Abstract
Objectives
Sickle cell disease (SCD) is associated with high healthcare utilization rates and poor outcomes in a subset of patients, although the underlying factors that predict this phenotype are poorly understood. Prior studies suggest that comorbid avascular necrosis (AVN) contributes to high healthcare utilization. We sought to clarify whether AVN independently predicts acute care utilization in adults with SCD and to identify characteristics of those with AVN that predict higher utilization.
Methods
We reviewed the medical records of 87 patients with SCD with symptomatic AVN and compared acute care utilization and clinical characteristics with 87 sex- and age-matched patients with SCD without symptomatic AVN. Patients with ≥2 years of follow-up were included. Outcomes were compared using bivariate analysis and multivariate regression.
Results
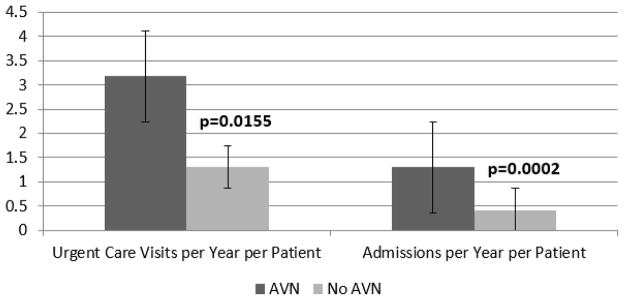
Our study included 1381 follow-up years, with a median of 7 years per patient. The AVN cohort had greater median rates of urgent care visits (3.2/year vs 1.3/year; P = 0.0155), admissions (1.3/year vs 0.4/year; P = 0.0002), and admission days (5.1 days/year vs 1.8 days/year; P = 0.0007). History of high utilization (odds ratio [OR] 4.28; P = 0.001), acute chest syndrome (OR 3.12; P = 0.005), pneumonia (OR 3.20; P = 0.023), hydroxyurea therapy (OR 2.23; P = 0.0136), and long-term transfusion (OR 2.33; P = 0.014) were associated with AVN. In a median regression model, AVN, acute chest syndrome, and pneumonia were independently associated with greater urgent care visits and admissions.
Conclusions
Symptomatic AVN was found to be an independent risk factor for acute care utilization in patients with SCD. Because this is a potentially modifiable factor, further studies are urgently needed to determine whether AVN prevention/early treatment interventions will alter utilization and improve outcomes for patients with SCD.
Keywords: sickle cell disease, osteonecrosis, avascular necrosis, hospitalization, utilization
People living with sickle cell disease (SCD) have high rates of healthcare utilization, with a small subpopulation of patients accounting for a disproportionate share of healthcare use and costs.1–4 The healthcare utilization of patients living with SCD is complex and not easily predicted.2,4 SCD severity is generally linked to higher utilization.1,2,5 An existing diagnosis of avascular necrosis (AVN) has been associated with high utilization.2,3,6
AVN is a complication of SCD characterized by death of bone tissue that is believed to be caused by a disruption in blood supply. AVN most commonly affects the femoral or humeral heads. Studies of patients with SCD with varying hemoglobinopathies and age ranges (0 to ≥45 years) report femoral and/or humeral head AVN prevalence in ranges from 3% to 27%, based on imaging and clinical diagnoses.5–11 The actual prevalence likely is higher; it has been reported that 41% of patients had silent AVN of at least one hip when evaluated by radiography and magnetic resonance imaging.12 Compared with other complications of SCD, AVN is the second most prevalent chronic complication in both HbSS and HbSC individuals.5,9 The presence of AVN has been associated with a higher number of hospitalized sickle cell pain crises, irreversible organ damage, and mortality.2,5 AVN typically is asymptomatic until late-stage disease, and once symptomatic, there is rapid progression to collapse, especially in AVN secondary to SCD as compared with other etiologies.7,13–15 Surgical intervention is one of the few treatment options available for AVN.13,16
To develop interventions to mitigate acute care utilization, we aimed to clarify the contribution of AVN to hospital utilization. By comparing utilization rates and the clinical characteristics of patients with SCD with and without AVN, our goals were to clarify whether AVN is an independent risk factor for utilization and to identify characteristics of those with AVN that predict higher acute care utilization.
Methods
Data Collection
We retrospectively reviewed medical records at the Johns Hopkins Sickle Cell Center for Adults (SCCA) to identify all patients with SCD diagnosed as having symptomatic AVN. These patients were then individually sex and age matched (±5 years) with patients with SCD without symptomatic AVN, resulting in a total sample of 174 patients (87 patients with SCD with symptomatic AVN, and 87 patients with SCD without symptomatic AVN). Symptomatic AVN was defined as chronic joint pain (femoral or humeral head) with confirmation of AVN by available imaging records. To identify symptomatic AVN, we first reviewed inpatient and outpatient provider clinical notes for mention of AVN, including “avascular necrosis,” “osteonecrosis,” “aseptic necrosis,” or “ischemic bone necrosis.” Symptomatic AVN diagnoses were then confirmed by existing radiographic, computed tomography scanning, and/or magnetic resonance imaging for all of the patients, except for five who had a history of AVN before establishing care at the SCCA. We included these cases in the study based on hematologist notes confirming the review of prior medical records and medical/surgical history of AVN. For controls, imaging was not consistently available but was reviewed where possible to verify that AVN was not identified. All of the patients were 18 years old or older as of January 31, 2014. Patients with a history of bone marrow transplant were excluded.
Follow-up periods, defined as the number of years from baseline to the study end date, were established for each patient. For those with AVN, baseline was defined as the date of AVN diagnosis, and if unknown, the date of established care at the SCCA. For those without AVN, baseline was defined as the date of established care at the SCCA. All of the patients had ≥2 years of follow-up; years without Johns Hopkins Hospital (JHH) encounters were considered breaks in follow-up care and excluded from the calculation of total years of follow-up per patient. From baseline to January 31, 2014 or, if earlier, date of death, we collected data on the frequency of hospital admissions and urgent care visits, defined as visits to the emergency department (ED) or Sickle Cell Infusion Center (SCIC). We defined acute care visits or acute care utilization to collectively include admissions and urgent care visits.
In addition, we recorded steady-state outpatient laboratory and clinical markers that were associated with AVN and/or high hospital utilization. Laboratory markers included hemoglobin and absolute reticulocyte count. The most recent steady-state values within the follow-up period were used, with steady-state values defined as well-visit values >3 weeks since a hospitalization or urgent care visit, and >3 months since surgery or pregnancy. For hemoglobin values, a mean of up to three steady-state values was used. Clinical markers included latest weight; tricuspid regurgitation peak velocity; transfusion history; hydroxyurea use; and history of cholecystectomy, acute chest syndrome (ACS), pneumonia (PNA), cerebrovascular accident, pulmonary embolism, leg ulcer, deep vein thrombosis, retinopathy, chronic kidney disease, psychiatric illness, and substance use.
Statistical Methods
STATA version 13.1 (StataCorp, College Station, TX) was used for all of the analyses. Utilization rates per patient were calculated as total urgent care visits (either ED or SCIC visits) divided by years of follow-up and total admissions divided by years of follow-up. Student t tests, Wilcoxon signed rank tests, and Mantel-Haenszel pooled odds ratios (ORs) were used for bivariate analyses of cases (AVN) and controls (no AVN). Variables from significant bivariate tests (P ≤ 0.05) and traditional AVN comorbidities were then used in a median regression risk factor model for urgent care and admission utilization rates. The Johns Hopkins University institutional review board approved this study protocol.
Results
Data for sample characteristics of cases and controls are shown in Table 1. The sample was 59.7% female patients, with a mean age of 41 years (range 24–71 years). The study included 1381 follow-up years, with a median of 7 years per patient (interquartile range 5–10 years). Thirty-one (18%) patients had at least 1 year without JHH encounters, which was then excluded from the respective number of follow-up years per patient. Patients with HbSS or HbSβ0 thalassemia (SS) constituted 72.4% of the sample and had a lower mean age of 39 years compared with 45 years for patients with other SCD genotypes (P = 0.0059). No other significant differences were found between cohorts regarding age or sex. SCD genotype and follow-up period distributions also were comparable for both cohorts.
Table 1.
Sample characteristics of cases (AVN) and controls (no AVN)
| Variable | Total, N = 174 | AVN, n = 87 | No AVN, n = 87 |
|---|---|---|---|
| Female sex (%) | 104 (59.8) | 52 (59.8) | 52 (59.8) |
| Mean (range) years olda | |||
| Start age | 32 ± 12 (7–68) | 32 ± 12 (7–61) | 33 ± 12 (18–68) |
| End age | 41 ± 11 (24–71) | 41 ± 12 (24–68) | 41 ± 11 (24–71) |
| SS genotype (%) | 126 (72.4) | 66 (75.9) | 60 (69.0) |
| Total follow-up years | 1381 | 698 | 683 |
| Median (IQR) follow-up years per patient | 7 (5–10) | 7 (5–10) | 7 (5–10) |
AVN, avascular necrosis; IQR, interquartile range; SS, Sβ0 thalassemia.
Start age corresponds with baseline date, defined as date of AVN diagnosis and/or date of established care (controls). End age corresponds with age of death or as of January 31, 2014; cases and controls were matched by end age.
The data for bivariate analysis of patient characteristics are shown in Tables 2 and 3. The AVN cohort had significantly greater rates of urgent care visits and admissions and admission days per year/per patient (Table 3, Fig.). In addition, a history of high utilization (mean acute care visits per year ≥4),2 ACS, PNA, hydroxyurea therapy, and chronic transfusions were significantly more prevalent in the AVN cohort (Table 2). Of note, we did not find meaningful differences in laboratory values or tricuspid regurgitation peak velocity between cohorts. When limiting this analysis to SS patients not receiving long-term transfusion therapy, however, higher steady-state hemoglobin levels were significantly associated with femoral head AVN (AVN cohort mean hemoglobin 8.7 g/dL, 95% confidence interval [CI] 8.3–9.0; no AVN cohort mean hemoglobin 8.1 g/dL, 95% CI 7.6–8.5; P = 0.046).
Table 2.
Bivariate analysis of binary variables and association with AVN
| Variable | OR | 95% CI |
|---|---|---|
| SS genotype | 1.43 | 0.72–2.83 |
| High utilizer (≥4 visits/y) | 4.28*** | 1.88–9.76 |
| Cholecystectomy | 1.86 | 0.97–3.56 |
| ACS | 3.12*** | 1.41–6.93 |
| Pneumonia | 3.20* | 1.17–8.73 |
| CVA | 0.80 | 0.32–2.03 |
| Pulmonary embolism | 1.00 | 0.35–2.85 |
| Leg ulcer | 1.86 | 0.74–4.65 |
| DVT | 2.11 | 0.96–4.67 |
| Retinopathy | 1.47 | 0.79–2.72 |
| Chronic kidney disease | 0.59 | 0.27–1.28 |
| Ever used hydroxyurea | 2.23* | 1.16–4.29 |
| Ever received long-term transfusion | 2.33* | 1.19–4.60 |
| Psychiatric illness | 1.43 | 0.72–2.83 |
| Substance use | 0.94 | 0.49–1.83 |
ACS, acute chest syndrome; AVN, avascular necrosis; CI, confidence interval; CVA, cerebrovascular accident; DVT, deep vein thrombosis; OR, odds ratio; SS, Sβ0 thalassemia.
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.005.
Table 3.
Bivariate analysis of continuous variables and association with AVN
| Variable | AVN | No AVN | P |
|---|---|---|---|
| Urgent care visits/y/patient | 3.2 (1.1–8.1) | 1.3 (0.5–3.4) | 0.016 |
| Admissions/y/patient | 1.3 (0.5–2.1) | 0.4 (0.1–1.2) | 0.000 |
| Admission d/y/patient | 5.1 (2.3–11.9) | 1.8 (0.3–5.7) | 0.0007 |
| Hemoglobin, g/dL | 9.4 (8.0–10.8) | 9.1 (8.1–10.5) | 0.913 |
| Absolute reticulocyte count (K/mm3) | 230.7 (165.6–324.6) | 255.1 (150.0–326.4) | 0.988 |
| Tricuspid regurgitation peak velocity (m/s) | 2.44 (2.26–2.73) | 2.32 (2.06–2.62) | 0.198 |
Values reported as median (interquartile range). AVN, avascular necrosis.
Fig.
Comparison of median acute care utilization rates by AVN status for patients with sickle cell disease. AVN, avascular necrosis.
In a regression model controlling for comorbidities and genotype, AVN, ACS, and PNA were independently associated with a greater number of urgent care visits and admissions per year (Table 4).
Table 4.
Median regression risk factor models for utilization rates
| Variable | Urgent care (ED or SCIC) visits/y | Admissions/y | ||
|---|---|---|---|---|
|
| ||||
| Coeff | 95% CI | Coeff | 95% CI | |
| Avascular necrosis | 0.98* | 0.17–1.78 | 0.36* | 0.01–0.70 |
| ACS | 1.95** | 0.58–3.32 | 0.78*** | 0.42–1.14 |
| Pneumonia | 0.67* | 0.08–1.26 | 0.28 | −0.00 to 0.56 |
| DVT | 0.31 | −2.07 to 2.68 | 0.03 | −0.35 to 0.40 |
| CVA | 0.00 | −1.07 to 1.08 | 0.06 | −0.24 to 0.36 |
| Retinopathy | 0.26 | −0.72 to 1.24 | 0.14 | −0.15 to 0.43 |
| Cholecystectomy | −0.51 | −1.17 to 0.14 | −0.06 | −0.35 to 0.23 |
| SS genotype | 0.26 | −0.54 to 1.05 | 0.14 | −0.17 to 0.45 |
ACS, acute chest syndrome; CI, confidence interval; coeff, coefficient; CVA, cerebrovascular accident; DVT, deep vein thrombosis; ED, emergency department; SCIC, Sickle Cell Infusion Center; SS, Sβ0 thalassemia.
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.005.
Within the AVN cohort, the mean age at symptomatic AVN diagnosis was lower for SS patients than for those with other genotypes, 28 years old as compared with 37 years old, respectively. Of 87 individuals in the cohort, 24 (28%) individuals had symptomatic femoral head AVN only, 15 (17%) individuals had symptomatic humeral head AVN only, and 48 (55%) individuals had both symptomatic femoral and humeral head AVN. The median number of affected joints was three. Forty-eight (55%) individuals had at least one surgical intervention for AVN, and 26 (30%) had more than one operation. We did not find a statistically significant association between rates of utilization and type of affected joint, number of affected joints, nor history of surgical intervention, respectively. In addition, type of affected joint was not associated with SCD genotype. Of those who underwent surgical correction, 37 (77%) were operated upon during the follow-up period. For these individuals, we did not find meaningful differences in utilization rates for either admissions (1.91, 95% CI 1.13–2.70 vs 1.08, 95% CI 0.51–1.65]) or ED/SCIC (5.68, 95% CI 1.07–10.28 vs 5.08, 95% CI 2.02–8.14) between the years before and after the year of their last documented surgery within the follow-up period. Lastly, when comparing history of hydroxyurea therapy, patients with AVN who had ever used hydroxyurea had greater mean urgent care visits per year (4.8/year vs 1.8/year; P < 0.0001) and greater mean admissions per year (1.7/year vs 0.48/year; P < 0.0001) than those with AVN who had never used hydroxyurea. When the analysis was limited to SS patients (hydroxyurea is primarily indicated for patients with SS disease), a trend toward significance was still noted for an association between AVN and ever using hydroxyurea (OR 2.5, 95% CI 0.970–6.443; P = 0.058).
Discussion
The finding that AVN is independently associated with increased acute care utilization raises important questions regarding the current prevention, diagnosis, and treatment of AVN in patients with SCD. To our knowledge, this is one of the first studies to specifically examine AVN as a predictor of acute healthcare utilization events. Although a growing number of studies have broadly examined rising SCD healthcare utilization and costs, few have evaluated specific predictors of utilization among these patients, especially of adults and the subset identified as high users.1–4,17–23 A bivariate analysis of 25 patients with SCD with femoral head AVN who were age and sex matched with 26 patients with SCD without femoral head AVN found a significantly higher mean number of admissions in a 1-year period for patients diagnosed as having femoral head AVN.6 Our analysis expands upon these findings by affirming the presence of AVN (humeral or femoral) as an independent predictor of utilization in a larger cohort during a long follow-up period.
Regarding acute complications, the finding that ACS and PNA independently predict greater utilization rates also affirms previous findings.3 The association found between ACS/PNA and AVN suggests that AVN may be a marker of more severe disease. The findings that ever using hydroxyurea therapy and/or chronic transfusions were more prevalent among the AVN cohort and that patients with AVN who had ever used hydroxyurea had greater utilization rates than those with AVN with no hydroxyurea history supports this hypothesis.
When comparing utilization rates of the AVN cohort to those without AVN, our average rates appear to be lower than previously reported.3 A 1-year study specifically comparing patients with SCD with AVN to those without AVN reported even higher admission rates for both the AVN and no-AVN cohorts.6 It is possible that our average rates are attenuated by longer follow-up periods per patient. In addition, our data could be influenced by the longitudinal nature of the study versus findings from cross-sectional studies.
The utility of SCD genotype as a predictor of AVN requires further investigation.24 Although we did not find differences in AVN prevalence among SCD genotypes, the resulting younger AVN diagnosis age and study age for SS patients agree with previous studies demonstrating that AVN tends to develop later in life for other genotypes.8,11 These data support the need for early intervention options in children and young adults.
Hematological markers also serve as a potential source of clinical predictors of AVN. Regarding hemoglobin and hematocrit, studies report varying associations with AVN. Some studies report associations between femoral head AVN and higher hematocrit and/or hemoglobin for SS patients.8,25 A smaller study also examining age- and sex-matched AVN and no-AVN cohorts did not find any difference in hematocrit levels.6 Similarly, in our study sample of diverse SCD genotypes and both femoral and humeral head AVN, we did not find meaningful differences in hemoglobin levels between those with AVN and those without. Although we used rigorous criteria for determining steady-state values, sample size and the retrospective design made it difficult to control for possible effects from comorbid chronic kidney disease, transfusions, and hydroxyurea therapy.
Although our study supports the need for predictors of AVN in SCD to improve prevention and early diagnosis, the paucity of data on interventions that effectively slow progression limits potential benefits of early monitoring. A full discussion of management options is outside the scope of this study, but our findings underscore the importance of further investigation of optimal management strategies for AVN in SCD. The treatment of AVN in SCD is not standardized.16 Operative and nonoperative treatments have been described with variable success.26 Savage et al highlighted major evidence gaps in AVN management, including randomized controlled trials to evaluate safety and efficacy of physical therapy versus surgical intervention at different stages of AVN, longitudinal studies to determine predictors of AVN at a younger age, and comparison studies of the incidence and prevalence of AVN among different types of SCD.24
Regarding operative treatment, it was not surprising to find that more than half of the AVN cohort had at least one surgical intervention indicated for AVN, because surgery remains one of the few treatment options. Unfortunately, AVN with SCD as the underlying etiology is associated with worse total hip arthroplasty outcomes and has higher revision rates than all-cause revision rates, although total hip replacement therapy has improved over time even in high-risk groups.16,26–28 We did not find significant differences in utilization between the periods before and after the year of surgery in this study. The interpretation of these results was challenging because of sample size, the unknown effects of surgery on utilization pattern in the short- and long-term postoperative period, the variation in the number of years with data before and following the year of surgery per patient, the variation in type of surgery (eg, core decompression, arthroplasty), and other unknown influences on utilization from comorbidities, prior treatments, or a history of multiple AVN-indicated surgeries. Future studies should prospectively evaluate utilization outcomes after surgery as well as optimal timing for surgical intervention.
We acknowledge several limitations to our study. There was likely an underestimate of admissions and acute visits because we missed visits that occurred outside JHH. It is unlikely that this would significantly affect our results because we have no reason to believe that those with or without AVN would use facilities outside JHH at differing rates. The retrospective nature of our study limited our ability to standardize the characterization of AVN in our inclusion criteria. For example, because we do not routinely screen asymptomatic patients in our program, negative imaging was not available for all of the controls. As such, it is possible that some control patients may have AVN that has not yet been clinically detected and/or diagnosed. Our analysis and conclusions should be limited to symptomatic AVN. Pain is another interesting factor associated with SCD, AVN, and utilization. Because of the retrospective nature of our study, we could not directly examine associations among pain, AVN, and utilization.
Despite these limitations, we collected available data on types of affected joints, number of affected joints, and AVN-specific surgical interventions in an attempt to characterize features of AVN that may be associated with higher utilization. Although we could not determine meaningful associations between these characteristics and acute care utilization rates among the AVN cohort, our analyses raised important questions concerning the definition and use of AVN as a marker for acute care utilization. The significance of our multiple regression model suggests that AVN independently predicts for acute care utilization rather than serving simply as a marker for disease severity. Prospective studies should specifically examine AVN severity, features of AVN itself, or treatments that may modulate acute care utilization.
The cost of not treating AVN is high, and our study raises the question of how we can better prevent AVN and support those with SCD living with this chronic, debilitating complication.21 To improve long-term outcomes, including acute care utilization, efficacious measures for preventing and/or slowing AVN progression must be identified.
Conclusions
AVN is an independent risk factor for acute care utilization in patients with SCD. Because AVN is a potentially modifiable risk factor, interventions that address prevention and early treatment for AVN in SCD are needed urgently.
Key Points.
Sickle cell disease (SCD) is associated with high healthcare utilization and poor outcomes in a subset of patients, although the underlying factors that predict this phenotype are poorly understood.
Our study found symptomatic avascular necrosis (AVN) to be an independent risk factor for acute care utilization in adults living with SCD.
Compared with adults with SCD without symptomatic AVN, these patients experience greater rates of urgent care visits to the emergency department and/or sickle cell infusion center, greater rates of hospital admissions, and greater lengths of stay in the hospital.
AVN is a potentially modifiable risk factor, and future studies should investigate whether early interventions can modify utilization outcomes and improve quality of life.
Acknowledgments
C.H. Jr has received funding from the National Heart, Lung, and Blood Institute (grant no. #5K-1HL1088332-04). L.M.S.R. has received institutional grants from the National Institutes of Health, ALSF, ALA, and the Maryland Stem Cell Research Fund.A J.J.S. has received institutional grants from the National Heart, Lung, and Blood Institute, the Health Resources and Services Administration, the Maryland Department of Health and Mental Hygiene, and serves as an expert witness for the Vaccine Adverse Event Trust Fund. S.L. has received funding from the National Heart, Lung, and Blood Institute (grant no. #K23HL083089), Pfizer, Selexys, Apopharma, and Novartis. The remaining authors have no financial relationships to disclose and no conflicts of interest to report.
Footnotes
Define ALSF, ALA, and check expansion of MSCRF.
To purchase a single copy of this article, visit sma.org/smj-home. To purchase larger reprint quantities, please contact reprints@wolterskluwer.com.
Early results of this study were published in an abstract and poster presentation at the American Society of Hematology meeting and exposition, December 7, 2014.
References
- 1.Blinder M, Vekeman F, Sasane M, et al. Age-related treatment patterns in sickle cell disease patients and the associated sickle cell complications and healthcare costs. Pediatr Blood Cancer. 2013;60:828–835. doi: 10.1002/pbc.24459. [DOI] [PubMed] [Google Scholar]
- 2.Carroll CP, Haywood C, Jr, Lanzkron S. Prediction of onset and course of high hospital utilization in sickle cell disease. J Hosp Med. 2011;6:248–255. doi: 10.1002/jhm.850. [DOI] [PubMed] [Google Scholar]
- 3.Carroll CP, Haywood C, Jr, Fagan P, et al. The course and correlates of high hospital utilization in sickle cell disease: evidence from a large, urban Medicaid managed care organization. Am J Hematol. 2009;84:666–670. doi: 10.1002/ajh.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein K, Yuen E, Riggio JM, et al. Utilization of the office, hospital and emergency department for adult sickle cell patients: a five-year study. J Natl Med Assoc. 2006;98:1109–1113. [PMC free article] [PubMed] [Google Scholar]
- 5.Powars DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84:363–376. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 6.Akinyoola AL, Adediran IA, Asaleye CM, et al. Risk factors for osteonecrosis of the femoral head in patients with sickle cell disease. Int Orthop. 2009;33:923–926. doi: 10.1007/s00264-008-0584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akinyoola AL, Adediran IA, Asaleye CM. Avascular necrosis of the femoral head in sickle cell disease in Nigeria: a retrospective study. Niger Postgrad Med J. 2007;14:217–220. [PubMed] [Google Scholar]
- 8.Milner PF, Kraus AP, Sebes JI, et al. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 9.Powars DR, Hiti A, Ramicone E, et al. Outcome in hemoglobin SC disease: a four-decade observational study of clinical, hematologic, and genetic factors. Am J Hematol. 2002;70:206–215. doi: 10.1002/ajh.10140. [DOI] [PubMed] [Google Scholar]
- 10.Iwegbu CG, Fleming AF. Avascular necrosis of the femoral head in sickle-cell disease. A series from the Guinea Savannah of Nigeria. J Bone Joint Surg Br. 1985;67:29–32. doi: 10.1302/0301-620X.67B1.3968138. [DOI] [PubMed] [Google Scholar]
- 11.Milner PF, Kraus AP, Sebes JI, et al. Osteonecrosis of the humeral head in sickle cell disease. Clin Orthop Relat Pres. 1993;289:136–143. [PubMed] [Google Scholar]
- 12.Ware HE, Brooks AP, Toye R, et al. Sickle cell disease and silent avascular necrosis of the hip. J Bone Joint Surg Br. 1991;73:947–949. doi: 10.1302/0301-620X.73B6.1955442. [DOI] [PubMed] [Google Scholar]
- 13.Hernigou P, Bachir D, Galacteros F. The natural history of symptomatic osteonecrosis in adults with sickle-cell disease. J Bone Joint Surg Am. 2003;85-A(3):500–504. doi: 10.2106/00004623-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Hernigou P, Habibi A, Bachir D, et al. The natural history of asymptomatic osteonecrosis of the femoral head in adults with sickle cell disease. J Bone Joint Surg Am. 2006;88:2565–2572. doi: 10.2106/JBJS.E.01455. [DOI] [PubMed] [Google Scholar]
- 15.Hernigou P, Poignard A, Nogier A, et al. Fate of very small asymptomatic stage-I osteonecrotic lesions of the hip. J Bone Joint Surg Am. 2004;86-A(12):2589–2593. doi: 10.2106/00004623-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Osunkwo I. An update on the recent literature on sickle cell bone disease. Curr Opin Endocrinal Diabetes Obes. 2013;20:539–546. doi: 10.1097/01.med.0000436192.25846.0b. [DOI] [PubMed] [Google Scholar]
- 17.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 18.Candrilli SD, O’Brien SH, Ware RE, et al. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;8:273–277. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- 19.Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–327. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 20.Lanzkron S, Haywood C, Jr, Segal JB, et al. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81:927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 21.Lanzkron S, Carroll CP, Haywood C., Jr The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85:797–799. doi: 10.1002/ajh.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okam MM, Shaykevich S, Ebert BL, et al. National trends in hospitalizations for sickle cell disease in the United States following the FDA approval of hydroxyurea, 1998–2008. Med Care. 2014;52:612–618. doi: 10.1097/MLR.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezenwa MO, Molokie RE, Wang ZJ, et al. Outpatient pain predicts subsequent one-year acute health care utilization among adults with sickle cell disease. J Pain Symptom Manage. 2014;48:65–74. doi: 10.1016/j.jpainsymman.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savage WJ, Buchanan GR, Yawn BP, et al. Evidence gaps in the management of sickle cell disease: a summary of needed research. Am J Hematol. 2015;90:273–275. doi: 10.1002/ajh.23945. [DOI] [PubMed] [Google Scholar]
- 25.Hawker H, Neilson H, Hayes RJ, et al. Haematological factors associated with avascular necrosis of the femoral head in homozygous sickle cell disease. Br J Haematol. 1982;50:29–34. doi: 10.1111/j.1365-2141.1982.tb01887.x. [DOI] [PubMed] [Google Scholar]
- 26.Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: current concepts. Indian J Orthop. 2015;49:28–45. doi: 10.4103/0019-5413.143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johannson HR, Zywiel MG, Marker DR, et al. Osteonecrosis is not a predictor of poor outcomes in primary total hip arthroplasty: a systematic literature review. Int Orthop. 2011;35:465–473. doi: 10.1007/s00264-010-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel YD, Szczech BW, Patel S, et al. Management strategies for total hip arthroplasty in sickle cell patients. J Long Term Eff Med Implants. 2014;24:219–224. doi: 10.1615/jlongtermeffmedimplants.2014010493. [DOI] [PubMed] [Google Scholar]