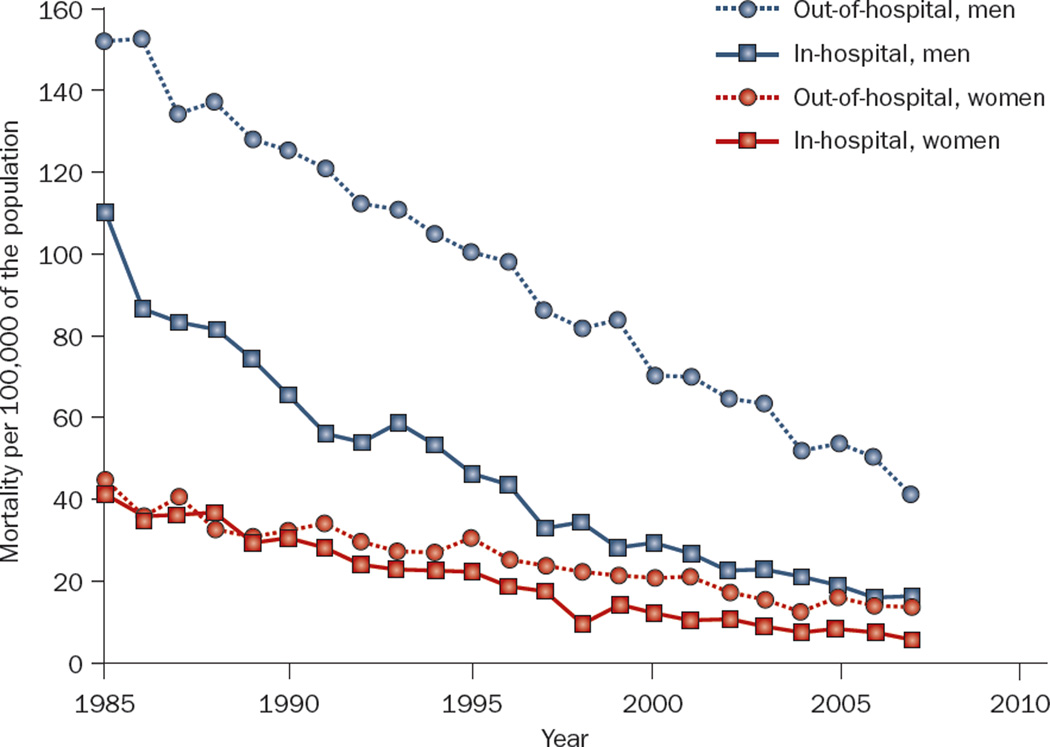
Deaths from CHD have declined markedly over the past several decades. This change is largely attributable to improvements in the primary and secondary prevention of CHD and to progress in acute treatment strategies.23–25 The incidence of SCD has also declined, in parallel with the decline in CHD mortality.14,21,23 A 49% decrease in the risk of SCD over 50 years was observed in the Framingham Heart Study.21 The temporal decline in SCD was more pronounced among patients with known CHD than in those without CHD,14 and was greater among men than women.1 Despite the reduction in the absolute rate of SCD, the incidence of SCD as a proportion of overall cardiovascular deaths has increased, because in-hospital mortality has declined more rapidly than out-of-hospital mortality (Figure 1). Therefore, SCD now accounts for more than half of all CHD deaths.1 These trends underscore the need for a renewed emphasis on primary prevention of CHD. Although survival after cardiac arrest has not changed appreciably in the past three decades, the long-term prognosis of those who survive to hospital discharge after a sudden cardiac arrest has improved.26 In Olmsted County, MN, USA, the 5-year survival of patients who had an out-of-hospital cardiac arrest with ventricular fibrillation and survived to be discharged from the hospital was 79%, equal to age, sex, and disease-matched individuals without cardiac arrest.26 These data highlight the successes of secondary prevention and ICD therapy.27
Figure 1.
Temporal trends in in-hospital and out-of-hospital cardiovascular mortality among men and women living in Minneapolis–St Paul, MN, USA.
Age, sex, and race
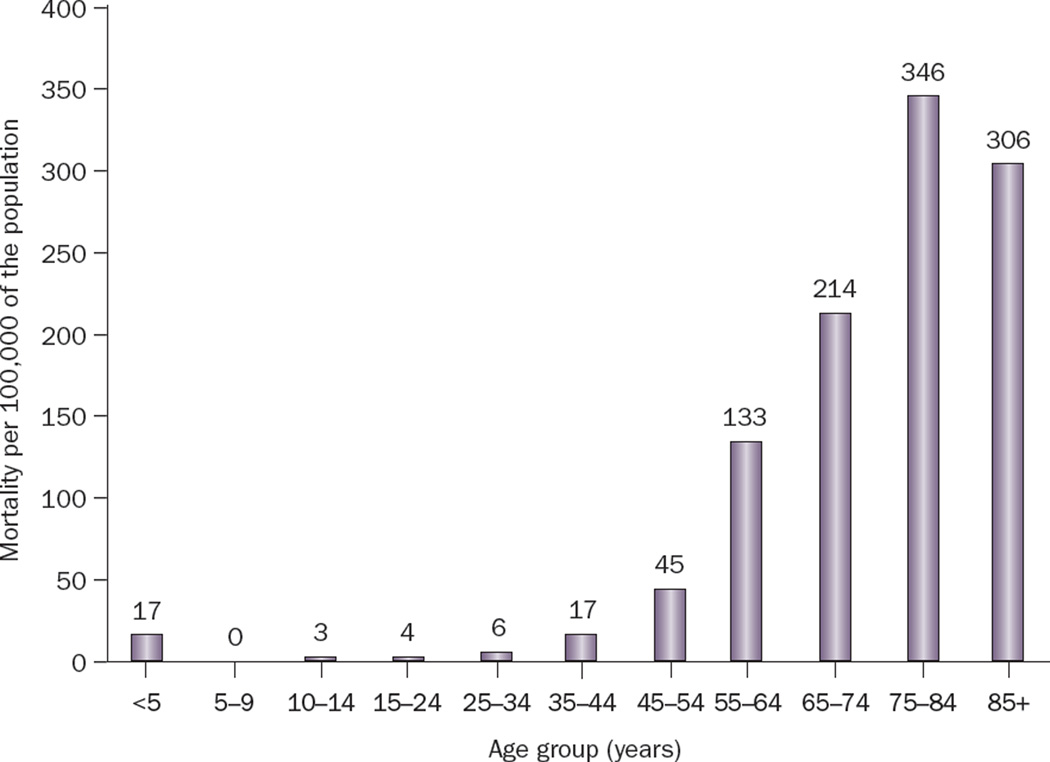
The age distribution of SCD demonstrates peaks during infancy and after the age of 45 years (Figure 2). In adults, the risk of SCD increases with age and mirrors the incidence of CHD.8,11 In the young (<30 years of age), however, the most common causes of SCD include cardiomyopathies, coronary anomalies, primary arrhythmogenic disorders, and drug abuse, rather than CHD.28 Middle-aged men have a fourfold greater risk of SCD when compared with women of the same age.8 However, this difference decreases with age, possibly as a result of the postmenopausal development of CHD in women. Furthermore, an increase in the proportion of out-of-hospital CHD deaths occurring in women has been observed since the 1970s.11,29 This shift is attributed to a lower rate of decline in total mortality and SCD incidence among women in comparison with men, for reasons that are as yet unclear (Figure 1).1,15
Figure 2.
Age distribution of sudden cardiac death among residents of Multnomah County, OR, USA (population 660,486) between 1 February 2002 and 31 January 2003. Reprinted from the Journal of the American College of Cardiology, 44(6), Chugh, S. S. et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large US community. 1268–1275 © 2004 with permission from Elsevier and the American College of Cardiology.
Racial differences in the incidence of SCD have not been well investigated. The available data from death certificates suggest that, for both sexes, SCD is more common among black Americans than white and Hispanic Americans.1,30 Moreover, in the US, black patients with in-hospital cardiac arrest are significantly less likely to survive to hospital discharge, are less likely to survive after cardiopulmonary resuscitation (CPR), and have lower rates of postresuscitation care than white patients.31 This racial difference in outcomes substantially decreases after adjustment for the effect of the hospital site at which patients received care.31
Temporality and variation in rhythm
Approximately 80% of all SCDs occur in the home and around 60% are witnessed.18,32–34 Several studies have demonstrated that there is a heightened risk of SCD on Mondays, in the early hours of the morning (0500 h to 0900 h), and during the winter months, with particular association with lower temperatures (<0 °C).35–38 These temporal variations are thought to be secondary to increased ischemia owing to factors such as increased adrenergic activity. Overall, relatively few people who experience a sudden cardiac arrest receive CPR from a bystander, and this action is more likely to occur in public places than in the home.18,32,39
In most cases, SCD is thought to be the consequence of ventricular tachycardia, degenerating to ventricular fibrillation and subsequent asystole. In a study of confirmed cardiac arrest, where a defibrillator was used within 3 min of cardiac arrest, the initial rhythm was ventricular tachycardia or fibrillation in 71% of individuals, asystole in 18%, and pulseless electrical activity in 11%.40 However, there was a 43% decline in the incidence of ventricular fibrillation as the causative rhythm disturbance between 1980 and 2000 among patients treated for out-of-hospital cardiac arrest in Seattle, WA, USA.15 In the year 2000, only 41% of the cardiac arrests were due to ventricular fibrillation.15 Similar reductions in the incidence of ventricular fibrillation in Finland and Sweden have also been reported.41,42 The reasons for this change are speculative, but could be related to aging of the population with a higher prevalence of comorbidities, including heart failure. Whereas ventricular fibrillation can be a manifestation of ischemia, asystole is often the initial rhythm in cardiac arrest caused by heart failure.
SCD after myocardial infarction
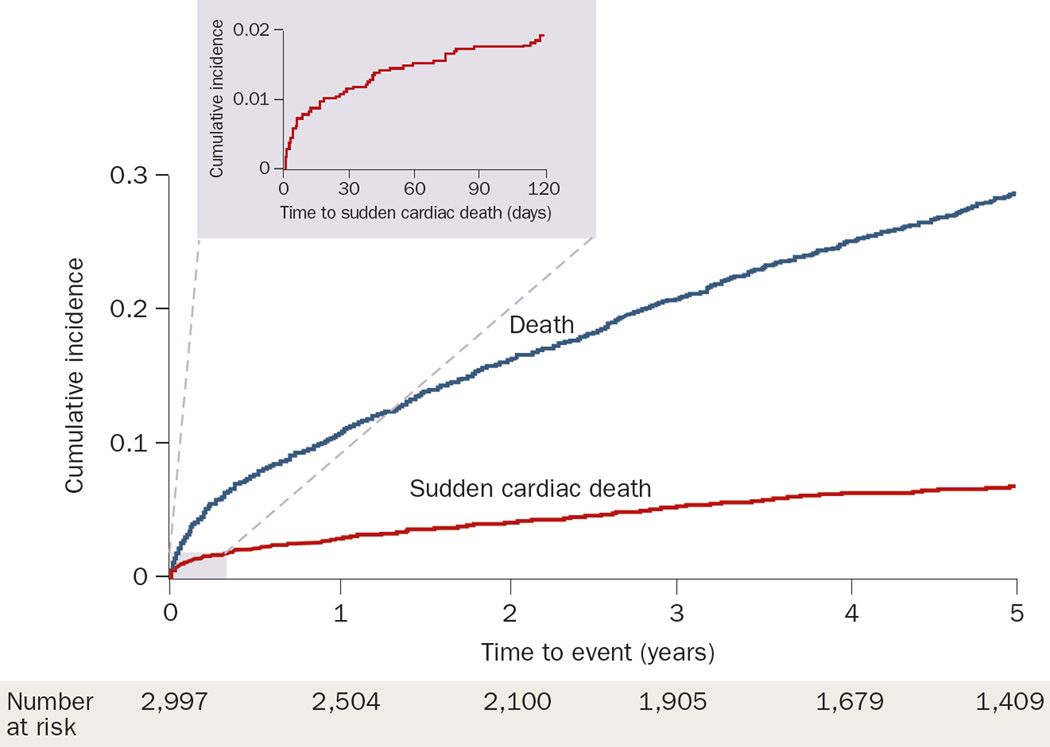
Acute ST-segment elevation myocardial infarction (MI) is associated with ventricular arrhythmias and cardiac arrest. The risk of SCD is highest in the first 30 days after MI, and decreases gradually with time.43–45 Among survivors of MI with left ventricular (LV) dysfunction or heart failure, the risk of SCD has been reported to be 1.4% in the first 30 days, but 0.14% per month after 2 years.44 In a community-based cohort, the risk of SCD was 1.2% within the first month after MI, markedly exceeding the incidence in the general population43 (Figure 3). Thereafter, however, the SCD risk declined markedly to 1.2% per year, which is lower than expected in the general population (~3% per year). This risk reduction is largely the result of secondary prevention measures, the early death of patients with severe disease, or both. Classic teaching is that arrhythmias in the first 12–24 h after MI do not predict SCD, although this notion has been challenged.46 Current practice guidelines recommend assessing LV function 6 weeks after MI to determine whether ICD implantation for primary prevention of SCD is recommended. Zaman et al. showed that ventricular tachycardia, induced during programmed electrical stimulation (which was performed to risk stratify patients early after MI and subsequent revascularization), identifies those at high risk of SCD who are likely to benefit from ICD implantation.47
Figure 3.
Cumulative incidence of sudden cardiac death and all-cause mortality after myocardial infarction among residents of Olmsted County, MN, USA. The shaded area represents the cumulative incidence of sudden cardiac death during the first 120 days after the index myocardial infarction. Reproduced from Adabag, A. S. et al. Sudden death after myocardial infarction. JAMA 5 November 2008; 300(17), 2022–2029. © 2008 American Medical Association. All rights reserved.
The incidence of SCD after MI has decreased over the years in parallel with the decline in CHD mortality and SCD in the general population.43 In a study by Marcus and colleagues, which was conducted in the 1980s, approximately 10% of MI survivors died suddenly during the 4-year follow-up period.48 This rate has now decreased to less than 1% per year among patients who receive optimal medical therapy and revascularization.49,50 Indeed, in Olmsted County, MN, USA, the risk of SCD after MI has declined by more than 40% over the past 25 years.43 This decline predates the widespread use of ICDs, but parallels the increased use of reperfusion therapy and secondary prevention measures after MI. In the 1980s, 40–50% of deaths after MI were caused by sudden cardiac arrest,48,51 but the proportion of SCDs has decreased to 20–30% in more contemporary cohorts.43,49,50
The concept of ‘dynamic risk profiling’ after MI relates to changes in the presence and power of risk markers over time, including LV remodeling, changes in the anatomical and electrophysiological properties of the myocardial scar, and progression of CHD.6 Indeed, the risk of SCD beyond the first 30 days after MI is markedly increased by the presence of concomitant heart failure and ischemic events, which occur frequently during follow-up.43,50–53 Among participants of MADIT-II52 who were randomly assigned to receive an ICD, heart failure and recurrent ischemia were associated with a 2.5-fold and 1.5-fold higher risk of appropriate shocks for ventricular tachycardia or fibrillation, respectively. Also, among residents of Olmsted County, MN, USA who survived an MI, the occurrence of heart failure was associated with a fourfold increase in SCD risk, with the majority of SCDs occurring within 30 days of the heart failure episode.43 These findings underscore the importance of continued surveillance and dynamic risk profiling of patients after MI.
Risk factors for SCD
Clinical risk predictors
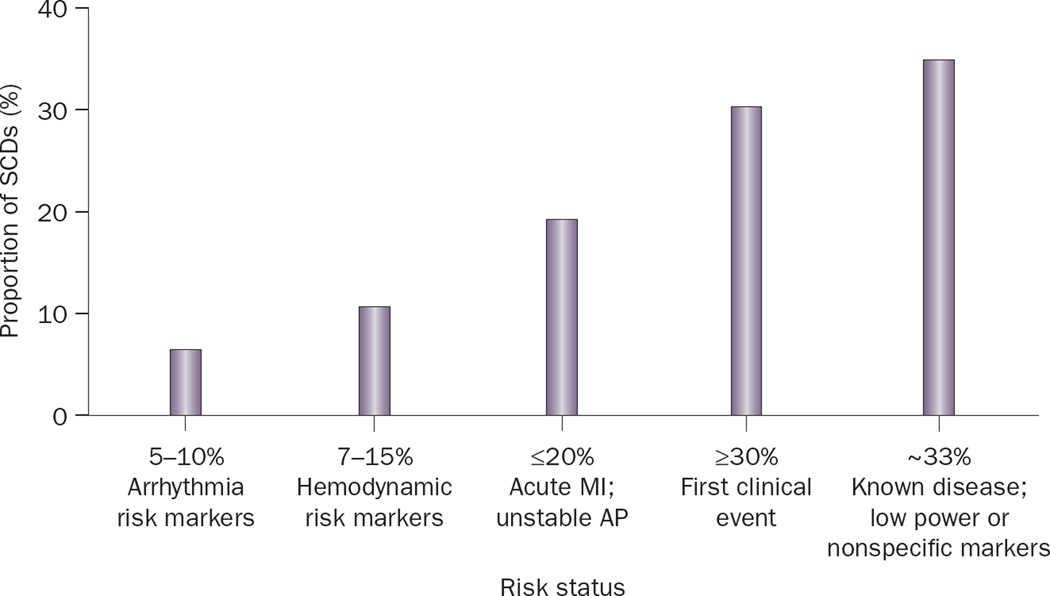
Most people who suffer SCD have CHD. In around 50% of cases, SCD is the first clinical manifestation of heart disease.18 Therefore, it is not surprising that clinical risk factors for SCD are also predictors of CHD-related death and all-cause mortality.6 Indeed, risk factors such as, older age, male sex, cigarette smoking, hypertension, diabetes mellitus, hypercholesterolemia, obesity, and family history of CHD have all been associated with an increased risk of SCD.8,22,53–55 Although these risk factors are powerful predictors at a population level, they are not specific enough to determine risk in an individual patient because of relatively low event rates (that is, low absolute risk). Additional SCD risk factors, such as LV dysfunction, history of heart failure, LV hypertrophy, poor functional status, elevated heart rate, an abnormal electrocardiogram, and abnormal autonomic markers, also lack the specificity to discriminate SCD from non-sudden death.3,8,56–59 Only a small proportion of SCDs occur in patients with known markers of high-risk for arrhythmia5 (Figure 4). Thus, in the absence of specific single markers, a multimarker strategy may be necessary to identify individuals with high SCD risk. Indeed, Buxton et al.56,57 and others have created multivariable risk algorithms for SCD that are based on retrospective analyses of large clinical trials.60–62 The markers in their algorithm were age, functional class, history of heart failure, nonsustained ventricular tachycardia, LV ejection fraction, LV conduction abnormalities, inducible sustained ventricular tachycardia, and atrial fibrillation. However, these algorithms are yet to be validated prospectively in large population studies.
Figure 4.
Distribution of clinical status of individuals who suffer sudden cardiac death. Abbreviations: AP, angina pectoris; MI, myocardial infarction; SCD, sudden cardiac death. Reprinted from Journal of the American College of Cardiology 54(9), Myerburg, R. J., Reddy, V. and Castellanos, A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. 747–763. Copyright (2009), with permission from Elsevier and the American College of Cardiology.
Autopsy findings
Autopsy studies have shown that approximately 80% of adults who suffer SCD have severe CHD,8,63 10–15% have dilated or hypertrophic cardiomyopathy, and 5–10% have structurally normal hearts, suggesting that a primary arrhythmogenic disorder was the cause of death.1 A substantial proportion (10–80%) of those with CHD have intracoronary plaque rupture, thrombus formation, or both, which are indicative of an acute coronary syndrome,63–65 and around 30% have myocardial scar from a previous MI, creating a substrate for ventricular tachycardia.65 LV hypertrophy, inflammation, infiltrative diseases (such as amyloidosis), and interstitial fibrosis are other structural abnormalities that create a potential substrate for ventricular arrhythmias.66–68 Although clinical reports suggest that cardiac structural abnormalities are absent in up to 10% of survivors of sudden cardiac arrest and those who died suddenly,2,3,8 careful evaluation of clinical history has revealed potential risks for SCD in the majority of these patients.66 For example, Shen et al. reported that 33% of 20–40 year-olds who suffered SCD had a history of cocaine abuse.28
Heart failure
More than 5 million people in the US have heart failure, and around 600,000 new cases occur annually.69 The process of remodeling in heart failure, which encompasses cellular, structural, and electrical changes in the myocardium, and neurohormonal activation, provide a suitable milieu for arrhythmogenesis.70 Consequently, clinical heart failure leads to a fivefold increase in SCD risk.43,71 Furthermore, SCD accounts for 30–50% of all deaths in patients with heart failure.71 With advancing symptoms and decompensation of heart failure, however, the proportion of deaths that are classified as SCD decrease whereas those caused by heart failure itself increase. Reports suggest that 64% of patients with mild symptoms of heart failure die suddenly as opposed to 33% of those with severe symptoms.5 Increased levels of brain natriuretic peptide—a biomarker secreted in response to ventricular stretch—was associated with an increased risk of SCD in patients with LV dysfunction and heart failure, and among women who participated in the Nurses’ Health Study.72–74
Severe LV dysfunction, caused by ischemic or non-ischemic cardiomyopathy, is also a marker of elevated SCD risk.75 In current practice guidelines, an LV ejection fraction of less than 35% is a major criterion for ICD therapy.75 However, only 20–30% of ICD recipients in randomized clinical trials receive appropriate ICD shocks over 4 years of follow-up, reducing the positive predictive value of LV dysfunction as a marker.76,77 Furthermore, in population cohort studies, approximately 65% of those who suffer SCD have either normal or mildly depressed LV function (that is, an ejection fraction of 35–50%).18,78,79 Therefore, severe LV dysfunction alone is not a sufficiently specific marker for SCD, but could be useful when used with other predictors or as part of a multivariable risk score.56,61,62
Electrocardiographic risk predictors
Abnormalities on a 12-lead electrocardiogram raise the suspicion of underlying structural or genetic heart diseases associated with SCD (Box 1). Pathologic Q waves or dynamic ST-segment changes on electrocardiography are indicative of CHD, whereas increased R-wave voltage or prolonged QRS duration are signs of LV hypertrophy and cardiomyopathy, respectively. Left bundle (but not right bundle) branch block or LV hypertrophy on electrocardiography are associated with a mildly increased risk of SCD (hazard ratio ~1.5 for both).58,80 In addition, prolonged QRS duration was associated with SCD and ventricular tachyarrhythmias in two large clinical trials,81,82 and Das et al. have suggested that fragmented QRS on electrocardiography is a marker of structural heart disease and predicts SCD.83
Box 1. Electrocardiographic markers of SCD risk.
12-lead electrocardiography
-
▪
Pathologic Q waves or dynamic ST-segment changes
-
▪
Prolonged QRS duration
-
▪
Increased R-wave voltage
-
▪
Fragmented QRS
-
▪
Prolonged QT interval
Exercise electrocardiography
-
▪
Reduced heart-rate recovery
-
▪
Reduced functional capacity
-
▪
Increased ventricular ectopy
-
▪
T-wave alternans
Signal-averaged electrocardiography
-
▪
Late potentials
Ambulatory Holter electrocardiography
-
▪
Reduced heart-rate variability
-
▪
Nonsustained ventricular tachycardia
The electrocardiogram is particularly helpful in diagnosing primary arrhythmogenic disorders, such as the long and short QT syndromes, Brugada syndrome, arrhythmogenic right ventricular cardiomyopathy, and Wolff–Parkinson–White syndrome, all of which are associated with SCD.8,84,85 These conditions are rare in the community; however, QT-interval prolongation and dispersion, which are more common and indicate prolonged repolarization, have also been associated with SCD in the general population.2,86,87 Individuals with a corrected QT interval of greater than 440 ms have a 2.3-fold higher risk of SCD than those with corrected QT interval of less than 440 ms, independent of age, sex, heart rate, and drug use.85 Furthermore, those with QT prolongation in the absence of QT prolonging drugs or diabetes have a fivefold increased risk of SCD.87
Abnormal heart rate profile on exercise electrocardiography, late potentials on signal-averaged electrocardiography, microvolt T-wave alternans, and reduced heart rate variability on Holter electrocardiography have all been shown to correlate with increased risk of SCD.46,88,89 However, these specialized markers have a high negative predictive value and a low positive predictive value. Thus, SCD risk is low with a negative test, but indeterminate with a positive test.
Socioeconomic and psychosocial risk predictors
Low socioeconomic status is associated with an increased prevalence of risk factors for cardiovascular disease, CHD, and cardiovascular mortality.90 The incidence of out-of-hospital cardiac arrest and SCD are also higher in areas of socioeconomic deprivation than in more affluent areas.2,91,92 The effect of socioeconomic status on SCD is more marked among individuals younger than 65 years of age.92 The mechanisms underlying the apparent association between socioeconomic status and SCD probably reflect a confluence of behavioral, environmental, and coronary risk factors, such as smoking and reduced access to health care.
Psychosocial risk factors, such as social isolation, a high degree of life stress, and substantial life changes have also been associated with SCD.8,93 Among men with complex premature ventricular contractions after MI, those with lower educational status had threefold higher mortality than those who were better educated.93 Furthermore, individuals who have suffered SCD have been reported to have experienced more life-changing events during the 6 months before SCD than controls.8 Hostility and history of psychiatric diseases were also associated with an increased prevalence of SCD.94 Whether the elevated risk of SCD is associated with the presence of CHD risk factors (such as smoking) in this population, or with the potential arrhythmic consequences of psychoactive medications, is yet to be established.94
Genetic risk predictors
The genetics of rare arrhythmogenic syndromes associated with SCD, such as long QT syndrome, have been recognized for several decades; however, the possibility of a genetic basis for SCD in the general population has only been proposed in the last 10 years.22,95–97 Jouven et al. found that SCD risk was twofold higher if an individual had one parent who died suddenly, and ninefold higher if both parents died suddenly.22 This effect was independent of parental history of MI. Dekker et al. reported that family history of SCD (odds ratio ~2.5) and cumulative ST-segment deviation (odds ratio ~1.5) were the only differences between patients who had cardiac arrest with acute MI and those who did not.95 These data, therefore, indicate that heritable factors play an important role in determining SCD risk, possibly because of shared genes that increase vulnerability to life-threatening arrhythmias. The selective effect of family history on SCD risk, independent of MI, suggests that at least some genetic factors may specifically predispose the individual to fatal arrhythmias, rather than the effect being mediated through an increased risk of CHD. Furthermore, the increase in SCD risk with a greater number of relatives affected is consistent with a complex genetic architecture, in which susceptibility alleles increase risk additively.98
The genetic origins of complex events, such as SCD, can be explained either by rare variants with strong effects, rare variants with modest effects, or common variants with modest effects.89 Rare variants with strong effects have been identified in genes that lead to uncommon inherited cardiac diseases associated with increased risk of ventricular arrhythmias and SCD, such as long and short QT syndromes, Brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia.89 Molecular characterization of these diseases has provided the evidence that mutations in specific genes predispose the individual to SCD and has increased our understanding of the mechanisms of arrhythmogenesis. However, these rare mutations are subject to negative selection of allele frequency and contribute little to the burden of SCD in the general population.89,98
Rare variants with modest effect are ‘less malignant’ variations in the genes responsible for the arrhythmogenic disorders, such as long QT syndrome. These relatively unimportant changes could increase susceptibility to arrhythmias in the general population. Indeed, evidence from autopsy series suggests that those who have suffered apparently idiopathic SCD might harbor mutations in these candidate genes, particularly those described in long QT syndrome.99–103 Chugh et al. identified a mutation in KCNH2 (HERG), which encodes a voltage-gated potassium channel, in 16.7% of 12 adults who had suffered SCD.99 Tester et al. showed that 30% of 49 individuals who died of unexplained sudden cardiac arrest harbored a mutation in one of the genes associated with long QT syndrome, and 14% had a mutation in the ryanodine receptor gene (RYR2).100,101 Furthermore, in the Nurses’ Health Study, rare missense variants in SCN5A, which encodes the sodium channel alpha subunit, were detected in 10% of participants who died suddenly versus 1.6% of matched controls.102 In a community-based study in Hennepin County, MN, USA, missense mutations in genes associated with long QT syndrome, all localized to SCN5A, were found in 6% of individuals who suffered SCD.103 These data support the concept that rare variants with modest effects might not produce an identifiable clinical syndrome in isolation, but could predispose the individual to acquired long QT syndrome and SCD after exposure to a secondary risk factor, such as a QT-interval-prolonging medication.89
Of potentially greater relevance to the general population are common genetic variants with modest effects that could contribute incrementally to SCD risk. Individuals with these gene variants may survive to reproductive age and, therefore, the variant remains unaffected by negative selection and can reach relatively high allele frequency in the population. Splawksi et al. identified the S1102Y variant of the sodium channel gene SCN5A in 57% of 23 black patients with an arrhythmia, syncope, and QT prolongation versus 13% of healthy control individuals.104 This variant allele accelerates sodium-channel activation, potentially increasing the likelihood of arrhythmia. In a follow-up autopsy study, the S1102Y allele was found in the SCN5A gene of 28% of black individuals without structural heart disease who had suffered SCD, and in only 5.6% of controls who had died suddenly from non-cardiac causes.105 Common genetic variants in isolation are unlikely to cause SCD because, if they did, strong selection pressure would reduce their allele frequency substantially.89,98 More probably, these variants contribute only incrementally to the overall risk of SCD by reducing ‘repolarization reserve’ and predisposing some individuals to SCD through interactions with other risk factors such as ischemia, hypokalemia, or drug exposure.
As discussed above, QT prolongation is a consistent risk factor for SCD in the general population.86,106 The QT interval—adjusted for heart rate, age, and sex—is normally distributed in the general population and around 35% of QT interval variability is attributable to genetic factors.107 Common variants in the KCNH2 (voltage-gated potassium channel) and NOS1AP (nitric oxide synthase 1 adaptor protein) genes influence QT interval duration.106,108–110 However, these variants have a modest effect on QT interval at baseline (ranging from 6 to 12 ms) and an external influence is also likely to be necessary to prolong the QT interval to a degree where the SCD risk increases substantially. Therefore, the available evidence suggests that inheritable factors are partly involved in the pathogenesis of SCD, but the genetic basis of these events in the population is multifactorial and potentially includes the genetics of atherothrombosis and plaque stability, among other factors.111,112
Triggers of SCD
The current paradigm in SCD requires the presence of an abnormal myocardial substrate, such as CHD, and a transient external or internal factor that triggers cardiac arrest. While the abnormal substrate is identifiable in most cases of SCD, the transient triggers are not because a substantial proportion of deaths are unwitnessed or the accounts of the witnesses are biased. The majority of individuals who suffer a witnessed SCD report angina, dyspnea, nausea or vomiting, dizziness or syncope, and ‘not feeling well’ before cardiac arrest.18,32 In a community-based SCD study in Hennepin County, MN, USA, we observed that 50% of those who died from a sudden cardiac arrest had taken analgesic and anti-inflammatory medications shortly before death, suggesting that they were not feeling well.103 In the same study, around 30% of those who suffered SCD had smoked in the hours before death, and this risk factor may have acted as a trigger. Indeed, cigarette smoking promotes platelet aggregation and catecholamine surges, increasing the likelihood of plaque rupture, coronary vasospasm, and thrombus formation.64,113 Symptoms, therefore, often seem to be present before cardiac arrest, but are tolerated for prolonged periods of time (~75 min) particularly when the individual is at home.32 These findings underscore the importance of educating patients with cardiovascular disease and their families about the warning signs of impending sudden cardiac arrest and promoting early intervention.
Public-access defibrillators
Prompt defibrillation of an individual who has suffered a sudden cardiac arrest is the most important determinant of survival. For every minute that passes between cardiac arrest and defibrillation, survival decreases by 7–10% without CPR, and by 3–4% with immediate CPR.114 After 10 min or longer without defibrillation, 95% of patients die. The response times for emergency medical services in most areas of the US are typically 8–15 min; therefore, overall survival after sudden cardiac arrest in most communities is only 5–10%.33,114 However, patients who are defibrillated within 10 min of cardiac arrest have a 40% chance of surviving to hospital discharge neurologically intact.26 The long-term survival and quality-of-life scores of these patients are equal to age-matched and sex-matched individuals in the general population.26
AEDs are portable, computerized devices that can analyze cardiac rhythm accurately and deliver a biphasic electrical shock in cases of ventricular tachycardia or fibrillation. Studies have shown that AEDs can be easily operated by untrained lay persons.115,116 Indeed, AED programs at airports and casinos have been associated with 50–75% survival among individuals who suffer an out-of-hospital cardiac arrest when immediate CPR is provided and defibrillation occurs within 3–5 min of cardiac arrest.40,114,116,117 In the Public Access Defibrillation Trial,118 volunteer responders from ~1,000 community locations, such as shopping malls or apartment complexes, were randomly assigned to undergo training in CPR alone or to training in CPR plus AED use. Individuals who suffered a cardiac arrest in these communities were twice as likely to survive to hospital discharge when the responder was trained in CPR plus AED use than if the responder was trained in CPR alone.118 Currently, all federal buildings and airports in the US, and passenger airplanes run by US-based airlines, are legally required to have AEDs. Health and fitness facilities, and schools are also recommended to have AEDs, and many states are passing laws mandating AEDs in public places.
Since most SCDs occur in the home, the Home Automated External Defibrillator Trial119 was performed to assess whether AED placement at homes of individuals who are at increased risk of SCD would save lives. About 7,000 patients with prior MI who were not candidates for an ICD were randomly assigned to have an AED in their home or to control response (calling the emergency medical services and performing CPR; both groups were trained in this response) in case of a cardiac arrest. After ~3 years, there was no difference in survival between the groups.119 AEDs were used in only 32 patients, of whom 14 received an appropriate shock and four survived to hospital discharge.119 Thus, at present, home usage of AEDs is not recommended as a general health policy. However, there is no reason why a strategy of AED use in the home should not work, when there is a willingness to use these devices in the household of the individual at risk.
Conclusions
In the last 60 years much progress has been made in the understanding of the mechanisms, risk factors, and management of SCD. However, despite the progress in knowledge and the temporal reduction in cardiovascular mortality, SCD remains a major public-health problem. One of the challenges is determining the true incidence of SCD in the community, a problem that is exacerbated by the inconsistency of the definition of SCD used by investigators. Thus, consensus on a specific definition for SCD and establishing prospective, community-based surveillance programs using multiple sources to identify cases of SCD would enable more accurate determination of SCD incidence. In addition, a statement by the AHA published in 2008 recommended that all out-of-hospital cardiac arrests should be reportable events.120
Another challenge is the accurate identification of the person at risk. Virtually all individual SCD risk markers, including LV ejection fraction, lack specificity. Multimarker SCD risk scores that include demographic, clinical, and laboratory variables could prove to be more useful in identifying persons at risk of SCD. Several such risk scores have been developed and retrospectively tested among participants of large clinical trials,56,57,61,62,75 but prospective testing in the general population has not been performed. Perhaps the greatest challenge in identifying the high-risk individual lies in the observation that SCD is the first manifestation of cardiac disease for the majority of those who suffer a sudden cardiac arrest. Heart disease surveillance programs and community-wide interventions for risk-factor reduction (for example, smoking cessation) are, therefore, extremely important in reducing the incidence of SCD. Indeed, a slower reduction in mortality from out-of-hospital SCD than for in-hospital SCD, and among those without known CHD than in patients with CHD, suggest that there is room for improvement in the primary prevention of SCD. Finally, survival after sudden cardiac arrest continues to be dismal at ~5% and many individuals do not receive bystander CPR or timely defibrillation. Thus, educating the general public to perform CPR and use AEDs, and increasing their willingness and competence to do so, will be vital to increase survival after sudden cardiac arrest.
Key points.
-
▪
Sudden cardiac death (SCD) is a common public health problem that causes more than 60% of all deaths from cardiovascular disease
-
▪
Coronary heart disease underlies 80% of SCD cases; SCD is the first manifestation of heart disease in 50% of these individuals
-
▪
Prospective surveillance programs, using multiple sources to identify cases of SCD, would enable more accurate determination of SCD burden in the community
-
▪
Demographic, clinical, structural, laboratory, and genetic risk factors lack the specificity to identify individuals at high risk for SCD when used alone; multimarker SCD risk scores may improve SCD prediction
-
▪
The risk of SCD after a coronary event changes with time, therefore, dynamic risk-profiling is important
-
▪
Survival after sudden cardiac arrest is ~5% and many individuals do not receive cardiopulmonary resuscitation or defibrillation; educating the public to use automated external defibrillators will be important to improving survival
Review criteria.
Articles were selected for inclusion in this Review by a search of the MEDLINE database. Key words used in the search were “sudden cardiac death”, and “epidemiology” in combination with additional keywords (such as “genetics”) relating to each subheading of the manuscript. The search was limited to English language articles and human data. No time limits were applied.
Acknowledgments
This work was funded in part by NIH grant number: NIH 5 R01 HL023727.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog. Cardiovasc. Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2251. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 4.Engdahl J, Holmberg M, Karlson BW, Luepker R, Herlitz J. The epidemiology of out-of-hospital ‘sudden’ cardiac arrest. Resuscitation. 2002;52:235–245. doi: 10.1016/s0300-9572(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 5.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J. Cardiovasc. Electrophysiol. 2002;13:709–723. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 6.Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J. Am. Coll. Cardiol. 2009;54:747–763. doi: 10.1016/j.jacc.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB. Clinical misconceptions dispelled by epidemiological research. Circulation. 1995;92:3350–3360. doi: 10.1161/01.cir.92.11.3350. [DOI] [PubMed] [Google Scholar]
- 8.Myerburg R, Castellanos A. In: Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine. 8th. Libby P, Bonow RO, Mann DL, Zipes DP, editors. Philadelphia: Saunders; 2007. pp. 933–974. [Google Scholar]
- 9.Pratt CM, Greenway PS, Schoenfeld MH, Hibben ML, Reiffel JA. Exploration of the precision of classifying sudden cardiac death. Implications for the interpretation of clinical trials. Circulation. 1996;93:519–524. doi: 10.1161/01.cir.93.3.519. [DOI] [PubMed] [Google Scholar]
- 10.Carlson MD. Classification of death in clinical trials: precision versus accuracy. J. Cardiovasc. Electrophysiol. 1999;10:1057–1059. doi: 10.1111/j.1540-8167.1999.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 11.Chugh SS, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U. S. community. J. Am. Coll. Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Iribarren C, Crow RS, Hannan PJ, Jacobs DR, Jr, Luepker RV. Validation of death certificate diagnosis of out-of-hospital sudden cardiac death. Am. J. Cardiol. 1998;82:50–53. doi: 10.1016/s0002-9149(98)00240-9. [DOI] [PubMed] [Google Scholar]
- 13.Fox CS, et al. A comparison of death certificate out-of-hospital coronary heart disease death with physician-adjudicated sudden cardiac death. Am. J. Cardiol. 2005;95:856–859. doi: 10.1016/j.amjcard.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Goraya TY, et al. Validation of death certificate diagnosis of out-of-hospital coronary heart disease deaths in Olmsted county, Minnesota. Mayo Clin. Proc. 2000;75:681–687. doi: 10.4065/75.7.681. [DOI] [PubMed] [Google Scholar]
- 15.Cobb LA, Fahrenbruch CE, Olsufka M, Copass MK. Changing incidence of out-of-hospital ventricular fibrillation, 1980–2000. JAMA. 2002;288:3008–3013. doi: 10.1001/jama.288.23.3008. [DOI] [PubMed] [Google Scholar]
- 16.Kass LE, et al. One-year survival after prehospital cardiac arrest: the Utstein style applied to a rural–suburban system. Am. J. Emerg. Med. 1994;12:17–20. doi: 10.1016/0735-6757(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi G, Gallagher J, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City. The Pre-Hospital Arrest Survival Evaluation (PHASE) Study. JAMA. 1994;271:678–683. [PubMed] [Google Scholar]
- 18.de Vreede-Swagemakers JJ, et al. Out-of-hospital cardiac arrest in the 1990s: a population-based study in the Maastricht area on incidence, characteristics and survival. J. Am. Coll. Cardiol. 1997;30:1500–1505. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 19.Hua W, et al. Incidence of sudden cardiac death in China: analysis of 4 regional populations. J. Am. Coll. Cardiol. 2009;54:1110–1118. doi: 10.1016/j.jacc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Byrne R, et al. Multiple source surveillance incidence and aetiology of out-of-hospital sudden cardiac death in a rural population in the west of Ireland. Eur. Heart J. 2008;29:1418–1423. doi: 10.1093/eurheartj/ehn155. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 22.Jouven X, Desnos M, Guerot C, Ducimetière P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 23.Ni H, et al. Trends from 1987 to 2004 in sudden death due to coronary heart disease: the Atherosclerosis Rsk in Communities (ARIC) Study. Am. Heart J. 2009;157:46–52. doi: 10.1016/j.ahj.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luepker RV. Decline in incident coronary heart disease: why are the rates falling? Circulation. 2008;117:592–593. doi: 10.1161/CIRCULATIONAHA.107.747477. [DOI] [PubMed] [Google Scholar]
- 25.McGovern PG, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 26.Bunch TJ, et al. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N. Engl. J. Med. 2003;348:2626–2633. doi: 10.1056/NEJMoa023053. [DOI] [PubMed] [Google Scholar]
- 27.Rea TD, Crouthamel M, Eisenberg MS, Becker LJ, Lima AR. Temporal patterns in long-term survival after resuscitation from out-of-hospital cardiac arrest. Circulation. 2003;108:1196–1201. doi: 10.1161/01.CIR.0000087403.24467.A4. [DOI] [PubMed] [Google Scholar]
- 28.Shen WK, et al. Sudden unexpected nontraumatic death in 54 young adults: a 30-year population-based study. Am. J. Cardiol. 1995;76:148–152. doi: 10.1016/s0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 29.Gerber Y, et al. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 30.Gillum RF. Sudden cardiac death in Hispanic Americans and African Americans. Am. J. Public Health. 1997;87:1461–1466. doi: 10.2105/ajph.87.9.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan PS, et al. Racial differences in survival after in-hospital cardiac arrest. JAMA. 2009;302:1195–1201. doi: 10.1001/jama.2009.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller D, Agrawal R, Arntz HR. How sudden is sudden cardiac death? Circulation. 2006;114:1146–1450. doi: 10.1161/CIRCULATIONAHA.106.616318. [DOI] [PubMed] [Google Scholar]
- 33.Weaver WD, Peberdy MA. Defibrillators in public places—one step closer to home. N. Engl. J. Med. 2002;347:1223–1224. doi: 10.1056/NEJMp020108. [DOI] [PubMed] [Google Scholar]
- 34.Becker L, Eisenberg M, Fahrenbruch C, Cobb L. Public locations of cardiac arrest. Implications for public access defibrillation. Circulation. 1998;97:2106–2109. doi: 10.1161/01.cir.97.21.2106. [DOI] [PubMed] [Google Scholar]
- 35.Muller D, Lampe F, Wegscheider K, Schultheiss HP, Behrens S. Annual distribution of ventricular tachycardias and ventricular fibrillation. Am. Heart J. 2003;146:1061–1065. doi: 10.1016/S0002-8703(03)00426-5. [DOI] [PubMed] [Google Scholar]
- 36.Arntz HR, et al. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur. Heart J. 2000;21:315–320. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 37.Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am. J. Cardiol. 1992;70:65–68. doi: 10.1016/0002-9149(92)91391-g. [DOI] [PubMed] [Google Scholar]
- 38.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Seasonality and daily weather conditions in relation to myocardial infarction and sudden cardiac death in Olmsted county, Minnesota, 1979 to 2002. J. Am. Coll. Cardiol. 2006;48:287–292. doi: 10.1016/j.jacc.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 39.Lopshire JC, Zipes DP. Sudden cardiac death: better understanding of risks, mechanisms, and treatment. Circulation. 2006;114:1134–1136. doi: 10.1161/CIRCULATIONAHA.106.647933. [DOI] [PubMed] [Google Scholar]
- 40.Valenzuela TD, et al. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N. Engl. J. Med. 2000;343:1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 41.Kuisma M, Repo J, Alaspaa A. The incidence of out-of-hospital ventricular fibrillation in Helsinki, Finland, from 1994 to 1999. Lancet. 2001;358:473–474. doi: 10.1016/S0140-6736(01)05634-3. [DOI] [PubMed] [Google Scholar]
- 42.Herlitz J, et al. Experiences from treatment of out-of-hospital cardiac arrest during 17 years in Göteborg. Eur. Heart J. 2000;21:1251–1258. doi: 10.1053/euhj.2000.2150. [DOI] [PubMed] [Google Scholar]
- 43.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon SD, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005;352:2581–2588. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]
- 45.Berger CJ, Murabito JM, Evans JC, Anderson KM, Levy D. Prognosis after first myocardial infarction. Comparison of Q-wave and non-Q-wave myocardial infarction in the Framingham Heart Study. JAMA. 1992;268:1545–1551. doi: 10.1001/jama.268.12.1545. [DOI] [PubMed] [Google Scholar]
- 46.Mehta RH, et al. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. JAMA. 2009;301:1779–1789. doi: 10.1001/jama.2009.600. [DOI] [PubMed] [Google Scholar]
- 47.Zaman S, et al. Outcomes of early risk stratification and targeted implantable cardioverter-defibrillator implantation after ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Circulation. 2009;120:194–200. doi: 10.1161/CIRCULATIONAHA.108.836791. [DOI] [PubMed] [Google Scholar]
- 48.Marcus FI, et al. Mechanism of death and prevalence of myocardial ischemic symptoms in the terminal event after acute myocardial infarction. Am. J. Cardiol. 1988;61:8–15. doi: 10.1016/0002-9149(88)91295-7. [DOI] [PubMed] [Google Scholar]
- 49.Huikuri HV, et al. Prediction of sudden cardiac death after myocardial infarction in the beta-blocking era. J. Am. Coll. Cardiol. 2003;42:652–658. doi: 10.1016/s0735-1097(03)00783-6. [DOI] [PubMed] [Google Scholar]
- 50.Makikallio TH, et al. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am. J. Cardiol. 2006;97:480–484. doi: 10.1016/j.amjcard.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 51.Mukharji J, et al. Risk factors for sudden death after acute myocardial infarction: two-year follow-up. Am. J. Cardiol. 1984;54:31–36. doi: 10.1016/0002-9149(84)90299-6. [DOI] [PubMed] [Google Scholar]
- 52.Singh JP, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the multicenter automatic defibrillator implantation trial II (MADIT-II) J. Am. Coll. Cardiol. 2005;46:1712–1720. doi: 10.1016/j.jacc.2005.05.088. [DOI] [PubMed] [Google Scholar]
- 53.Goldenberg I, et al. Current smoking, smoking cessation, and the risk of sudden cardiac death in patients with coronary artery disease. Arch. Intern. Med. 2003;163:2301–2305. doi: 10.1001/archinte.163.19.2301. [DOI] [PubMed] [Google Scholar]
- 54.Jouven X, et al. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 55.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85(1 Suppl.):I11–I18. [PubMed] [Google Scholar]
- 56.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6:836–847. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Buxton AE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J. Am. Coll. Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 58.McLenachan JM, Dargie HJ. Left ventricular hypertrophy as a factor in arrhythmias and sudden death. Am. J. Hypertens. 1989;2:128–131. doi: 10.1093/ajh/2.2.128. [DOI] [PubMed] [Google Scholar]
- 59.Adabag AS, et al. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am. J. Cardiol. 2008;101:1437–1443. doi: 10.1016/j.amjcard.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson KP. Risk assessment for defibrillator therapy: II Trittico. J. Am. Coll. Cardiol. 2007;50:1158–1160. doi: 10.1016/j.jacc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 61.Goldenberg I, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2008;51:288–296. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 62.Levy WC, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992;85(1 Suppl.):I19–I24. [PubMed] [Google Scholar]
- 64.Burke AP, et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 65.Farb A, et al. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1709. doi: 10.1161/01.cir.92.7.1701. [DOI] [PubMed] [Google Scholar]
- 66.Chugh SS, Kelly KL, Titus JL. Sudden cardiac death with apparently normal heart. Circulation. 2000;102:649–654. doi: 10.1161/01.cir.102.6.649. [DOI] [PubMed] [Google Scholar]
- 67.Kwong RY, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 68.Adabag AS, et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2008;51:1369–1374. doi: 10.1016/j.jacc.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 69.Lloyd-Jones D, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 70.Saxon LA. Sudden cardiac death: epidemiology and temporal trends. Rev. Cardiovasc. Med. 2005;6(Suppl. 2):S12–S20. [PubMed] [Google Scholar]
- 71.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham study. Am. Heart J. 1988;115:869–875. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 72.Chugh SS, Reinier K. Predicting sudden death in the general population: another step, N terminal B-type natriuretic factor levels. Circulation. 2009;119:2863–2864. doi: 10.1161/CIRCULATIONAHA.109.865436. [DOI] [PubMed] [Google Scholar]
- 73.Korngold EC, et al. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. 2009;119:2868–2876. doi: 10.1161/CIRCULATIONAHA.108.832576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger R, et al. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002;105:2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]
- 75.Goldberger JJ, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J. Am. Coll. Cardiol. 2008;52:1179–1799. doi: 10.1016/j.jacc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Bardy GH, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 77.Moss AJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 78.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur. Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 79.Stecker EC, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J. Am. Coll. Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 80.Zimetbaum PJ, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–769. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 81.Dhar R, et al. Association of prolonged QRS duration with ventricular tachyarrhythmias and sudden cardiac death in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) Heart Rhythm. 2008;5:807–813. doi: 10.1016/j.hrthm.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morin DP, et al. QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: The LIFE study. Eur. Heart J. 2009;30:2908–2914. doi: 10.1093/eurheartj/ehp321. [DOI] [PubMed] [Google Scholar]
- 83.Das MK, Zipes DP. Fragmented QRS: a predictor of mortality and sudden cardiac death. Heart Rhythm. 2009;6(3 Suppl.):S8–S14. doi: 10.1016/j.hrthm.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 84.Ott P, Marcus FI. Electrocardiographic markers of sudden death. Cardiol. Clin. 2006;24:453–469. doi: 10.1016/j.ccl.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Al Aloul B, Adabag AS, Houghland MA, Tholakanahalli V. Brugada pattern electrocardiogram associated with supratherapeutic phenytoin levels and the risk of sudden death. Pacing Clin. Electrophysiol. 2007;30:713–715. doi: 10.1111/j.1540-8159.2007.00734.x. [DOI] [PubMed] [Google Scholar]
- 86.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 87.Chugh SS, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stein KM. Noninvasive risk stratification for sudden death: signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog. Cardiovasc. Dis. 2008;51:106–117. doi: 10.1016/j.pcad.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Noseworthy PA, Newton-Cheh C. Genetic determinants of sudden cardiac death. Circulation. 2008;118:1854–1863. doi: 10.1161/CIRCULATIONAHA.108.783654. [DOI] [PubMed] [Google Scholar]
- 90.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 91.Soo L, Huff N, Gray D, Hampton JR. Geographical distribution of cardiac arrest in Nottinghamshire. Resuscitation. 2001;48:137–147. doi: 10.1016/s0300-9572(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 92.Reinier K, et al. Incidence of sudden cardiac arrest is higher in areas of low socioeconomic status: a prospective two year study in a large United States community. Resuscitation. 2006;70:186–192. doi: 10.1016/j.resuscitation.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 93.Ruberman W, Weinblatt E, Goldberg JD, Chaudhary BS. Psychosocial influences on mortality after myocardial infarction. N. Engl. J. Med. 1984;311:552–559. doi: 10.1056/NEJM198408303110902. [DOI] [PubMed] [Google Scholar]
- 94.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J. Am. Coll. Cardiol. 2005;45:637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 95.Dekker LR, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case–control study in acute myocardial infarction patients. Circulation. 2006;114:1140–1145. doi: 10.1161/CIRCULATIONAHA.105.606145. [DOI] [PubMed] [Google Scholar]
- 96.Friedlander Y, et al. Sudden death and myocardial infarction in first degree relatives as predictors of primary cardiac arrest. Atherosclerosis. 2002;162:211–216. doi: 10.1016/s0021-9150(01)00701-8. [DOI] [PubMed] [Google Scholar]
- 97.Kaikkonen KS, Kortelainen ML, Linna E, Huikuri HV. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation. 2006;114:1462–1467. doi: 10.1161/CIRCULATIONAHA.106.624593. [DOI] [PubMed] [Google Scholar]
- 98.Prutkin JM, Sotoodehnia N. Genetics of sudden cardiac arrest. Prog. Cardiovasc. Dis. 2008;50:390–403. doi: 10.1016/j.pcad.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 99.Chugh SS, et al. Postmortem molecular screening in unexplained sudden death. J. Am. Coll. Cardiol. 2004;43:1625–1629. doi: 10.1016/j.jacc.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 100.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J. Am. Coll. Cardiol. 2007;49:240–246. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 101.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner’s cases. Mayo Clin. Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 102.Albert CM, et al. Cardiac sodium channel gene variants and sudden cardiac death in women. Circulation. 2008;117:16–23. doi: 10.1161/CIRCULATIONAHA.107.736330. [DOI] [PubMed] [Google Scholar]
- 103.Adabag AS, et al. Etiology of sudden death in the community: results of anatomic, metabolic and genetic evaluation. Am. Heart J. 2010;159:33–39. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Splawski I, et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science. 2002;297:1333–1336. doi: 10.1126/science.1073569. [DOI] [PubMed] [Google Scholar]
- 105.Burke A, et al. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation. 2005;112:798–802. doi: 10.1161/CIRCULATIONAHA.104.482760. [DOI] [PubMed] [Google Scholar]
- 106.Newton-Cheh C, et al. Common genetic variation in KCNH2 is associated with QT interval duration: the Framingham Heart Study. Circulation. 2007;116:1128–1136. doi: 10.1161/CIRCULATIONAHA.107.710780. [DOI] [PubMed] [Google Scholar]
- 107.Newton-Cheh C, et al. QT interval is a heritable quantitative trait with evidence of linkage to chromosome 3 in a genome-wide linkage analysis: the Framingham Heart Study. Heart Rhythm. 2005;2:277–284. doi: 10.1016/j.hrthm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 108.Pfeufer A, et al. Common variants in myocardial ion channel genes modify the QT interval in the general population: results from the KORA study. Circ. Res. 2005;96:693–701. doi: 10.1161/01.RES.0000161077.53751.e6. [DOI] [PubMed] [Google Scholar]
- 109.Aarnoudse AJ, et al. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam study. Circulation. 2007;116:10–16. doi: 10.1161/CIRCULATIONAHA.106.676783. [DOI] [PubMed] [Google Scholar]
- 110.Arking DE, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat. Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 111.Spooner PM, et al. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a National Heart, Lung, and Blood Institute workshop, part I. Circulation. 2001;103:2361–2364. doi: 10.1161/01.cir.103.19.2361. [DOI] [PubMed] [Google Scholar]
- 112.Spooner PM, et al. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a National Heart, Lung, and Blood Institute workshop, part II. Circulation. 2001;103:2447–2452. doi: 10.1161/01.cir.103.20.2447. [DOI] [PubMed] [Google Scholar]
- 113.Hung J, Lam JY, Lacoste L, Letchacovski G. Cigarette smoking acutely increases platelet thrombus formation in patients with coronary artery disease taking aspirin. Circulation. 1995;92:2432–2436. doi: 10.1161/01.cir.92.9.2432. [DOI] [PubMed] [Google Scholar]
- 114.Hazinski MF, et al. Lay rescuer automated external defibrillator (“public access defibrillation”) programs: lessons learned from an international multicenter trial: Advisory statement from the American Heart Association Emergency Cardiovascular Committee; the Council on Cardiopulmonary, Perioperative, and Critical Care; and the Council on Clinical Cardiology. Circulation. 2005;111:3336–3340. doi: 10.1161/CIRCULATIONAHA.105.165674. [DOI] [PubMed] [Google Scholar]
- 115.Gundry JW, Comess KA, DeRook FA, Jorgenson D, Bardy GH. Comparison of naive sixth-grade children with trained professionals in the use of an automated external defibrillator. Circulation. 1999;100:1703–1707. doi: 10.1161/01.cir.100.16.1703. [DOI] [PubMed] [Google Scholar]
- 116.Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. N. Engl. J. Med. 2002;347:1242–1247. doi: 10.1056/NEJMoa020932. [DOI] [PubMed] [Google Scholar]
- 117.White RD, Hankins DG, Bugliosi TF. Seven years’ experience with early defibrillation by police and paramedics in an emergency medical services system. Resuscitation. 1998;39:145–151. doi: 10.1016/s0300-9572(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 118.Hallstrom AP, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N. Engl. J. Med. 2004;351:637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 119.Bardy GH, et al. Home use of automated external defibrillators for sudden cardiac arrest. N. Engl. J. Med. 2008;358:1793–1804. doi: 10.1056/NEJMoa0801651. [DOI] [PubMed] [Google Scholar]
- 120.Nichol G, et al. Essential features of designating out-of-hospital cardiac arrest as a reportable event: a scientific statement from the American Heart Association Emergency Cardiovascular Care Committee; Council on Cardiopulmonary, Perioperative, and Critical Care; Council on Cardiovascular Nursing; Council on Clinical Cardiology; and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117:2299–2308. doi: 10.1161/CIRCULATIONAHA.107.189472. [DOI] [PubMed] [Google Scholar]