Abstract
Neutrophil gelatinase associated lipocalin (NGAL), was originally identified in neutrophil granules as a heterodimer complex with gelatinase B (matrix metalloproteinase 9, MMP9), but more recently has been found to be secreted by damaged epithelial cells. Ngal is a member of the lipocalin family and subsequently named as lipocalin 2 on the basis of structural similarity with other members of the lipocalin family and its potential association with hydrophobic retinol and cholesterol oleate more strongly than their hydrophilic counterparts. In 2002, a landmark paper suggested that Ngal is a bacteriostatic agent which blocks iron acquisition by interacting with a number of bacterial siderophores, especially enterobactin. Since then, more siderophore-carrying functions have been reported than the possibility of hydrophobic ligand transport. In this setting, Ngal was renamed Siderocalin. Functions of siderocalin include not only bacteriostatic activity but potentially as a mediator of cell growth and differentiation; some of these functions appear to be referable to the holo siderocalin:siderophore:iron complex and recent work suggests that metabolic products may act as mammalian siderophores bound by Ngal. While still speculative, it may be that the mammalian siderophores can establish the missing link between Ngal and a number of its functions in vivo. This review provides an overview of the discoveries of the different ligands of Ngal and consequently related functions. Hydrophobic ligands, bacterial siderophores as well as their modified structures (synthetic siderophores), and mammalian siderophores are summarized.
Keywords: Ngal, bacteriostatic, lipocalin-2, siderocalin, siderophore, ligand, mammalian
Graphical Abstract
Ligands of Ngal: hydrophobic, bacterial siderophores together with their modified structures, mammalian siderophores and consequently related functions were summarized.
1. Introduction
Neutrophil gelatinase-associated lipocalin (NGAL, human NGAL also known as LCN2, SCN denoted by upper case, its mouse homolog Ngal, lipocalin 2, Lcn-2, Scn, 24p3, uterocalin denoted by lower case) is expressed in neutrophil granules and was first purified from the neutrophil as a 25-kDa glycoprotein covalently complexed to the 92-kDa gelatinase B (matrix metalloproteinase 9, MMP9).1–4 More recently, Ngal was identified as a major product of epithelial cells synthesized in response to bacterial infection [1] or ischemic and toxic damage.4 Ngal was later found to be a bacteriostatic agent through sequestering the siderophore-iron complex which bacteria use for acquiring iron from environmental sources or from the host1,5 in iron limiting conditions. Additional functions were also associated including activation of differentiation and promotion of renal cell epitheliazation from mesenchymal cells of the kidney6,7 and repair of damaged epithelial cells8. Most importantly, Ngal is highly expressed even 100–1000 fold and appears early in both the urine and the serum at the onset of acute injury to kidney tubular cells (AKI). As a result, NGAL is now used as an early biomarker for AKI9–11. Additional functions of Ngal were reviewed in detail by several papers12–20.
Structurally, Ngal is a member of lipocalin family, featured with the characteristic cup-shape calyx formed by eight anti-parallel β sheets which are hydrogen bonded with one another21. The hydrophobic amino acid residues at the low region of the calyx provide binding sites for lipophilic small molecules through hydrophobic interaction22. Since lipocalins were classically named following the ligands they bound, Ngal was thus named as Lipocalin 2. However, Ngal can also bind macromolecules and hydrophilic molecules due to the much larger and shallower mouth of the calyx which is uncharacteristically lined with polar and positively charged residues. The latter is distinct feature of Ngal and differs from other lipocalins, which can only bind hydrophobic molecules such as lipocalins: epididymal retinoic acid-binding protein (ERBP), β-lactoglobulin (βLG), or nitrophorin-type lipocalins (NP)21. A very important hydrophilic small molecule, enterobactin (enterochelin, Ent), was found to be associated with Ngal and with iron5. Ent is a key siderophore of many Gram negative bacteria (especially Escherichia coli) which they use to sequester iron from the host. When bound to Ngal, the Ngal:siderophore:iron complex can inhibit iron acquisition by bacteria and in turn inhibit their growth. Because many different ligands of Ngal have been reported, from the original macromolecule MMP9 and small lipophilic molecules to more and more hydrophilic siderophores such as Ent, catechols in scattered literature, a review is needed to summarize the different ligands. In addition, new data and new interpretations have contributed to a better understanding of the functions of this protein. This review seeks to present a comprehensive overview of the ligands of Ngal and different functions of Ngal are also addressed.
2. The ligand binding sites of NGAL
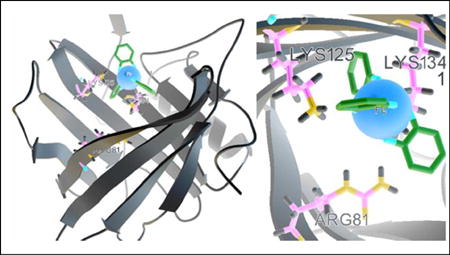
Using liquid NMR spectroscopy, Coles established the secondary, tertiary and quaternary structures of solution NGAL23. The side chains cluster of the conserved residues W31, T113 and R140 together with the 310-helix, closed the smaller end of the barrel at the bottom of the calyx (Fig. 1A). While the bottom end is sealed off by the short 310-helix, the top end of the calyx is open, and can be accessed by other molecules.
Fig. 1.
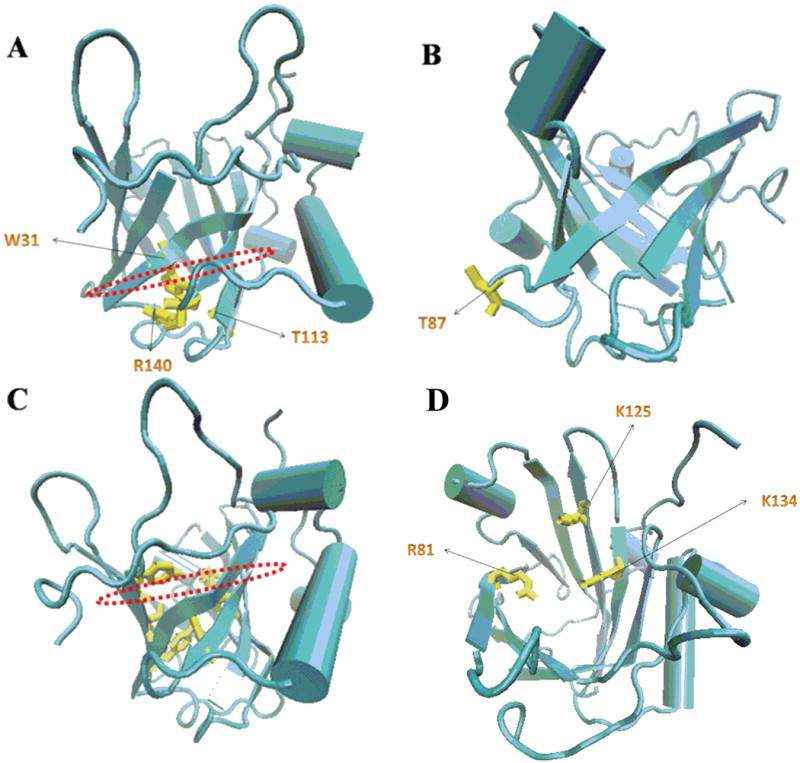
(A) The side chains cluster of the conserved residues W31, T113 and R140 (Yellow) together with the 310-helix closed the smaller end of the barrel at the bottom of the calyx. (B) The free SH group of residue C87 (Yellow) which associated with MMP-9 lied in an inter-strand loop at the closed end of the calyx. (C) The putative binding sites for lipophilic ligands were those hydrophobic aromatic and aliphatic residues including F27 (from the 310-helix), W31 and V33 (β1), V66 (β3), F83 (β4), F92 and L94 (β5), V108 and V110 (β6), and V121 and F123 (β7) (Yellow) at the base of the barrel. (D) Positively charged side-chains of two lysine residues (K125 and K134) and that of R81 were projected into the top open end of the barrel, which bind hydrophilic siderophores such as catecholates.
The free SH group of residue C87, which was found to be associated with MMP-9, lies in an inter-strand loop at the closed end of the calyx (Fig. 1B).
The putative binding sites for lipophilic ligands are hydrophobic aromatic and aliphatic residues including F27 (from the 310-helix), W31 and V33 (at the first β strand, βA), V66 (βC), F83 (βD), F92 and L94 (βE), V108 and V110 (βF), and V121 and F123 (βG) at the base of the barrel (Fig. 1C).
Positively charged side-chains of two lysine residues (K125 and K134) and R81 are projected into the top open end of the barrel (Fig. 1D), where are the binding sites for hydrophilic ligands are located and which have been studied in great depth bringing an epochal change in the field of NGAL research and differentiating NGAL from other lipocalins, even though many aspects of the rest of the calyx are similar.
3. The ligands of NGAL
3.1 Neutrophil gelatinase-associated lipocalin (Ngal) complexed to MMP9
Ngal was originally reported to be associated with the enzyme MMP9 as a covalently linked, disulfide-bridged heterodimer 125-kDa form isolated from human polymorphonuclear leukocytes (PMNL) [2]. The 25-kDa protein was prepared under reducing condition (together with the 95-kDa protein MMP9) from the progelatinase complex, and was further found to have homology to α2-microglobulin-related protein24. The α2-microglobulin-related protein was assigned to the lipocalin family according to the similarity in tertiary structure albeit the absence of similarity in whole amino acid sequence, as is typical of the comparison of one lipocalin to another. The 25-kDa protein was first named as α2-microglobulin-related protein in the paper published in 19922. Almost at the same time, another paper (1993) described the same form of the protein associated with human neutrophil gelatinase and presented the whole amino acid sequence and gave the protein the name Neutrophil gelatinase-associated lipocalin (NGAL) also termed p25 according to its molecular weight3. The primary structure of the glycoprotein NGAL was determined as a 178 residues protein. In addition, the paper pointed out that human NGAL mainly existed as uncomplexed form either as a monomer or a homodimeric and only a part of neutrophil gelatinase was covalently linked to NGAL. Thus, both of the proteins (MMP9 and NGAL) existed mainly in forms unrelated to each other and NGAL did not affect the function of MMP9. More recently, Ngal has been found to stabilize MMP9 and prevent its decomposition25, preserving its function.
Since the tertiary structure of NGAL defines it as a member of lipocalin family, its potential, albeit debated function of binding hydrophobic ligands is presented bellow.
3.2 Ngal may bind hydrophobic molecules as do other members of the lipocalin family
Lipocalins are a group of small (about 20-kDa) secreted proteins acting as carriers of lipophilic compounds. As a member of the lipocalin family, NGAL was assumed to be a carrier for small hydrophobic molecules in analogy to retinol-binding protein which binds and transports vitamin A26, the lipocalin α1-microglobulin which scavenges heme27, and the NP carry heme groups complexed with nitric oxide28. In this light, several studies were conducted on the hydrophobic ligands of Ngal. Chu et al. reported that 24p3 (another name for mouse Ngal) can bind cholesteryl oleate (1), oleic acid (2), retinol (3), retinoic acid (4), α-aminocaprylic acid (5), undecanoic acid (6) by CD spectrum and fluorescence quenching methods (Fig. 2). These authors pointed out that hydrophobic molecules bound more tightly than their hydrophilic counterparts, for example, retinol > retinoic acid. The binding activity of all the above molecules was in the following order: α-aminocaprylic acid < retinoic acid < undecanoic acid < oleic acid < retinol < cholesteryl oleate.22 Chu further identified their binding site at residue W31 in the β strand A (Fig. 1A), which provided the hydrophobic interaction between lipophilic ligands and the hydrophobic aromatic amino acid residues inside the β sheet core. Through thorough analysis of CD spectra, Chu described the secondary structure of the protein which contained one short 310 helix, one α helix and nine β strands (designated as β A-I) and predicted that these structural elements formed an eight β stranded barrel. According to above structural analysis, they suggested that the possible function of the protein was cellular regulation through transport of lipophilic molecules.22
Fig. 2.
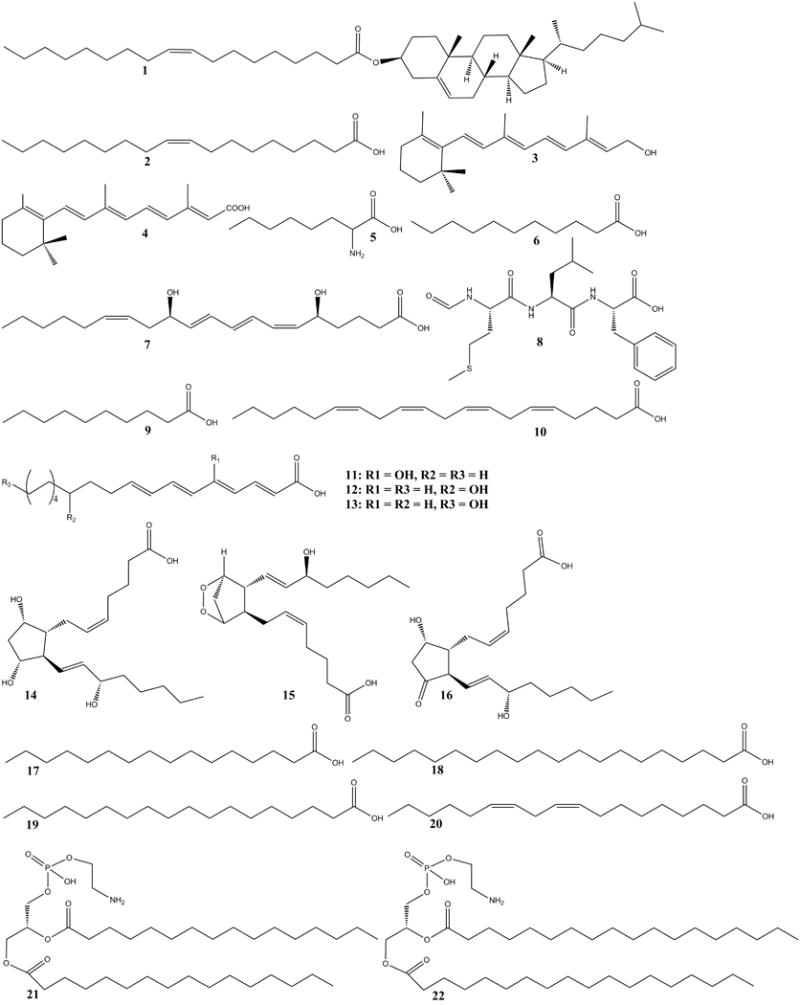
The structure of lipophilic ligands (1–22) of Ngal
In 1999, Bratt identified binding activities between human NGAL and natural lipophilic ligands, platelet activating factor (PAF), leukotriene B4 (7) (LTB4) and the bacterial hydrophobic peptide N-formyl-Met-Leu-Phe (8) (fMLP) by weak affinity chromatography (Fig. 2). This paper pointed out that NGAL binding to small lipophilic ligands was too weak to be detected by fluorescence quenching, gel filtration, and/or immunoprecipitation, which suggested that NGAL might not bind lipophilic ligands such as the above mentioned compounds 1–6.29
In fact, according to the crystal structure of NGAL, none of the proposed ligands such as N-formylated tripeptides, fatty acids, etc. were likely to be the preferred ligand of this protein except for decanoic acid (9) or n-capric acid (NCA) (Fig. 2), leaving the physiologic function of NGAL in question. However, two recent papers concerning lipophilic ligands and related complex functions may cause a renewed interest in trying to define lipophilic ligands of NGAL. Song et al. showed that Ngal could bind several fatty acids (10–20) especially linoleic acid (20) (Fig. 2), which they suggest may increase the circulating levels and the arterial accumulation of deaminated NGAL, resulting in vascular inflammation and endothelial dysfunction and hypertension in mice.30 NGAL was also found to modulate fertility acquisition in sperm perhaps by binding membrane phosphatidylethanolamines (21–22) (Fig. 2).31,32
While awaiting additional crystal structures and despite these interesting leads, an earlier paper showed that the presence of NGAL in neutrophils and in tissues liable to encounter microorganisms indicated that NGAL might have a role in the immune system.33 The unveiling of the ligand of NGAL marked the beginning of a new era in the field of NGAL, which will be introduced below.5
3.3 Siderocalin binds siderophores and its related functions (Fig. 3)
Fig. 3.
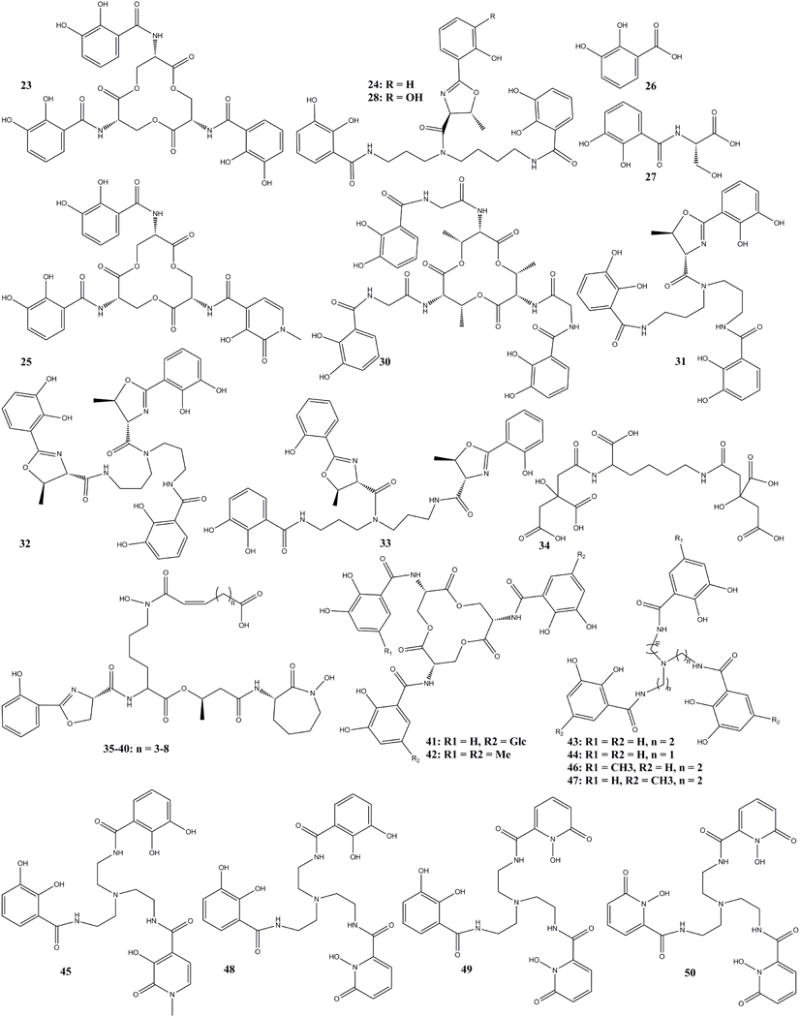
The structure of siderocalin binding siderophores (23–40), stealthy siderophores (41–42), and synthetic siderocalin binding siderophores (43–50)
After elucidating the structure of NGAL by the NMR23 and X-ray21 techniques, the structure of the ligand of NGAL was urgently needed to determine the functions of NGAL in a wide range of pathologies including acute kidney injury4, nephropathy2, inflammation, apoptosis and important immunomodulatory functions21, which were all based on phenomenology at that time. In 2002, Goetz reported their breakthrough study on the copurified ligand of NGAL and established the structure as a known bacterial siderophore Ent (23) (Fig. 3). This study identified the ligand based on crystallographic analyses and also identified one important function of the mysterious protein was bacteriostasis in line with iron chelation activities in the innate immune system, previously noted by lactoferrin. The Goetz paper has influenced all related studies till now5. NGAL was found to bind ferric Ent (FeEnt) and related siderophores by interaction with the three positively charged residues (R81, K125 and K134, Fig. 1D). Based on their prominent structural studies, another paper from one of the collaborating groups in the same issue of Molecular Cell6 demonstrated that Ngal had a growth and inductive activity in embryonic mesenchymal cells. The two papers reported that the function of NGAL was related to form a ternary complex with ferric iron through a ligand. The Goetz research established the important functions against bacterial growth through competition of iron. NGAL participated in the iron-depletion strategy of the innate immune system along with other antibacterial proteins and reactive oxygen species released from neutrophil recruited to infected or injured tissues. Generally, lipocalins derived the names from the bound ligands, Siderocalin was thus proposed as the new name of NGAL in recognition of its chelation of bacterial siderophores.
Since the identification of Ent was published, related microbial siderophores were screened for NGAL-Siderocalin binding. Parabactin (24) (Paracoccus denitrificans) and cepabactin (25) (Pseudomonas cepacia), were reported together with the basic catecholate unit, including 2,3-dihydroxybenzoic acid (26) (DHBA) and 2,3-dihydroxybenzoyl-serine (27) (DHBS)5. Cepabactin (25) is a similar catecholate siderophore like Ent with the same skeleton but a hydroxypyridinone (HOPO) group replacing one catecholate group. Parabactin is also a catecholate siderophore with different skeleton and an oxazoline group replacing one phenolate group.
Agrobactin (28) (Agrobacterium tumefaciens), brucebactin (29) (Brucella abortus), bacillibactin (30, reported as corynebactin from Bacillus subtilis),34–36 fluvibactin (31) (Vibrio fluvialis), vibriobactin (32) (Vibrio. cholerae), and vulnibactin (33) (Vibrio vulnificus) were reported as possible NGAL siderophores according to their structural similarity to Ent.14 The structure of brucebactin was not established at that time. The structure of corynebactin was actually the same as that of bacillibactin37 and corynebactin38 may have been a misnomer because in the species Corynebacterium glutamicum there is no synthetic gene for corynebactin according to comparative studies on siderophore-dependent iron uptake in B. subtilis and C. glutamicum.37,39–41 Rather authentic corynebactin (34) has been found structurally related to staphyloferrin A from Staphylococcus aureus and rhizoferrin from Rhizopus microsporus42 a set of siderophores that are not relevant to NGAL. Fluvibactin (31), vibiobactin (32), vulnibactin (33) are all similar to parabactin (24) with the same skeleton (Fig. 3).
Although siderocalin with the open end of the calyx, lined with positive residues seems optimized for binding three aromatic rings in the three binding pockets, the protein can also accommodate siderophores and iron-siderophore complexes of varying structures, so long as steric and electrostatic/cation-pi requirements are met.43 Some mycobactins were first considered to be NGAL binding siderophores in 20041. The structures of NGAL binding carboxymycobactins (35–40) (Fig. 3) with different methylene length (n = 3–8) were published in next year.19,44,45 NGAL’s ability to bind the carboxymycobactins suggested that NGAL could recognize different types of siderophores in addition to the catecholate ones and that it could be used as potential therapeutic agent against M. tubercuosis.
3.4 Stealthy siderophores evading siderocalin binding
Siderocalin was found to inhibit bacterial growth in some species and unable to function as bacteriostatic agent in other circumstances, which has drawn interest from both microbiologists and biochemists. It was found that bacteria have evolved to introduce structural modifications into siderophores including new skeletal elements in order to sequester iron from environment but evade binding by NGAL-Siderocalin.46–49 These siderophores are called stealthy siderophores. Most of the modifications introduce steric hindrance such as that Salmonella typhimurium LT2 and E. coli CFT073 could produce glycosylated Ent analogues, salmochelin S4 (41) to preclude binding to Ngal. Alternatively, the introduction of a methyl group SERMECAM (42) (Fig. 3) could also completely inhibit the NGAL binding.50–52
3.5 Synthetic siderocalin binding siderophores
Many synthetic siderophores have been synthesized based on the known siderophores. These include Tren(CAM)3 (43)52, MeCAM (44), TrenCAM-3,2-HOPO (45)44, Tren(CAM)2(MECAM) (46), Tren(CAM)(MECAM)2 (47)46, Tren(CAM)2(1,2-HOPO) (48), Tren(CAM)(1,2-HOPO)2 (49), Tren(1,2-HOPO)3 (50)52 (Fig. 3).
Through synthetic approaches, the effects of steric hinderance and electrostatic interactions on ligand binding could be thoroughly studied. Synthesis of siderophore derivatives helped to more thoroughly understand the interactions between the protein and its ligands. Iron chelators for possible use in iron overload diseases could be developed through the synthesis of different siderophores. Iron chelation therapy has been seen as an effective treatment in a number of serious clinical conditions.
3.6 Mammalian siderocalin binding siderophores
Besides for blocking bacterial growth through sequestering FeEnt, NGAL is also highly expressed in noninfectious condition, implying that it binds ligands other than Ent and serves additional functions such as growth activity for example induce the renal cell differentiation through transporting iron in a transferrin-independent pathway6,7,53,54. NGAL is also reported to regulate diverse process such as cell apoptosis55, and to rescue ischemic53,56 and immunological injury8. All the functions have been shown to be related to a siderophore-like ligand because NGAL does not bind iron directly in the absence of a siderophore. It was supposed that an endogenous siderophore could work as an iron carrier through forming a ternary Scn:ligand:iron (III) complex. Therefore, attempts to find the mammalian NGAL binding siderophore have become the most urgent task in the area especially after structure of the bacterial ligand Ent was elucidated.
Efforts to find mammalian equivalents of bacterial siderophores have languished for decades since the hypothesis was firstly posited57. A 1500 Da mammalian siderophore was first isolated from horse liver in 1980 together with its capability to bind iron and to stimulate bacterial growth by promoting iron uptake by the bacteria58. Several groups have launched related explorations. Three papers were published in three different influential journals in 2010.54,59,60
The catechols were first mentioned at two different academic conferences61,62 by Barasch’s group. Detailed reviews and comments on the catechols could be found in different journals17,64–67. In the study, simple catechols such as catechol (51), 3-methylcatechol (52), 4-methylcatechol (53), and pyrogallol (54) (Fig. 4) were found to bind NGAL and could form the classic ternary NGAL:catechols:iron (III) complex by 55Fe ultrafiltration assay. Large scale screening with filtration and chromotgraphy assays could be satisfactorily explained by the effect of steric hindrance or the effects of electron donating or electron withdrawal from the ring. However, only two crystal structures of the ternary complex (catechol, 4-methycatechol) were successfully identified by R. Strong. The overall structure of NGAL was not significantly affected by these two catechols. At acidic pH (which blocks the binding of the catechols to NGAL), a single catechol or 4-methycatechol occupied pocket 1 interacted with the positively charged residues K125 and K134.
Fig. 4.
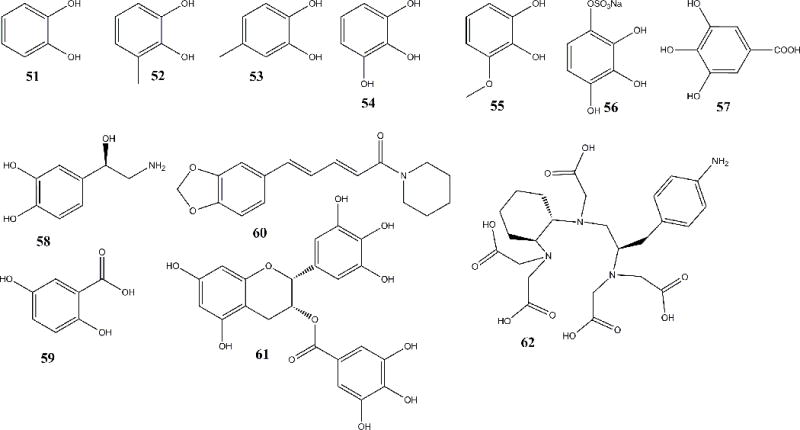
Mammalian siderocalin binding siderophores (51–59), plant-origin Ligands of NGAL (60–61), and NGAL mutant binding ligand that complexed to DTPA which can bind rare earth elements (62)
The binding of the catecholFe (CatFe) complex to NGAL ligand binding pocket occurred in a two-step process: the first step occurred at physiological pH (7.4), during which two catechols were complex to one iron molecule and the complex was recruited to the calyx. The third catechol moiety was then added to the two catechol-iron complexes to form a tris-catecholate complex. This configuration led to the iron molecule being stabilized in a hexadentate co-ordination complex owing to the formation of stable cationic-π interactions and coulombic (electrostatic) interactions between the ferric iron and the catechols. Through forming the ternary complex with these simple catechols, NGAL could traffic and clear iron. Free catechol was directly purified from pooled human urine with multiple columns and definitely identified by HPLC, HR-ESI MS, and NMR spectroscopic methods. This report directly confirmed free catechol was present in the urine as well as its capacity to serve as an iron trap within the NGAL-Siderocalin calyx.66 Comparison of the binding strengths of different catechols demonstrated that the vicinal-dihydroxyl groups were the key functional groups and that steric compatibilities of the catechol ring had the strongest effect on the binding. In addition to catechol (51), 3-methylcatechol (52), 4-methylcatechol (53), and pyrogallol (54), more simple catechols were reported: 3-methoxycatechol (55), pyrogallol-4-sulfate (56), gallic acid (57) (Fig. 4). These studies, combining chemical screening54 and natural product approaches proved the putative ligands as naturally occurring iron “buffers” in the human urine66. Using a LC-MS based metabolomic analysis, catechols have been further confirmed existing in human urine and controlling urinary NGAL antimicrobial activity recently67. The combination of the three studies support the notion that simple catechols should be considered naturally occurring binding partners of NGAL. The mammalian catecholate siderophores can be used by NGAL to shuttle iron or act as a growth factor to participate in a wide range of cellular processes.
Two ligands (58, 59) reported in additional papers59,60 almost at the same time are perhaps not as suitable as the catechols to occupy the calyx of NGAL based on the following: the ligand binding domain or calyx, is shallow, broad and lined with polar and positively charged residues (R81, K125, K134); it is also quite rigid, with three binding pockets inside the calyx that imposed a steric limitation on which ligands are NGAL-Siderocalin-compatible; furthermore, Siderocalin resists any conformational change when it is exposed to changes in pH, ionic strength or upon ligand binding. In addition, bidentate chelators generally are not superior to transferrin in binding iron. Our filtration assay showed that neither the catecholamine L-norepinephrine (58)59 nor 2,5-dihydroxybenzoic acid (59) (2,5-DHBA)60 (Fig. 4) could form a ternary complex with NGAL. Furthermore, our studies showed that the para-substituted (such as 58 and 59) catecholate metabolites had strong steric effects inhibiting binding to NGAL-Siderocalin66. 2,5-DHBA was also not confirmed by four related papers, including two reviews and two verification research papers.18,43,68,69 Siderocalin:Fe:2,5-DHBA mediated apoptosis experiments, have shown that 2,5-DHBA-based siderophores do not chelate iron strongly enough to generate an apoptotic response in hematopoietic cell lines. However, several papers on new functions of 2,5-DHBA were also successively published70–72, but it is not clear if their function is due to a siderophore-like activity.
3.7 Plant-origin Ligands of NGAL (Fig. 4)
In 2005, a paper suggested that piperine (60), an alkaloid isolated from pepper, could bind NGAL by CD spectrum, which was the first plant originated natural product reported to bind NGAL.73
Bao’s recent studies showed that epigallocatechin gallate (EGCG) (61), a major catechin (flavan-3-ol) in green tea, could also bind NGAL and form a ternary complex with ferric iron.74 EGCG could enter the same binding sites K125 and K134. However, the pH dependent experiment showed two binding peaks at pH 6.5 and 5.5, respectively, which was different from both Ent (whose complex was stable even at pH 4) and catechol (descending all the way below the peak at pH 7), which was very interesting since it might release iron in two steps in the late endosome and lysosome which had pH of ≤6.0 and ≤5.5, respectively, among the cellular organelle. EGCG and iron (III) were both unstable in physiological condition and could easily enhance oxidative stress once released. However, the ternary NGAL:EGCG:iron complex could inhibit the reactivity of both chemicals and transport them to tissues for their utilization especially the protective effect of EGCG on kidney.74 It is important to note however, that any new ligand including EGCG must be eventually supported by structural studies.
In summary, there are five different functional groups of siderophores for chelating iron: the catecholate group, oxazolines, hydroxamates, α-hydroxycarboxylic acids, and imidazole groups can provide iron chelating activity (Fig. 5).14 All these siderophores can interact with NGAL if they can provide negative charge complementarities and aromatic alcohol cation-interactions.
Fig. 5.
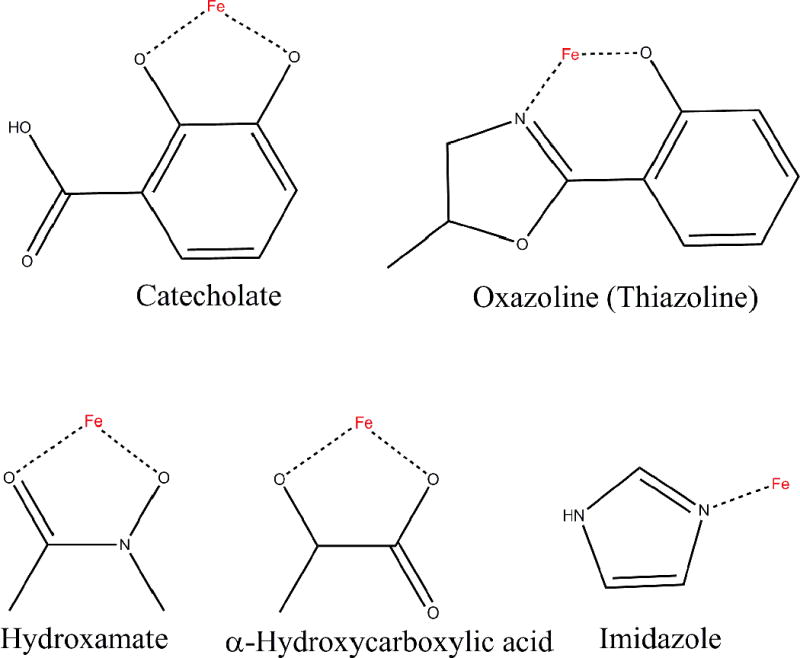
The functional groups of siderophores for chelating iron.
The protein images were made with VMD software support. VMD is developed with NIH support by the Theoretical and Computational Biophysics group at the Beckman Institute,University of Illinois at Urbana-Champaign http://www.ks.uiuc.edu/Overview/acknowledge.html.
The structure of ligands (1–62) were drawn by chembiodraw ultra 12.0
4. Conclusion and outlook
Cell differentiation, embryogenesis, inflammation, cancer, and other diseases have been associated with the expression of NGAL. The role of NGAL in above-mentioned processes is still unknown, but the elucidation of its ligands provides the first molecular insights of its function. In fact, research on NGAL has progressed with the discovery of its ligands.
NGAL was deduced to bind hydrophobic ligands in parallel with other lipocalins and was thus called as lipocalin-2.22 The putative binding sites for lipophilic ligands were those hydrophobic aromatic and aliphatic residues including F27 (from the 310-helix), W31 and V33 (βA), V66 (βC), F83 (βD), F92 and L94 (βE), V108 and V110 (βF), and V121 and F123 (βG) at the base of the barrel (Fig. 1A and C). An epochal paper on its bacteriostatic effects based on binding with the bacterial siderophore Ent gave it the new name Siderocalin5 and disclosed its novel function as a bacteriostatic agent. The binding sites of siderophores were those positively charged side-chains of two lysine residues (K125 and K134) and that of R81, which were projected into the top open end of the barrel (Fig. 1D). After binding through the ternary complex, the NGAL:ligand:iron (III) could be excreted into urine like a therapeutic iron chelating medication, such as that the siderophore drug desferrioxamine. The catechols were confirmed to be NGAL binding mammalian siderophores by two recent papers66,67 and might participate in creating a urinary buffer to handle iron in the kidney lumen as part of the iron reclamation process.
Since NGAL is a small secreted protein, it can also easily be modified. Modification of the ligand binding pocket of NGAL to enhance its iron deletion ability to treat iron overload syndromes or to suppress bacterial infection may be future uses of NGAL technology75–77. Additionally, more speculative uses might include extraction and purification of rare earth metal ions through binding rare earth related metal ions as chelating complexes with [(R)-2-amino-3-(4-aminophenyl) propyl]-trans-(S,S)-cyclohexane-1,2-diaminepenta acetic acid (p-NH2-Bn-CHX-A″- DTPA) (62) (Fig. 4) and rare earth DTPA- complexed Y3+, Tb3+, Gd3+, and Lu3+Y(III)–DTPA.78
NGAL was also associated with cell growth as a result of the association with its siderophores53 and potentially as a result of iron delivery. Whether these activities are ultimately due to iron chelation at their target site or due to iron delivery to their target site, for example to mitochondria, remains to be seen. Many speculative ideas are now being tested. For example, an area of inquiry is whether apo-NGAL has an independent effect or simply the converse effect to the siderophore:iron loaded molecule. Some support for independent effects comes from the fact that apo- and holo-NGAL have different effects on stimulating epithelia to express IL-88. In the case of cell growth one could imagine that if NGAL bound siderophore, it could induce and enhance growth while conversely it could induce iron deficiency by chelating siderophore:iron.16,79–82 These ideas are speculative but maybe related to some endogenous catechol derivative.
Although exponential papers on NGAL have been published year by year, its source, regulation of expression, and its functions remain to be discovered and placed in cellular contexts. Barasch’s group has recently reported that α-intercalated cells, not β-intercalated or principal cells within the collection duct (CD) of kidney secreted NGAL to control the urinary tract infection through iron sequestration. The pathway begins with the activation of TLR-4 in the CDs and results in cell specific responses.83 Hence the site of expression, and its relationship to iron binding has demonstrated a new site of innate immune defense.
NGAL was also reported to be associated with obesity related disease.30,84–89 Because NGAL was causally involved in obesity-related vascular dysfunctions, it might represent a promising target for discovery of agents against obesity-associated cardiac vascular disease (CVD).30 Most critical in this work, is to identify new receptors that bind NGAL and transmit signaling to adipose cells or other cells controlling food intake and metabolism. The ligands binding to NGAL might also be an approach for designing novel inhibitors88 but in both cancer research and obesity/metabolic research it is not at this time clear whether the reported activities are siderophore dependent.
Supplementary Material
Acknowledgments
Financial assistance was received with appreciation from National Natural Science Foundation of China 81170654/H0507, Anhui Agricultural University Talents Foundation (YJ2011-06), Undergraduate Innovation Project from Anhui Province and Anhui Agricultural University 2013, Major Program of Acquisition of Academy Lead Talent and Team in Anhui Province. Financial support also derived from NIH 5R01DK073462 (JB).
Abbreviations
- Ngal
Neutrophil gelatinase associated lipocalin
- MMP9
matrix metalloproteinase 9
- AKI
acute kidney injury
- Ent
Enterobactin, Enterochelin
- NMR
Nuclear Magnetic Resonance
- PMNL
polymorphonuclear leukocytes
- DHBA
dihydroxybenzoic acid
- DHBS
dihydroxybenzoyl-serine
- EGCG
epigallocatechin gallate
- CD
collection duct collection duct collection ductcollection duct
- ERBP
epididymal retinoic acid-binding protein
- βLG
β-lactoglobulin
- NP
nitrophorin-type lipocalins
References
- 1.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 2.Triebel S, Blaser J, Reinke H, Tschesche H. A 25kDa alpha 2-microglobulin related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. doi: 10.1016/0014-5793(92)81511-j. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 4.Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-Siderocalin in kidney disease. Biochim Biophys Acta. 2012;1823(9):1451–1458. doi: 10.1016/j.bbamcr.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, et al. The neutrophil Lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Goetz DH, Li JY, Wang W, Mori K, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 8.Ashraf MI, Schwelberger HG, Brendel KA, Feurle J, Andrassy J, et al. Exogenous Lipocalin 2 ameliorates acute rejection in a mouse model of renal transplantation. Am J Transplant. 2015 doi: 10.1111/ajt.13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, et al. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 11.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 12.Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2005;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- 13.Kalousek I, Röselová P, Otevrelová P. NGAL-neutrophil gelatinase associated lipocalin in biochemistry, physiology and clinical praxis. Cas Lek Cesk. 2006;145:373–376. [PubMed] [Google Scholar]
- 14.Strong RK. Lipocalins, Chapter Siderocalins. Landes Bioscience; Austin, USA: 2006. pp. 83–98. [Google Scholar]
- 15.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Bolignano D, Donato V, Lacquaniti A, Fazio MR, Bono C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in human neoplasias: a new protein enters the scene. Cancer Lett. 2010;288:10–16. doi: 10.1016/j.canlet.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. BBA-rev cancer. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correnti C, Strong RK. Mammalian siderophores, siderophore-binding lipocalins and the labile iron pool. J Biol Chem. 2012;287:13524–13531. doi: 10.1074/jbc.R111.311829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allred BE, Sia AK, Raymond KN. Chapter 4 Siderocalin Combats Mycobacterial Infections. In: Byers BR, editor. Springer Briefs in Biometals. Vol. 10. 2013. pp. 53–63. Iron Acquisition by the Genus Mycobacterium. [Google Scholar]
- 20.Schiefner A, Skerra A. The Menagerie of human lipocalins: a natural protein scaffold for molecular recognition of physiological compounds. Acc Chem Res. 2015;48:976–985. doi: 10.1021/ar5003973. [DOI] [PubMed] [Google Scholar]
- 21.Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, et al. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39:1935–1941. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 22.Chu ST, Lin HJ, Huang HL, Chen YH. The hydrophobic pocket of 24p3 protein from mouse uterine luminal fluid: Fatty acid and retinol binding activity and predicted structural similarity to lipocalins. J Pept Res. 1998;52:390–397. doi: 10.1111/j.1399-3011.1998.tb00663.x. [DOI] [PubMed] [Google Scholar]
- 23.Coles M, Diercks T, Muehlenweg B, Bartsch S, Zölzer V, et al. The Solution Structure and Dynamics of Human Neutrophil Gelatinase-associated Lipocalin. J Mol Biol. 1999;289:139–157. doi: 10.1006/jmbi.1999.2755. [DOI] [PubMed] [Google Scholar]
- 24.Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000;1482(1–2):272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 25.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276(40):37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 26.Newcomer ME, Ong DE. Plasma retinol binding protein: Structure and function of the prototypic lipocalin. Biochim Biophys Acta. 2000;1482:57–64. doi: 10.1016/s0167-4838(00)00150-3. [DOI] [PubMed] [Google Scholar]
- 27.Larsson J, Allhorn M, Kerstrom B. The lipocalin alpha(1)-microglobulin binds heme in different species. Arch Biochem Biophys. 2004;432:196–204. doi: 10.1016/j.abb.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Weichsel A, Andersen JF, Champagne DE, Walker FA, Montfort WR. Crystal structures of a nitric oxide transport protein from a blood-sucking insect. Nat Struct Biol. 1998;5:304–309. doi: 10.1038/nsb0498-304. [DOI] [PubMed] [Google Scholar]
- 29.Bratt T, Ohlson S, Borregaard N. Interactions between neutrophil gelatinase-associated lipocalin and natural lipophilic ligands. Biochem Biophys Acta. 1999;1472:262–269. doi: 10.1016/s0304-4165(99)00131-2. [DOI] [PubMed] [Google Scholar]
- 30.Song E, Fan P, Huang B, Deng HB, Cheung BMY, et al. Deamidated Lipocalin-2 Induces Endothelial Dysfunction and Hypertension in Dietary Obese Mice. J Am Heart Assoc. 2014;3:e000837. doi: 10.1161/JAHA.114.000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe H, Takeo T, Tojo H, Sakoh K, Berger T, et al. Lipocalin 2 binds to membrane phosphatidylethanolamine to induce lipid raft movement in a PKA-dependent manner and modulates sperm maturation. Development. 2014;141:2157–2164. doi: 10.1242/dev.105148. [DOI] [PubMed] [Google Scholar]
- 32.Linwood D. Lipocalin 2 as a Membrane-Reorganizing Agent. Sci Signal. 2014;7:pe19. doi: 10.1126/scisignal.2005563. [DOI] [PubMed] [Google Scholar]
- 33.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 34.Ong SA, Peterson T, Neilands JB. Agrobactin, a siderophore from Agrobacterium tumefaciens. J Biol Chem. 1979;254:1860–1865. [PubMed] [Google Scholar]
- 35.May JJ, Wendrich TM, Marahiel MA. Corynebactin and a serine trilactone based analogue: chirality and molecular modeling of ferric complexes. Inorg Chem. 2002;41(21):5475–5478. doi: 10.1021/ic025531y. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MK, Abergel RJ, Raymond KN, Arceneaux JEL, Byers BR. Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem Biophys Res Commun. 2006;348:320–325. doi: 10.1016/j.bbrc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 37.May JJ, Wendrich TM, Marahiel MA. The dhb operon of Bacillus subtilis encodes the biosynthetic template for the catecholic siderophore 2,3-dihydroxybenzoate-glycine-threonine trimeric ester bacillibactin. J Biol Chem. 2001;276:7209–7217. doi: 10.1074/jbc.M009140200. [DOI] [PubMed] [Google Scholar]
- 38.Budzikiewicz H, Bossenkamp A, Taraz K, Pandey A, Meyer JM. Corynebactin, a cyclic catecholate siderophore from Corynebacterium glutamicum ATCC 14067 (Brevibacterium sp. DSM 20411) Z Naturforsch C biosci. 1997;52:551–554. [Google Scholar]
- 39.Dertz EA, Stintzi A, Raymond KN. Siderophore-mediated iron transport in Bacillus subtilis and Corynebacterium glutamicum. J Biol Inorg Chem. 2006;11:1087–1097. doi: 10.1007/s00775-006-0151-4. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda M, Nakagawa S. The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol. 2003;62:99–109. doi: 10.1007/s00253-003-1328-1. [DOI] [PubMed] [Google Scholar]
- 41.Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, et al. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol. 2003;104:5–25. doi: 10.1016/s0168-1656(03)00154-8. [DOI] [PubMed] [Google Scholar]
- 42.Zajdowicz S, Haller JC, Krafft AE, Hunsucker SW, Mant CT, et al. Purification and structural characterization of siderophore (corynebactin) from Corynebacterium diphtheriae. PLoS One. 2012;7:e34591. doi: 10.1371/journal.pone.0034591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sia AK, Allred BE, Raymond KN. Siderocalins: Siderophore binding proteins evolved for primary pathogen host defense. Curr Opin Chem Biol. 2013;17(2):150–157. doi: 10.1016/j.cbpa.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes MA, Paulsene W, Xu J, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13(1):29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Hoette TM, Clifton MC, Zawadzka AM, Holmes MA, Strong RK, Raymond KN. Immune interference in mycobacterium tuberculosis intracellular iron acquisition through siderocalin recognition of carboxymycobactins. ACS Chem Biol. 2011;6:1327–1331. doi: 10.1021/cb200331g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abergel RJ, Moore EG, Strong RK, Raymond KN. Microbial evasion of the immune system: structural modifications of enterobactin impair siderocalin recognition. J Am Chem Soc. 2006;128:10998–10999. doi: 10.1021/ja062476+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abergel RJ, Wilson MK, Arceneaux JEL, Hoette TM, Strong RK. Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci USA. 2006;103:18499–18503. doi: 10.1073/pnas.0607055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abergel RJ, Zawadzka AM, Hoette TM, Raymond KN. Enzymatic hydrolysis of trilactone siderophores: where chiral recognition occurs in enterobactin and bacillibactin iron transport. J Am Chem Soc. 2009;131:12682–12692. doi: 10.1021/ja903051q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abergel RJ, Warner JA, Shuh DK, Raymond KN. Enterobactin protonation and iron release: structural characterization of the salicylate coordination shift in ferric enterobactin. J Am Chem Soc. 2006;128:8920–8931. doi: 10.1021/ja062046j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allyson KS, Benjamin EA, Kenneth NR. Siderocalins: Siderophore binding proteins evolved for primary athogen host defense. Curr Opin Chem Biol. 2013;17:150–157. doi: 10.1016/j.cbpa.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valdebenito M, Müller SI, Hantke K. Special conditions allow binding of the siderophore salmochelin to siderocalin (NGAL-lipocalin) FEMS Microbiol Lett. 2007;277:182–187. doi: 10.1111/j.1574-6968.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoette TM, Abergel RJ, Xu J, Strong RK, Raymond KN. The role of electrostatics in siderophore recognition by the immunoprotein siderocalin. J Am Chem Soc. 2008;130:17584–17592. doi: 10.1021/ja8074665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, et al. Endocytic delivery of lipocalin–siderophore–iron complex rescues the kidney from ischemia–reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao G, Clifton M, Hoette TM, Mori K, Deng SX, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–834. doi: 10.1126/science.1061075. [DOI] [PubMed] [Google Scholar]
- 56.Mishra J, Mori K, Ma Q, Kelly C, Yang J, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase–associated lipocalin. J Am Soc Nephrol. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Pol JA. Isolation and characterization of a siderophore-like growth factor from mutants of SV40-transformed cells adapted to picolinic acid. Cell. 1978;14:489–499. doi: 10.1016/0092-8674(78)90235-0. [DOI] [PubMed] [Google Scholar]
- 58.Jones RL, Peterson CM, Grady RW, Cerami A. Low molecular weight iron-binding factor from mammalian tissue that potentiates bacterial growth. J Exp Med. 1980;151:418–428. doi: 10.1084/jem.151.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miethke M, Skerra A. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with L-Norepinephrine-mediated bacterial iron acquisition. Antimicrob. Agents Chemother. 2010;54(4):1580–1589. doi: 10.1128/AAC.01158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141(6):1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barasch J, Bao G, Mori K, Clifton M, Strong RK. NGAL Derives from the renal tubule and binds a urinary siderophore; American Society of Nephrology Annual meeting; San Francisco. 2007. [Google Scholar]
- 62.Bao G, Barasch J, Clifton M, Strong RK. Catechol, a Urinary Ngal Binding Siderophore. Planta Med. 2008;74(3) [Google Scholar]; 74 7th Annual Oxford International Conference on the Science of Botanicals & American Society of Pharmacognosy 4th Interim Meeting; Oxford: University of Mississippi; [Google Scholar]
- 63.Barasch J, Deng SX, Bao G, Landry DW. Lipocalin NGAL-binding mammalian siderophores and use thereof to treat iron deficiency and iron overload and lipocalin detection. PCT Int. 2010;128:WO 2010033847. [Google Scholar]
- 64.Philpott C. Bioinorganic chemistry: Getting a grip on iron. Nat Chem Biol. 2010;6:68–570. doi: 10.1038/nchembio.411. [DOI] [PubMed] [Google Scholar]
- 65.Gómez-Casado C, Roth-Walter F, Jensen-Jarolim E, Díaz-Perales A, Pacios LF. Modeling iron-catecholates binding to NGAL protein. J Mol Graph Model. 2013;45C:111–121. doi: 10.1016/j.jmgm.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 66.Bao GH, Barasch J, Xu J, Wang W, Hu FL, Deng SX. Purification and structural characterization of “simple catechol”, the NGAL-Siderochelin siderophore in human urine. RSC Advances. 2015;5:28527–28535. doi: 10.1039/C5RA02509E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shields-Cutler RR, Crowley JR, Hung CS, Stapleton AE, Aldrich C, et al. Human urinary composition controls siderocalin’s antibacterial activity. J Biol Chem. 2015;290(26):15949–15960. doi: 10.1074/jbc.M115.645812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shvartsman M, Ioav CZ. Intracellular iron trafficking: role of cytosolic ligands. Biometals. 2012;25:711–723. doi: 10.1007/s10534-012-9529-7. [DOI] [PubMed] [Google Scholar]
- 69.Correnti C, Richardson V, Sia AK, Bandaranayake AD, Ruiz M, Suryo YR. Siderocalin/Lcn2/NGAL/24p3 does not drive apoptosisthrough gentisic acid mediated iron withdrawal in hematopoietic cell lines. PLoS One. 2012;7(8):43696. doi: 10.1371/journal.pone.0043696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu ZM, Reba S, Chen WD, Porwal SK, Boom WH, Petersen RB, et al. Regulation of mammalian siderophore 2,5-DHBA in the innate immune response to infection. J Exp Med. 2014;211:1197–1213. doi: 10.1084/jem.20132629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ananth S, Gnana-Prakasam JP, Yangzom DB, Veeranan-Karmegam R, Martin P, Sylvia BS. Regulation of the cholesterol efflux transporters ABCA1 and ABCG1 in retina in hemochromatosis and by the endogenous siderophore 2,5-dihydroxybenzoic acid. BBA-Mol Basis Dis. 2014;1842(4):603–612. doi: 10.1016/j.bbadis.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zughaier SM, Kandler JL, Shafer WM. Neisseria gonorrhoeae modulates iron-limiting innate immune defense in macrophages. PloS one. 2014;9:e87688. doi: 10.1371/journal.pone.0087688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zsila F, Hazai E, Sawyer L. Binding of the pepper alkaloid piperine to bovine β-lactoglobulin: circular dichroism spectroscopy and molecular modeling study. J Agric Food Chem. 2005;53:10179–10185. doi: 10.1021/jf051944g. [DOI] [PubMed] [Google Scholar]
- 74.Bao GH, Xu J, Hu FL, Wan XC, Deng SX, Barasch J. EGCG inhibit chemical reactivity of iron through forming an Ngal–EGCG–iron complex. Biometals. 2013;26:1041–1050. doi: 10.1007/s10534-013-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu AD, Barasch J. Mutant NGAL proteins and uses there of. WO2013US71344, WO2014081980(A2) [Google Scholar]
- 76.Schönfeld D, Matschiner G, Chatwell L, Trentmann S, Gille H, Hülsmeyer M, Brown N, et al. An engineered lipocalin specific for CTLA-4 reveals a combining site with structural and conformational features similar to antibodies. Proc Natl Acad Sci USA. 2009;106:8198–8203. doi: 10.1073/pnas.0813399106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gebauer M, Schiefner A, Matschiner G, Skerra A. Combinatorial design of an anticalin directed against the extra-domain B for the specific targeting of oncofetal fibronectin. J Mol Biol. 2013;425:780–802. doi: 10.1016/j.jmb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Kim HJ, Eichinger A, Skerra A. High-Affinity Recognition of Lanthanide(III) Chelate Complexes by a Reprogrammed Human Lipocalin 2. J Am Chem Soc. 2013;131:3565–3576. doi: 10.1021/ja806857r. [DOI] [PubMed] [Google Scholar]
- 79.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 80.Devarajan P. Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle. Cancer Ther. 2007;5(B):463–470. [PMC free article] [PubMed] [Google Scholar]
- 81.Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem. 2014;64:179–219. doi: 10.1016/b978-0-12-800263-6.00004-5. [DOI] [PubMed] [Google Scholar]
- 82.Yang J, Moses MA. Lipocalin 2: A multifaceted modulator of human cancer. Cell Cycle. 2009;8:2347–2352. doi: 10.4161/cc.8.15.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, et al. α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124(7):2963–2976. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreno-Navarrete JM, Manco M, Ibáñez J, García-Fuentes E, Ortega F, et al. Metabolic endotoxemia and saturated fat contribute to circulating NGAL concentrations in subjects with insulin resistance. Int J Obesity. 2010;34:240–249. doi: 10.1038/ijo.2009.242. [DOI] [PubMed] [Google Scholar]
- 85.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes Metab Res Rev. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moreno-Navarrete JM, Fernández-Real JM. Antimicrobial-sensing proteins in obesity and type 2 diabetes. Diabetes Care. 2011;34(Suppl):335–341. doi: 10.2337/dc11-s238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fried SK, Greenberg AS. Lipocalin 2: a “sexy” adipokine that regulates 17β-estradiol and obesity. Endocrinology. 2012;153:1582–1584. doi: 10.1210/en.2012-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y. Small lipid-binding proteins in regulating endothelial and vascular functions: focusing on adipocyte fatty acid binding protein and lipocalin-2. Brit J Pharmacol. 2012;165:603–621. doi: 10.1111/j.1476-5381.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao P, Elks CM, Stephens JM. The induction of lipocalin-2 protein expression in vivo and in vitro. J Biol Chem. 2014;289:5960–5969. doi: 10.1074/jbc.M113.532234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


