Abstract
Purpose
The aims of the present study were to update the previous report from two randomized clinical trials, now with median follow-up of 16 years, to analyze the effect of radiotherapy timing on local failure and disease-free survival.
Methods and Materials
From July 1986 to April 1993 the International Breast Cancer Study Group (IBCSG) Trial VI randomly assigned 1475 pre/perimenopausal women with node-positive breast cancer to receive 3 or 6 cycles of initial chemotherapy (CT). IBCSG Trial VII randomly assigned 1212 postmenopausal women with node-positive breast cancer to receive tamoxifen for 5 years, or tamoxifen for 5 years with three early cycles of initial CT. For patients who received breast-conserving surgery (BCS), radiotherapy (RT) was delayed until initial CT was completed; 4 or 7 months after BCS for Trial VI and 2 or 4 months for Trial VII. We compared RT-timing groups among 433 patients on Trial VI and 285 patients on Trial VII who received BCS plus RT. Endpoints were local failure, regional/distant failure, and disease-free survival (DFS).
Results
Among pre/perimenopausal patients there were no significant differences in disease-related outcomes. 15-year DFS was 48.2% in the group allocated 3 months initial CT and 44.9% in the group allocated 6 months initial CT (HR=1.12; 95% CI:0.87–1.45). Among postmenopausal patients, 15-year DFS was 46.1% in the no-initial-CT group and 43.3% in the group allocated 3 months initial CT (HR=1.11; 95% CI:0.82–1.51). Corresponding HRs for local failures were 0.94 (95% CI: 0.61–1.46) in Trial VI and 1.51 (95% CI:0.77–2.97) in Trial VII. For regional/distant failures, the respective HRs were 1.15 (95% CI:0.80–1.63) and 1.08 (95% CI:0.69–1.68).
Conclusions
This study confirms that, after more than 15 years of follow-up, it is reasonable to delay radiotherapy until after the completion of standard chemotherapy.
Summary
This study looks at the 16-year results of two clinical trials that required radiotherapy after surgery and chemotherapy for patients who received breast-conserving surgery. Patients received radiotherapy after initial chemotherapy within 2 months, 4 months, and 7 months after surgery. Our results confirm no increased risk of local failures in patients delaying their radiotherapy until after systemic treatment.
Introduction
Postoperative radiotherapy to the breast after breast-conserving surgery increases local control rates and long term survival (1). The optimal timing of this common radiotherapy is still not known. To start postoperative adjuvant treatment as soon as reasonably possible seems logical since the aim of the adjuvant treatment is to eradicate remaining clonogenic cells after surgery, and the probability of success would theoretically be better if the time for these cells to proliferate after surgery were shorter (2). However, the risk of waiting before initiating adjuvant radiotherapy differs among cancers and cancer subtypes (3).
Several retrospective breast cancer studies on the effect of delay of radiotherapy have yielded variable results (4–8). Often the time to start irradiation is not independent of the primary tumor characteristics, making interpretations difficult. Generally an increased risk for local failure with longer delay between surgery and radiotherapy was seen in univariate analysis, but this effect was not observed after adjusting for prognostic factors in multivariate analysis. In a report from the International Breast Cancer Study Group (IBCSG), no increased risk was observed with a delay of up to 20 weeks for patients with hormone receptor-positive breast cancer receiving endocrine therapy (9).
Three review articles indicate an increased risk for local failures with a longer delay than 8 weeks between surgery and radiotherapy (3, 10, 11). These reviews, however, include a variety of different study designs and various breast cancer subtypes.
A previous IBCSG publication reported the effect of delaying radiotherapy in patients randomized to different chemotherapy regimens (12). In this study, no significant difference was observed in 4-year local failure rate among pre/peri-menopausal patients starting radiotherapy after 3 or 6 courses of chemotherapy or among postmenopausal patients starting radiotherapy within 3 months after surgery or after 3 courses of chemotherapy. An advantage with this study was that the delay of radiotherapy was predefined by the study protocol and independent of primary tumor characteristics. However, the median follow-up was only 4 years and the power too low to exclude clinically relevant differences in outcome.
The aims of the present study were to update the previous report (12) with a median follow-up of 16 years, to analyze the effect of radiotherapy timing on local failure rates and disease-free survival, and to further study possible modifying effects of estrogen-receptor status, nodal status and age.
Patients and Methods
The clinical trial designs and treatment details have been previously published (12,13,14). In brief, patients were recruited to IBCSG Trial VI (pre/peri-menopausal) and IBCSG Trial VII (postmenopausal) from July 1986 to April 1993 and were randomized to the treatment groups shown in Table 1. A total of 2687 patients meeting eligibility criteria were enrolled in the two trials (1475 in Trial VI and 1212 in Trial VII). Of these, 718 (27%) elected to undergo breast-conserving surgery and radiation therapy subsequent to chemotherapy (as required by protocol in both trials). Thus, the differing chemotherapy schedules (Table 1) resulted in four radiotherapy-timing groups: pre/peri-menopausal patients assigned to receive radiation therapy beginning 4 months from surgery (3 months initial CT then radiotherapy, CT×3→RT); pre/peri-menopausal patients assigned to receive radiation therapy beginning 7 months from surgery (6 months initial CT then radiotherapy, CT×6→RT); postmenopausal patients assigned to receive radiation therapy within 2 months after surgery (no initial CT, radiotherapy after surgery); and postmenopausal patients assigned to receive radiation therapy at 4 months (3 months initial CT then radiotherapy, CT×3→RT). The protocols were approved by the relevant ethics committees as required by the governing bodies in each country at the time.
Table 1.
Treatment Groups and Patient Characteristics in Trials VI and VII
| Pre/perimenopausal (Trial VI) |
Postmenopausal (Trial VII) |
|||
|---|---|---|---|---|
| CMF×3 | CMF×6 | No Initial CMF |
CMF × 3 | |
| Randomized | 725 | 750 | 614 | 598 |
| Treatment Assignment | ||||
| CT×3→RT | 360 | |||
| CT×3→RT→CT×1 at mos 6, 9, 12 | 364 | |||
| CT×6→RT | 375 | |||
| CT×6→RT→CT×1 at months 9, 12, 15 | 375 | |||
| Tamoxifen + RT | 306 | |||
| Tamoxifen + RT→CT×1 mos 9, 12, 15 | 308 | |||
| Tamoxifen+CT×3→RT | 302 | |||
| Tamoxifen+CT×3→RT→CT×1 mos 9, 12, 15 | 296 | |||
| Breast-conserving surgery+RT (analytic cohort) | 213 | 220 | 146 | 139 |
| Age median (range) | 44 (27–56) | 44 (26–56) | 60 (45–76) | 60 (46–76) |
| Age <40 (%) | 51 (24) | 50 (23) | ||
| Age ≥40 (%) | 162 (76) | 170 (77) | ||
| Age <65 (%) | 112 (77) | 108 (78) | ||
| Age ≥65 (%) | 34 (23) | 31 (22) | ||
| ER Negative* (%) | 54 (25) | 64 (29) | 30 (21) | 22 (16) |
| ER Positive (%) | 159 (75) | 156 (71) | 116 (79) | 117 (84) |
| 1–3 Nodes positive (%) | 165 (77) | 164 (77) | 111 (76) | 98 (71) |
| ≥4 Nodes positive (%) | 48 (23) | 56 (25) | 35 (24) | 41 (29) |
<10 fmol/mg cytosol protein
According to the protocol, radiotherapy was to be given by two opposing tangential fields encompassing the entire breast parenchyma. None of the regional lymph node areas were specifically included in the target volume. However, no special attempt was made to exclude part of the internal mammary or lower axillary lymph nodes if these interfered with the irradiation of the breast. A planned dose of 50 Gy, in a specification point in the middle of the breast along the central axis of the two opposing tangential fields, was prescribed. The radiotherapy was given with daily 2 Gy fractions 5 times a week. A boost was not prescribed according to the protocol, but was left to discretion of each radiotherapist.
We compared outcomes and patterns of failure for the radiation-therapy groups based on intention to treat. Outcomes were disease-free survival (DFS) and time to recurrence. Disease-free survival was defined as the time from randomization until the first occurrence of invasive breast cancer in any site (including ipsilateral breast, contralateral breast, regional, distant), second (non-breast) primary tumor, or death from any cause. Time to recurrence was defined as the time from randomization to diagnosis of recurrent breast cancer or, if confirmed later, when it was first suspected. Site of recurrence was defined as local, regional, or distant. Recurrence sites were considered to be simultaneous if they were diagnosed within 2 months of one another.
We also investigated the timing of RT in an as-treated analysis. This analysis provided results very similar to the intention-to-treat analysis (data not shown) and did not alter our conclusions in any way.
We analyzed DFS using the Kaplan-Meier product limit method and the log-rank test to compare the radiotherapy groups. We assessed time to recurrence according to type of recurrence using methods for competing risks analysis (15). We used proportional-hazards regression and competing-risks regression to evaluate the impact of covariates on outcome (16). Covariates were estrogen-receptor status (defined as negative for <10 fmol/mg cytosol protein, otherwise positive), number of positive lymph nodes involved (1–3 or ≥4), and age (grouped as <40 vs. ≥40 among pre/peri-menopausal patients and <65 vs. ≥65 among postmenopausal patients). A two-sided p-value less than 0.05 was considered statistically significant. Statistical analysis was performed using SAS version 9.3 and R version 3.0.0.
Results
The characteristics of the 718 patients included in this analysis have been published previously (12). In brief, among the 433 pre/peri-menopausal patients, 213 were in the CT×3→RT group and 220 were in the CT×6→RT group; the median age was 44 years; 73% had estrogen receptor-positive disease; 24% had ≥4 positive lymph nodes; and 96% received radiotherapy. Among the 285 postmenopausal patients, 146 were allocated no initial CT and 139 were in the CT×3→RT group; the median age was 60 years; 82% had estrogen receptor-positive disease; 27% had ≥4 positive lymph nodes; and 95% received radiotherapy.
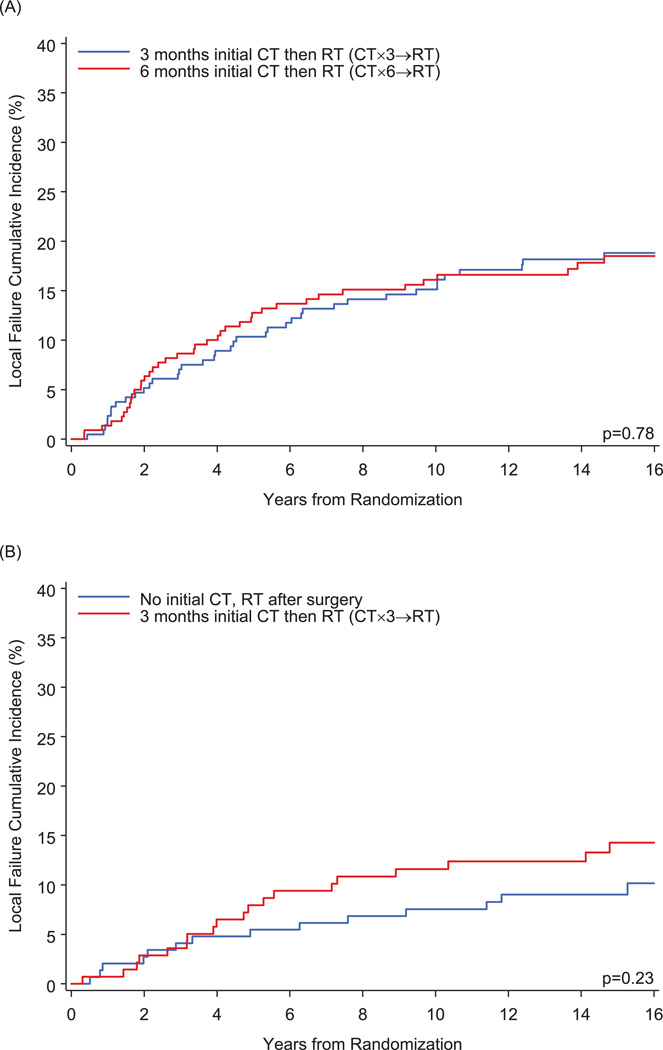
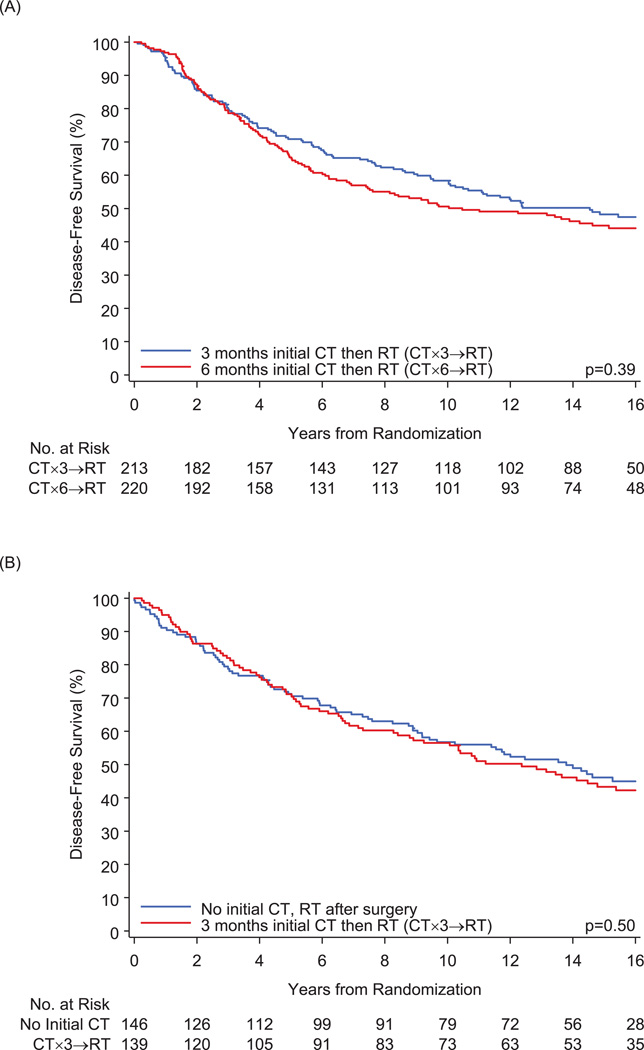
The median follow-up for the entire cohort was 16 years (range, 0.3–21.3). Table 2 shows the numbers of events observed. A total of 398 patients had a DFS event. There were a total of 114 local failures (with or without other type of failure) and 188 distant failures (with or without regional failure). Figure 1 illustrates the cumulative incidence of local failures (with or without other events). Among pre/peri-menopausal patients, the 15-year cumulative incidence was 18.8±2.7% in the CT×3→RT group and 18.5±2.7% in the CT×6→RT group (HR = 0.94; 95% CI: 0.61–1.46). Among postmenopausal patients, 15-year cumulative incidence was 9.0±2.4% in the no-initial-CT group and 14.3±3.1% in the CT×3→RT group (HR = 1.51; 95% CI: 0.77–2.97). Figure 2 illustrates the cumulative incidence of regional/distant (regional or distant or both) failures. Among pre/peri-menopausal patients, the 15-year cumulative incidence was 26.4±3.1% in the CT×3→RT group and 29.7±3.1% in the CT×6→RT group (HR = 1.15; 95% CI: 0.80–1.63). Among postmenopausal patients, 15-year cumulative incidence was 24.7±3.6% in the no-initial-CT group and 27.3±3.9% in the CT×3→RT group (HR = 1.08; 95% CI: 0.69–1.68). Figure 3 shows no significant differences in DFS when comparing the radiotherapy groups. Among pre/peri-menopausal patients, 15-year DFS was 48.2±3.5% (±standard error) in the CT×3→RT group and 44.9±3.5% in the CT×6→RT group (hazard ratio [HR] = 1.12; 95% confidence interval [CI]: 0.87–1.45). Among postmenopausal patients, 15-year DFS was 46.1±4.3% in the no-initial-CT group and 43.3±4.4% in the CT×3→RT group (HR = 1.11; 95% CI: 0.82–1.51).
Table 2.
Numbers of Events Observed
| Pre/perimenopausal | Postmenopausal | ||||
|---|---|---|---|---|---|
| CT×3→RT | CT×6→RT | No initial CT, RT after surgery |
CT×3→RT | Total | |
| Number of patients | 213 | 220 | 146 | 139 | 718 |
| Number of DFS events | 112 | 122 | 80 | 84 | 398 |
| Local only | 13 | 9 | 1 | 5 | 28 |
| Local ± other events | 41 | 39 | 14 | 20 | 114 |
| Regional only | 3 | 4 | 2 | 1 | 10 |
| Distant ± regional | 54 | 62 | 35 | 37 | 188 |
| Distant or regional | 57 | 66 | 37 | 38 | 198 |
| Contralateral breast | 7 | 4 | 3 | 3 | 17 |
| Second malignancy, non-breast | 7 | 11 | 11 | 12 | 41 |
| Death without recurrence | 0 | 2 | 15 | 11 | 28 |
Abbreviations: CT, chemotherapy; CT×3, 3 months initial chemotherapy; CT×6, 6 months initial chemotherapy; DFS, disease-free survival; RT radiotherapy
Figure 1.
Cumulative Incidence of Local Failures for (A) Pre/peri-menopausal Patients and (B) Postmenopausal Patients
Figure 2.
Cumulative Incidence of Regional/Distant Failures for (A) Pre/peri-menopausal Patients and (B) Postmenopausal Patients
Figure 3.
Disease-Free Survival for (A) Pre/peri-menopausal Patients and (B) Postmenopausal Patients
We performed multivariable analyses of DFS, local failure and regional/distant failure (Table 3). After adjustment for nodal status, estrogen-receptor status and age, radiotherapy timing was not significantly associated with outcome. We also evaluated interaction effects in the multivariable models. In all cases, the radiotherapy-timing effect was not significantly modified by nodal status, estrogen-receptor status, or age. For DFS in the pre/peri-menopausal group, the interaction p-values were 0.32, 0.89 and 0.56 for the modifying effect of nodal status, estrogen-receptor status, and age, respectively. For DFS in the postmenopausal group, the respective interaction p-values were 0.12, 0.15 and 0.96.
Table 3.
Multivariable Analysis of DFS, Local Failure, and Regional or Distant Failure
| Local ± Other | Regional or Distant Failure | Disease-Free Survival | ||||
|---|---|---|---|---|---|---|
| Factor | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value |
| Pre/peri-menopausal patients | ||||||
| Treatment | ||||||
| CT×3→RT | 1.00 | 1.00 | 1.00 | |||
| CT×6→RT | 0.95 (0.61–1.48) | 0.82 | 1.11 (0.78–1.59) | 0.57 | 1.09 (0.84–1.41) | 0.50 |
| Nodal status | ||||||
| 1–3 positive nodes | 1.00 | 1.00 | 1.00 | |||
| ≥4 positive nodes | 1.09 (0.65–1.82) | 0.74 | 1.96 (1.34–2.87) | 0.0005 | 1.74 (1.31–2.30) | 0.0001 |
| ER status | ||||||
| Negative | 1.00 | 1.00 | 1.00 | |||
| Positive | 0.80 (0.50–1.28) | 0.35 | 0.96 (0.61–1.48) | 0.84 | 0.87 (0.65–1.16) | 0.34 |
| Age | ||||||
| < 40 years | 1.00 | 1.00 | 1.00 | |||
| ≥ 40 years | 0.50 (0.32–0.80) | 0.003 | 0.76 (0.50–1.16) | 0.21 | 0.63 (0.47–0.84) | 0.002 |
| Postmenopausal patients | ||||||
| Treatment | ||||||
| No initial CT | 1.00 | 1.00 | 1.00 | |||
| CT×3→RT | 1.53 (0.77–3.01) | 0.22 | 1.04 (0.66–1.64) | 0.87 | 1.07 (0.79–1.46) | 0.65 |
| Nodal status | ||||||
| 1–3 positive nodes | 1.00 | 1.00 | 1.00 | |||
| ≥4 positive nodes | 1.35 (0.65–2.78) | 0.42 | 2.05 (1.28–3.27) | 0.003 | 1.83 (1.32–2.54) | 0.0003 |
| ER status | ||||||
| Negative | 1.00 | 1.00 | 1.00 | |||
| Positive | 0.70 (0.31–1.58) | 0.39 | 0.87 (0.49–1.58) | 0.66 | 0.69 (0.48–1.01) | 0.06 |
| Age | ||||||
| < 65 years | 1.00 | 1.00 | 1.00 | |||
| ≥ 65 years | 1.49 (0.70–3.15) | 0.30 | 0.92 (0.52–1.64) | 0.78 | 1.42 (1.00–2.03) | 0.05 |
Abbreviations: CT, chemotherapy; ER, estrogen receptor; CT×3, 3 months initial chemotherapy; CT×6, 6 months initial chemotherapy; RT, radiotherapy
Discussion
With a median follow-up of 16 years and with greater statistical power we detected no significantly increased risk of local failures in patients delaying their radiotherapy until after systemic treatment. In multivariable analysis age < 40 years was significantly associated with increased risk for local recurrence and nodal status, age <40 years (premenopausal) and >65 years (postmenopausal) were significantly associated with decreased disease-free survival. However, no modifying effect of nodal status, estrogen receptor status or age on the risk of delaying radiotherapy in the different radiotherapy-timing groups could be observed.
This is in line with a randomized trial in which 244 patients were randomized between 12 weeks of chemotherapy (CAMFP-cyclophosphamide, doxorubicin, methotrexate, fluorouracil, prednisone) before or after radiotherapy (17, 18). With a median follow-up of 135 months 11% of the patients randomized to CT-first versus 10% in patients having radiotherapy first developed a local recurrence. The corresponding rates for distant failures at 10 years were 35% and 36% in the two treatment arms, respectively (17). The authors concluded that the low power of the study could not exclude clinically relevant differences in outcome between the arms.
In a previous up-date of trial VI, disease free survival in patients receiving 3 or 6 courses of CMF were equal. Three courses were shown to be adequate for patients at least 40 years old and with estrogen positive tumors (19).
In the present study the possible preventive effect of longer duration of adjuvant chemotherapy on risk for local recurrence could counteract a possible increased risk of delaying the start of radiotherapy and the nature of this study could not sort out these two opposing effects On the other hand, one can hypothesize that systemic treatment might be reduced by giving radiotherapy earlier, as there was no difference at all between the two treatment arms regarding all endpoints (Figures 1–3). However, for patient care it is important to consider the sum of these effects in an unbiased way. An advantage of this trial was the independence between primary tumor characteristics and delay of starting radiotherapy. The main limitation of the current study is the relatively low total number of patients, so no firm conclusions can be drawn for different subgroups of patients. For instance in the postmenopausal group the 15 year local failure rate was 9.0% versus 14.3% in patients starting radiotherapy within 2 months or after 4 months from surgery (HR = 1.51; 95% CI: 0.77–2.97).
Nevertheless, this study further confirms that it is reasonable to delay radiotherapy until after the completion of standard chemotherapy even if small differences in local failure rates in specific subgroups still cannot be excluded.
Acknowledgments
The International Breast Cancer Study Group (IBCSG) received no industry support for the conduct or the long-term follow-up of these trials. IBCSG received funding from the Swedish Cancer Society, The Cancer Council Australia, Australian New Zealand Breast Cancer Trials Group, Frontier Science and Technology Research Foundation, Swiss Group for Clinical Cancer Research (SAKK), US National Institute of Health Grant CA-75362, Cancer Research Switzerland/Oncosuisse, and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None of the authors have any conflicts.
Contributor Information
Per Karlsson, Department of Oncology, Institute of Clinical Sciences, Sahgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Gothenburg, Sweden, per.karlsson@oncology.gu.se.
Bernard F. Cole, Department of Mathematics and Statistics, University of Vermont, Burlington, VT 05401, USA bernard.cole@uvm.edu.
Karen N. Price, International Breast Cancer Study Group Statistical Center, Frontier Science and Technology Research Foundation, Boston, MA, price@jimmy.harvard.edu.
Richard D. Gelber, International Breast Cancer Study Group Statistical Center, Department of Biostatistics and Computatonal Biology, Dana-Farber Cancer Institute, Frontier Science and Technology Research Foundation, Harvard TF Chan School of Public Health, Boston, MA. gelber@jimmy.harvard.edu.
Alan S. Coates, International Breast Cancer Study Group and University of Sydney, Sydney, Australia. alan.coates@ibcsg.org.
Aron Goldhirsch, Program for Breast Health (Senology), European Institute of Oncology and International Breast Cancer Study Group, Milan, Italy. aron.goldhirsch@ibcsg.org.
Monica Castiglione, International Breast Cancer Study Group (retired), Bern, Switzerland. monica.castiglione@bluewin.ch.
Marco Colleoni, Division of Medical Senology, European Institute of Oncology and International Breast Cancer Study Group, Milan, Italy. marco.colleoni@ieo.it.
Günther Gruber, Insitute of Radiotherapy, Klinik Hirslanden, Witellikerstrasse 40, 8032 Zürich, Switzerland. guenther.gruber@hirslanden.ch.
References
- 1.Early Breast Cancer Trialists' Collaborative G. Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackillop WJ, Bates JH, O'Sullivan B, Withers HR. The effect of delay in treatment on local control by radiotherapy. International journal of radiation oncology, biology, physics. 1996;34(1):243–250. doi: 10.1016/0360-3016(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2008;87(1):3–16. doi: 10.1016/j.radonc.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Benk V, Joseph L, Fortin P, Zhang G, Belisle P, Levinton C, et al. Effect of delay in initiating radiotherapy for patients with early stage breast cancer. Clinical oncology. 2004;16(1):6–11. doi: 10.1016/j.clon.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Corradini S, Niemoeller OM, Niyazi M, Manapov F, Haerting M, Harbeck N, et al. Timing of radiotherapy following breast-conserving surgery: outcome of 1393 patients at a single institution. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2014;190(4):352–357. doi: 10.1007/s00066-013-0540-x. [DOI] [PubMed] [Google Scholar]
- 6.Livi L, Borghesi S, Saieva C, Meattini I, Rampini A, Petrucci A, et al. Radiotherapy timing in 4,820 patients with breast cancer: university of florence experience. International journal of radiation oncology, biology, physics. 2009;73(2):365–369. doi: 10.1016/j.ijrobp.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 7.Froud PJ, Mates D, Jackson JS, Phillips N, Andersen S, Jackson SM, et al. Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. International journal of radiation oncology, biology, physics. 2000;46(2):363–372. doi: 10.1016/s0360-3016(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 8.Hebert-Croteau N, Freeman CR, Latreille J, Brisson J. Delay in adjuvant radiation treatment and outcomes of breast cancer--a review. Breast cancer research and treatment. 2002;74(1):77–94. doi: 10.1023/a:1016089215070. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson P, Cole BF, Colleoni M, Roncadin M, Chua BH, Murray E, et al. Timing of radiotherapy and outcome in patients receiving adjuvant endocrine therapy. International journal of radiation oncology, biology, physics. 2011;80(2):398–402. doi: 10.1016/j.ijrobp.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(3):555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 11.Tsoutsou PG, Koukourakis MI, Azria D, Belkacemi Y. Optimal timing for adjuvant radiation therapy in breast cancer: a comprehensive review and perspectives. Critical reviews in oncology/hematology. 2009;71(2):102–116. doi: 10.1016/j.critrevonc.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Wallgren A, Bernier J, Gelber RD, Goldhirsch A, Roncadin M, Joseph D, et al. Timing of radiotherapy and chemotherapy following breast-conserving surgery for patients with node-positive breast cancer. International Breast Cancer Study Group. International journal of radiation oncology, biology, physics. 1996;35(4):649–659. doi: 10.1016/0360-3016(96)00186-1. [DOI] [PubMed] [Google Scholar]
- 13.The International Breast Cancer Study Group. Duration and reintroduction of adjuvant chemotherapy for node-positive premenopausal breast cancer patients. J Clin Oncol. 1996;14:1885–1894. doi: 10.1200/JCO.1996.14.6.1885. [DOI] [PubMed] [Google Scholar]
- 14.The International Breast Cancer Study Group. Effectiveness of adjuvant chemotherapy in combination with tamoxifen for node-positive postmenopausal breast cancer patients. J Clin Oncol. 1997;15:1385–1393. doi: 10.1200/JCO.1997.15.4.1385. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Bellon JR, Harris JR. Chemotherapy and radiation therapy for breast cancer: what is the optimal sequence? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(1):5–7. doi: 10.1200/JCO.2005.09.962. [DOI] [PubMed] [Google Scholar]
- 18.Recht A, Come SE, Henderson IC, Gelman RS, Silver B, Hayes DF, et al. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. The New England journal of medicine. 1996;334(21):1356–1361. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 19.Colleoni M, Litman HJ, Castiglione-Gertsch M, Sauerbrei W, Gelber RD, Bonetti M, et al. Duration of adjuvant chemotherapy for breast cancer: a joint analysis of two randomised trials investigating three versus six courses of CMF. Br J Cancer. 2002;86(11):1705–1714. doi: 10.1038/sj.bjc.6600334. [DOI] [PMC free article] [PubMed] [Google Scholar]